Head Trajectory Diagrams for Gait Symmetry Analysis Using a Single Head-Worn IMU
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participtants and Data Colection
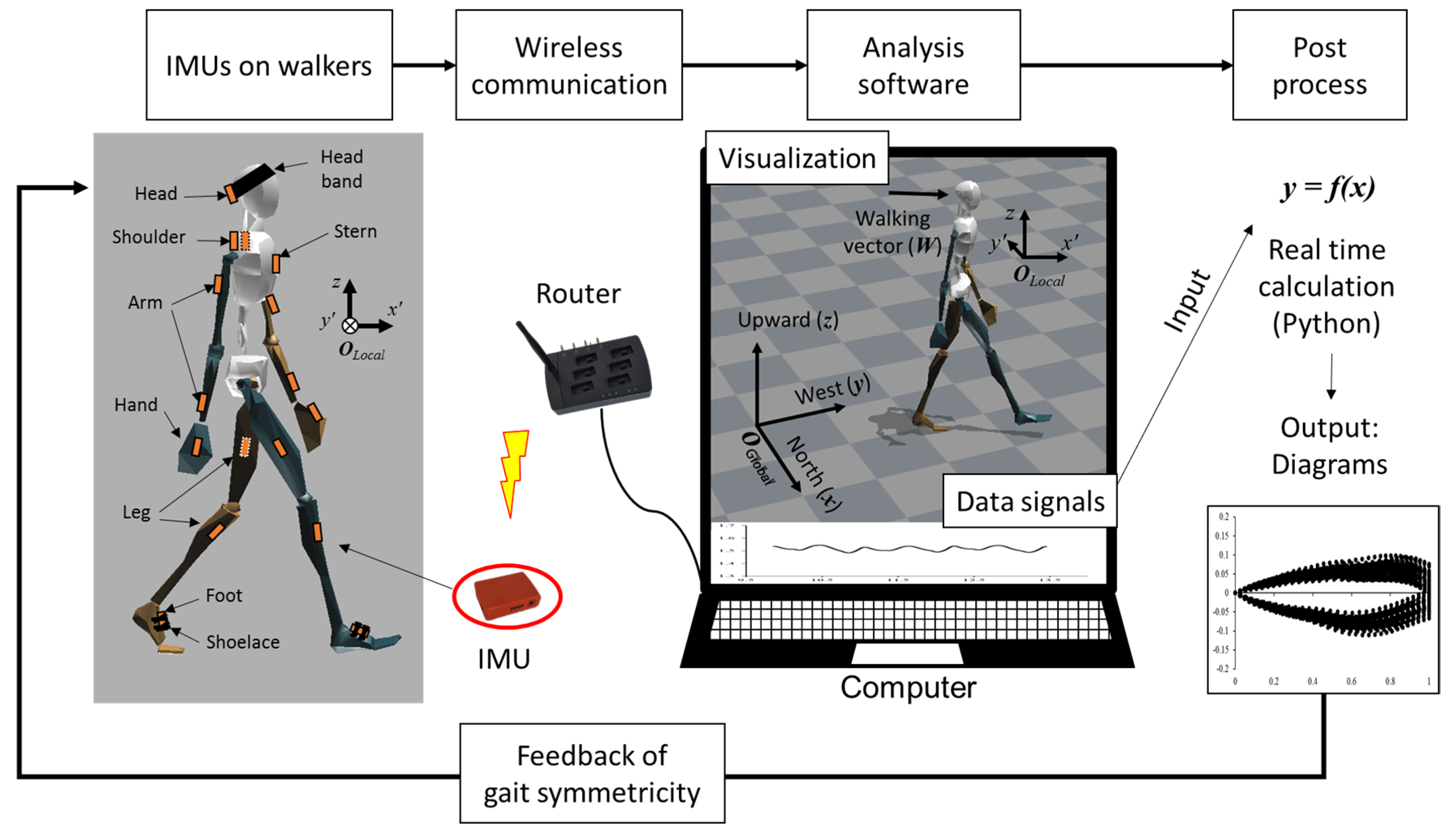
2.2. Sensor Placement and Orientations of Coordination Systems
2.3. Anatomical Planes in Observation
2.4. Temporal Alignment: Step Time
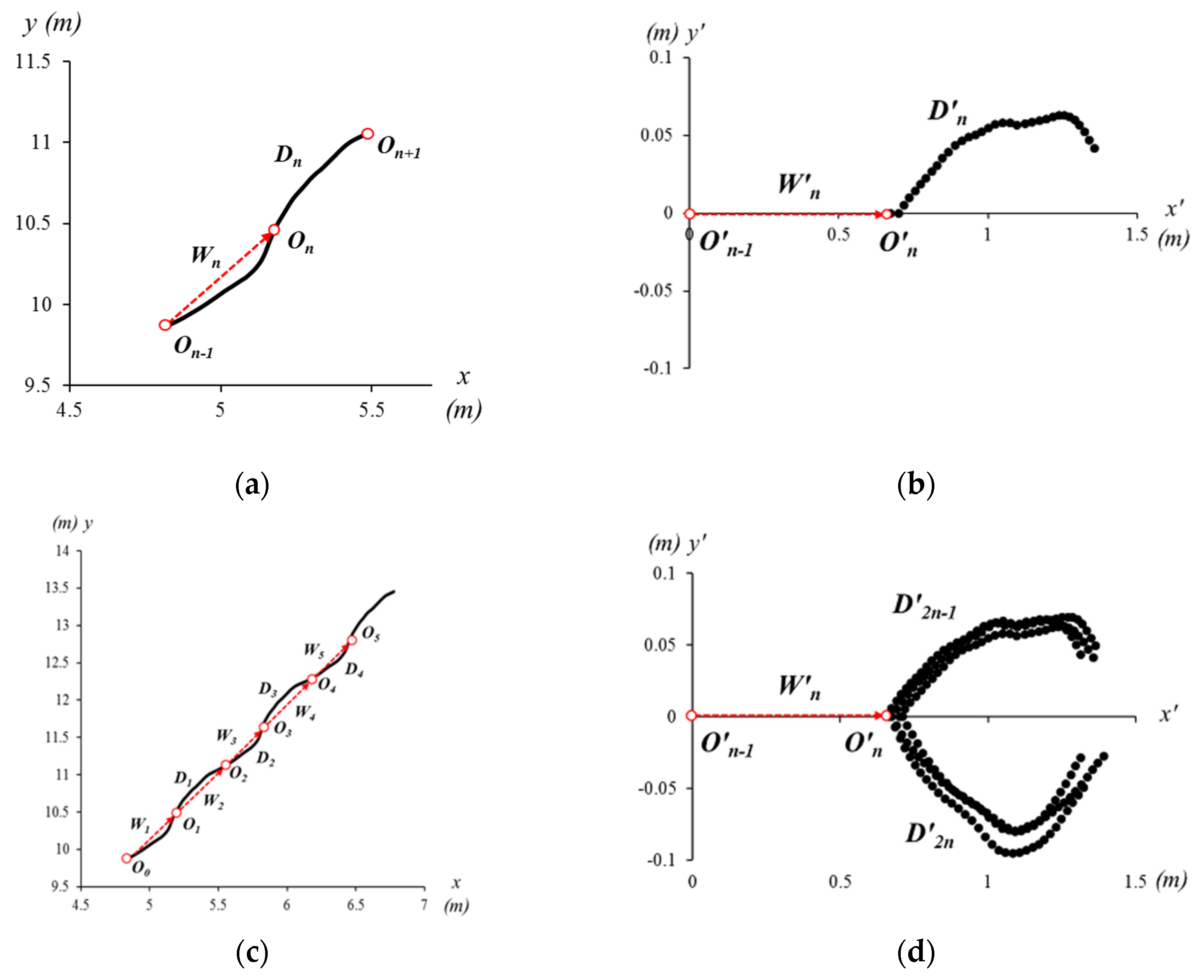
2.5. Spatial Alignment and Walking Vector
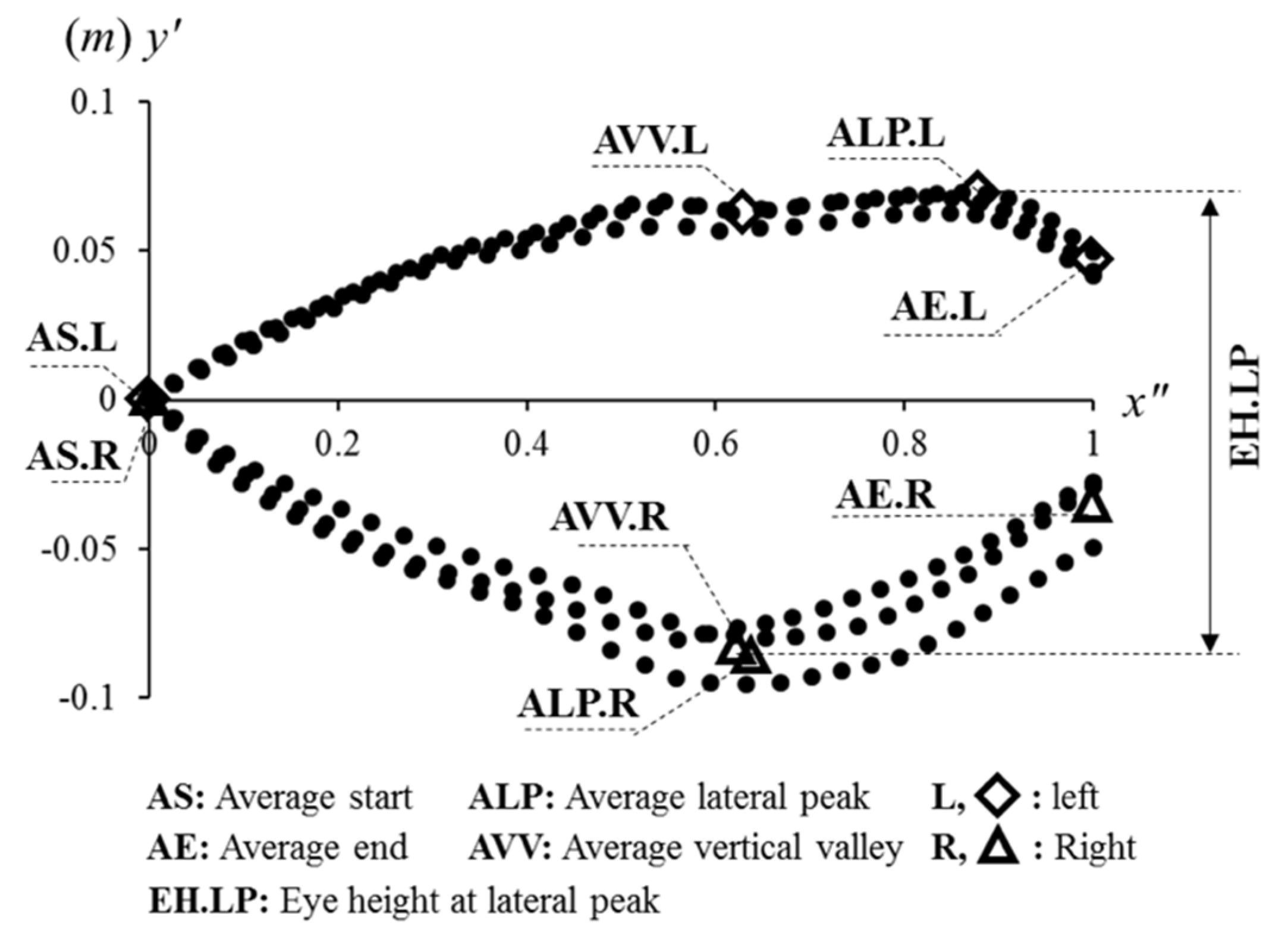
