3D Dosimetry Based on LiMgPO4 OSL Silicone Foils: Facilitating the Verification of Eye-Ball Cancer Proton Radiotherapy
Abstract
1. Introduction
2. Materials and Methods
2.1. D OSL Silicone Foils
2.2. Proton Treatment Plan Using the Clinical Treatment Planning System (TPS)
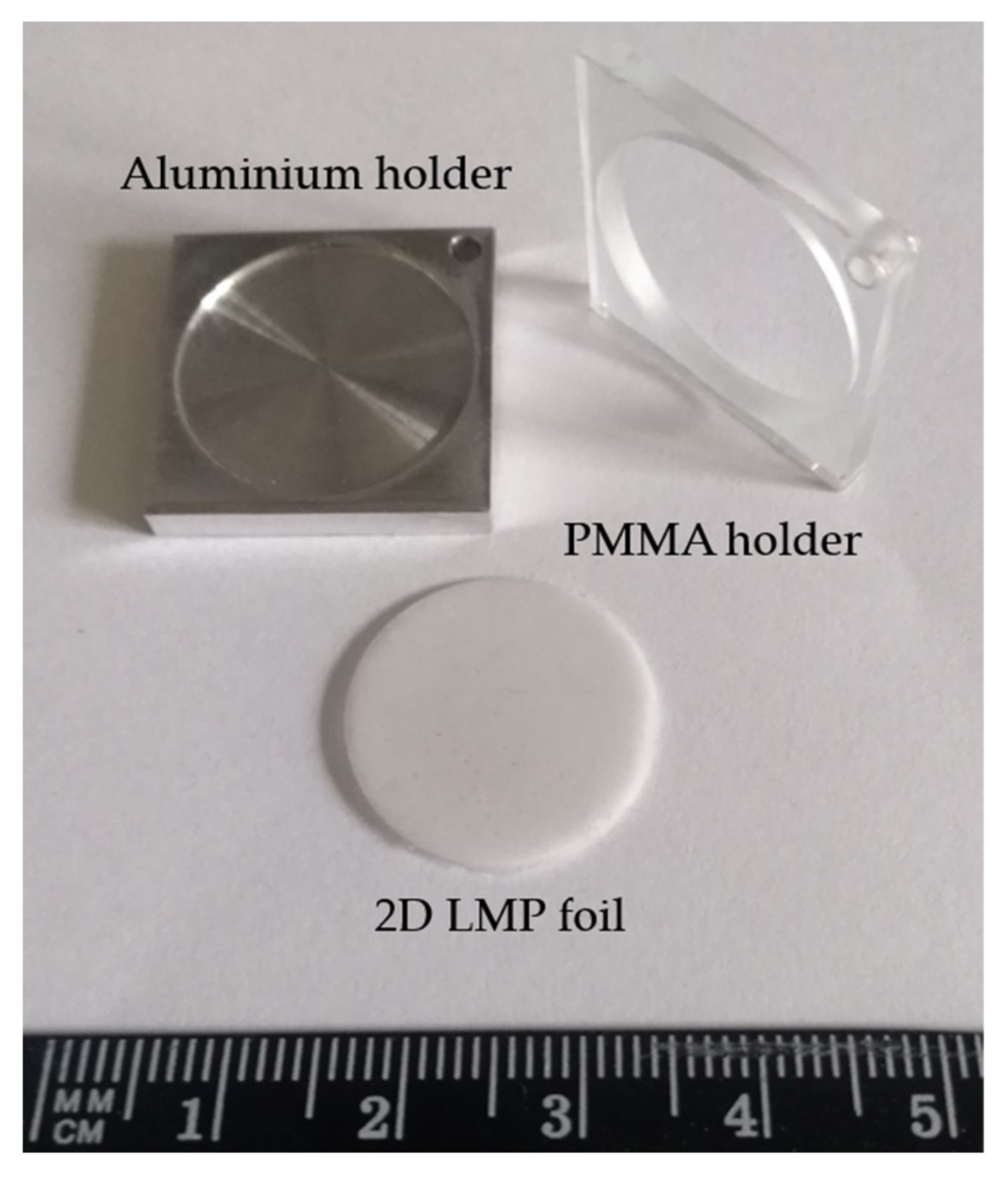
2.3. Eye-Ball Phantom Construction for TPS Verification
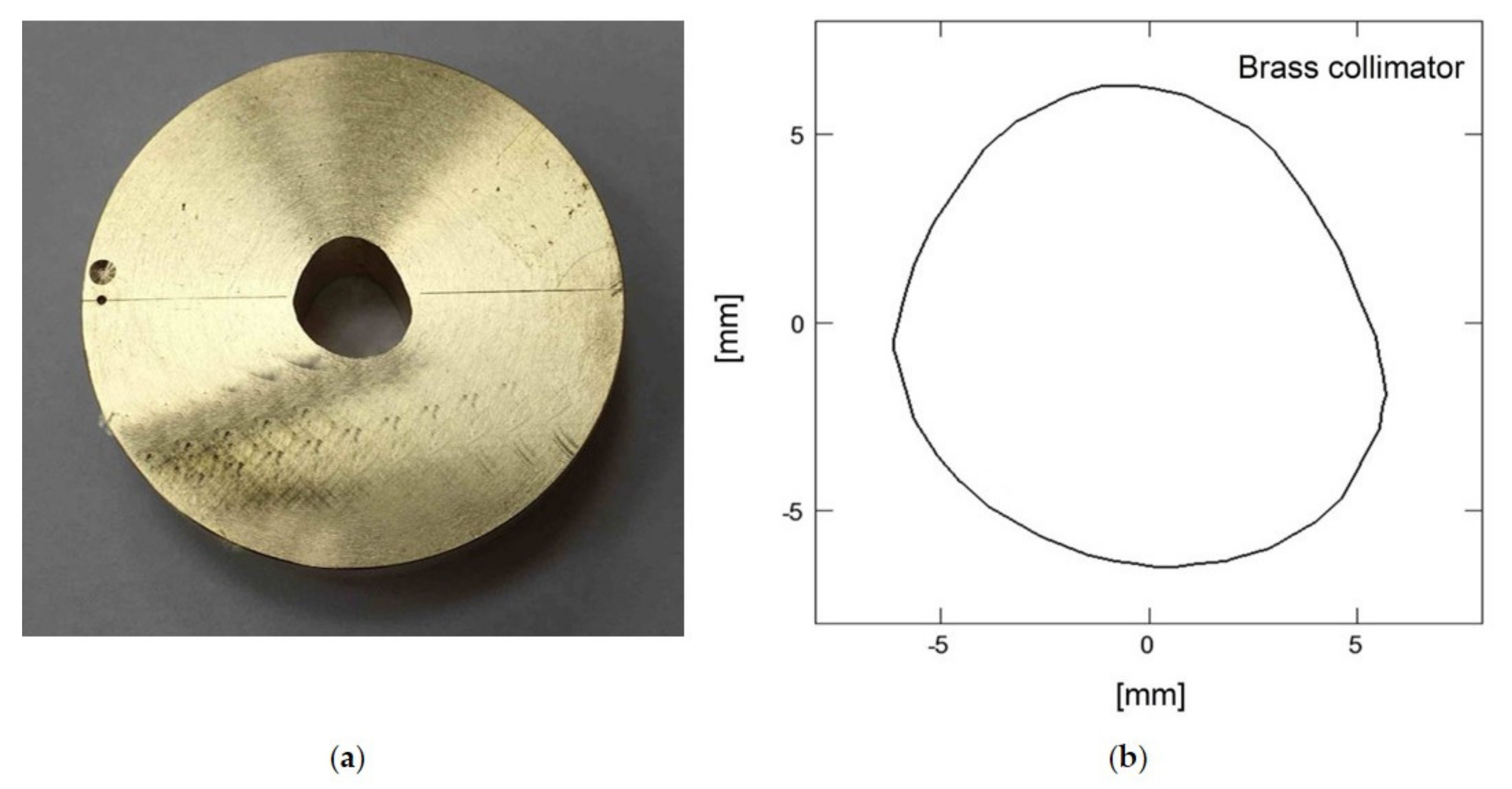
2.4. Proton Beam Dosimetry
2.5. Image Readout System
2.6. Image Data Acquisition
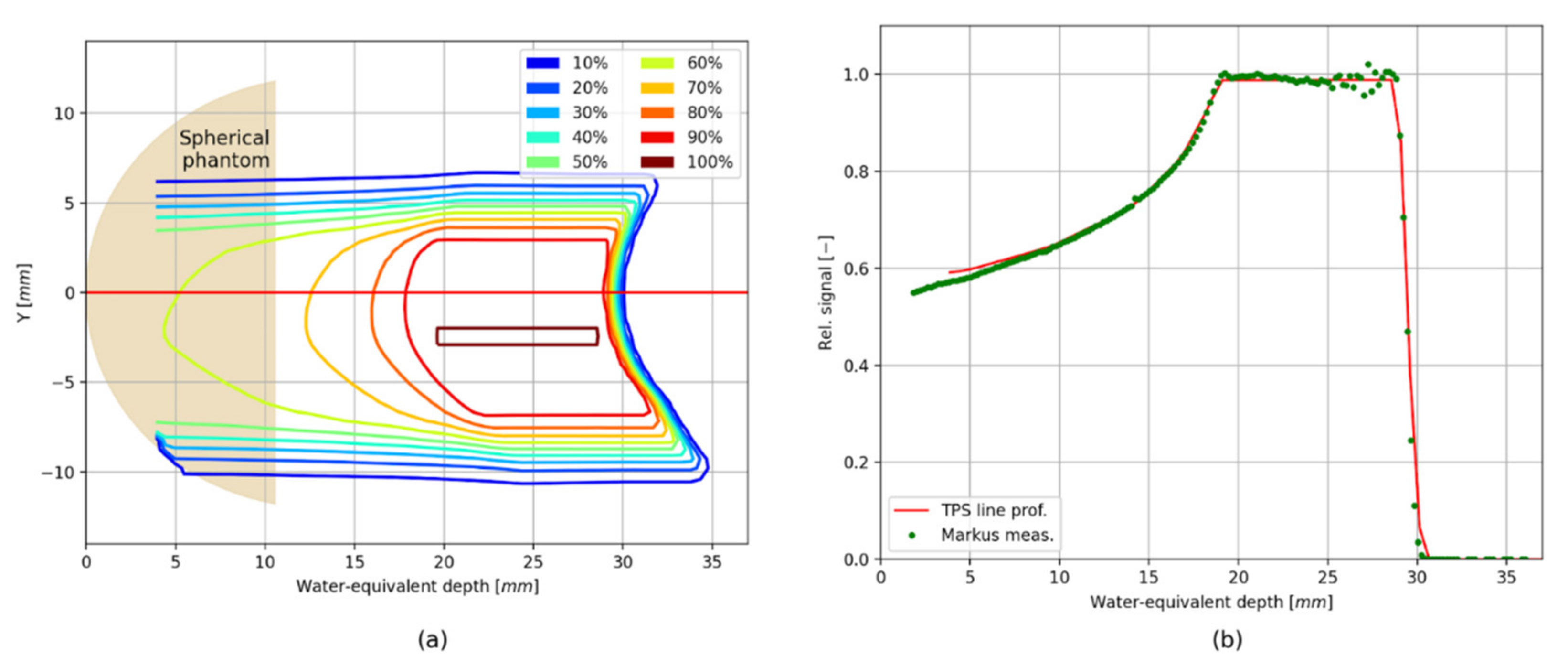
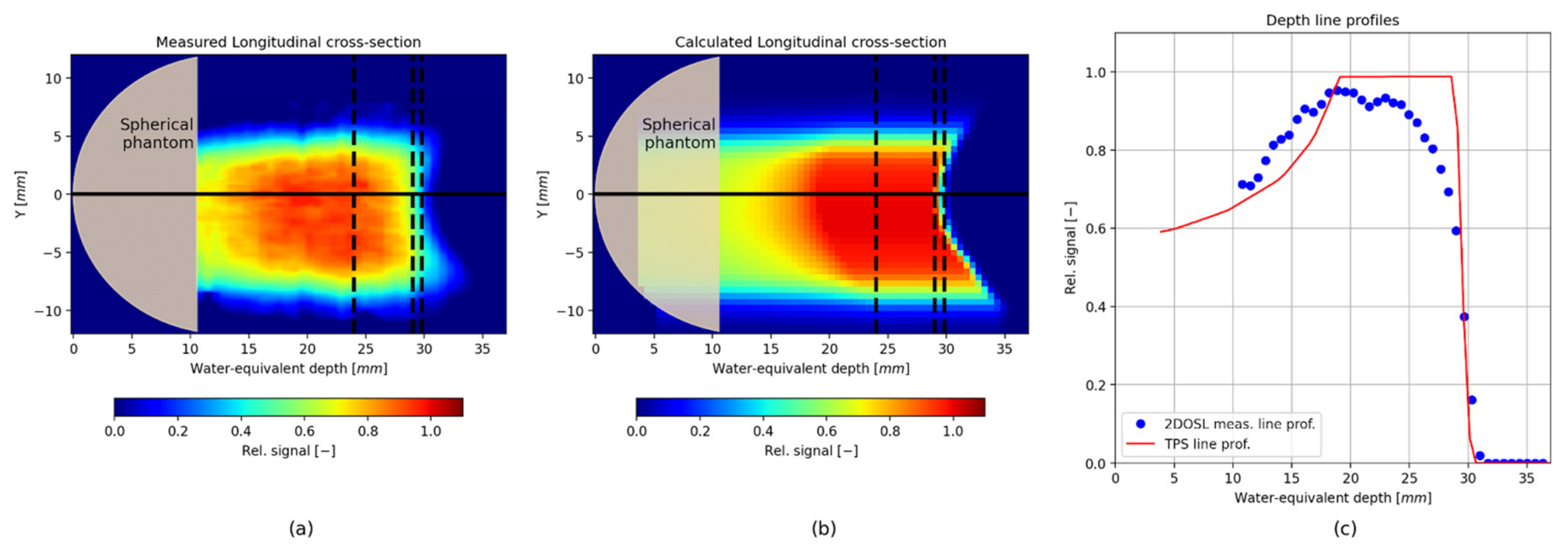
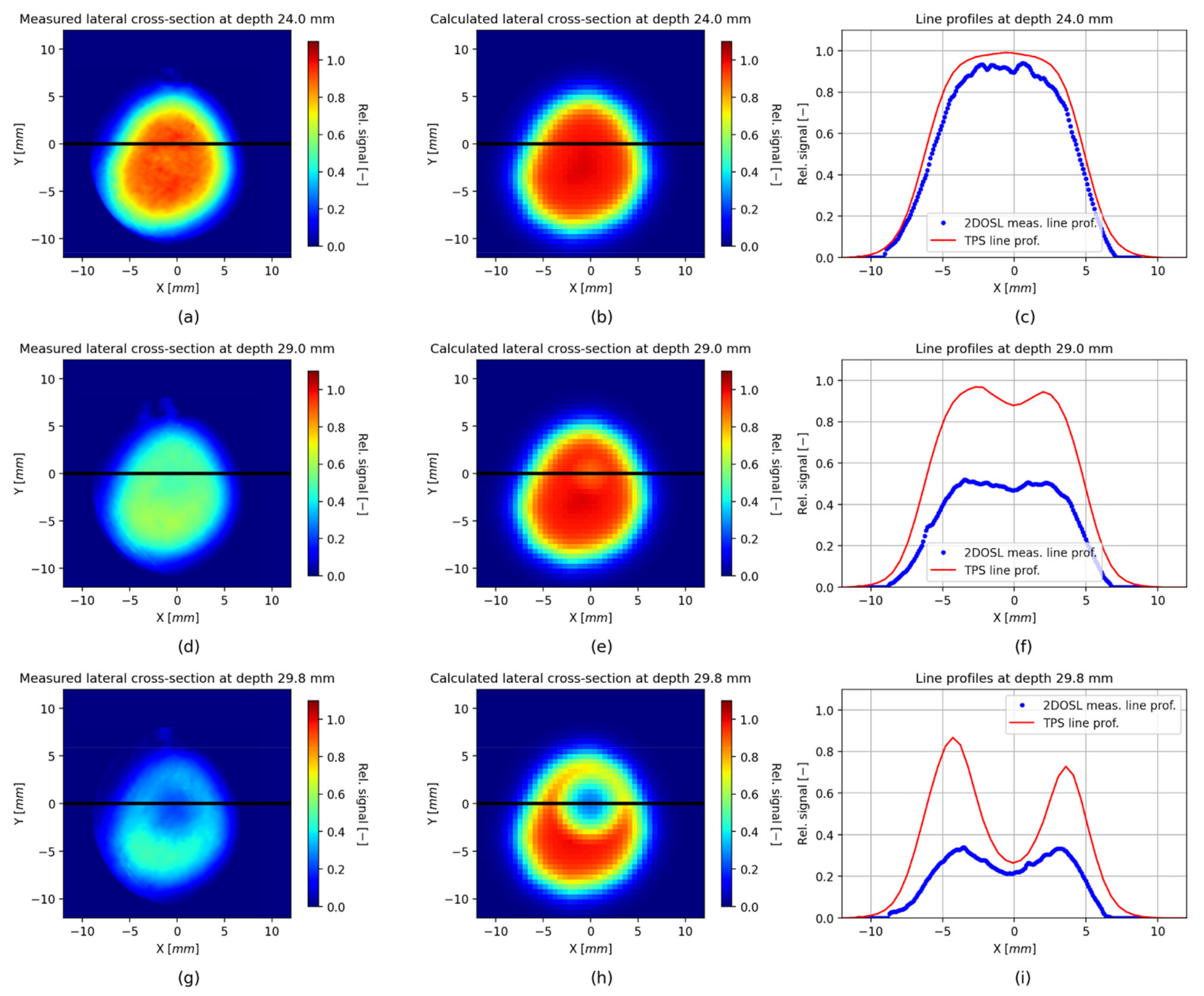
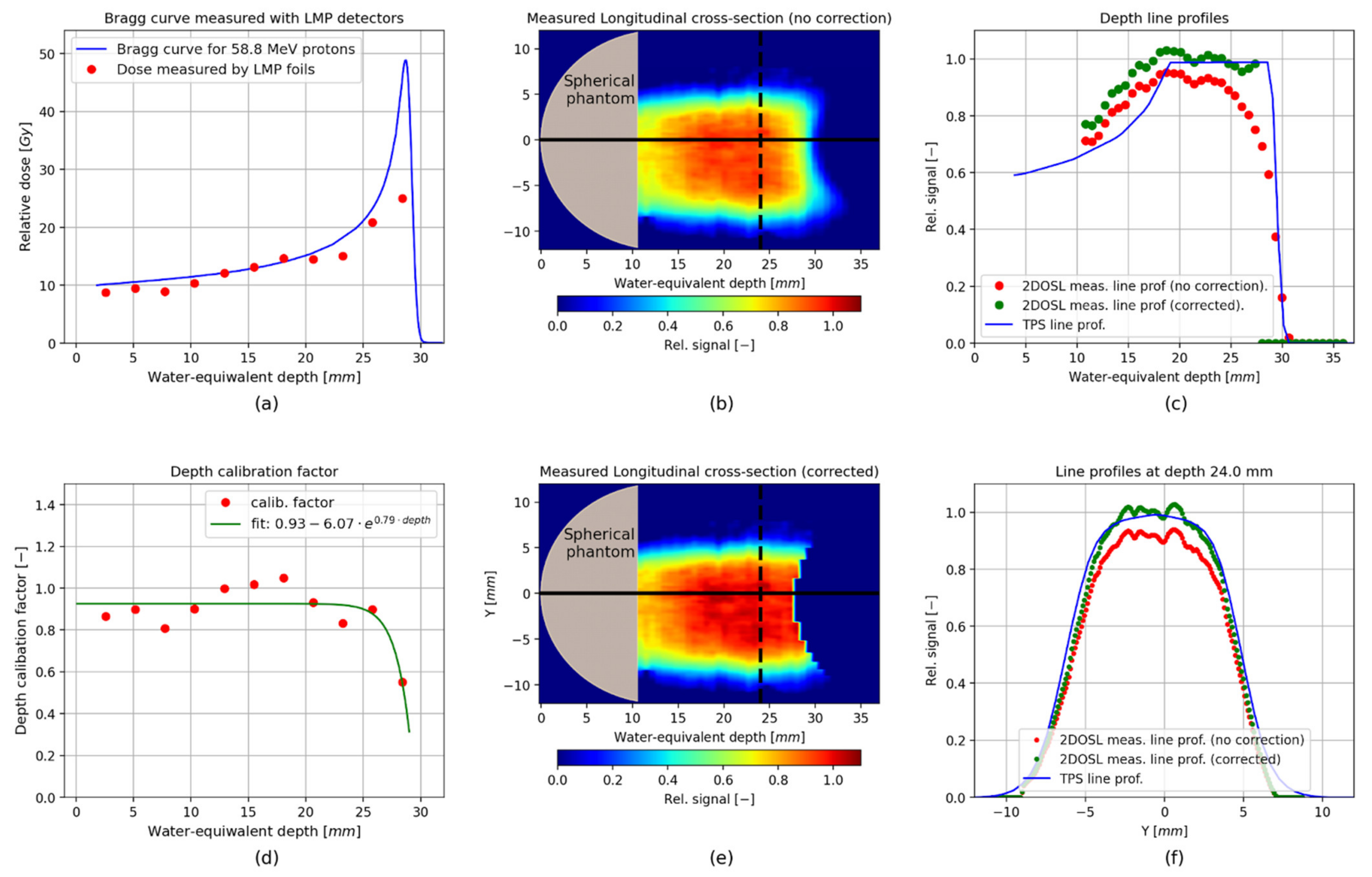
3. Results
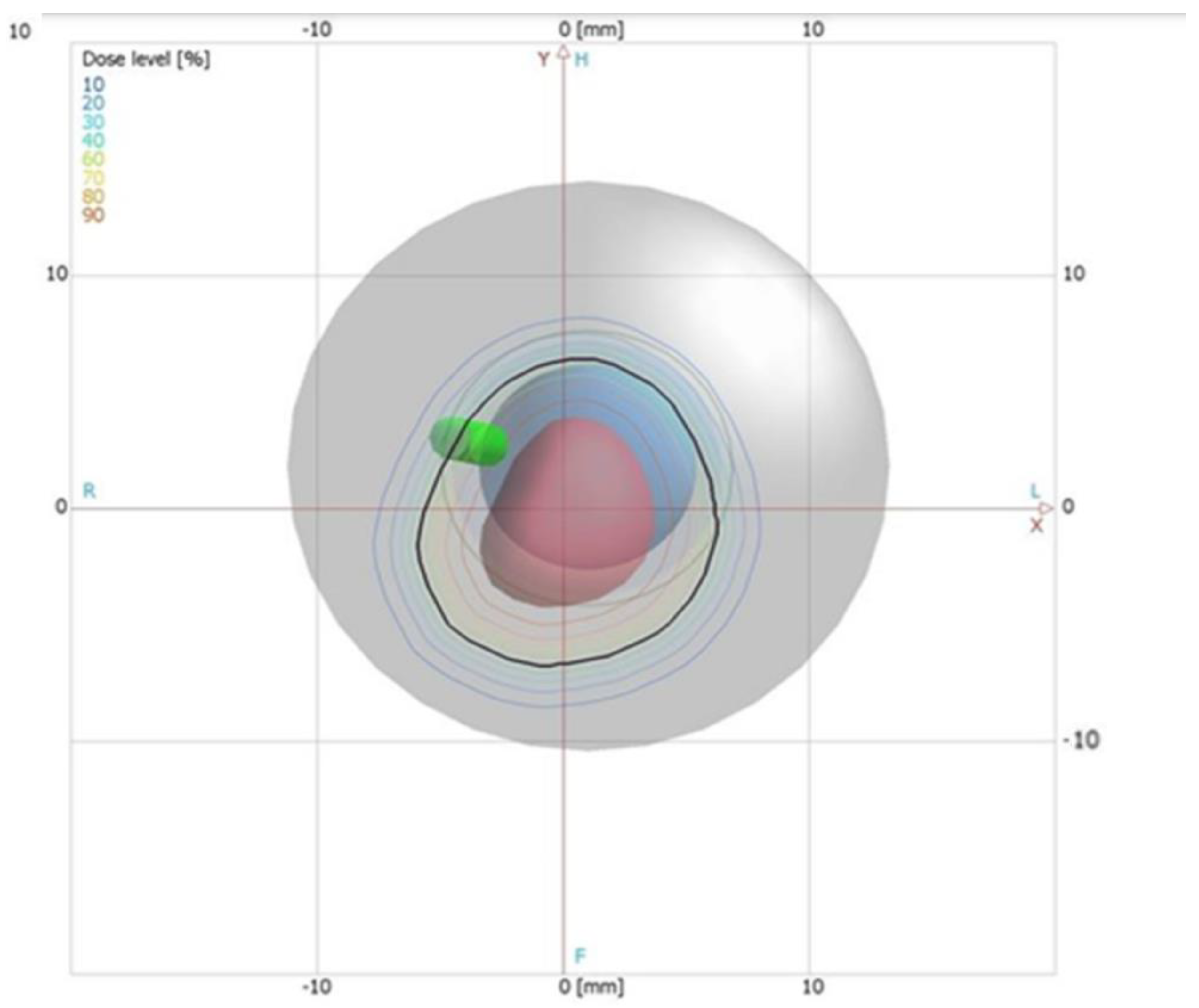
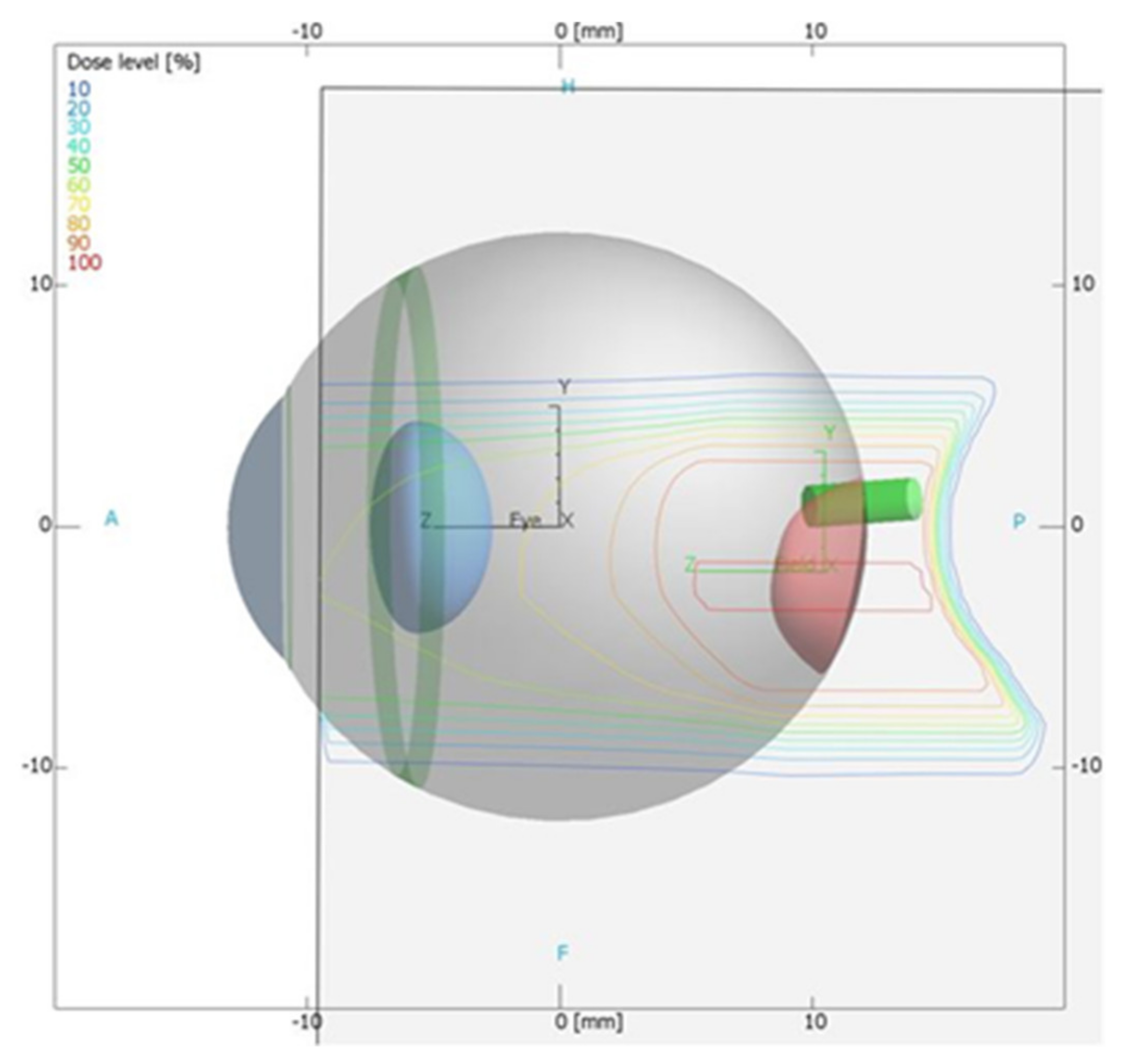
Verification of the TPS Using the 2D LMP Foils
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Jursinic, P.A. Characterization of optically stimulated luminescent dosimeters, OSLDs, for clinical dosimetric measurements. Med. Phys. 2007, 34, 4594–4604. [Google Scholar] [CrossRef]
