Tablet Technology for Writing and Drawing during Functional Magnetic Resonance Imaging: A Review
Abstract
1. Introduction
2. Tablet Technology for fMRI Studies of Writing and Drawing
3. Tablet Applications
3.1. Neuropsychological Tests
3.1.1. The Trail Making Test
3.1.2. The Clock Drawing Test
3.1.3. Letter Cancellation Test
3.2. Neurosurgery
3.2.1. Awake Craniotomy Application to Brain Tumours
3.2.2. MR-Guided Focused Ultrasound Treatment of Essential Tremor
3.3. Neurolinguistics
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Belliveau, J.W.; Kennedy, D.N.; McKinstry, R.C.; Buchbinder, B.R.; Weisskoff, R.M.; Cohen, M.S.; Vevea, J.M.; Brady, T.J.; Rosen, B.R. Functional Mapping of the Human Visual Cortex by Magnetic Resonance Imaging. Science 1991, 254, 716–719. [Google Scholar] [CrossRef] [PubMed]
- Bandettini, P.A.; Wong, E.C.; Hinks, R.S.; Tikofsky, R.S.; Hyde, J.S. Time Course EPI of Human Brain Function during Task Activation. Magn. Reson. Med. 1992, 25, 390–397. [Google Scholar] [CrossRef] [PubMed]
- Ogawa, S.; Tank, D.W.; Menon, R.; Ellermann, J.M.; Kim, S.G.; Merkle, H.; Ugurbil, K. Intrinsic Signal Changes Accompanying Sensory Stimulation: Functional Brain Mapping with Magnetic Resonance Imaging. Proc. Natl. Acad. Sci. USA 1992, 89, 5951–5955. [Google Scholar] [CrossRef] [PubMed]
- Baddeley, A.D.; Logie, R.H. Working memory: The multiple-component model. In Models of Working Memory: Mechanisms of Active Maintenance and Executive Control; Cambridge University Press: New York, NY, USA, 1999; pp. 28–61. ISBN 978-0-521-58325-1. [Google Scholar]
- Christoffels, I.K.; Formisano, E.; Schiller, N.O. Neural Correlates of Verbal Feedback Processing: An FMRI Study Employing Overt Speech. Hum. Brain Mapp. 2007, 28, 868–879. [Google Scholar] [CrossRef]
- Gauvin, H.S.; De Baene, W.; Brass, M.; Hartsuiker, R.J. Conflict Monitoring in Speech Processing: An FMRI Study of Error Detection in Speech Production and Perception. NeuroImage 2016, 126, 96–105. [Google Scholar] [CrossRef]
- Tam, F.; Churchill, N.W.; Strother, S.C.; Graham, S.J. A New Tablet for Writing and Drawing during Functional MRI. Hum. Brain Mapp. 2011, 32, 240–248. [Google Scholar] [CrossRef]
- Katanoda, K.; Yoshikawa, K.; Sugishita, M. A Functional MRI Study on the Neural Substrates for Writing. Hum. Brain Mapp. 2001, 13, 34–42. [Google Scholar] [CrossRef]
- Makuuchi, M.; Kaminaga, T.; Sugishita, M. Both Parietal Lobes Are Involved in Drawing: A Functional MRI Study and Implications for Constructional Apraxia. Cogn. Brain Res. 2003, 16, 338–347. [Google Scholar] [CrossRef]
- Moll, J.; De Oliveira-Souza, R.; Moll, F.T.; Bramati, I.E.; Andreiuolo, P.A. The Cerebral Correlates of Set-Shifting: An FMRI Study of the Trail Making Test. Arq. Neuropsiquiatr. 2002, 60, 900–905. [Google Scholar] [CrossRef]
- Jacobson, S.C.; Blanchard, M.; Connolly, C.C.; Cannon, M.; Garavan, H. An FMRI Investigation of a Novel Analogue to the Trail-Making Test. Brain Cogn. 2011, 77, 60–70. [Google Scholar] [CrossRef]
- Allen, M.D.; Owens, T.E.; Fong, A.K.; Richards, D.R. A Functional Neuroimaging Analysis of the Trail Making Test-B: Implications for Clinical Application. Behav. Neurol. 2011, 24, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Reithler, J.; Reithler, H.; van den Boogert, E.; Goebel, R.; van Mier, H. Resistance-Based High Resolution Recording of Predefined 2-Dimensional Pen Trajectories in an FMRI Setting. J. Neurosci. Methods 2006, 152, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Zakzanis, K.K.; Mraz, R.; Graham, S.J. An FMRI Study of the Trail Making Test. Neuropsychologia 2005, 43, 1878–1886. [Google Scholar] [CrossRef] [PubMed]
- Karimpoor, M.; Churchill, N.W.; Tam, F.; Fischer, C.E.; Schweizer, T.A.; Graham, S.J. Tablet-Based Functional MRI of the Trail Making Test: Effect of Tablet Interaction Mode. Front. Hum. Neurosci. 2017, 11, 496. [Google Scholar] [CrossRef] [PubMed]
- Vinci-Booher, S.; Sturgeon, J.; James, T.; James, K. The MRItab: A MR-Compatible Touchscreen with Video-Display. J. Neurosci. Methods 2018, 306, 10–18. [Google Scholar] [CrossRef]
- Karimpoor, M.; Tam, F.; Strother, S.C.; Fischer, C.E.; Schweizer, T.A.; Graham, S.J. A Computerized Tablet with Visual Feedback of Hand Position for Functional Magnetic Resonance Imaging. Front. Hum. Neurosci. 2015, 9, 150. [Google Scholar] [CrossRef]
- Braadbaart, L.; Buchan, G.; Williams, J.H.G.; Waiter, G.D. An FMRI Compatible Touchscreen to Measure Hand Kinematics During a Complex Drawing Task. Br. J. Appl. Sci. Technol. 2015, 9, 346–353. [Google Scholar] [CrossRef]
- Longcamp, M.; Lagarrigue, A.; Nazarian, B.; Roth, M.; Anton, J.-L.; Alario, F.-X.; Velay, J.-L. Functional Specificity in the Motor System: Evidence from Coupled FMRI and Kinematic Recordings during Letter and Digit Writing. Hum. Brain Mapp. 2014, 35, 6077–6087. [Google Scholar] [CrossRef]
- Braadbaart, L.; Waiter, G.; Williams, J. Neural Correlates of Individual Differences in Manual Imitation Fidelity. Front. Integr. Neurosci. 2012, 6, 91. [Google Scholar] [CrossRef]
- Hanakawa, T.; Dimyan, M.A.; Hallett, M. Motor Planning, Imagery, and Execution in the Distributed Motor Network: A Time-Course Study with Functional MRI. Cereb. Cortex 2008, 18, 2775–2788. [Google Scholar] [CrossRef]
- Planton, S.; Longcamp, M.; Péran, P.; Démonet, J.; Jucla, M. How Specialized Are Writing-Specific Brain Regions? An FMRI Study of Writing, Drawing and Oral Spelling. Available online: https://pubmed.ncbi.nlm.nih.gov/28081451/ (accessed on 19 May 2020).