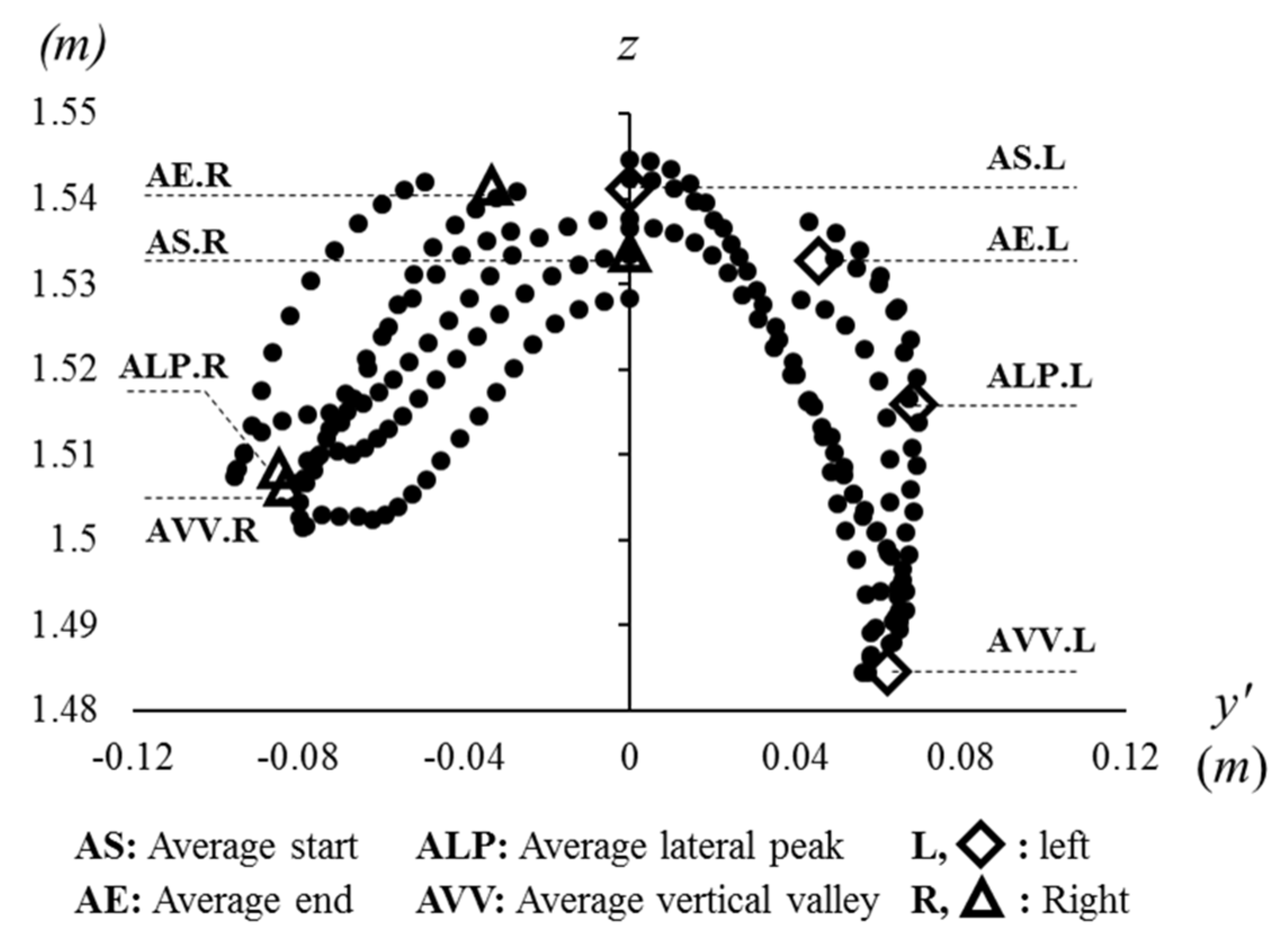
2.6. Parameters in the Concepts of the Eye Diagram
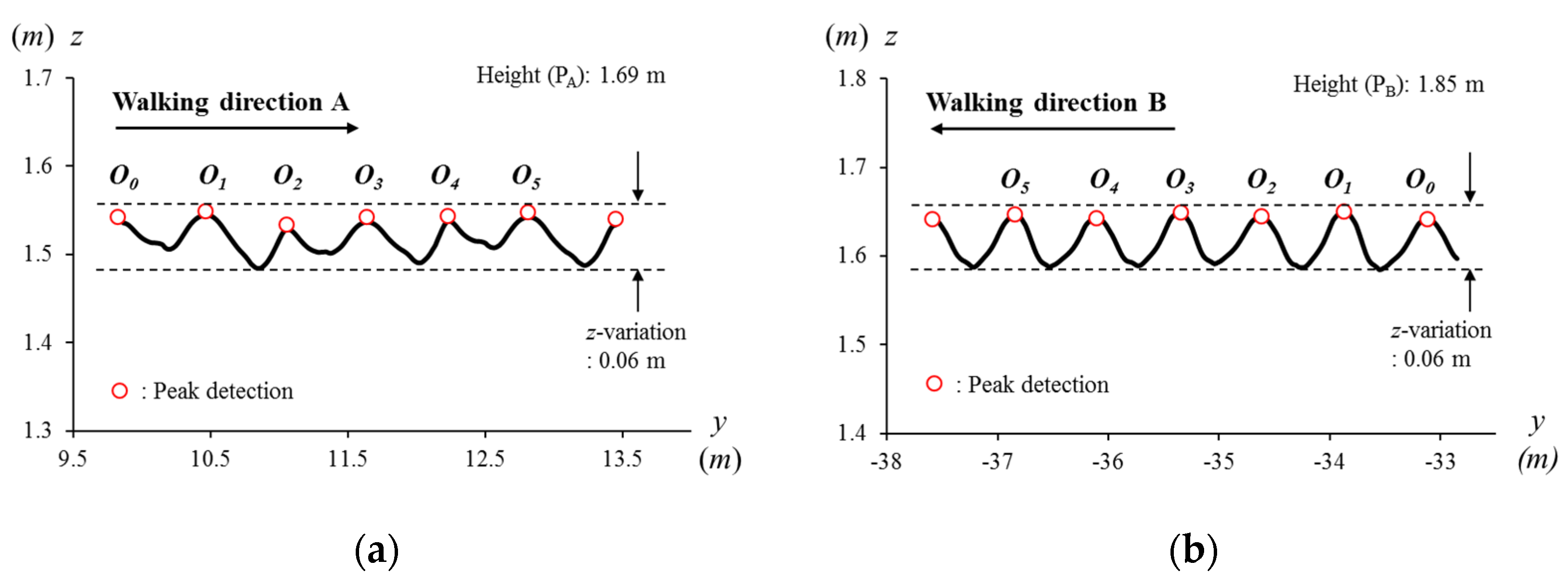
2.6.1. Eye Height
2.6.2. Jitter
2.6.3. Bandwidth
2.6.4. Signal-to-Noise Ratio
2.6.5. Vertical Movement
2.7. Gait Symmetry Indices
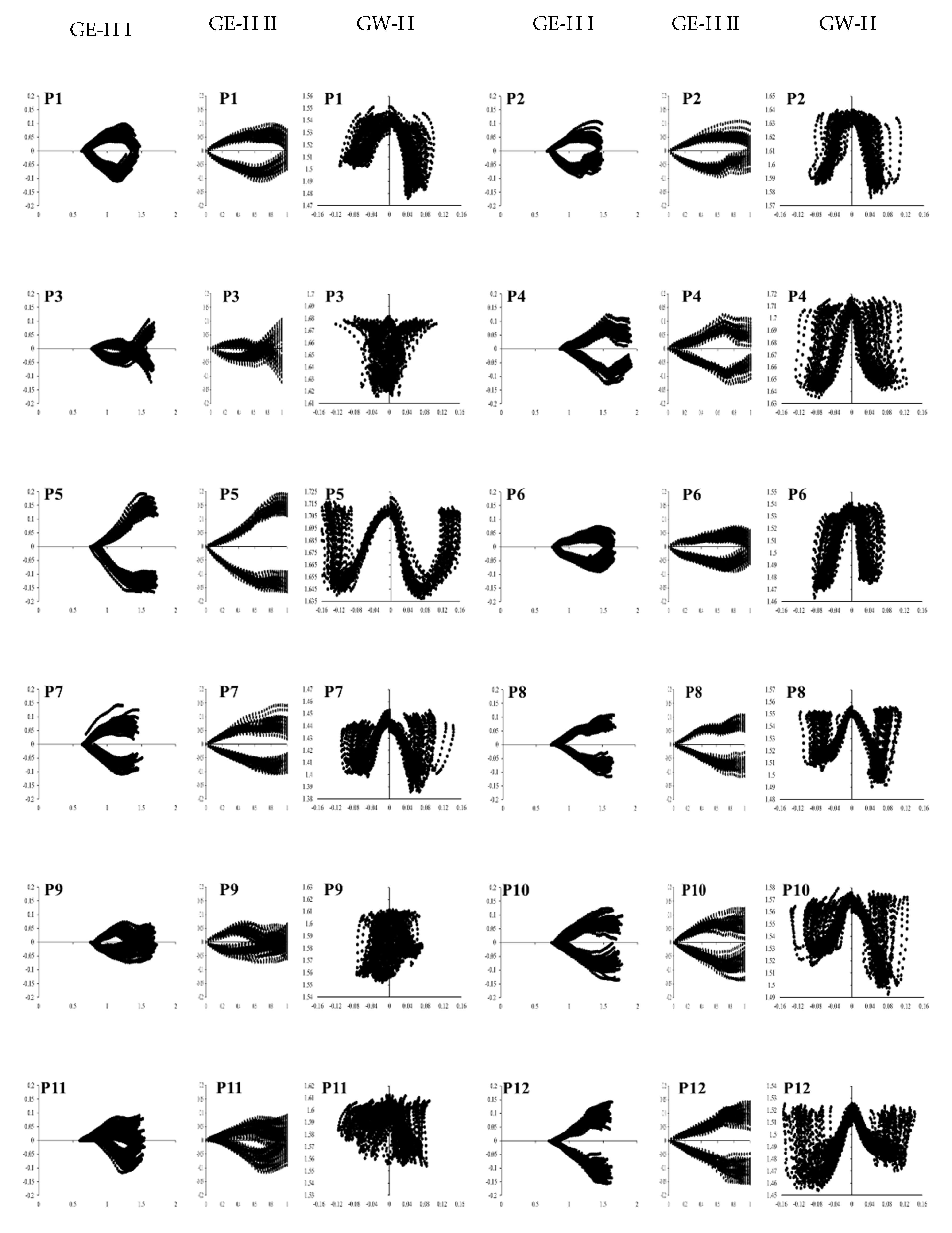
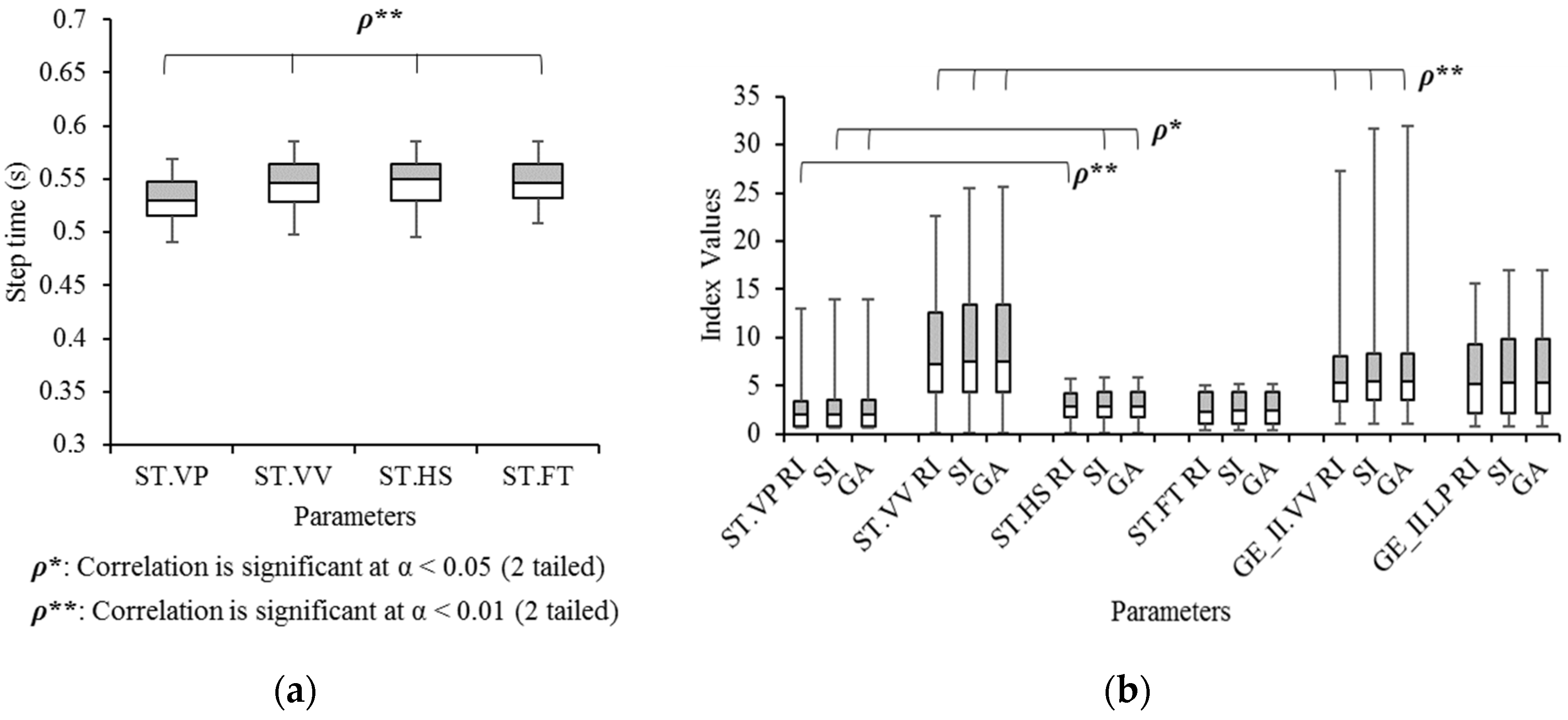
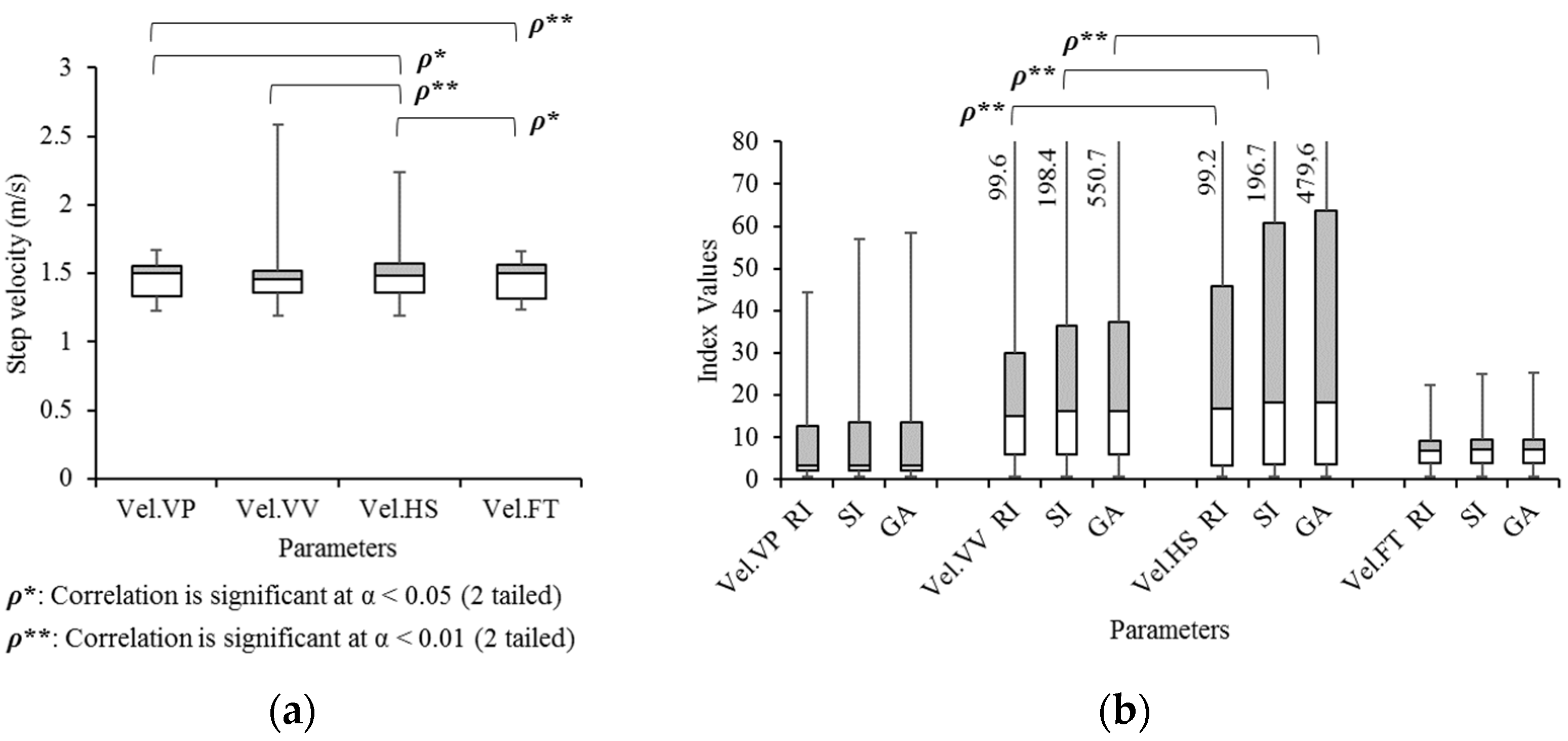
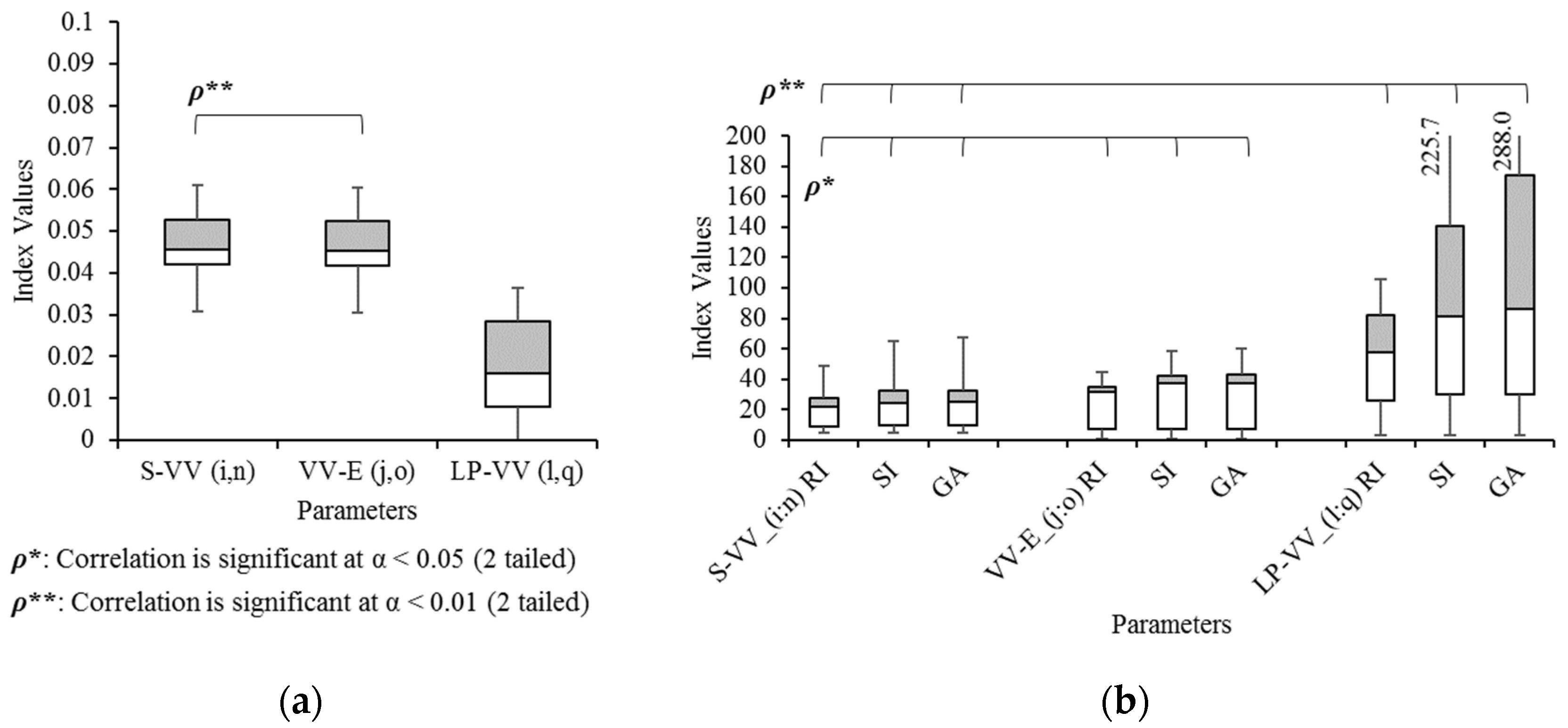
3. Results
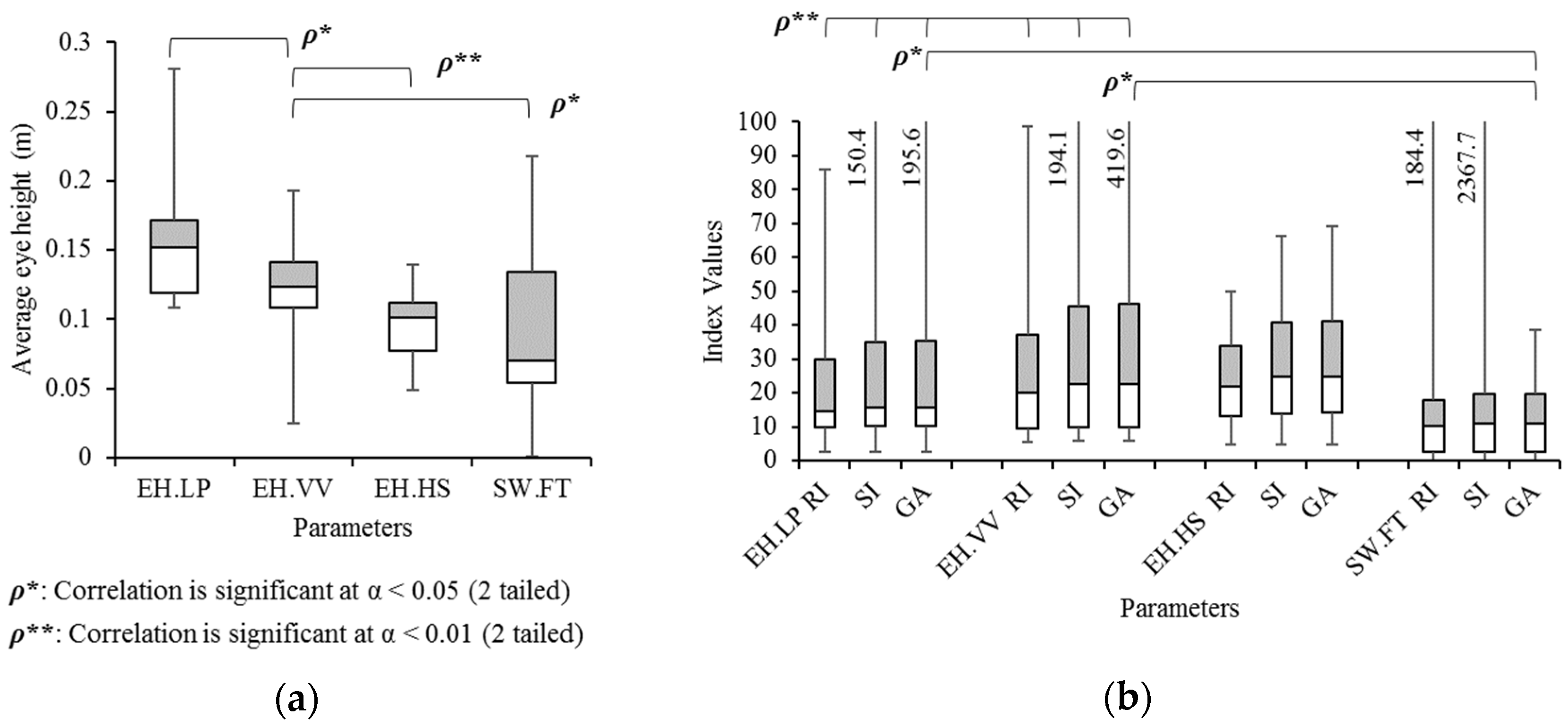
3.1. Concept: Eye Height