- Mrčela, I.; Bokulić, T.; Izewska, J.; Budanec, M.; Fröbe, A.; Kusić, Z. Optically stimulated luminescencein vivodosimetry for radiotherapy: Physical characterization and clinical measurements in60Co beams. Phys. Med. Biol. 2011, 56, 6065–6082. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, A.H.; Olch, A.J. Validation of OSLD and a treatment planning system for surface dose determination in IMRT treatments. Med. Phys. 2014, 41, 081720. [Google Scholar] [CrossRef]
- Tariq, M.; Gomez, C.; Riegel, A.C. Dosimetric impact of placement errors in optically stimulated luminescent in vivo dosimetry in radiotherapy. Phys. Imaging Radiat. Oncol. 2019, 11, 63–68. [Google Scholar] [CrossRef]
- Idri, K.; Santoro, L.; Charpiot, E.; Herault, J.; Costa, A.; Ailleres, N.; Delard, R.; Vaille, J.; Fesquet, J.; Dusseau, L. Quality control of intensity modulated radiation therapy with optically stimulated luminescent films. IEEE Trans. Nucl. Sci. 2004, 51, 3638–3641. [Google Scholar] [CrossRef]
- Jahn, A.; Sommer, M.; Liebmann, M.; Henniger, J. Progress in 2D-OSL-dosimetry with beryllium oxide. Radiat. Meas. 2011, 46, 1908–1911. [Google Scholar] [CrossRef]
- Li, H.H.; Driewer, J.P.; Han, Z.; Low, D.A.; Yang, D.; Xiao, Z. Two-dimensional high spatial-resolution dosimeter using europium doped potassium chloride: A feasibility study. Phys. Med. Biol. 2014, 59, 1899–1909. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ahmed, M.; Eller, S.; Schnell, E.; Ahmad, S.; Akselrod, M.; Hanson, O.; Yukihara, E. Development of a 2D dosimetry system based on the optically stimulated luminescence of Al2O3. Radiat. Meas. 2014, 71, 187–192. [Google Scholar] [CrossRef]
- Ahmed, M.F.; Shrestha, N.; Schnell, E.; Ahmad, S.; Akselrod, M.S.; Yukihara, E.G. Characterization of Al2O3optically stimulated luminescence films for 2D dosimetry using a 6 MV photon beam. Phys. Med. Biol. 2016, 61, 7551–7570. [Google Scholar] [CrossRef]
