Multiphoton Laser Fabrication of Hybrid Photo-Activable Biomaterials
Abstract
:1. Introduction
2. Light Interaction with Soft Matter
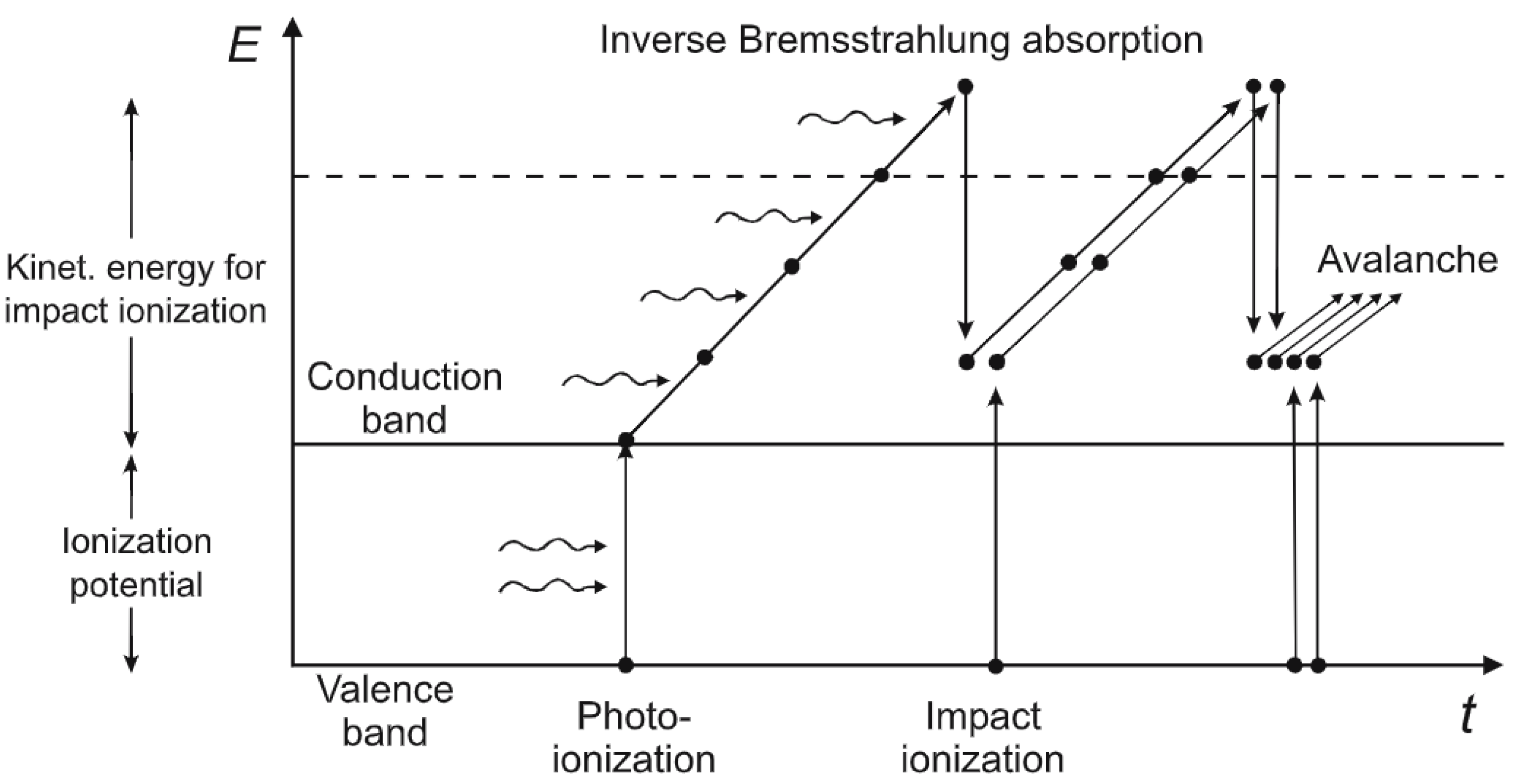
2.1. Photo-Excitation and Photo-Ionization
2.2. Multiphoton Polymerization
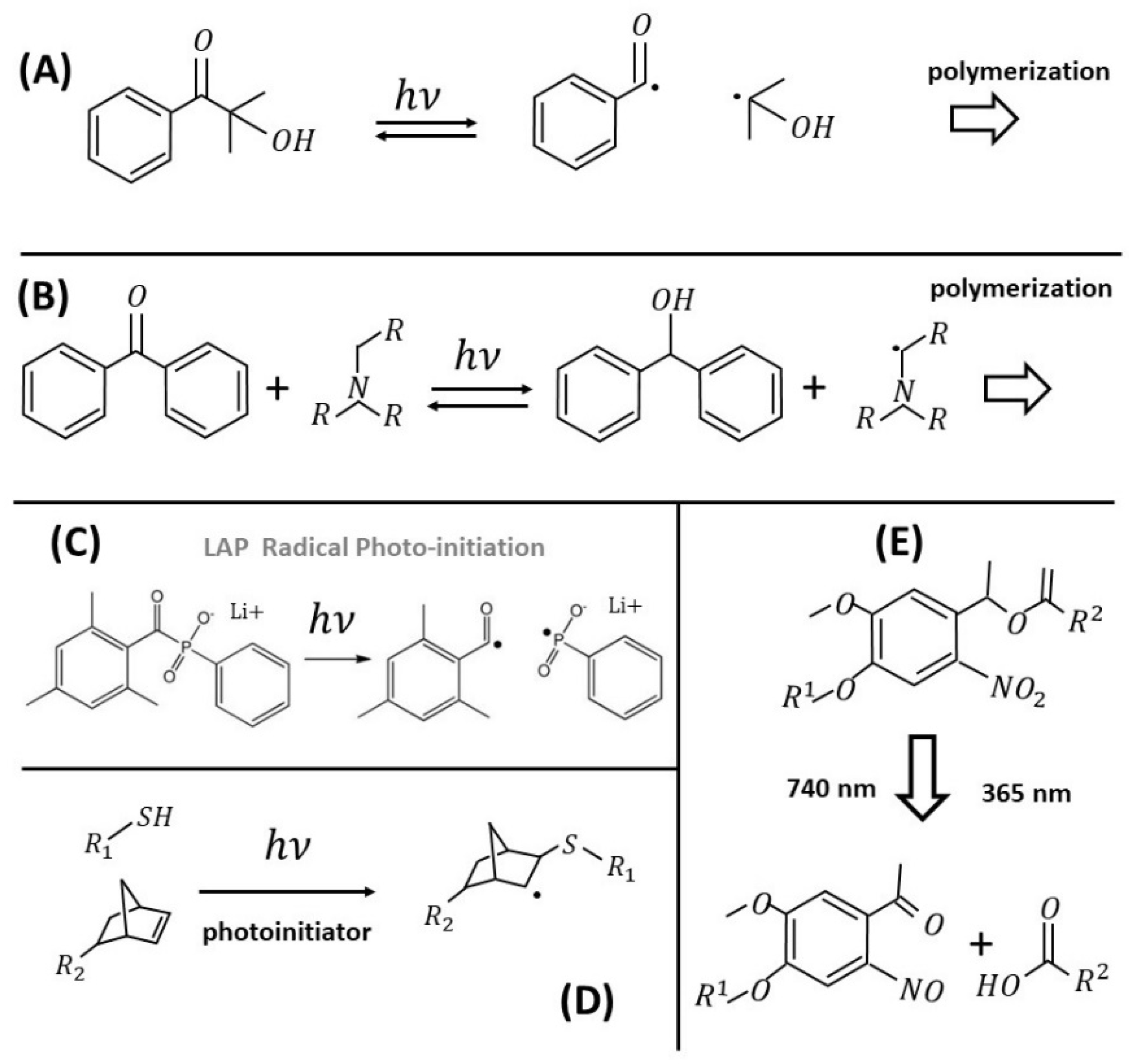
2.2.1. Radical Photoinitiators
2.2.2. Cationic Photoinitiators
2.2.3. Materials for MAP
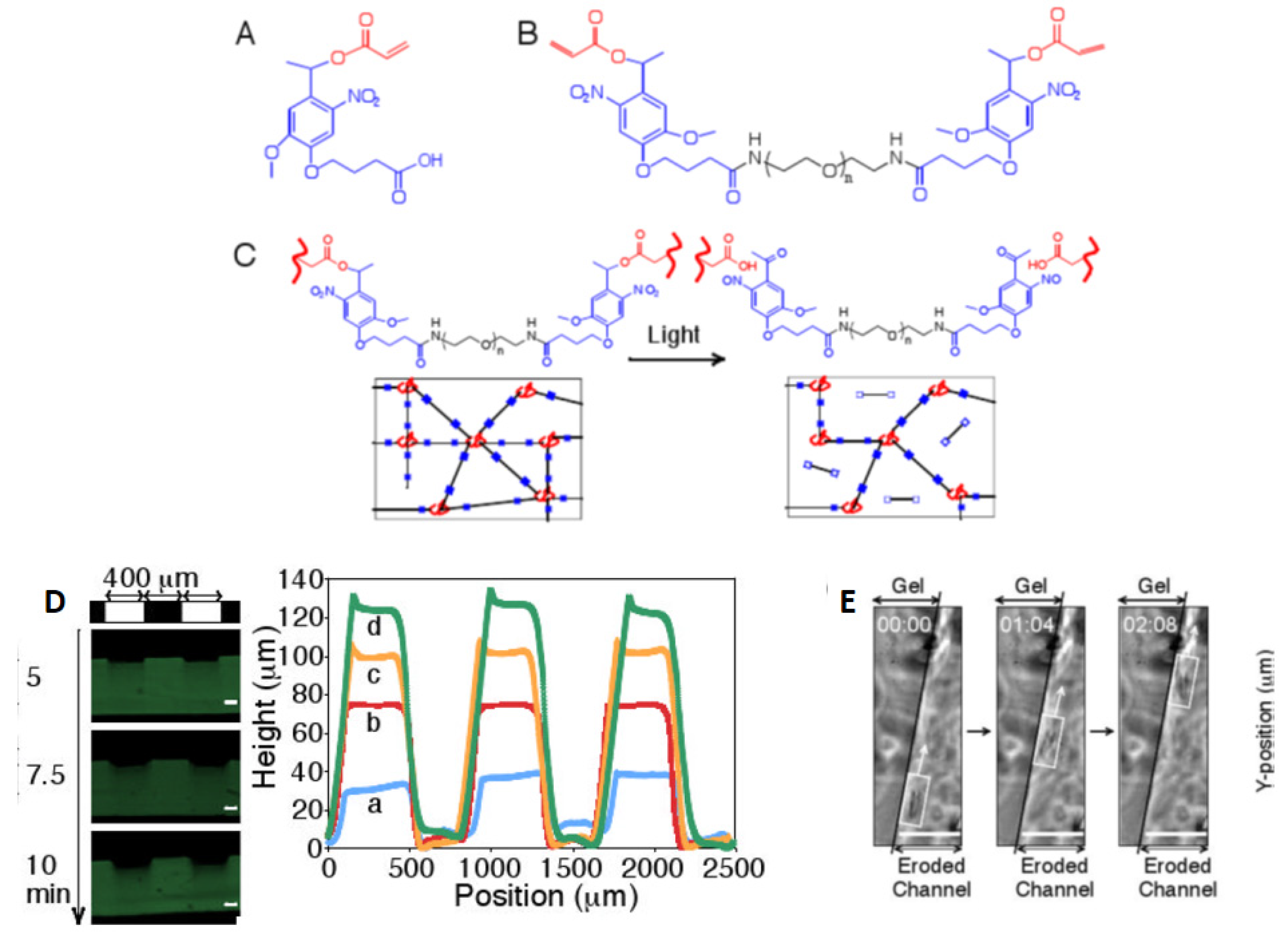
2.3. Multiphoton Polymer Ablation/Degradation
3. Laser-Fabricated Active Microstructured Hydrogels
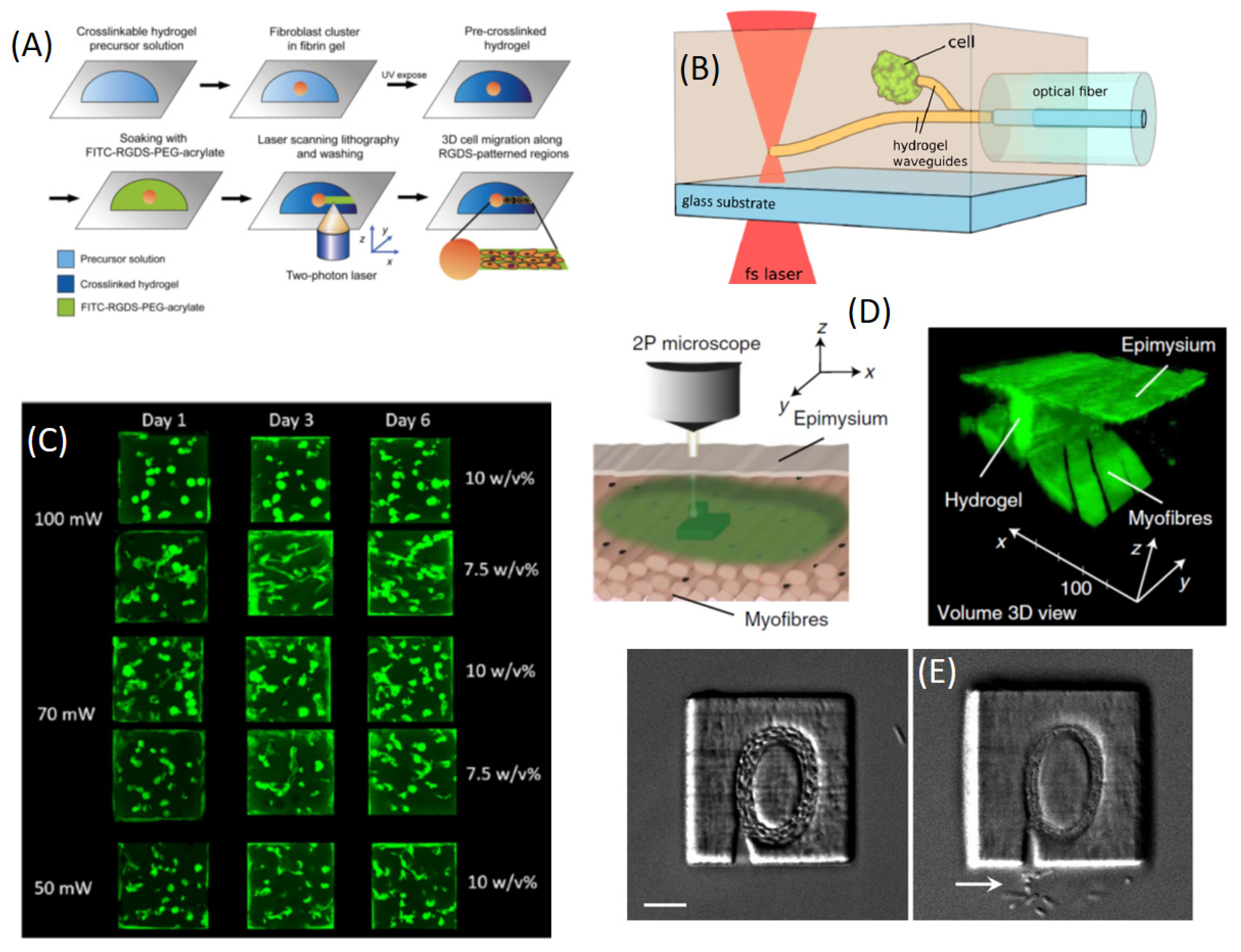
3.1. Chemical and Topographic Patterning to Probe and Guide Cells
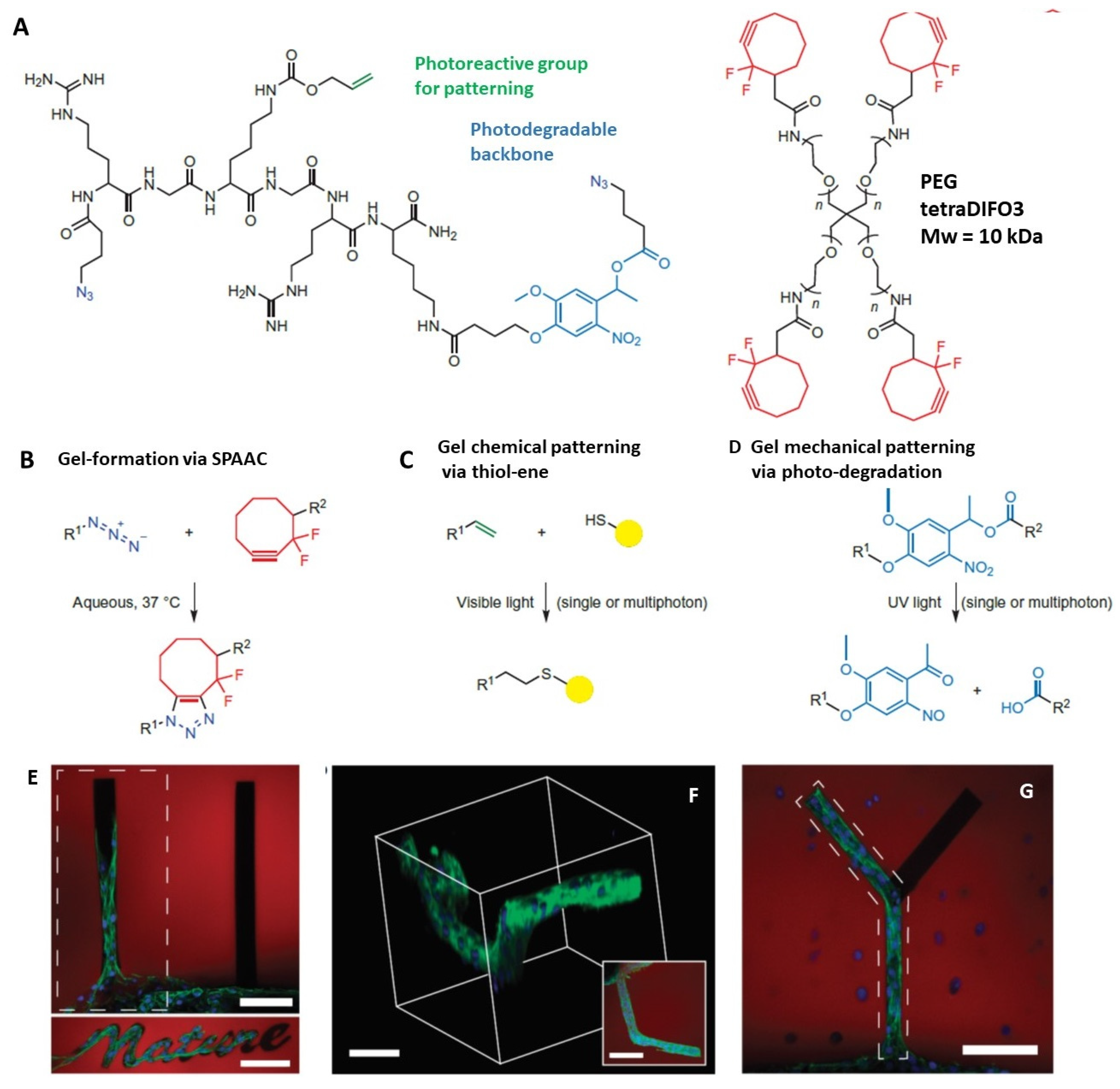
3.2. Four-Dimensional Printing and Click Photochemistry
3.3. Microfabrication for Tissue Engineering
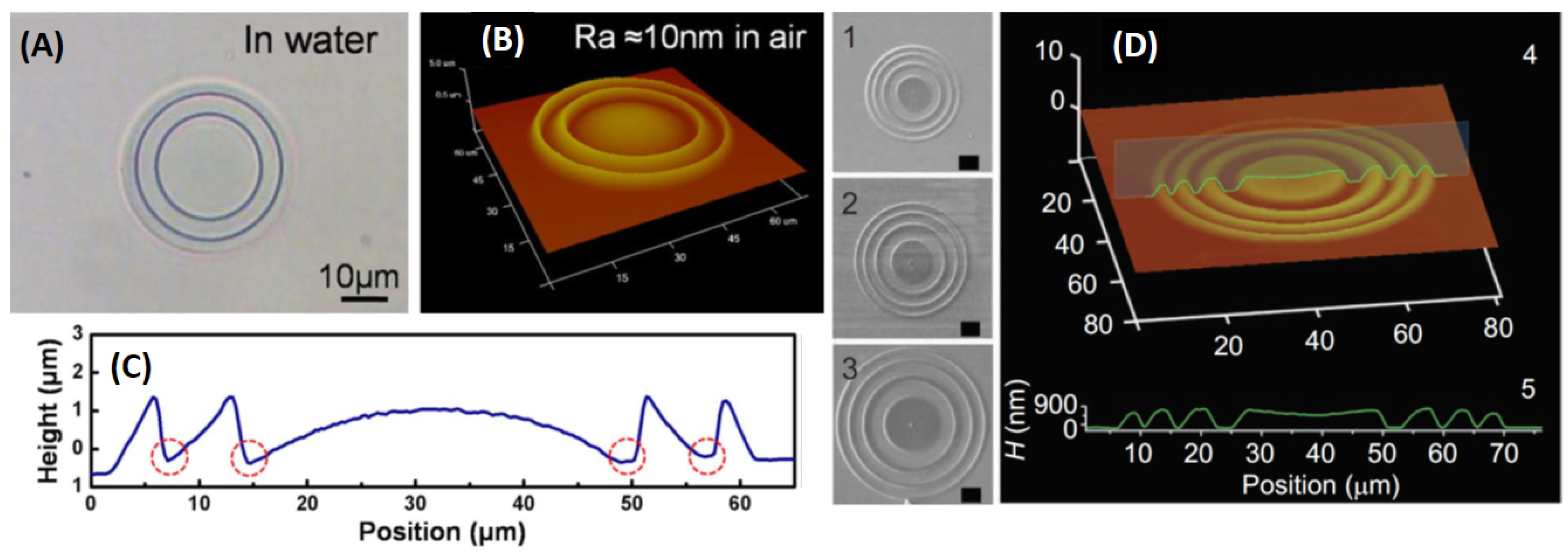
3.4. Hydrogel-Based Micro-Optics
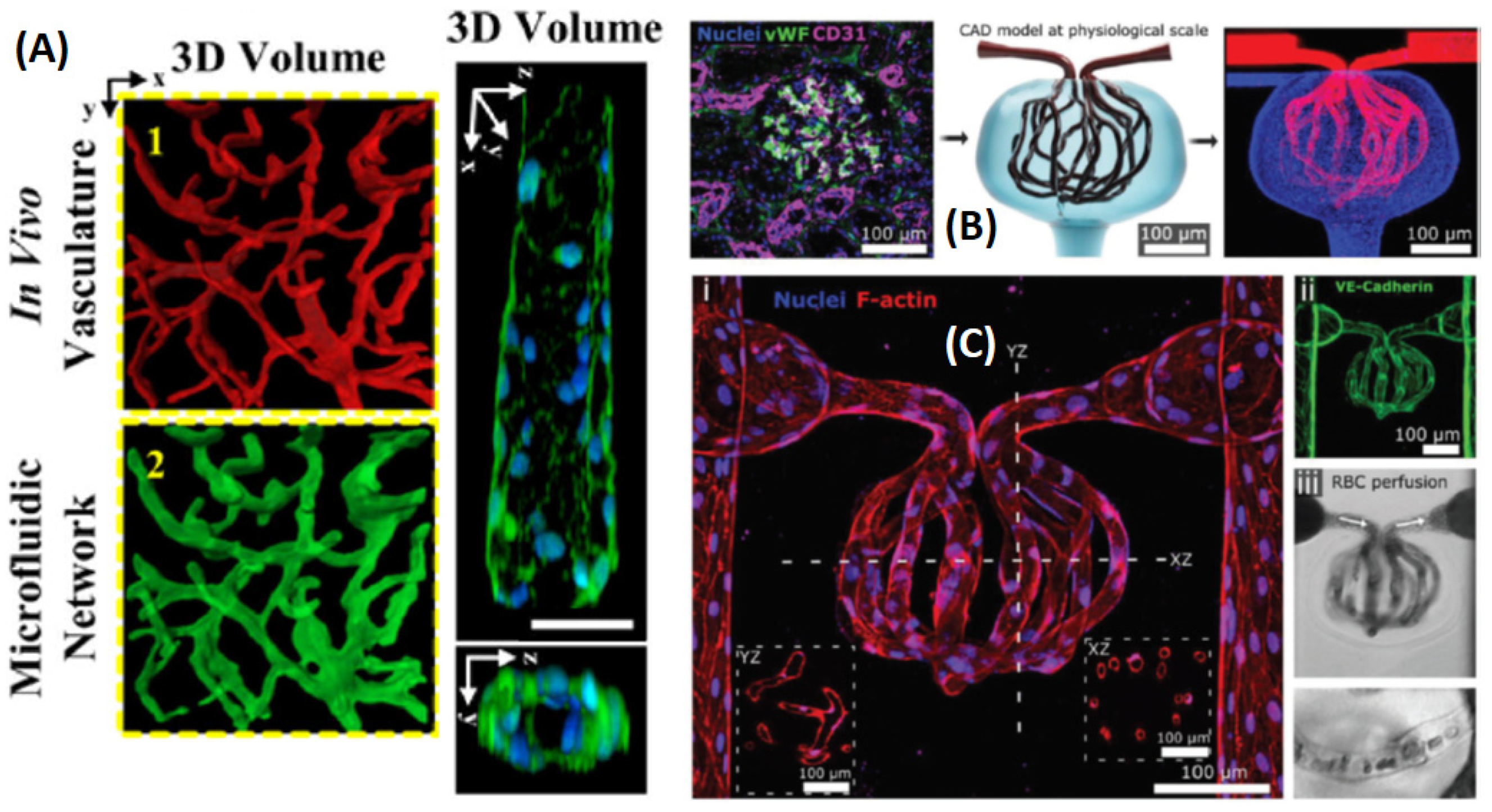
3.5. Microvasculature 3D and 4D Printing
3.6. 3D Microfabrication/Ablation in Living Organisms and Cell Cultures
3.7. Biomimetic Responsive Microstructures
3.7.1. pH-Dependent Micro-Hydrogels
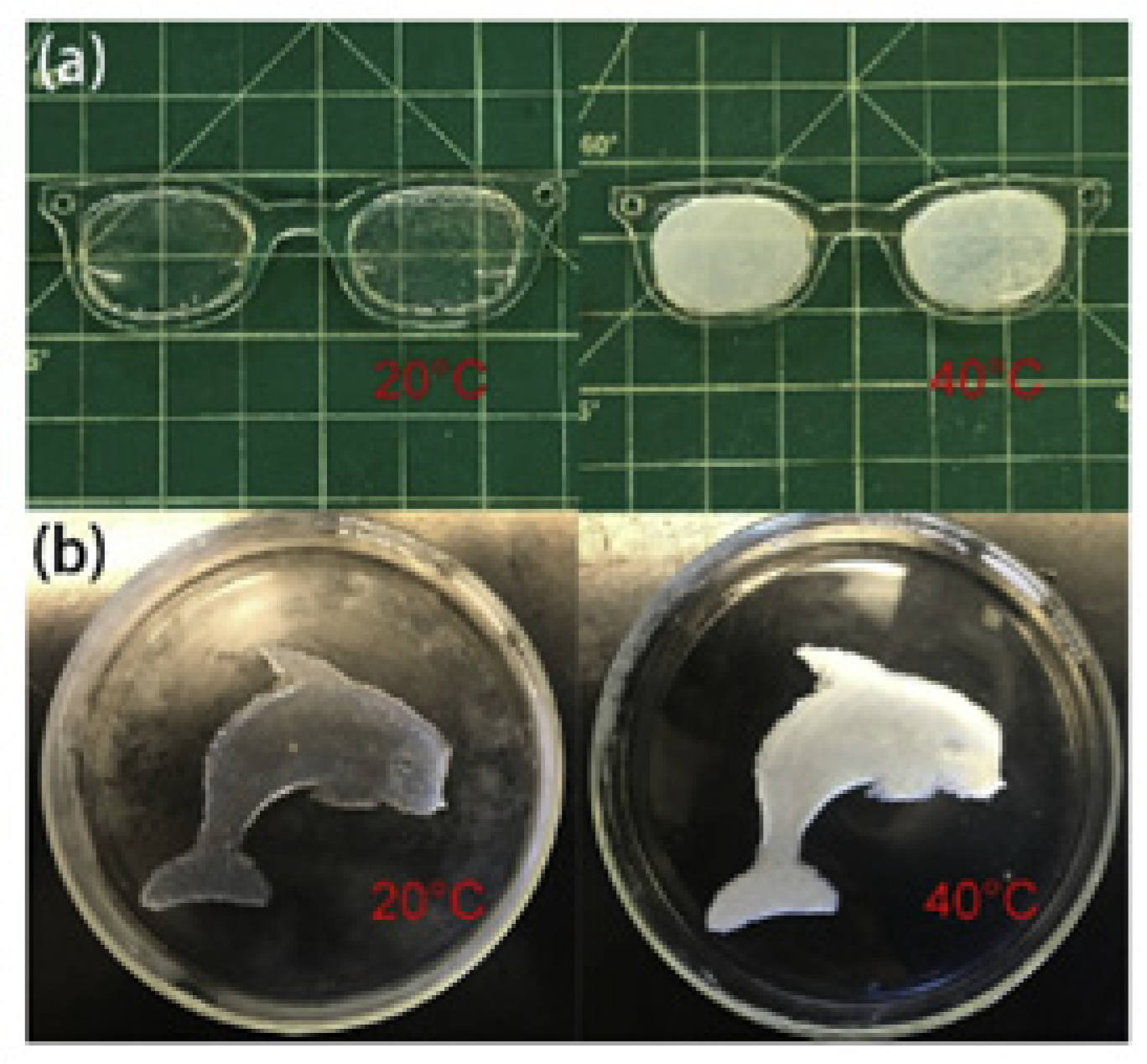
3.7.2. Photothermally Responsive Microstructured Hydrogels
| Material | Technique | Source | Application | Ref. |
|---|---|---|---|---|
| ACRL-PEG-peptide or low-MW PEGDA, 300 mg/mL 2,2-dimethoxy-2-phenyl-acetophenone in N-vinyl-pyrrolidone (NVP) | Mask photo-lythography (single photon) and MPL | For MPL, Ti:sapphire, 720 nm, average I = 225 mW/ pixel dwell time = 120 | Softening the matrix; used to guide cell migration | [84] |
| PEGDA (575 kDa) 9%, 1 nM AuNRs, 0.05% (w/v) LAP | MPL in prefabricated hydrogel by thermal erosion | , P = = 1.0 mm/s | Modulation of stiffness | [87] |
| Irgacure 819, (1 wt%), 20 wt % allyl-functional PNIPAm, 20 wt % NIPAm (Iso-propyl-acrylamide), and 2wt % N,N′-methylene bis-acrylamide | MPL | , P = = 1000 to 10,000 μm/s | 3D soft actuator composed of thermoresponsive polymers | [88] |
| PEGdiPDA linked to a monoacrylated PEG macromer through a photolabile ortho-nitrobenzylether (NBE) | MPL fabrication and erosion | 740 nm, pixel dwell time = 1.58 ms, P = 0.1 W | Photodegradation of hydrogel to guide hMSC migration | [67] |
