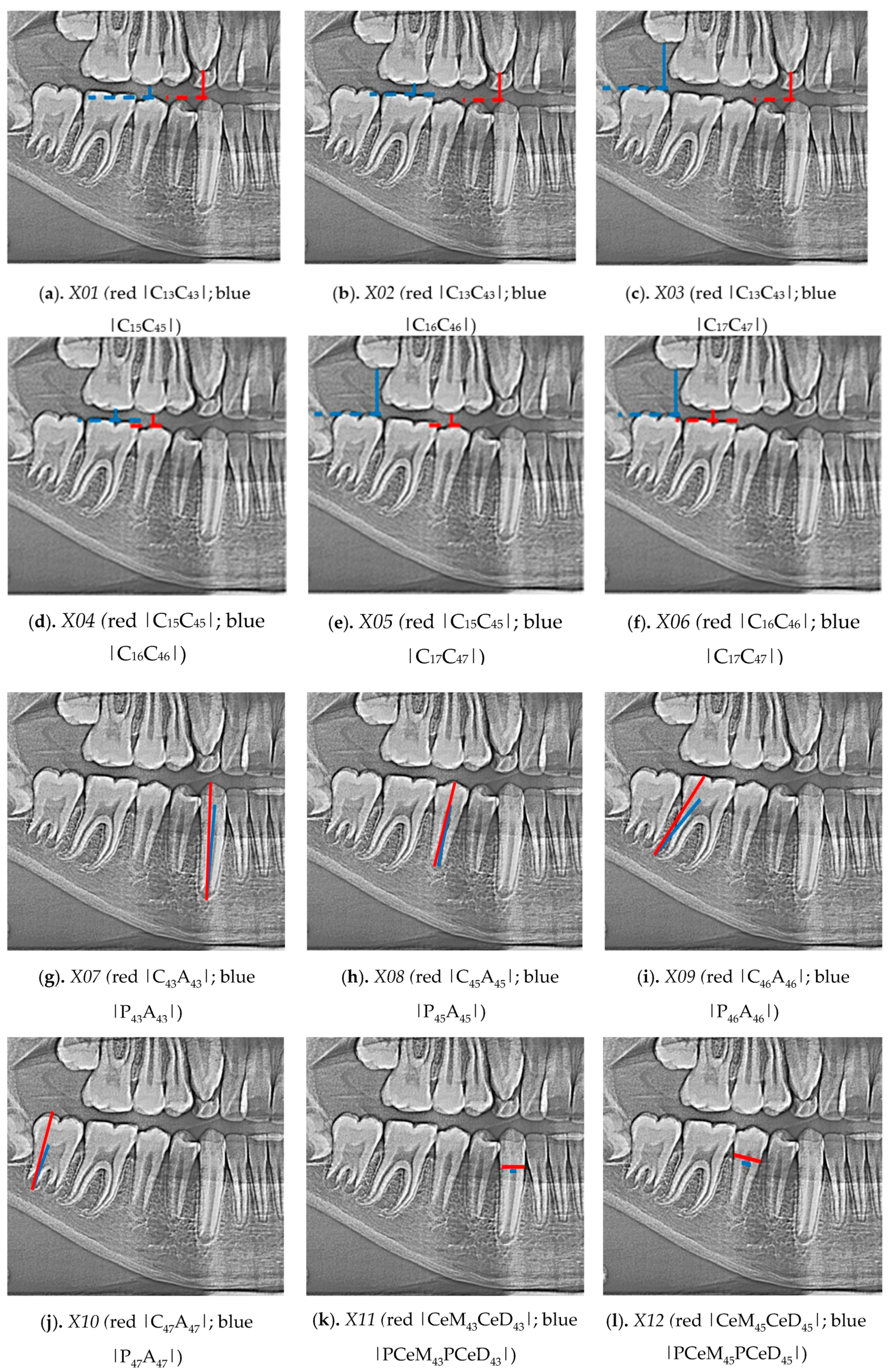
1. Introduction
The first part of this paper reviews the literature beginning with issues related to dental and metric age assessment. The commonly used methods are referred to and their advantages and disadvantages indicated. The following paragraphs present the possibility of using computer and neuronal image analysis methods in medicine and indicate the area of using artificial intelligence methods in dentistry. Then the material and research methods are described, a set of 21 original indicators is presented, which were the basis of the research and further analysis. Moreover, the three best generated network models that accomplish the task of metric age assessment are presented. The whole was crowned with a discussion and conclusion.