- Sadel, M.; Høye, E.M.; Skyt, P.S.; Muren, L.P.; Petersen, J.B.B.; Balling, P. Three-dimensional radiation dosimetry based on optically-stimulated luminescence. J. Phys. Conf. Ser. 2017, 847, 012044. [Google Scholar] [CrossRef]
- Sądel, M.; Bilski, P.; Kłosowski, M. Optically stimulated luminescence of LiF: Mg,Cu,P with different dopant concentrations. Radiat. Meas. 2019, 123, 58–62. [Google Scholar] [CrossRef]
- Sądel, M.; Bilski, P.; Kłosowski, M. Optically stimulated luminescence of LiF: Mg, Cu, P powder—influence of thermal treatment. Radiat. Prot. Dosim. 2019, 186, 488–495. [Google Scholar] [CrossRef]
- Sądel, M.; Bilski, P.; Kłosowski, M.; Sankowska, M. A new approach to the 2D radiation dosimetry based on optically stimulated luminescence of LiF: Mg,Cu,P. Radiat. Meas. 2020, 133, 106293. [Google Scholar] [CrossRef]
- Wróbel, D.K.; Gieszczyk, W.; Bilski, P.; Marczewska, B.; Kłosowski, M. New OSL detectors based on LiMgPO4 crystals grown by micro pulling down method. Dosimetric properties vs. growth parameters. Radiat. Meas. 2016, 90, 303–307. [Google Scholar] [CrossRef]