| Four-arm PEG tetracyclooctyne | Thermal gelation + MPL erosion | Ti:Sapphire, ) | ); outgrowth of 3T3 fibroblast cells | [3] |
| Collagen/fibrin functionalized with a photocaged alkoxyamine. Photocaging through a 2-(2-nitrophenyl) propoxycarbonyl group. | Fabrication by photomask UV lithography + MPL erosion | , 10 min exp. time; MPL with lympus FV1000 MPE BX61 Microscope (λ = 740 nm, 0 to 100 laser power, a.u.) | Decoration of cell-laden natural hydrogels with growth factors | [96] |
| Norbornene-functionalized PVA 22 kDa, 7% substitution; 5–20% in mass, dithiothreitol (DTT) | ) and MPL degradation | Photochemical construction and manipulation of 3D cellular microenvironments of kidney epithelial cells | [100] | |
| Acrylated photolabile moiety to PEG-bis-amine to create a photocleavable cross-linking diacrylate macromer; TEMED with ammonium persulfate | Spontaneous ammonium sulfate polymerization and MPL erosion | Two-photon 740nm laser (3W laser, 20× objective NA~ 0.75, 1 μm scan intervals over~ 150 μm thickness, laser power = 50%, scan speed setting = 8) | Migration of fibrosarcoma cells along the edge of a channel | [83] |
| Barnase complexed agarose hydrogel modified with coumarin-caged thiols | UV (365 nm) photolithography followed by MPL photodegradation/activation | 740 nm | 3D patterns of proteins and growth factors cultures, to guide cells | [104] |
| 75% v/v of native bovine dermis type-I collagen solution and 25% v/v Matrigel | Thermal polymerization followed by MPL erosion | = 100-Hz, at 355 nm), 10 ×/ 0.25 NA objective | Induction of intestinal stem cells to form tube-shaped epithelia | [35] |
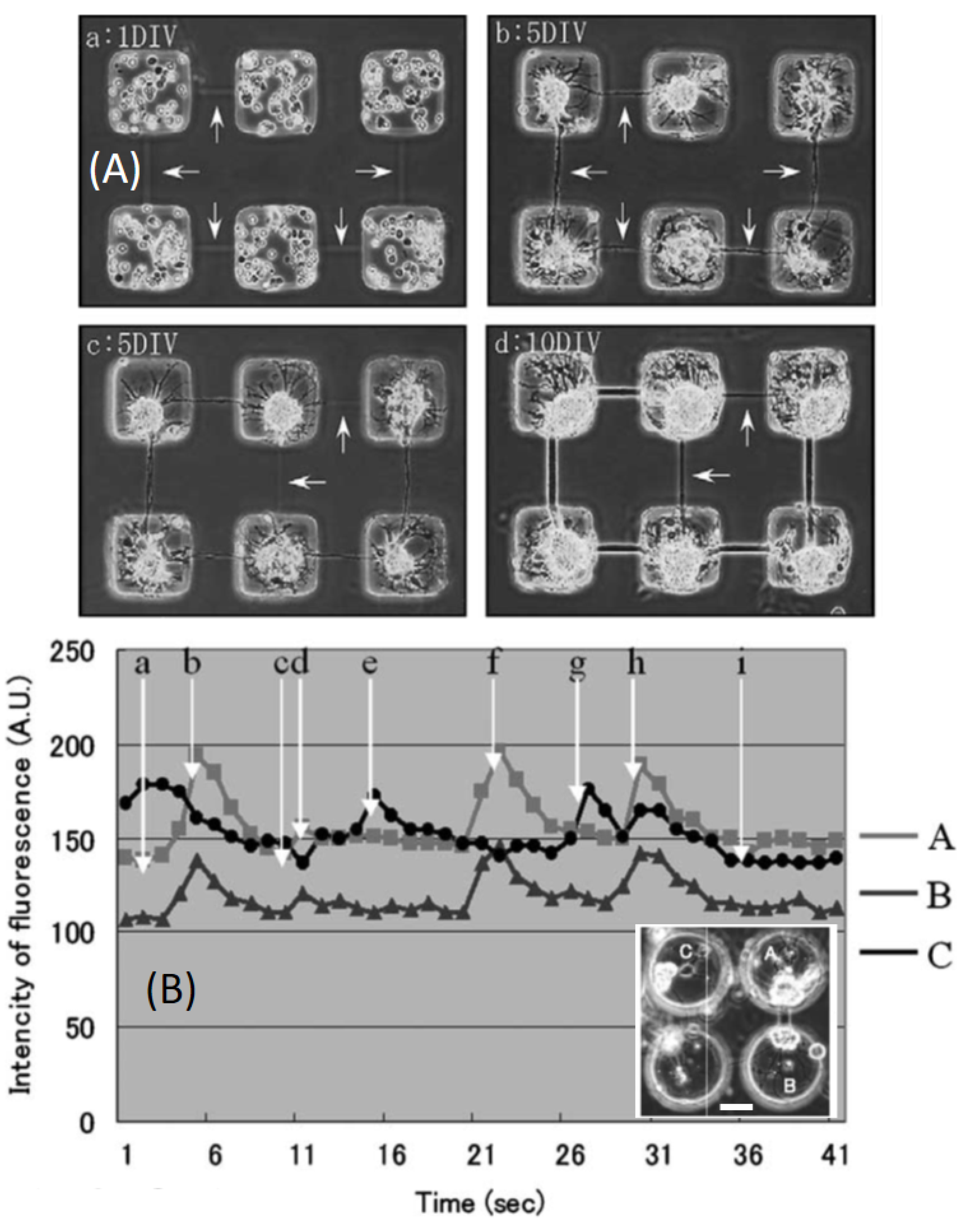
| Agarose | Cold gelation and thermal laser-induced erosion of agarose hydrogels | Rat hippocampal cells forming fiber connections between chambers | [36] | |
| Agarose | Cold gelation and thermal laser induced erosion of agarose hydrogels | 10 Hz | Artificial growth of neural tissue | [72] |
| (3-trimethoxy-silylpropyl) diethylene triamine in a uniform layer of octadecylsilane | Laser ablation of self-assembled monolayers in presence of laminin-1 | 1 kHz, P = 0.5 mW | length | [109] |
| Hybrid poly(N-isopropylacrylamide) (PNIPAm)/cellulose nanofibrils (CNFs) hydrogel, (TEGDMA) as a cross-linker | UV photolithography | UV radiation (365 nm) | Hydrogel-based micro-optics | [114] |