- Bisio, A.; Pedullà, L.; Bonzano, L.; Ruggeri, P.; Brichetto, G.; Bove, M. Evaluation of Handwriting Movement Kinematics: From an Ecological to a Magnetic Resonance Environment. Front. Hum. Neurosci. 2016, 10, 488. [Google Scholar] [CrossRef] [PubMed]
- Diciotti, S.; Cecchi, P.; Ginestroni, A.; Mazzoni, L.N.; Pesaresi, I.; Lombardo, S.; Boni, E.; Cosottini, M.; Soricelli, A.; De Stefano, N.; et al. MR-Compatible Device for Monitoring Hand Tracing and Writing Tasks in FMRI with an Application to Healthy Subjects. Concepts Magn. Reson. Part A 2010, 36A, 139–152. [Google Scholar] [CrossRef]
- Reitz, F.; Richards, T.; Wu, K.; Boord, P.; Askren, M.; Lewis, T.; Berninger, V. A Low-Cost, Computer-Interfaced Drawing Pad for FMRI Studies of Dysgraphia and Dyslexia. Sensors 2013, 13, 5099–5108. [Google Scholar] [CrossRef] [PubMed]
- Stuss, D.T.; Bisschop, S.M.; Alexander, M.P.; Levine, B.; Katz, D.; Izukawa, D. The Trail Making Test: A Study in Focal Lesion Patients. Psychol. Assess. 2001, 13, 230–239. [Google Scholar] [CrossRef] [PubMed]
- Price, C.J.; Friston, K.J. Degeneracy and Cognitive Anatomy. Trends Cogn. Sci. 2002, 6, 416–421. [Google Scholar] [CrossRef]
- Lezak, M.D.; Howieson, D.B.; Bigler, E.D.; Tranel, D. Neuropsychological Assessment, 5th ed.; Oxford University Press: New York, NY, USA, 2012; ISBN 978-0-19-539552-5. [Google Scholar]
- Rorden, C.; Karnath, H.-O. Using Human Brain Lesions to Infer Function: A Relic from a Past Era in the FMRI Age? Nat. Rev. Neurosci. 2004, 5, 813–819. [Google Scholar] [CrossRef] [PubMed]
- Strauss, E.; Sherman, E.M.S.; Spreen, O. A Compendium of Neuropsychological Tests: Administration, Norms, and Commentary; Oxford University Press: Oxford, UK; New York, NY, USA, 2006; ISBN 978-0-19-515957-8. [Google Scholar]
- Corrigan, J.D.; Hinkeldey, N.S. Relationships between Parts A and B of the Trail Making Test. J. Clin. Psychol. 1987, 43, 402–409. [Google Scholar] [CrossRef]
- Bowie, C.R.; Harvey, P.D. Administration and Interpretation of the Trail Making Test. Nat. Protoc. 2006, 1, 2277–2281. [Google Scholar] [CrossRef]
- Stuss, D.T.; Stethem, L.L.; Poirier, C.A. Comparison of Three Tests of Attention and Rapid Information Processing across Six Age Groups. Clin. Neuropsychol. 1987, 1, 139–152. [Google Scholar] [CrossRef]
- Stuss, D.T.; Stethem, L.L.; Pelchat, G. Three Tests of Attention and Rapid Information Processing: An Extension. Clin. Neuropsychol. 1988, 2, 246–250. [Google Scholar] [CrossRef]
- Corbetta, M.; Shulman, G.L. Spatial Neglect and Attention Networks. Annu. Rev. Neurosci. 2011, 34, 569–599. [Google Scholar] [CrossRef] [PubMed]
- Churchill, N.W.; Raamana, P.; Spring, R.; Strother, S.C. Optimizing FMRI Preprocessing Pipelines for Block-Design Tasks as a Function of Age. NeuroImage 2017, 154, 240–254. [Google Scholar] [CrossRef] [PubMed]
- Churchill, N.W.; Spring, R.; Afshin-Pour, B.; Dong, F.; Strother, S.C. An Automated, Adaptive Framework for Optimizing Preprocessing Pipelines in Task-Based Functional MRI. PLoS ONE 2015, 10, e0131520. [Google Scholar] [CrossRef] [PubMed]
- Richter, W.; Somorjai, R.; Summers, R.; Jarmasz, M.; Menon, R.S.; Gati, J.S.; Georgopoulos, A.P.; Tegeler, C.; Ugurbil, K.; Kim, S.-G. Motor Area Activity During Mental Rotation Studied by Time-Resolved Single-Trial FMRI. J. Cogn. Neurosci. 2000, 12, 310–320. [Google Scholar] [CrossRef]
- Nobre, A.C.; Coull, J.T.; Walsh, V.; Frith, C.D. Brain Activations during Visual Search: Contributions of Search Efficiency versus Feature Binding. NeuroImage 2003, 18, 91–103. [Google Scholar] [CrossRef] [PubMed]
- Brown, J.W.; Braver, T.S. Learned Predictions of Error Likelihood in the Anterior Cingulate Cortex. Science 2005, 307, 1118–1121. [Google Scholar] [CrossRef]
- Talwar, N.; Churchill, N.W.; Hird, M.A.; Tam, F.; Graham, S.J.; Schweizer, T.A. Functional Magnetic Resonance Imaging of the Trail-Making Test in Older Adults. PLoS ONE 2020, 15, e0232469. [Google Scholar] [CrossRef]
- Freedman, M.; Leech, L.; Kaplan, E.; Winocur, G.; Shulman, K.; Delis, D. Clock Drawing: A Neuropsychological Analysis; Oxford University Press: Oxford, UK; New York, NY, USA, 1994; ISBN 978-0-19-505906-9. [Google Scholar]
- Rouleau, I.; Salmon, D.P.; Butters, N.; Kennedy, C.; McGuire, K. Quantitative and Qualitative Analyses of Clock Drawings in Alzheimer’s and Huntington’s Disease. Brain Cogn. 1992, 18, 70–87. [Google Scholar] [CrossRef]
- Talwar, N.A.; Churchill, N.W.; Hird, M.A.; Pshonyak, I.; Tam, F.; Fischer, C.E.; Graham, S.J.; Schweizer, T.A. The Neural Correlates of the Clock-Drawing Test in Healthy Aging. Front. Hum. Neurosci. 2019, 13, 25. [Google Scholar] [CrossRef]
- Matsuoka, T.; Narumoto, J.; Okamura, A.; Taniguchi, S.; Kato, Y.; Shibata, K.; Nakamura, K.; Okuyama, C.; Yamada, K.; Fukui, K. Neural Correlates of the Components of the Clock Drawing Test. Int. Psychogeriatr. 2013, 25, 1317–1323. [Google Scholar] [CrossRef]