- Kulig, D.; Gieszczyk, W.; Marczewska, B.; Bilski, P.; Kłosowski, M.; Malthez, A. Comparative studies on OSL properties of LiMgPO4:Tb,B powders and crystals. Radiat. Meas. 2017, 106, 94–99. [Google Scholar] [CrossRef]
- Gieszczyk, W.; Bilski, P.; Kłosowski, M.; Nowak, T.; Malinowski, L. Thermoluminescent response of differently doped lithium magnesium phosphate (LiMgPO4, LMP) crystals to protons, neutrons and alpha particles. Radiat. Meas. 2018, 113, 14–19. [Google Scholar] [CrossRef]
- Marczewska, B.; Sas-Bieniarz, A.; Bilski, P.; Gieszczyk, W.; Kłosowski, M.; Sądel, M. OSL and RL of LiMgPO4 crystals doped with rare earth elements. Radiat. Meas. 2019, 129, 106205. [Google Scholar] [CrossRef]
- Sądel, M.; Bilski, P.; Sankowska, M.; Gajewski, J.; Swakoń, J.; Horwacik, T.; Nowak, T.; Kłosowski, M. Two-dimensional radiation dosimetry based on LiMgPO4 powder embedded into silicone elastomer matrix. Radiat. Meas. 2020, 133, 106255. [Google Scholar] [CrossRef]