| BSA and methylene blue | MPL fabrication | Hydrogel-based micro-optics, kinoform lenses | [52] | |
| BSA and methylene blue | MPL fabrication | Microlenses with pH-dependent focal length | [115] | |
| BSA and methylene blue | MPL fabrication | Tunable harmonic diffractive lenses (HDL) | [116] | |
| PEGylated fibrinogen hydrogels | Multiphoton LA in hydrogels | Design of nerve guidance conduits | [122] | |
| PEGDA hydrogel | Multiphoton LA in hydrogels | Vascular bed created by degradation of the gel and seeding of endothelial cells (ECs) | [69] | |
| ), and a hydrogel containing 2% (w/v) each of agarose and gelatin | Multiphoton LA in hydrogels | Connections among channels | [123] | |
| Collagen hydrogels | Multiphoton LA in hydrogels | Study of metastasis infiltration | [71] | |
| PEG hydrogels: acrylate-PEG-(GGGLGPAGGK-PEG)n-acrylate | Multiphoton LA in hydrogels | scanning rate | Controlled HDF migration | [124] |
| PEGDA hydrogel (with IrgacureTM PIs) or BSA with Riboflavin | Multiphoton LA in hydrogels | E. coli trapping | [125,126] | |
| PEGda, 700Da) 50 wt%, 2 wt% PI: 2PCK or DAS | MPL fabrication | 1–100 mm/s | Trapping of cells: osteosarcoma and endothelial cells | [128] |
| Methacrylamide (RCPhC1-MA), norbornene (RCPhC1-NB), or thiol (RCPhC1-SH) + DAS | MPL fabrication | 1000 mm/s | Encapsulation of human adipose stem cells | [129] |
| PEGDa and a water-soluble PI | MPL fabrication | 10 mm/s | Encapsulation of Caenorhabditis elegans (C. elegans) | [132] |
| PEG–coumarin hydrogels | MPL fabrication | 0.8 mm/s | In vivo subcutaneous fabrication of hydrogels | [130] |
| BSA hydrogels | MPL fabrication | 30 μm/s. | pH-sensitive deformable structures | [136,137] |
| 2-carboxyethyl acrylate (CEA) and PEGDA (10 kDa), PI = Irgacure-2959 | MPL fabrication | Ti:Sapphire, 780 nm, P = 17 mW, 100 μm/s. | pH-sensitive deformable structures | [139] |
| BSA and Rose Bengal and gold nanostars | MPL fabrication | /s | In vivo subcutaneous fabrication of hydrogels | [130] |
4. Conclusions. Two in One—Light Brush and Light Chisel for Hydrogels