Metric age assessment is particularly useful for doctors to plan and evaluate the results of treatment, in anthropology and forensic medicine to determine the metric age of human remains, and to determine the age of children in the case of international adoptions or in individuals illegally staying in a given country.
The analog methods used in the clinical assessment of the patient’s chronological age, based on the development of his or her dentition, are subjective and characterized by low accuracy. These methods are not reliable because there are noticeable discrepancies between the chronological age and the predicted age determined using relevant scientifically developed tables, charts, and atlases [
1,
2,
3,
4,
5,
6,
7,
8,
9,
10,
11,
12,
13,
14,
15,
16,
17,
18]. The variations in determining the chronological age may be significant [
1,
2] as they can be as high as 36 months [
3]. Moreover, the methods currently used are time-consuming: the dentist has to assess the stage of development of the tooth buds of the whole or half of the dentition himself.
Methods of assessing the dental age are divided into clinical methods, comparing the time of eruption of specific tooth groups and pantomographic methods, assessing the mineralization process of tooth buds.
Clinical methods, which assess the presence of tooth groups or individual teeth in a patient, allow to determine the dental age during a routine dental check-up. These methods are considered by dentists to be easy to use, relatively fast and non-invasive. However, the main drawbacks of these methods include inaccuracy, due to the difficulty of determining whether a tooth in the process of eruption should be counted among teeth that have already reached the occlusal plane, and the presence of factors disturbing the time of eruption [
4].
Clinical methods of assessing dental age determine the age on the basis of tables and growth charts, from which the average time of eruption is determined. The most frequently quoted methods come from 1928 (Matiegka and Lukasova method) and 1968 (Charzewski and Panek method) [
5]. Due to the phenomenon of acceleration, i.e., accelerated tooth eruption in the population, these methods are nowadays rarely used for precise assessment of the chronological age. They are mainly used for the initial assessment of the patient’s age and the detection of possible dental abnormalities.
Pantomographic methods of dental age assessment are methods based on the assessment of mineralization of tooth buds. They are used for more precise assessment of dental age than that using tables and growth charts. Many systems have been developed which differ in the number and type of evaluated teeth and the number of individual stages of teeth development [
6,
7,
8,
9].
Most methods of assessing dental age are based on the teeth of the mandible. This is due to the fact that the tooth buds develop earlier in the mandible. The exceptions are the third molars, which are formed first in the maxilla [
9,
10]. Additionally, the presence of maxillary sinuses on pantomographic images makes it difficult to assess the development of tooth buds in the superior arch.
Among the many methods of assessing the dental age [
6,
7,
8,
9,
11,
12,
13,
14], the Demirjian method is the most popular. It is used to assess the shape of the cavity and canal, as well as the degree of enamel and dentin formation. Due to the magnification of 3–10% occurring in the pantomographic picture, the tooth size is not analyzed. In this method, 7 permanent teeth on the left side of mandible are evaluated. There are eight stages of development obtained directly from the tables—separate for the male sex, separate for the female sex [
15,
16].
Another commonly used method is the Schour and Massler method, sometimes referred to as the atlas method, in which, based on the developmental pattern of deciduous and permanent teeth, 21 stages are distinguished and all teeth are assessed simultaneously [
12].
In 1944, the Ubelaker method was developed, which, similarly to the Schour and Massler method, distinguishes 21 stages of development and is currently used worldwide in patients between 5 months and 35 years of age [
17].
The latest analog method of assessing dental age is the London Atlas, which was developed in 2009 and presents 31 stages of development and eruption of teeth from 30 weeks in utero to 23 years of age. The data for the Atlas were obtained from 72 prenatal and 104 postnatal skeletal remains of children and young adults of known age-at-death. These data were supplemented by the analysis of archival radiological pictures of living individuals. The median stage for each age category was used to prepare the Atlas [
17,
18].
It should be noted that medical science is increasingly using innovations in the field of information technology, with particular emphasis on artificial intelligence methods, including neural modelling. These methods help to improve the effectiveness of treatment and accuracy of diagnosis of medical conditions [
19,
20].
The use of artificial neural networks in the processing of medical information and images helps to generalize the data contained even in X-rays with noise [
21,
22], thus enabling more efficient diagnostics and avoiding incorrect diagnoses [
23]. Neural modelling can be the core of an expert system that assists the physician in the daily management of patient information, including the management of data during procedures, such as in the Da Vinci Robot [
24,
25].