- Dhabekar, B.; Menon, S.; Raja, E.A.; Bakshi, A.; Singh, A.; Chougaonkar, M.; Mayya, Y. LiMgPO4:Tb,B—A new sensitive OSL phosphor for dosimetry. Nucl. Instrum. Methods Phys. Res. Sect. B Beam Interact. Mater. Atoms 2011, 269, 1844–1848. [Google Scholar] [CrossRef]
- Høye, E.M.; Skyt, P.S.; Yates, E.S.; Muren, L.; Petersen, J.B.B.; Balling, P. A new dosimeter formulation for deformable 3D dose verification. J. Phys. Conf. Ser. 2015, 573, 012067. [Google Scholar] [CrossRef]
- Swakon, J.; Olko, P.; Adamczyk, D.; Cywicka-Jakiel, T.; Dabrowska, J.; Dulny, B.; Grzanka, L.; Horwacik, T.; Kajdrowicz, T.; Michalec, B.; et al. Facility for proton radiotherapy of eye cancer at IFJ PAN in Krakow. Radiat. Meas. 2010, 45, 1469–1471. [Google Scholar] [CrossRef]
- IAEA TRS 398. Absorbed Dose Determination in External Beam Radiotherapy; Absorbed Dose Water IAEA: Vienna, Austria, 2000; ISSN 1011-4289. [Google Scholar]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Gajewski, J. FREDtools—Radiotherapy Image Analysis in Python. Available online: www.fredtools.ifj.edu.pl (accessed on 1 August 2021).