- Tranel, D.; Rudrauf, D.; Vianna, E.P.M.; Damasio, H. Does the clock drawing test have focal neuroanatomical correlates? Neuropsychology 2008, 22, 553–562. [Google Scholar] [CrossRef] [PubMed]
- Royall, D.R.; Mulroy, A.R.; Chiodo, L.K.; Polk, M.J. Clock Drawing Is Sensitive to Executive Control: A Comparison of Six Methods. J. Gerontol. Ser. B 1999, 54B, P328–P333. [Google Scholar] [CrossRef] [PubMed]
- Desmond, J.E.; Gabrieli, J.D.E.; Wagner, A.D.; Ginier, B.L.; Glover, G.H. Lobular Patterns of Cerebellar Activation in Verbal Working-Memory and Finger-Tapping Tasks as Revealed by Functional MRI. J. Neurosci. 1997, 17, 9675–9685. [Google Scholar] [CrossRef] [PubMed]
- Picard, N.; Strick, P.L. Activation of the Supplementary Motor Area (SMA) during Performance of Visually Guided Movements. Cereb. Cortex 2003, 13, 977–986. [Google Scholar] [CrossRef]
- Diller, L.; Ben-Yishay, Y.; Gerstman, L.; Goodin, R.; Gordon, W. Studies in Cognition and Rehabilitation in Hemiplegia; Rehabilitation Monograph No. 50; New York University Medical Center, Institute of Rehabilitation Medicine: New York, NY, USA, 1974. [Google Scholar]
- Deng, I.D.; Chung, L.; Talwar, N.; Tam, F.; Churchill, N.W.; Schweizer, T.A.; Graham, S.J. Functional MRI of Letter Cancellation Task Performance in Older Adults. Front. Hum. Neurosci. 2019, 13, 97. [Google Scholar] [CrossRef]
- Li, Y.; Li, C.; Wu, Q.; Xu, Z.; Kurata, T.; Ohno, S.; Kanazawa, S.; Abe, K.; Wu, J. Decreased Resting-State Connections within the Visuospatial Attention-Related Network in Advanced Aging. Neurosci. Lett. 2015, 597, 13–18. [Google Scholar] [CrossRef]
- Posner, M.I.; Petersen, S.E. The Attention System of the Human Brain. Annu. Rev. Neurosci. 1990, 13, 25–42. [Google Scholar] [CrossRef]
- Culham, J.C.; Brandt, S.A.; Cavanagh, P.; Kanwisher, N.G.; Dale, A.M.; Tootell, R.B.H. Cortical FMRI Activation Produced by Attentive Tracking of Moving Targets. J. Neurophysiol. 1998, 80, 2657–2670. [Google Scholar] [CrossRef]
- Ruff, C.C.; Driver, J.; Bestmann, S. Combining TMS and FMRI. Cortex J. Devoted Study Nerv. Syst. Behav. 2009, 45, 1043–1049. [Google Scholar] [CrossRef]
- Morrison, M.A.; Churchill, N.W.; Cusimano, M.D.; Schweizer, T.A.; Das, S.; Graham, S.J. Reliability of Task-Based FMRI for Preoperative Planning: A Test-Retest Study in Brain Tumor Patients and Healthy Controls. PLoS ONE 2016, 11, e0149547. [Google Scholar] [CrossRef]
- Morrison, M.A.; Tam, F.; Garavaglia, M.M.; Golestanirad, L.; Hare, G.M.T.; Cusimano, M.D.; Schweizer, T.A.; Das, S.; Graham, S.J. A Novel Tablet Computer Platform for Advanced Language Mapping during Awake Craniotomy Procedures. J. Neurosurg. 2016, 124, 938–944. [Google Scholar] [CrossRef] [PubMed]
- Morrison, M.A.; Tam, F.; Garavaglia, M.M.; Hare, G.M.T.; Cusimano, M.D.; Schweizer, T.A.; Das, S.; Graham, S.J. Sources of Variation Influencing Concordance between Functional MRI and Direct Cortical Stimulation in Brain Tumor Surgery. Front. Neurosci. 2016, 10, 461. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Altaye, M.; Ret, J.; Schmithorst, V.; Byars, A.W.; Plante, E.; Holland, S.K. Quantification of Head Motion in Children during Various FMRI Language Tasks. Hum. Brain Mapp. 2008, 30, 1481–1489. [Google Scholar] [CrossRef]
- Jezzard, P.; Clare, S. Sources of Distortion in Functional MRI Data. Hum. Brain Mapp. 1999, 8, 80–85. [Google Scholar] [CrossRef]
- Duffau, H.; Peggy Gatignol, S.T.; Mandonnet, E.; Capelle, L.; Taillandier, L. Intraoperative Subcortical Stimulation Mapping of Language Pathways in a Consecutive Series of 115 Patients with Grade II Glioma in the Left Dominant Hemisphere. J. Neurosurg. 2008, 109, 461–471. [Google Scholar] [CrossRef] [PubMed]
- Golestanirad, L.; Das, S.; Schweizer, T.A.; Graham, S.J. A Preliminary FMRI Study of a Novel Self-Paced Written Fluency Task: Observation of Left-Hemispheric Activation, and Increased Frontal Activation in Late vs. Early Task Phases. Front. Hum. Neurosci. 2015, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Lurito, J.T.; Kareken, D.A.; Lowe, M.J.; Chen, S.H.; Mathews, V.P. Comparison of Rhyming and Word Generation with FMRI. Hum. Brain Mapp. 2000, 10, 99–106. [Google Scholar] [CrossRef]
- Zec, R.F.; Landreth, E.S.; Fritz, S.; Grames, E.; Hasara, A.; Fraizer, W.; Belman, J.; Wainman, S.; McCool, M.; O’Connell, C.; et al. A Comparison of Phonemic, Semantic, and Alternating Word Fluency in Parkinson’s Disease. Arch. Clin. Neuropsychol. Off. J. Natl. Acad. Neuropsychol. 1999, 14, 255–264. [Google Scholar] [CrossRef]
- Fu, C.H.Y.; Morgan, K.; Suckling, J.; Williams, S.C.R.; Andrew, C.; Vythelingum, G.N.; McGuire, P.K. A Functional Magnetic Resonance Imaging Study of Overt Letter Verbal Fluency Using a Clustered Acquisition Sequence: Greater Anterior Cingulate Activation with Increased Task Demand. NeuroImage 2002, 17, 871–879. [Google Scholar] [CrossRef][Green Version]