3.2. Concept: Jitter
3.3. Concept: Bandwidth
3.4. Concept: Signal-to-Noise Ratio
3.5. Vertical Movement in Gait W-Diagram
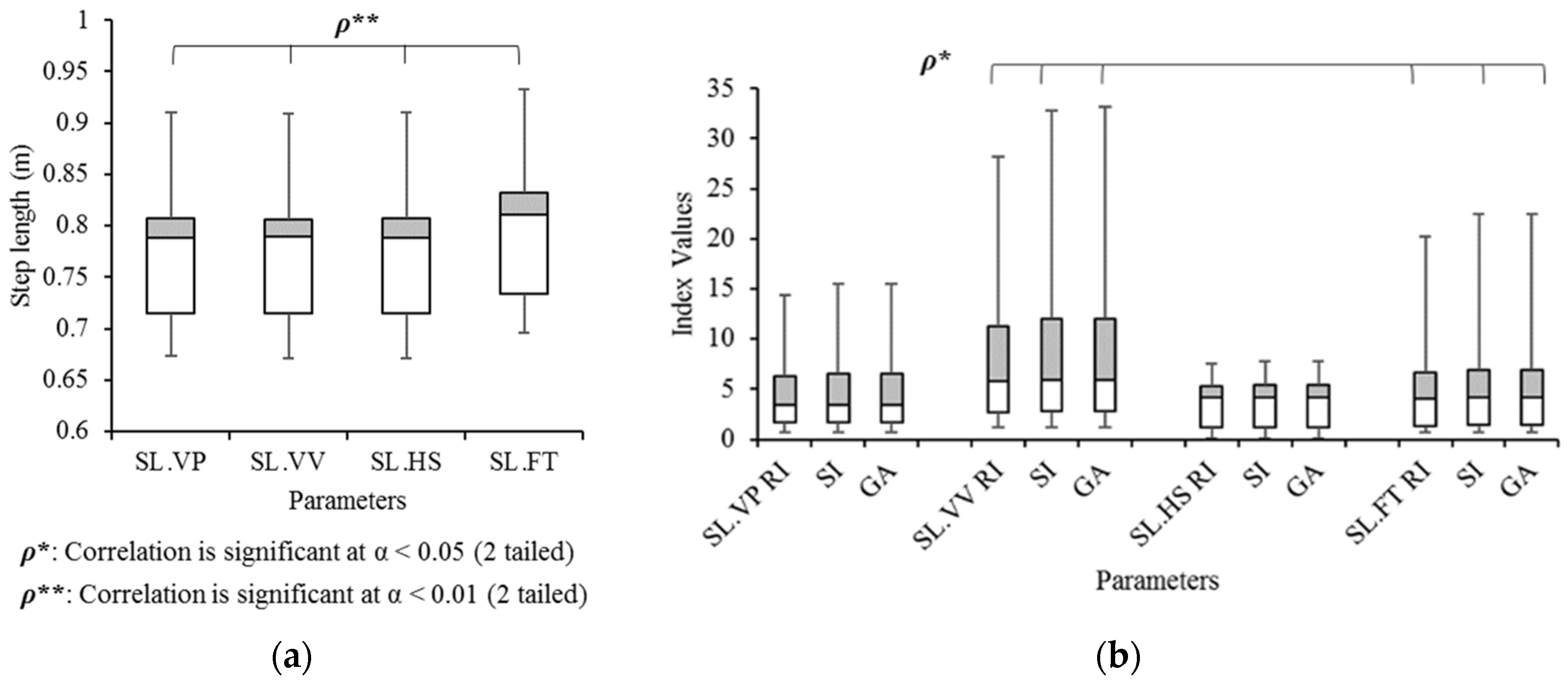
3.6. Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Whittle, M.W. Pathological and Other Abnormal Gaits, In Gait Analysis: An Introduction, 4th ed.; Butterworth-Heinemann Elsevier: Amsterdam, The Netherlands, 2007; pp. 101–136. [Google Scholar]