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Chirico, G.; Dacarro, G.; O’Regan, C.; Peltonen, J.; Sarfraz, J.; Taglietti, A.; Borzenkov, M.; Pallavicini, P. Photothermally responsive inks for inkjet-printing secure information. Part. Part. Syst. Charact. 2018, 5, 1800095. [Google Scholar] [CrossRef]
- Cha, C.; Oh, J.; Kim, K.; Qiu, Y.; Joh, M.; Shin, S.R.; Wang, X.; Camci-Unal, G.; Wan, K.-T.; Liao, R.; et al. Microfluidics-assisted fabrication of gelatin-silica core–shell microgels for injectable tissue constructs. Biomacromolecules 2014, 15, 283–290. [Google Scholar] [CrossRef] [PubMed]
- DeForest, C.A.; Anseth, K.S. Cytocompatible click-based hydrogels with dynamically tunable properties through orthogonal photoconjugation and photocleavage reactions. Nat. Chem. 2011, 3, 925–931. [Google Scholar] [CrossRef]
- Skliutas, E.; Lebedevaite, M.; Kasetaite, S.; Rekštytė, S.; Lileikis, S.; Ostrauskaite, J.; Malinauskas, M. A bio-based resin for a multi-scale optical 3D printing. Sci. Rep. 2020, 10, 9758. [Google Scholar] [CrossRef]
- Pradhan, S.; Keller, K.A.; Sperduto, J.L.; Slater, J.H. Fundamentals of laser-based hydrogel degradation. Adv. Healthc. Mater. 2017, 6, 1700681. [Google Scholar] [CrossRef]
- Munoz-Robles, B.G.; Kopyeva, I.; DeForest, C.A. Surface patterning of hydrogel biomaterials to probe and direct cell-matrix interactions. Adv. Mater. Interfaces 2020, 7, 2001198. [Google Scholar] [CrossRef]
- Lee, M.; Rizzo, R.; Surman, F.; Zenobi-Wong, M. Guiding lights: Tissue bioprinting using photoactivated materials. Chem. Rev. 2020, 120, 10950–11027. [Google Scholar] [CrossRef]
- Allonas, X.; Lalevée, J.; Fouassier, J.P. New advances in the investigation of photoinitiator reactivity. Tech. Conf. Proc. UVEB Technol. Expo Conf. 2004, 1, 547–553. [Google Scholar]
- Fischer, J.; Mueller, J.B.; Kaschke, J.; Wolf, T.J.A.; Unterreiner, A.N.; Wegener, M. Three-dimensional multi-photon direct laser writing with variable repetition rate. Opt. Express 2013, 21, 26244–26260. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wayner, D.D.M.; Clark, K.B.; Rauk, A.; Yu, D.; Armstrong, D.A. C−H bond dissociation energies of alkyl amines: Radical structures and stabilization energies. J. Am. Chem. Soc. 1997, 119, 8925–8932. [Google Scholar] [CrossRef]