- Halari, R.; Sharma, T.; Hines, M.; Andrew, C.; Simmons, A.; Kumari, V. Comparable FMRI Activity with Differential Behavioural Performance on Mental Rotation and Overt Verbal Fluency Tasks in Healthy Men and Women. Exp. Brain Res. 2006, 169, 1–14. [Google Scholar] [CrossRef]
- Birn, R.M.; Kenworthy, L.; Case, L.; Caravella, R.; Jones, T.B.; Bandettini, P.A.; Martin, A. Neural Systems Supporting Lexical Search Guided by Letter and Semantic Category Cues: A Self-Paced Overt Response FMRI Study of Verbal Fluency. NeuroImage 2010, 49, 1099–1107. [Google Scholar] [CrossRef] [PubMed]
- Lipsman, N.; Mainprize, T.G.; Schwartz, M.L.; Hynynen, K.; Lozano, A.M. Intracranial Applications of Magnetic Resonance-Guided Focused Ultrasound. Neurotherapeutics 2014, 11, 593–605. [Google Scholar] [CrossRef] [PubMed]
- Elias, W.J.; Huss, D.; Voss, T.; Loomba, J.; Khaled, M.; Zadicario, E.; Frysinger, R.C.; Sperling, S.A.; Wylie, S.; Monteith, S.J.; et al. A Pilot Study of Focused Ultrasound Thalamotomy for Essential Tremor. N. Engl. J. Med. 2013, 369, 640–648. [Google Scholar] [CrossRef] [PubMed]
- Elias, W.J.; Lipsman, N.; Ondo, W.G.; Ghanouni, P.; Kim, Y.G.; Lee, W.; Schwartz, M.; Hynynen, K.; Lozano, A.M.; Shah, B.B.; et al. A Randomized Trial of Focused Ultrasound Thalamotomy for Essential Tremor. N. Engl. J. Med. 2016, 375, 730–739. [Google Scholar] [CrossRef] [PubMed]
- Lipsman, N.; Schwartz, M.L.; Huang, Y.; Lee, L.; Sankar, T.; Chapman, M.; Hynynen, K.; Lozano, A.M. MR-Guided Focused Ultrasound Thalamotomy for Essential Tremor: A Proof-of-Concept Study. Lancet Neurol. 2013, 12, 462–468. [Google Scholar] [CrossRef]
- Fahn, S.; Tolosa, E.; Marin, C. Clinical Rating Scale for Tremor. In Parkinson’s Disease and Movement Disorders; Jankovik, J., Tolosa, E., Eds.; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 1993; pp. 271–280. [Google Scholar]
- Stacy, M.A.; Elble, R.J.; Ondo, W.G.; Wu, S.-C.; Hulihan, J. Assessment of interrater and intrarater reliability of the Fahn–Tolosa–Marin Tremor Rating Scale in essential tremor. Mov. Disord. 2007, 22, 833–838. [Google Scholar] [CrossRef]
- Elble, R.J.; Brilliant, M.; Leffler, K.; Higgins, C. Quantification of Essential Tremor in Writing and Drawing. Mov. Disord. 1996, 11, 70–78. [Google Scholar] [CrossRef]
- Elble, R.; Bain, P.; Forjaz, M.J.; Haubenberger, D.; Testa, C.; Goetz, C.G.; Leentjens, A.F.G.; Martinez-Martin, P.; Traon, A.P.-L.; Post, B.; et al. Task Force Report: Scales for Screening and Evaluating Tremor: Critique and Recommendations. Mov. Disord. 2013, 28, 1793–1800. [Google Scholar] [CrossRef]
- Tam, F.; Huang, Y.; Schwartz, M.L.; Schweizer, T.A.; Hynynen, K.; Graham, S.J. A Computerized Tablet System for Evaluating Treatment of Essential Tremor by Magnetic Resonance Guided Focused Ultrasound. BMC Neurol. 2017, 17, 74. [Google Scholar] [CrossRef]
- Haubenberger, D.; Kalowitz, D.; Nahab, F.B.; Toro, C.; Ippolito, D.; Luckenbaugh, D.A.; Wittevrongel, L.; Hallett, M. Validation of Digital Spiral Analysis as Outcome Parameter for Clinical Trials in Essential Tremor. Mov. Disord. Off. J. Mov. Disord. Soc. 2011, 26, 2073–2080. [Google Scholar] [CrossRef]
- Bookheimer, S. Functional MRI of Language: New Approaches to Understanding the Cortical Organization of Semantic Processing. Annu. Rev. Neurosci. 2002, 25, 151–188. [Google Scholar] [CrossRef] [PubMed]
- Keller, T.A.; Carpenter, P.A.; Just, M.A. The Neural Bases of Sentence Comprehension: A FMRI Examination of Syntactic and Lexical Processing. Cereb. Cortex 2001, 11, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.L.; Grabowski, T.J.; Boord, P.; Yagle, K.; Askren, M.; Mestre, Z.; Robinson, P.; Welker, O.; Gulliford, D.; Nagy, W.; et al. Contrasting Brain Patterns of Writing-Related DTI Parameters, FMRI Connectivity, and DTI–FMRI Connectivity Correlations in Children with and without Dysgraphia or Dyslexia. NeuroImage Clin. 2015, 8, 408–421. [Google Scholar] [CrossRef] [PubMed]
- Richards, T.L.; Berninger, V.W.; Yagle, K.J.; Abbott, R.D.; Peterson, D.J. Changes in DTI Diffusivity and FMRI Connectivity Cluster Coefficients for Students with and without Specific Learning Disabilities In Written Language: Brain’s Response to Writing Instruction. J. Nat. Sci. 2017, 3, e350. [Google Scholar]
- Roux, F.; Dufor, O.; Giussani, C.; Wamain, Y.; Draper, L.; Longcamp, M.; Démonet, J. The Graphemic/Motor Frontal Area Exner’s Area Revisited. Available online: https://pubmed.ncbi.nlm.nih.gov/19847902/ (accessed on 19 May 2020).
- Planton, S.; Jucla, M.; Roux, F.; Démonet, J. The “Handwriting Brain”: A Meta-Analysis of Neuroimaging Studies of Motor Versus Orthographic Processes. Available online: https://pubmed.ncbi.nlm.nih.gov/23831432/ (accessed on 19 May 2020).