- Reh, J.; Hwang, T.-H.; Schmitz, G.; Effenberg, A.O. Dual Mode Gait Sonification for Rehabilitation after unilateral Hip Arthroplasty. Brain Sci. 2019, 9, 66. [Google Scholar] [CrossRef] [Green Version]
- Howell, D.; Osternig, L.; Chou, L.S. Monitoring recovery of gait balance control following concussion using an accelerometer. J. Biomech. 2015, 48, 3364–3368. [Google Scholar] [CrossRef]
- Olney, S.J.; Richards, C. Hemiparetic gait following stroke. Part I: Characteristics. Gait Posture 1996, 4, 136–148. [Google Scholar] [CrossRef]
- Morris, M.E.; Huxham, F.; McGinley, J.; Dodd, K.; Iansek, R. The biomechanics and motor control of gait in Parkinson disease. Clin. Biomech. 2001, 16, 459–470. [Google Scholar] [CrossRef]
- Shull, P.B.; Jirattigalachote, W.; Hunt, M.A.; Cutkosky, M.R.; Delp, S.L. Quantified self and human movement: A review on the clinical impact of wearable sensing and feedback for gait analysis and intervention. Gait Posture 2014, 40, 11–19. [Google Scholar] [CrossRef] [PubMed]
- Brajdic, A.; Harle, R. Walk detection and step counting on unconstrained smartphones. In Proceedings of the 2013 ACM International Joint Conference on Pervasive and Ubiquitous Computing, Zurich, Switzerland, 8 September 2013; pp. 225–234. [Google Scholar]
- Tai, K.Y.; Chiang, D.L.; Chen, T.S.; Shen, V.R.; Lai, F.; Lin, F.Y. Smart fall prediction for elderly care using iPhone and Apple watch. Wirel. Pers. Commun. 2020, 114, 347–365. [Google Scholar] [CrossRef]
- Lin, F.; Wang, A.; Zhuang, Y.; Tomita, M.R.; Xu, W. Smart insole: A wearable sensor device for unobtrusive gait monitoring in daily life. IEEE Trans. Ind. Inform. 2016, 12, 2281–2291. [Google Scholar] [CrossRef]
- Identifying Pressure Sensor Problems: How Barometric Pressure, Installation, and Drift affect Sensor Performance. Available online: https://www.piprocessinstrumentation.com/instrumentation/pressure-measurement/article/15556560/identifying-pressure-sensor-problems (accessed on 2 April 2021).