- Kuebler, S.M.; Braun, K.L.; Zhou, W.; Cammack, J.; Yu, T.; Ober, C.K.; Marder, S.R.; Perry, J.W. Design and application of high-sensitivity two-photon initiators for three-dimensional microfabrication. J. Photochem. Photobiol. A Chem. 2003, 158, 163–170. [Google Scholar] [CrossRef]
- LaFratta, C.N.; Fourkas, J.T.; Baldacchini, T.; Farrer, R.A. Multiphoton fabrication. Angew. Chem. Int. Ed. 2007, 46, 6238–6258. [Google Scholar] [CrossRef]
- Basu, S.; Campagnola, P.J. Enzymatic activity of alkaline phosphatase inside protein and polymer structures fabricated via multiphoton excitation. Biomacromolecules 2004, 5, 572–579. [Google Scholar] [CrossRef]
- Sun, S.-M.; Sun, Y.-L.; Zheng, B.-Y.; Wang, P.; Hou, Z.-S.; Dong, W.-F.; Zhang, L.; Chen, Q.-D.; Tong, L.-M.; Sun, H.-B. Protein-based Y-junction optical micro-splitters with environment-stimulus-actuated adjustments. Sens. Actuators B Chem. 2016, 232, 571–576. [Google Scholar] [CrossRef]
- Lay, C.L.; Lee, Y.H.; Lee, M.R.; Phang, I.Y.; Ling, X.Y. Formulating an ideal protein photoresist for fabricating dynamic microstructures with high aspect ratios and uniform responsiveness. ACS Appl. Mater. Interfaces 2016, 8, 8145–8153. [Google Scholar] [CrossRef]
- Albota, M.; Beljonne, D.; Brédas, J.-L.; Ehrlich, J.E.; Fu, J.-Y.; Heikal, A.A.; Hess, S.E.; Kogej, T.; Levin, M.D.; Marder, S.R.; et al. Design of organic molecules with large two-photon absorption cross sections. Science 1998, 281, 1653–1656. [Google Scholar] [CrossRef] [Green Version]
- Lu, Y.; Hasegawa, F.; Ohkuma, S.; Goto, T.; Fukuhara, S.; Kawazu, Y.; Totani, K.; Yamashita, T.; Watanabe, T. Highly efficient two-photon initiated polymerization in solvent by using a novel two-photon chromophore and co-initiators. J. Mater. Chem. 2004, 14, 1391–1395. [Google Scholar] [CrossRef]
- Cumpston, B.H.; Ananthavel, S.P.; Barlow, S.; Dyer, D.L.; Ehrlich, J.E. Two-photon polymerization initiators for three-dimensional optical data storage and microfabrication. Nature 1999, 398, 51–54. [Google Scholar] [CrossRef]
- Joshi, M.P.; Pudavar, H.E.; Swiatkiewicz, J.; Prasad, P.N.; Reianhardt, B.A. Three-dimensional optical circuitry using two-photon-assisted polymerization. Appl. Phys. Lett. 1999, 74, 170–172. [Google Scholar] [CrossRef]