- Purcell, J.J.; Turkeltaub, P.E.; Eden, G.F.; Rapp, B. Examining the Central and Peripheral Processes of Written Word Production through Meta-Analysis. Front. Psychol. 2011, 2, 239. [Google Scholar] [CrossRef]
- Nakamura, K.; Kuo, W.-J.; Pegado, F.; Cohen, L.; Tzeng, O.J.L.; Dehaene, S. Universal Brain Systems for Recognizing Word Shapes and Handwriting Gestures during Reading. Proc. Natl. Acad. Sci. USA 2012, 109, 20762–20767. [Google Scholar] [CrossRef]
- Longcamp, M.; Hlushchuk, Y.; Hari, R. What Differs in Visual Recognition of Handwritten vs. Printed Letters? An FMRI Study. Hum. Brain Mapp. 2011, 32, 1250–1259. [Google Scholar] [CrossRef]
- Roeltgen, D.P.; Sevush, S.; Heilman, K.M. Phonological Agraphia. Neurology 1983, 33, 755. [Google Scholar] [CrossRef]
- Kersey, A.J.; James, K.H. Brain Activation Patterns Resulting from Learning Letter Forms through Active Self-Production and Passive Observation in Young Children. Front. Psychol. 2013, 4, 567. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, J.; Meng, Z.-L.; Qin, L.; Liu, Y.-F.; Bi, H.-Y. Neural Correlates of Orthographic Access in Mandarin Chinese Writing: An FMRI Study of the Word-Frequency Effect. Front. Behav. Neurosci. 2018, 12, 288. [Google Scholar] [CrossRef]
- Yang, Y.; Zuo, Z.; Tam, F.; Graham, S.J.; Tao, R.; Wang, N.; Bi, H.-Y. Brain Activation and Functional Connectivity during Chinese Writing: An FMRI Study. J. Neurolinguistics 2019, 51, 199–211. [Google Scholar] [CrossRef]