- Gajewski, J.; Schiavi, A.; Krah, N.; Vilches-Freixas, G.; Rucinski, A.; Patera, V.; Rinaldi, I. Implementation of a Compact Spot-Scanning Proton Therapy System in a GPU Monte Carlo Code to Support Clinical Routine. Front. Phys. 2020, 8, 8. [Google Scholar] [CrossRef]
- Gajewski, J.; Garbacz, M.; Chang, C.-W.; Czerska, K.; Durante, M.; Krah, N.; Krzempek, K.; Kopeć, R.; Lin, L.; Mojżeszek, N.; et al. Commissioning of GPU–Accelerated Monte Carlo Code FRED for Clinical Applications in Proton Therapy. Front. Phys. 2021, 8, 403. [Google Scholar] [CrossRef]
- Sądel, M.; Bilski, P.; Swakoń, J. Relative TL and OSL efficiency to protons of various dosimetric materials. Radiat. Prot. Dosim. 2013, 161, 112–115. [Google Scholar] [CrossRef] [PubMed]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sądel, M.; Gajewski, J.; Sowa, U.; Swakoń, J.; Kajdrowicz, T.; Bilski, P.; Kłosowski, M.; Pędracka, A.; Horwacik, T. 3D Dosimetry Based on LiMgPO4 OSL Silicone Foils: Facilitating the Verification of Eye-Ball Cancer Proton Radiotherapy. Sensors 2021, 21, 6015. https://doi.org/10.3390/s21186015