- Belfield, K.D.; Ren, X.; Van Stryland, E.W.; Hagan, D.J.; Dubikovsky, V.; Miesak, E.J. Near-IR two-photon photoinitiated polymerization using a fluorone/amine initiating system. J. Am. Chem. Soc. 2000, 122, 1217–1218. [Google Scholar] [CrossRef]
- Lemercier, G.; Mulatier, J.-C.; Martineau, C.; Anémian, R.; Andraud, C.; Wang, I.; Stéphan, O.; Amari, N.; Baldeck, P. Two-photon absorption: From optical power limiting to 3D microfabrication. C.R. Chimie 2005, 8, 1308–1316. [Google Scholar] [CrossRef]
- Miwa, M.; Juodkazis, S.; Kawakami, T.; Matsuo, S.; Misawa, H. Femtosecond two-photon stereo-lithography. Appl. Phys. A 2001, 73, 561–566. [Google Scholar] [CrossRef]
- Sun, H.B.; Kawata, S. Two-photon laser precision microfabrication and its applications to micro—Nano devices and systems. J. Light. Technol. 2003, 21, 624–633. [Google Scholar] [CrossRef]
- Belfield, K.D.; Schafer, K.J.; Liu, Y.; Liu, J.; Ren, X.; Van Stryland, E.W. Multiphoton-absorbing organic materials for microfabrication, emerging optical applications and non-destructive three-dimensional imaging. J. Phys. Org. Chem. 2000, 13, 837–849. [Google Scholar] [CrossRef]
- Decker, C.; Zahouily, K.; Decker, D.; Nguyen, T.; Viet, T. Performance analysis of acylphosphine oxides in photoinitiated polymerization. Polymer 2001, 42, 7551–7560. [Google Scholar] [CrossRef]
- Baldacchini, T. Acrylic-based resin with favorable properties for three-dimensional two-photon polymerization. J. Appl. Phys. 2004, 95, 6072–6076. [Google Scholar] [CrossRef] [Green Version]
- Campagnola, P.J.; DelGuidice, D.M.; Epling, G.A.; Hoffacker, K.D.; Howell, A.; Pitts, J.D.; Goodman, S.L. 3-Dimensional submicron polymerization of acrylamide by multiphoton excitation of xanthene dyes. Macromolecules 2000, 33, 1511–1513. [Google Scholar] [CrossRef]
- Pitts, J.; Campagnola, P.; Epling, G.; Goodman, S. Submicron multiphoton free-form fabrication of proteins and polymers: Studies of reaction efficiencies and applications in sustained release. Macromolecules 2000, 33, 1514–1533. [Google Scholar] [CrossRef]
- Li, C.; Luo, L.; Wang, S.; Huang, W.; Gong, Q.; Yang, Y.; Feng, S. Two-photon microstructure-polymerization initiated by a coumarin derivative/iodonium salt system. Chem. Phys. Lett. 2001, 340, 444–448. [Google Scholar] [CrossRef]