- Yang, Y.; Tam, F.; Graham, S.J.; Sun, G.; Li, J.; Gu, C.; Tao, R.; Wang, N.; Bi, H.-Y.; Zuo, Z. Men and Women Differ in the Neural Basis of Handwriting. Hum. Brain Mapp. 2020, 41, 2642–2655. [Google Scholar] [CrossRef] [PubMed]
- Roux, S.; McKeeff, T.J.; Grosjacques, G.; Afonso, O.; Kandel, S. The Interaction between Central and Peripheral Processes in Handwriting Production. Cognition 2013, 127, 235–241. [Google Scholar] [CrossRef] [PubMed]
- Kandel, S.; Perret, C. How Does the Interaction between Spelling and Motor Processes Build up during Writing Acquisition? Cognition 2015, 136, 325–336. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Feng, C. The Interaction between Central and Peripheral Processing in Chinese Handwritten Production: Evidence from the Effect of Lexicality and Radical Complexity. Front. Psychol. 2017, 8, 334. [Google Scholar] [CrossRef]
- Vinci-Booher, S.; Cheng, H.; James, K.H. An Analysis of the Brain Systems Involved with Producing Letters by Hand. J. Cogn. Neurosci. 2019, 31, 138–154. [Google Scholar] [CrossRef]
- Berninger, V.; Richards, T. Inter-Relationships among Behavioral Markers, Genes, Brain and Treatment in Dyslexia and Dysgraphia. Future Neurol. 2010, 5, 597–617. [Google Scholar] [CrossRef]