Neural modelling is becoming increasingly popular in the biological and medical community [
26]. It can be used in many diagnostic aspects like detection of pulmonary tumor in chest radiographs [
27], in the classification of medical data [
28], in the analysis of microscopic images [
29]. Neural modeling has also been used in determining age and sex [
30] and in clinical diagnostics related to endoscopy [
31]. Neural modeling is also used in the determination of biomarkers [
32] as well as in the detection of brain tumors [
27,
28,
29,
30,
31,
32,
33]. Artificial intelligence methods are also used in the analysis of ECG-electrocardiogram-signal analysis [
34,
35], in Alzheimer’s and Parkinson’s diagnosis [
36,
37,
38], urology [
39], and oncology [
40,
41,
42,
43,
44,
45].
In dentistry, the Seok-Ki and Tae-Woo team [
46] developed a neural model for the diagnosis of tooth extraction during orthodontic treatment. Neural models are also used in prosthetic [
47] and conservative treatment [
48]. In 2017, the Bunyarit team used the Demirijan method indicators in neural modelling to more accurately estimate the dental age of Malaysian children [
49].
It should be noted that the early 2021 papers touch on age determination for children, adolescents, and adults using artificial neural networks. The paper by Mauer and team [
50] presents the possibility of age estimation using a 3D image of the knee. However, even for the use of deep learning algorithms, the quality of the model is about 90% and the MAE error (mean absolute error) +/− is half a year. It should also be noted that this type of age determination is quite time consuming and expensive. The validity of using artificial neural network method is questioned [
51]. From a study of a population of more than 3000 cases aged from 4 to 40 years, the method presented in this paper gives better results. However, it should be noted the fact of a large age range of the studied cases and the use of compiled indicators. Moreover, the method of convolutional networks differs from deep learning and traditional methods. Measurements on cephalometric images are of a different nature and concern not only teeth but also other bone parameters. Nearly 300 images were tested, but the results, although indicating a correlation, were not satisfactory.
The study of age in relation to tooth deterioration was conducted by [
52]. In a study dating back to 2017, the team of researchers described a study on the use of CBCT (cone beam computed tomography) assessment of tooth destruction. In this study, the authors estimate age by determining the structural changes of the tooth. The CBCT technique used involves more expensive equipment. However, it should be emphasized that CBCT is a much more accurate examination, although it is not common in less developed countries. In contrast, the method itself does not use metrics to measure and automatic image analysis methods. The authors indicated a statistical R-value of 0.85.
The analysis of the literature in terms of the assessment of the chronological age, artificial intelligence methods, including the use of neural modelling methods, has shown that so far no highly effective and accurate methodology has been developed and published, which uses modern computer systems and tools to determine the chronological age on the basis of digital pantomographic images. Currently used analog methods are rather imprecise and subjective, which has created the necessity to search for new, more effective techniques and methods of determining the chronological age.
Therefore, work has been carried out in determining the features of pantomographic images that support the determination of metric age, and neural models were produced to support the process of identifying the age of children and adolescents. The whole conducted work was a new methodology of metric age assessment from pantomographic images.
3. Results
Various network topologies (PNN, GRNN, RBF, and MLP) were tested during the research. RBF networks were characterized by the best quality indicators, therefore the three best models determining the chronological age were obtained: 1. women and men, 2. women, 3. men. The results of the research are three neural models that determine the chronological age. Three possibilities were tested on different groups to identify which model is the most accurate and to determine which variables are characteristic of gender groups and which variables affect the accuracy of the modelling result.
In the process of neural modelling for 619 cases of women and men, 23 input variables were involved (X01-X21, gender and age expressed in months). The output variable was “MONTHS”, the remaining variables were input variables.
The sex “SEX” was a categorized variable. Months are a continuous variable, defining the number of months from birth to the date of the study. Variables from X01 to X21 were continuous in nature and marked the calculated indicators characterized above.
In the process of neural modelling for 296 female cases 22 variables were involved (the gender variable was eliminated). The output variable was “MONTHS”, the other variables were input variables.
In the process of neural modelling for 323 cases of men 22 variables were involved (the gender variable was eliminated). The output variable was “MONTHS”, the remaining variables were input variables.
The division into learning, validation, and testing set was carried out at random for each data collection. The learning set takes part in the process of generating the network, the validation set takes part in tuning the model, while the test set is excluded from the process of neural modelling—these are raw, previously unused data to check and evaluate the artificial neural network. As a standard, the data set is divided into a 2:1:1 ratio (50%, 25%, 25%).