- Zeynali, A.; Marini, M.; Chirico, G.; Bouzin, M.; Borzenkov, M.; Sironi, L.; D’Alfonso, L.; Pallavicini, P.; Cassina, V.; Mantegazza, F.; et al. Multiphoton fabrication of proteinaceous nanocomposite microstructures with photothermal activity in the infrared. Adv. Opt. Mater. 2020, 8, 2000584. [Google Scholar] [CrossRef]
- Huang, X.; Wang, X.; Zhao, Y. Study on a series of water-soluble photoinitiators for fabrication of 3D hydrogels by two-photon polymerization. Dyes Pigm. 2017, 141, 413–419. [Google Scholar] [CrossRef]
- Boiko, Y.; Costa, J.M.; Wang, M.; Esener, S. Cationic two-photon induced polymerization with high dynamic range. Opt. Express 2001, 8, 571–584. [Google Scholar] [CrossRef] [PubMed]
- Ober, C.K.; Liu, Z.; Cordero, R.; Cintora, A. Materials Overview for 2-Photon 3D Printing Applications. J. Photopolym. Sci. Technol. 2018, 31, 425–429. [Google Scholar] [CrossRef] [Green Version]
- Zhou, W.; Kuebler, S.M.; Braun, K.L.; Yu, T.; Cammack, J.K.; Ober, C.K.; Perry, J.W.; Marder, S.R. An efficient two-photon-generated photoacid applied to positive-tone 3D microfabrication. Science 2002, 296, 1106–1109. [Google Scholar] [CrossRef] [PubMed]
- Nikolaev, M.; Mitrofanova, O.; Broguiere, N.; Geraldo, S.; Dutta, D.; Tabata, Y.; Elci, B.; Brandenberg, N.; Kolotuev, I.; Gjorevski, N.; et al. Homeostatic mini-intestines through scaffold-guided organoid morphogenesis. Nature 2020, 585, 574–578. [Google Scholar] [CrossRef] [PubMed]
- Sugio, Y.; Komjima, K.; Moriguchi, H.; Takahashi, K.; Kaneko, T.; Yasuda, K. An agar-based on-chip neural-cell-cultivation system for stepwise control of network pattern generation during cultivation. Sens. Actuators B Chem. 2004, 99, 156–162. [Google Scholar] [CrossRef]
- Serbin, J.; Egbert, A.; Ostendorf, A.; Chichkov, B.N.; Houbertz, R.; Domann, G.; Schulz, J.; Cronauer, C.; Fröhlich, L.; Popall, M. Femtosecond laser-induced two-photon polymerization of inorganic–organic hybrid materials for applications in photonics. Opt. Lett. 2003, 28, 301–303. [Google Scholar] [CrossRef]
- Haas, K.H.; Rose, K. Hybrid inorganic/organic polymers with nanoscale building blocks: Precursors, processing, properties and applications. Rev. Adv. Mater. Sci. 2003, 5, 47–52. [Google Scholar]