- Berninger, V.W.; Richards, T.; Abbott, R.D. Differential Diagnosis of Dysgraphia, Dyslexia, and OWL LD: Behavioral and Neuroimaging Evidence. Read. Writ. 2015, 28, 1119–1153. [Google Scholar] [CrossRef] [PubMed]
- Graham, S.J.; Mraz, R.; Baker, N.; Clarkson, I.; Tam, F. Method and System for Computerized Drawing and Writing during Functional Magnetic Resonance Imaging. U.S. Patent 8,073,526, 6 December 2011. [Google Scholar]
- Graham, S.J.; Schweizer, T.A.; Strother, S.; Tam, F.; Karimpoor, M. Systems and Methods for Providing Visual Feedback of Touch Panel Input during Magnetic Resonance Imaging. U.S. Patent 10,582,878, 10 March 2020. [Google Scholar]
- Graham, S.J.; Morrison, M.A.; Tam, F.; Schweizer, T.A.; Das, S.; Garavaglia, M. System and Method for Intraoperative Characterization of Brain Function Using Input from a Touch Panel Device. U.S. Patent 10,506,962, 7 December 2019. [Google Scholar]
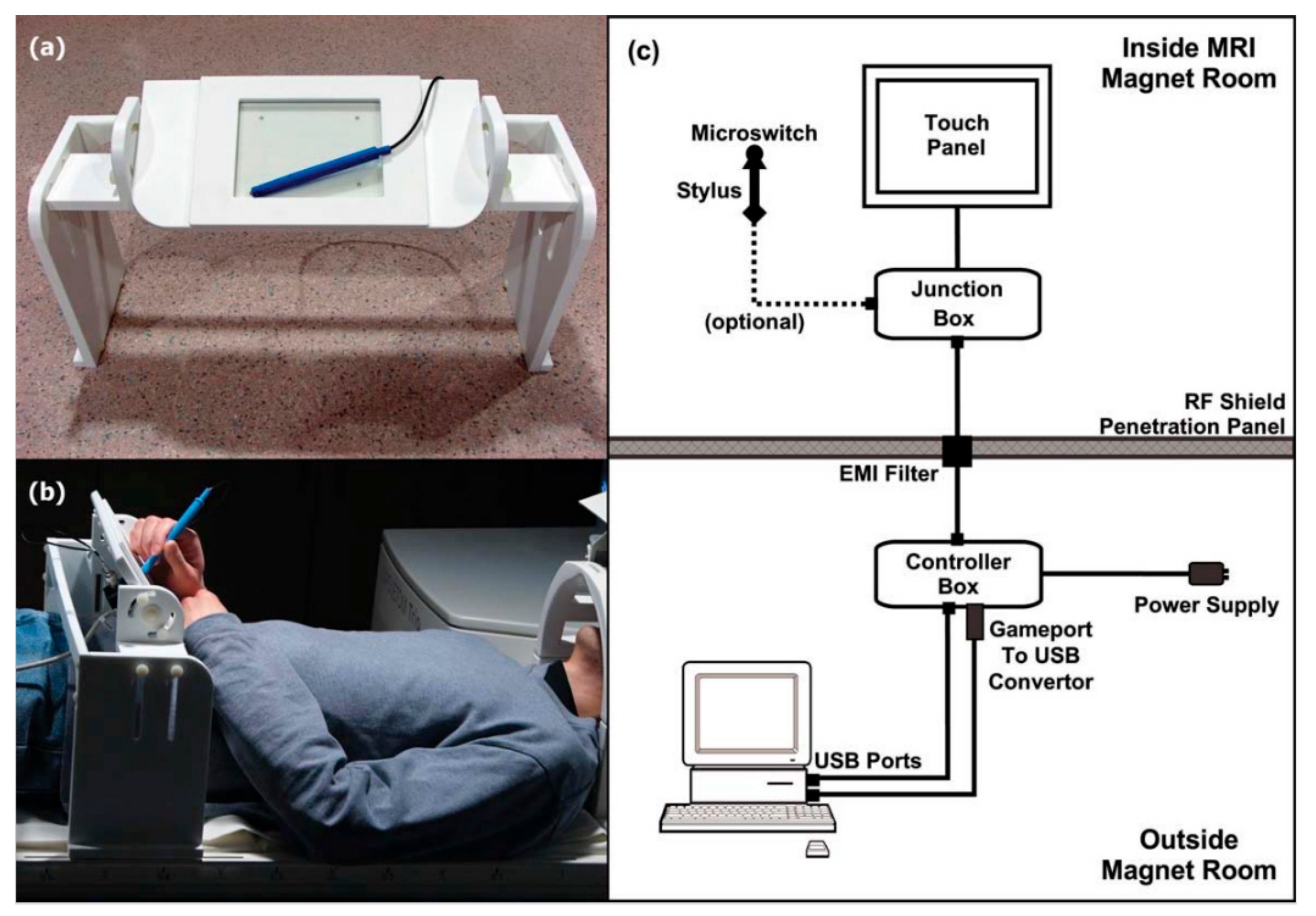


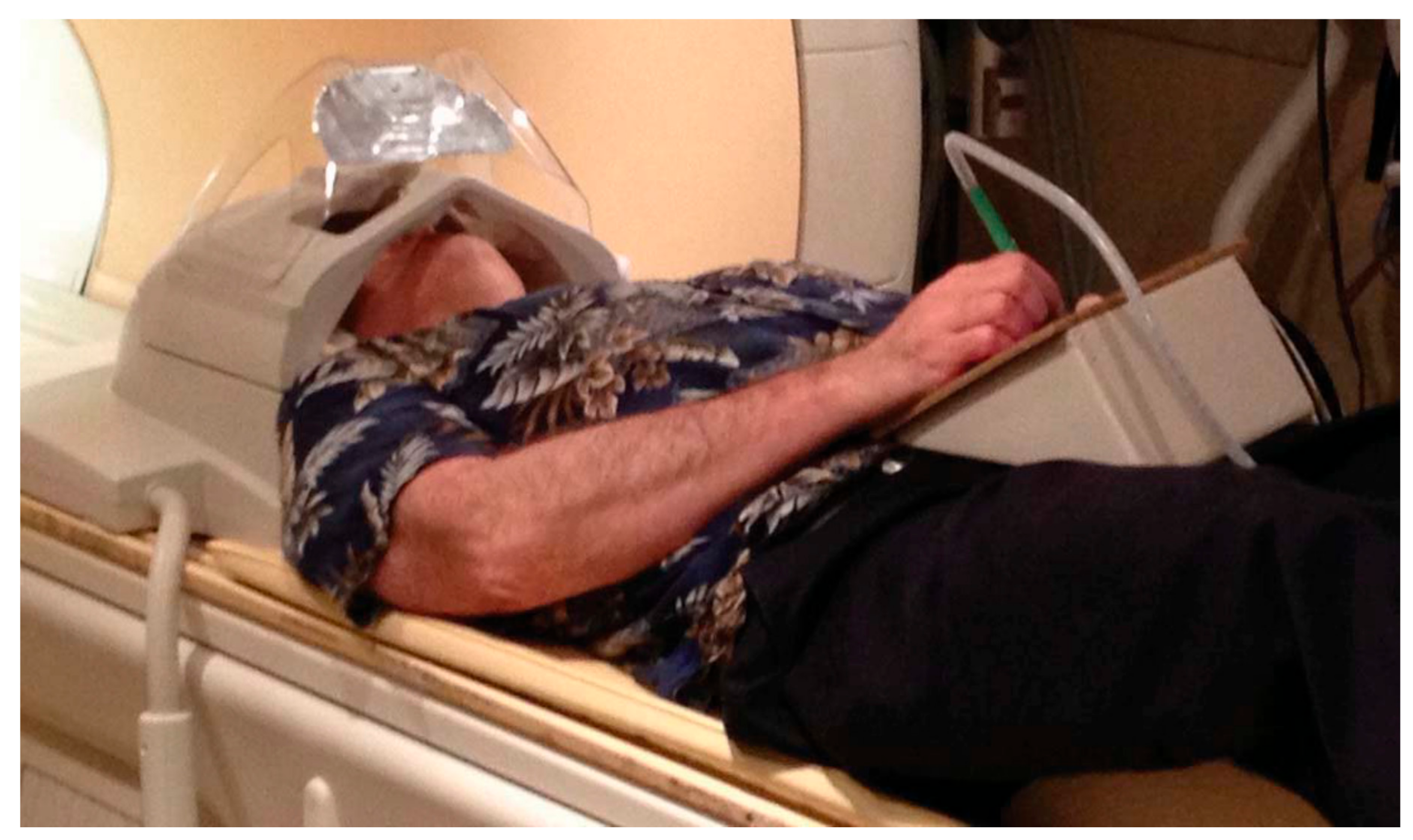

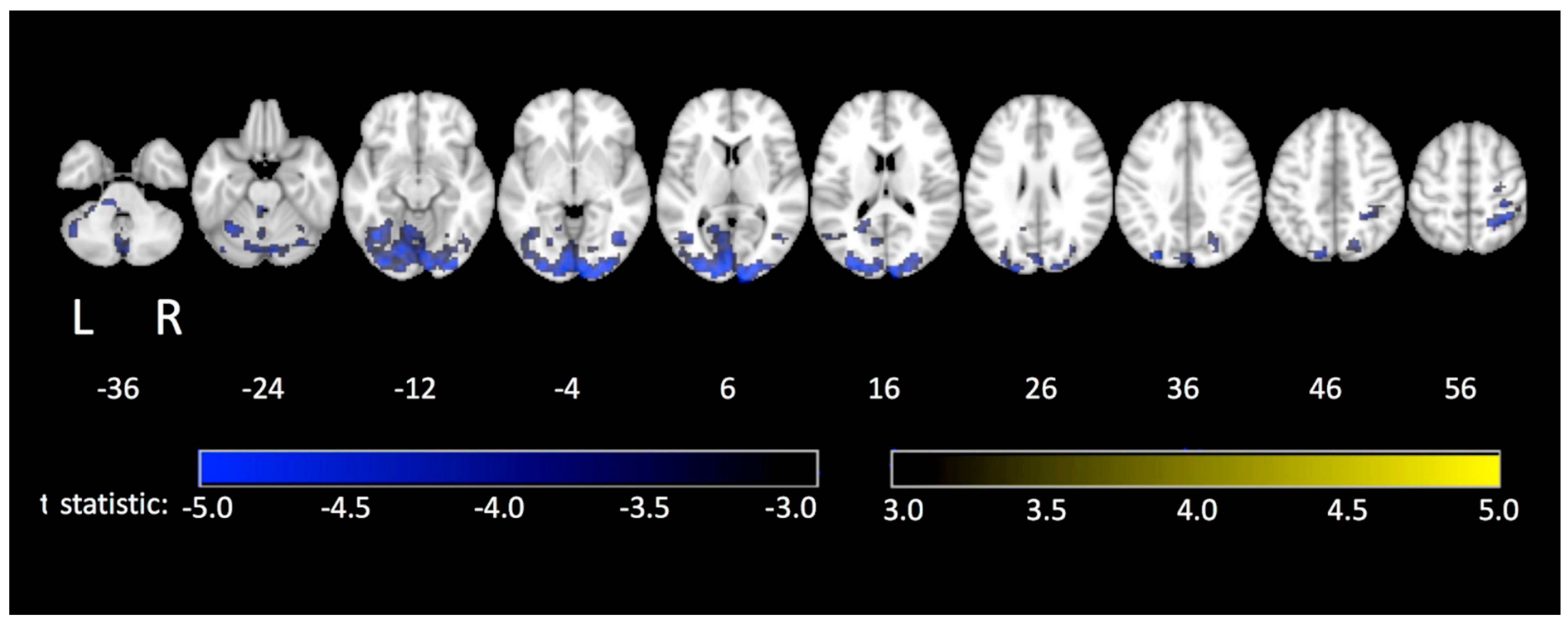
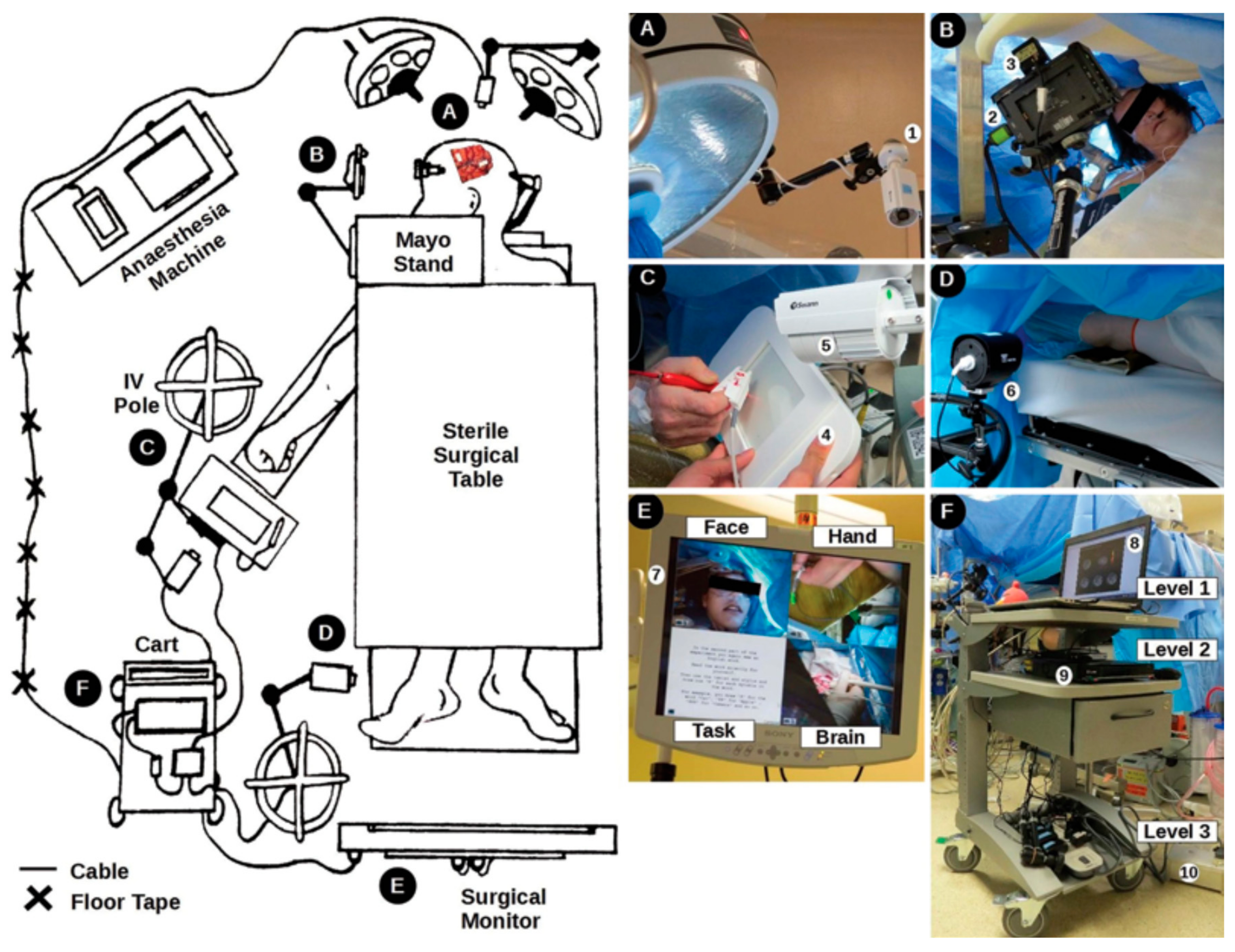
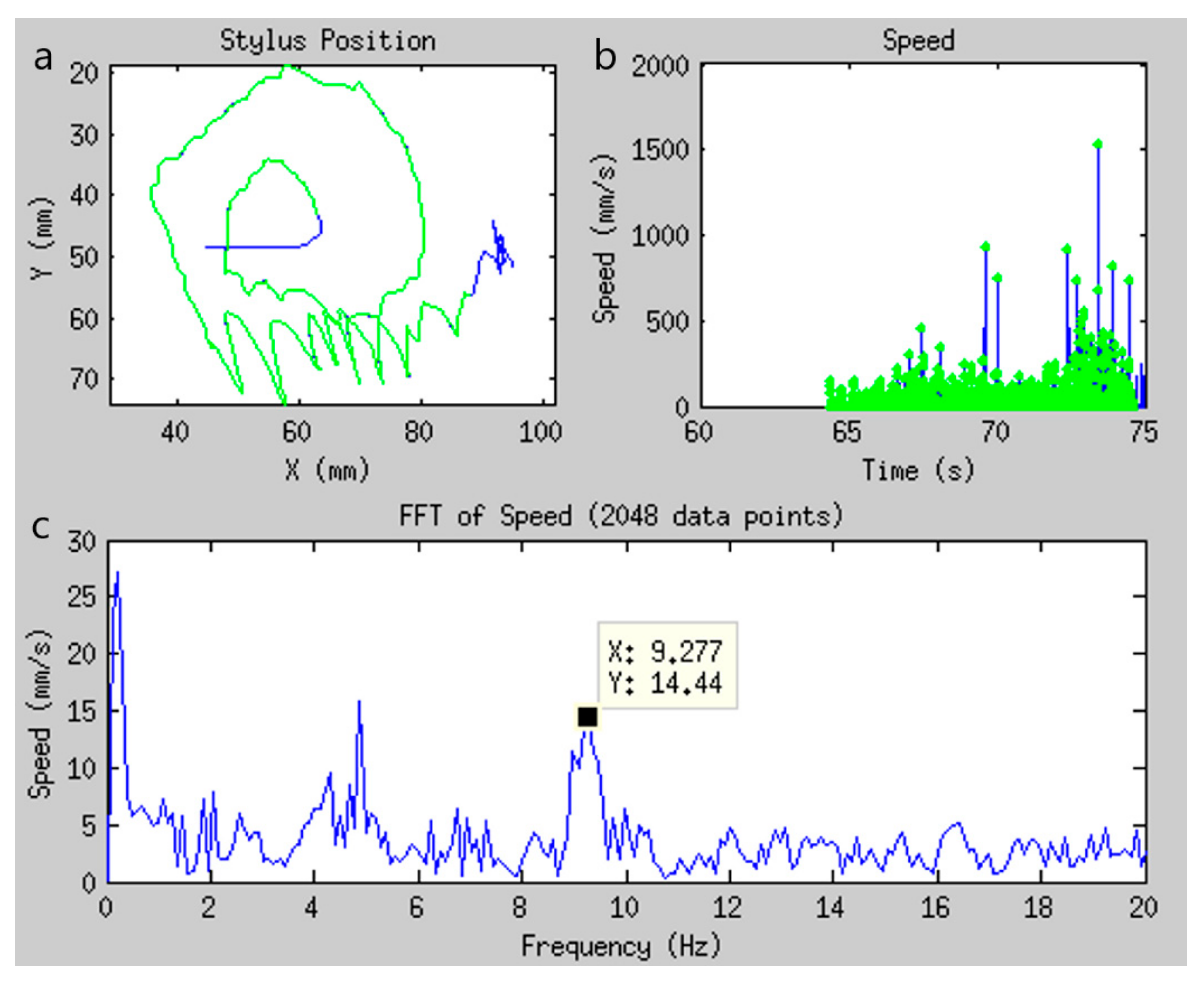
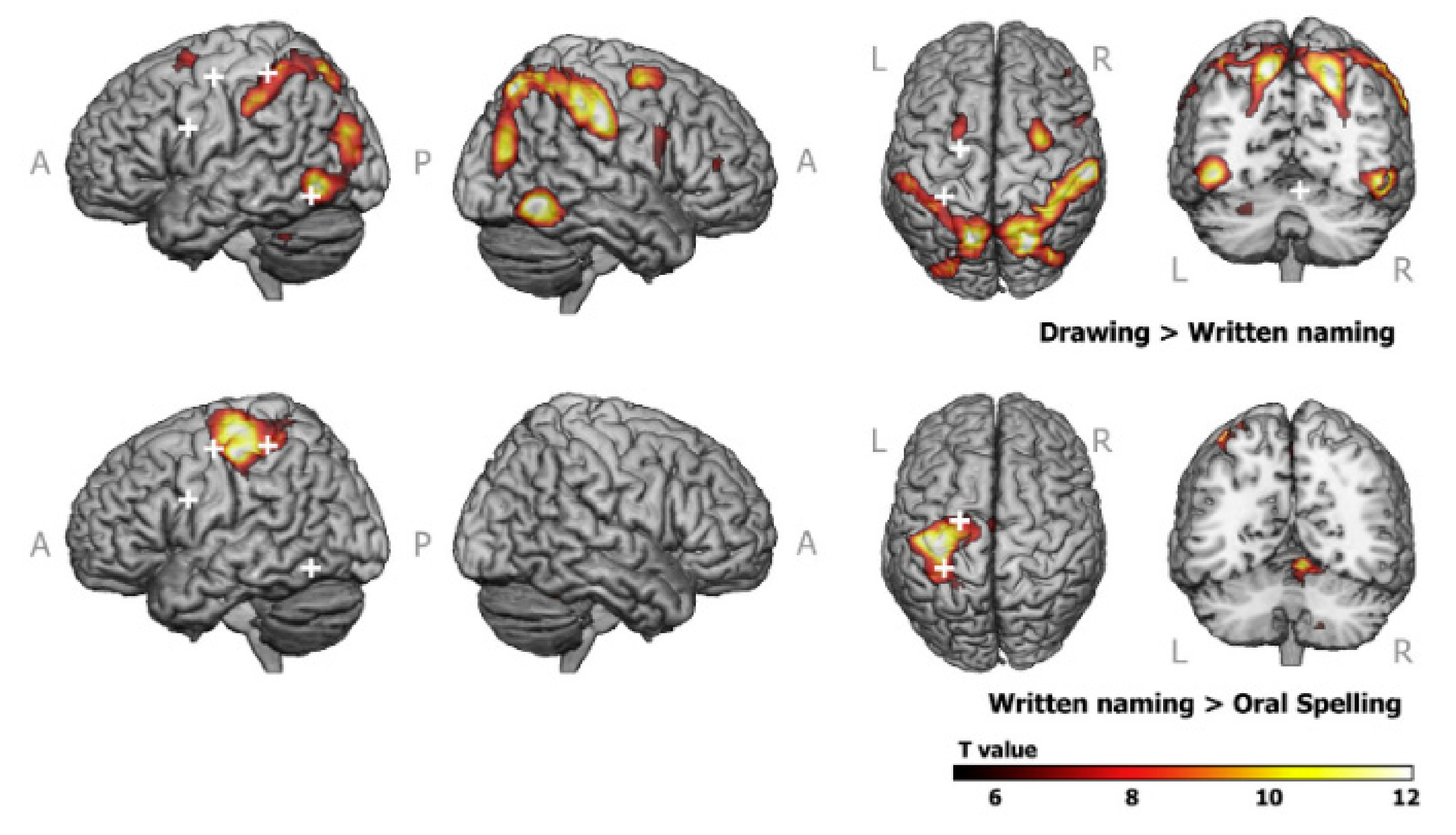
| Technique | Tablet Prototype 1 | Tablet Prototype 2 | Mid-Air Finger Drawing [8] |
|---|---|---|---|
| Pros | Good ecological validity | Enhanced ecological validity (VFHP 1) | Naturalistic finger movement |