Sądel M, Gajewski J, Sowa U, Swakoń J, Kajdrowicz T, Bilski P, Kłosowski M, Pędracka A, Horwacik T. 3D Dosimetry Based on LiMgPO4 OSL Silicone Foils: Facilitating the Verification of Eye-Ball Cancer Proton Radiotherapy. Sensors. 2021; 21(18):6015. https://doi.org/10.3390/s21186015
Chicago/Turabian StyleSądel, Michał, Jan Gajewski, Urszula Sowa, Jan Swakoń, Tomasz Kajdrowicz, Paweł Bilski, Mariusz Kłosowski, Anna Pędracka, and Tomasz Horwacik. 2021. "3D Dosimetry Based on LiMgPO4 OSL Silicone Foils: Facilitating the Verification of Eye-Ball Cancer Proton Radiotherapy" Sensors 21, no. 18: 6015. https://doi.org/10.3390/s21186015
APA StyleSądel, M., Gajewski, J., Sowa, U., Swakoń, J., Kajdrowicz, T., Bilski, P., Kłosowski, M., Pędracka, A., & Horwacik, T. (2021). 3D Dosimetry Based on LiMgPO4 OSL Silicone Foils: Facilitating the Verification of Eye-Ball Cancer Proton Radiotherapy. Sensors, 21(18), 6015. https://doi.org/10.3390/s21186015