Neural model RBF 22:22-22-1:1 (
Figure 2) (
Table 1), determining the chronological age for women and men. The network sensitivity analysis is shown in
Table 2.
Neural model RBF 13:13-1-1:1 (
Figure 3) (
Table 3), determining the chronological age for women. The network sensitivity analysis is shown in
Table 4.
Neural model RBF 18:18-1-1:1 (
Figure 4) (
Table 5) determining the chronological age for men. The network sensitivity analysis is shown in
Table 6.
4. Discussion
The result of the conducted research is the development of a new, original methodology for determining the chronological age of children between the ages of 4 and 15 (from 48 to 144 months). The paper identifies and presents an original set of 21 tooth and bone parameters, indicators, owing to which, on the basis of digital pantomographic images, using neural modelling methods, it is possible to accurately and effectively assess the chronological age. The accuracy of the proposed method of chronological age assessment is 99.7%.
During the study, three optimal neural models were developed to determine the chronological age: female and male RBF 22:22-15-1:1 with test set quality of 0.9974 and test set error of 0.0365, female only RBF 13:13-1-1:1 with test set quality of 0.9631 and test set error of 0.0336, and male only RBF 18:18-1-1:1 with test set quality of 0.9993, and test set error of 0.0398. The measure of error is RMSE (Root Mean Square Error). A summary of the models is presented in the
Table 7.
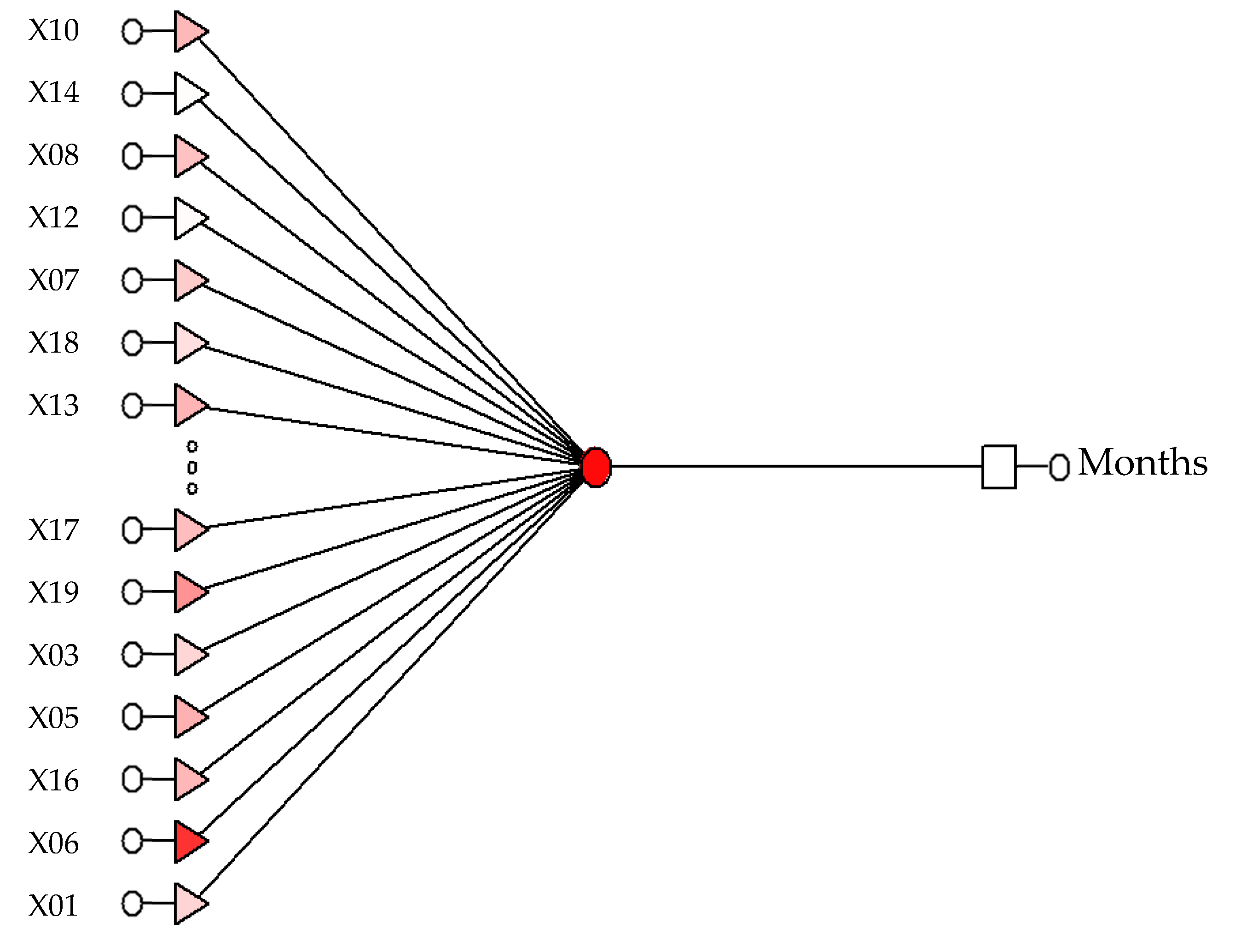
To work properly, each model needs appropriate input variables and indicators that have been defined in the sensitivity analysis process. For the model RBF 22:22-15-1:1 these are the indicators: X04; X02; X15; X18; X11; X12; X20; X14; X03; X16; X08; X19; X17; X10; SEX; X06; X01; X07; X09; X21; X05; X13. For model RBF 13:13-1-1:1, these are the indicators: X07; X14; X08; X12; X19; X20; X10; X13; X03; X01; X18; X06; X05. For the model RBF 18:18-1-1:1, these are the indicators: X18; X20; X16; X10; X03; X19; X14; X01; X07; X09; X05; X06; X17; X13; X08; PLEC; X12; X11.
Variables relevant for each of the analyzed models are X01; X03; X05; X06; X07; X08; X10; X12; X13; X14; X18; X19. A detailed analysis of the models produced is shown in
Table 8.
The newly developed methodology is based on: taking a digital pantomographic picture; making measurements of fixed sections on digital pantomographic pictures (measurements with ImageJ software), exporting a set of these measurements to a spreadsheet, e.g., MS Excel, and calculating the original indicators-proportions-proposed in the course of the conducted tests; subsequently, the calculated values should be transformed into a *.csv file and entered into the neural model used to determine the chronological age.