- Hirasaki, E.; Kubo, T.; Nozawa, S.; Matano, S.; Matsunaga, T. Analysis of head and body movements of elderly people during locomotion. Acta Oto-Laryngol. 1993, 113 (Suppl. 501), 25–30. [Google Scholar] [CrossRef]
- Atallah, L.; Aziz, O.; Lo, B.; Yang, G.Z. Detecting walking gait impairment with an ear-worn sensor. In Proceedings of the IEEE 2009 Sixth International Workshop on Wearable and Implantable Body Sensor Networks, Berkeley, CA, USA, 3 June 2009; pp. 175–180. [Google Scholar]
- Atallah, L.; Wiik, A.; Lo, B.; Cobb, J.P.; Amis, A.A.; Yang, G.Z. Gait asymmetry detection in older adults using a light ear-worn sensor. Physiol. Meas. 2014, 35, N95. [Google Scholar] [CrossRef]
- Jarchi, D.; Wong, C.; Kwasnicki, R.M.; Heller, B.; Tew, G.A.; Yang, G.Z. Gait parameter estimation from a miniaturized ear-worn sensor using singular spectrum analysis and longest common subsequence. IEEE Trans. Biomed. Eng. 2014, 61, 1261–1273. [Google Scholar] [CrossRef]
- Hwang, T.-H.; Reh, J.; Effenberg, A.O.; Blume, H. Real-Time Gait Analysis Using a Single Head-Worn Inertial Measurement Unit. IEEE Trans. Consum. Electron. 2018, 64, 240–248. [Google Scholar] [CrossRef]
- Kavanagh, J.J.; Morrison, S.; Barrett, R.S. Coordination of head and trunk accelerations during walking. Eur. J. Appl. Physiol. 2005, 94, 468–475. [Google Scholar] [CrossRef]
- Mentiplay, B.F.; Banky, M.; Clark, R.A.; Kahn, M.B.; Williams, G. Lower limb angular velocity during walking at various speeds. Gait Posture 2018, 65, 190–196. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kolstad, K.; Wigren, A.; Öberg, K. Gait analysis with an angle diagram technique: Application in healthy persons and in studies of Marmor knee arthroplasties. Acta Orthop. Scand. 1982, 53, 733–743. [Google Scholar] [CrossRef] [PubMed]
- Pedotti, A. Simple equipment used in clinical practice for evaluation of locomotion. IEEE Trans. Biomd. Eng. 1977, BME-24, 456–461. [Google Scholar] [CrossRef] [PubMed]
- Glover, I.; Grant, P.M. Baseband transmission and line cording. In Digital Communications, 4th ed.; Pearson Education: London, UK, 2010; pp. 534–582. [Google Scholar]
- Tektronix, Anatomy of an Eye Diagram. Available online: https://www.tek.com/application-note/anatomy-eye-diagram (accessed on 15 September 2021).
- Hwang, T.-H.; Reh, J.; Effenberg, A.O.; Blume, H. Validation of Real Time Gait Analysis Using a Single Head-Worn IMU. In Proceedings of the Europe-Korea Conference on Science and Technology, Vienna, Austria, 15 July 2019; pp. 87–97. [Google Scholar]
- Ganguli, S.; Mukherji, P.; Bose, K.S. Gait evaluation of unilateral below-knee amputees fitted with patellar-tendon-bearing prostheses. J. Indian Med. Assoc. 1974, 63, 256–259. [Google Scholar] [PubMed]
- Andres, R.O.; Stimmel, S.K. Prosthetic alignment effects on gait symmetry: A case study. Clin. Biomech. 1990, 5, 88–96. [Google Scholar] [CrossRef]
- Sadeghi, H.; Allard, P.; Prince, F.; Labelle, H. Symmetry and limb dominance in able-bodied gait: A review. Gait Posture 2000, 12, 34–45. [Google Scholar] [CrossRef]
- Robinson, R.O.; Herzog, W.; Nigg, B.M. Use of force platform variables to quantify the effects of chiropractic manipulation on gait symmetry. J. Manip. Physiol. Ther. 1987, 10, 172–176. [Google Scholar]
- Plotnik, M.; Giladi, N.; Hausdorff, J.M. A new measure for quantifying the bilateral coordination of human gait: Effects of aging and Parkinson’s disease. Exp. Brain Res. 2007, 181, 561–570. [Google Scholar] [CrossRef]
- Błażkiewicz, M.I.; Wiszomirska, I.; Wit, A. Comparison of four methods of calculating the symmetry of spatial-temporal parameters of gait. Acta Bioeng. Biomech. 2014, 16. [Google Scholar] [CrossRef]
- Paulich, M.; Schepers, M.; Rudigkeit, N.; Bellusci, G. Xsens MTw Awinda: Miniature Wireless Inertial-Magnetic Motion Tracker for Highly Accurate 3D Kinematic Applications; Xsens Technologies: Enschede, The Netherlands, 2018. [Google Scholar]
- Joshi, S.; Shenoy, D.; Simha, G.V.; Rrashmi, P.L.; Venugopal, K.R.; Patnaik, L.M. Classification of Alzheimer’s disease and Parkinson’s disease by using machine learning and neural network methods. In Proceedings of the IEEE 2010 Second International Conference on Machine Learning and Computing, Bangalore, India, 9–11 February 2010; pp. 218–222. [Google Scholar]
- Effenberg, A.O.; Fehse, U.; Schmitz, G.; Krueger, B.; Mechling, H. Movement sonification: Effects on motor learning beyond rhythmic adjustments. Front. Neurosci. 2016, 10, 219. [Google Scholar] [CrossRef] [PubMed]
- Ghai, S.; Ghai, I.; Schmitz, G.; Effenberg, A.O. Effect of rhythmic auditory cueing on parkinsonian gait: A systematic review and meta-analysis. Sci. Rep. 2018, 8, 506. [Google Scholar] [CrossRef] [PubMed]


| Side | Parameters | VP | VV | HS | Ground Truth |
|---|---|---|---|---|---|
| Both | Steps (Mean ± Std.) | 85.6 ± 2.9 | 85.3 ± 3.0 | 85.0 ± 2.7 | 85.6 ± 3.1 |
| 1 MAPE (%) | 0.19 | 0.48 | 0.65 | ||
| Left | Steps (Mean ± Std.) | 42.8 ± 1.8 | 42.7 ± 1.9 | 42.3 ± 1.3 | 42.7 ± 1.4 |
| 1 MAPE (%) | 0.99 | 1.59 | 0.93 | ||
| Right | Steps (Mean ± Std.) | 42.8 ± 1.2 | 42.7 ± 1.2 | 42.8 ± 1.6 | 42.9 ± 1.8 |
| 1 MAPE (%) | 1.36 | 2.13 | 0.37 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hwang, T.-H.; Effenberg, A.O. Head Trajectory Diagrams for Gait Symmetry Analysis Using a Single Head-Worn IMU. Sensors 2021, 21, 6621. https://doi.org/10.3390/s21196621
Hwang T-H, Effenberg AO. Head Trajectory Diagrams for Gait Symmetry Analysis Using a Single Head-Worn IMU. Sensors. 2021; 21(19):6621. https://doi.org/10.3390/s21196621
Chicago/Turabian StyleHwang, Tong-Hun, and Alfred O. Effenberg. 2021. "Head Trajectory Diagrams for Gait Symmetry Analysis Using a Single Head-Worn IMU" Sensors 21, no. 19: 6621. https://doi.org/10.3390/s21196621
APA StyleHwang, T.-H., & Effenberg, A. O. (2021). Head Trajectory Diagrams for Gait Symmetry Analysis Using a Single Head-Worn IMU. Sensors, 21(19), 6621. https://doi.org/10.3390/s21196621