| Digitized behavioural recording | Digitized behavioural recording | No hardware required | |
| Cons | Hardware complexity | More hardware complexity | No interaction with writing surface |
| More reliance on proprioception than Prototype 2 | VFHP can obstruct visual stimuli | Participant receives no written performance feedback | |
| No behavioural recording |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, Z.; Tam, F.; Churchill, N.W.; Schweizer, T.A.; Graham, S.J. Tablet Technology for Writing and Drawing during Functional Magnetic Resonance Imaging: A Review. Sensors 2021, 21, 401. https://doi.org/10.3390/s21020401
Lin Z, Tam F, Churchill NW, Schweizer TA, Graham SJ. Tablet Technology for Writing and Drawing during Functional Magnetic Resonance Imaging: A Review. Sensors. 2021; 21(2):401. https://doi.org/10.3390/s21020401
Chicago/Turabian StyleLin, Zhongmin, Fred Tam, Nathan W. Churchill, Tom A. Schweizer, and Simon J. Graham. 2021. "Tablet Technology for Writing and Drawing during Functional Magnetic Resonance Imaging: A Review" Sensors 21, no. 2: 401. https://doi.org/10.3390/s21020401
APA StyleLin, Z., Tam, F., Churchill, N. W., Schweizer, T. A., & Graham, S. J. (2021). Tablet Technology for Writing and Drawing during Functional Magnetic Resonance Imaging: A Review. Sensors, 21(2), 401. https://doi.org/10.3390/s21020401