The novelty of the developed method lies in the use of a pantomogarphy photograph and the use of a proprietary set of developed indicators. Currently, there is no scientific or commercial method that allows to estimate metric age in this way, using neural modeling methods.
Comparing the conducted study with other works [
46,
47,
48,
49], it should be emphasized that the works performed were dedicated to children of a certain age. The teaching set included a representative research group, which probably influenced the neural modeling process. In comparison with other cited methods, the assessment of metric age operates on two-dimensional pantomographic images. The set of indicators used to describe the image is the author’s. It was developed as a result of the clinical experience of the research team and forms the basis of the work carried out.
A limitation in the application of the developed method and technique is the use of 2D images (although it should be noted that taking a pantomographic image is inexpensive and common in clinical practice). It should also be emphasized that the models produced are dedicated to children and adolescents aged 48 to 144 months. The presented method is simpler than others cited in this work.
Mention should be made of the research of the Bunyarit [
57] team, who used the artificial neural network method to estimate the age of children and adolescents aged 5 to 18 years. The accuracy of their model based on Chaillet and Demirjian’s 8-tooth method for dental age estimation was 0.938 to 0.951 (fit coefficient value).
The paper by Galibourg and team [
58] uses the use of deep learning methods and Demirjian’s method to estimate the dental age. The authors report that the study was conducted on a group of 3605 images of people aged 2 to 24 years. The MAE error for their method was 0.811 years, which was more accurate than methods previously used.
In Pan and team’s work [
59], a modified method of Demirjan and Willems was presented, which increased the accuracy of metric age estimation to about 83%. The study was conducted on a population of 2367 Chinese children aged 5 to 16 years. Traditional statistical methods were used to process the results.
Wallraff’s team [
60] obtained promising results from an analysis of pantomographic images of 14,000 patients aged 11 to 20 years. The error of the developed model was more than 17%, but deep learning methods and convolutional networks seem to be an ideal tool in solving this type of issues.
Most recent work on metric age assessment is based on traditional, atlas-based methods [
61,
62]. There are also works on adult age assessment [
63,
64,
65]. It seems that the challenge of the 21st century is to develop modern tools that would allow measurements and classifications to be made automatically. Therefore, it is reasonable to develop neural image analysis methods in medical imaging problems.
The next stage of metric age estimation work should be related to the application of deep learning methods or using convolutional networks.