- Parkatzidis, K.; Chatzinikolaidou, M.; Koufakis, E.; Kaliva, M.; Farsari, M.; Vamvakaki, M. Multi-photon polymerization of bio-inspired, thymol-functionalized hybrid materials with biocompatible and antimicrobial activity. Polym. Chem. 2020, 11, 4078–4083. [Google Scholar] [CrossRef]
- Zandrini, T.; Shan, O.; Parodi, V.; Cerullo, G.; Raimondi, M.T.; Osellame, R. Multi-foci laser microfabrication of 3D polymeric scaffolds for stem cell expansion in regenerative medicine. Sci. Rep. 2019, 9, 11761. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Whitesides, G.M.; Xia, Y. Soft lithography. Angew. Chem. Int. Ed. Engl. 1998, 37, 550–575. [Google Scholar]
- Panusa, G.; Pu, Y.; Wang, J.; Moser, C.; Psaltis, D. Photoinitiator-free multi-photon fabrication of compact optical waveguides in polydimethylsiloxane. Opt. Mater. Express 2019, 9, 128–138. [Google Scholar] [CrossRef]
- Coenjarts, C.A.; Ober, C.K. Two-photon three-dimensional microfabrication of poly(dimethylsiloxane) elastomers. Chem. Mater. 2004, 16, 5556–5558. [Google Scholar] [CrossRef]
- Panusa, G.; Pu, Y.; Wang, J.; Moser, C.; Psaltis, D. Fabrication of sub-micron polymer waveguides through two-photon polymerization in polydimethylsiloxane. Polymers 2020, 12, 2485. [Google Scholar] [CrossRef] [PubMed]
- Watanabe, T.; Akiyama, M.; Totani, K.; Kuebler, S.M.; Stellacci, F.; Wenseleers, W.; Braun, K.; Marder, S.R.; Perry, J.W. Photoresponsive hydrogel microstructure fabricated by two-photon initiated polymerization. Adv. Funct. Mater. 2002, 12, 611–614. [Google Scholar] [CrossRef]
- Koskela, J.E.; Turunen, S.; Ylä-Outinen, L.; Narkilahti, S.; Kellomäki, M. Two-photon microfabrication of poly(ethylene glycol) diacrylate and a novel biodegradable photopolymer-comparison of processability for biomedical applications. Polym. Adv. Technol. 2012, 23, 992–1001. [Google Scholar] [CrossRef]
- Takayama, I.; Katayama, A.; Terakawa, M. Fabrication of hollow channels surrounded by gold nanoparticles in hydrogel by femtosecond laser irradiation. Nanomaterials 2020, 10, 2529. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-H.; Gruber, P.M.; Markovic, M.; Plochberger, B.; Klotzsch, E.; Stampfl, J.; Ovsianikov, A.; Liska, R. Enzymatic synthesis of hyaluronic acid vinyl esters for two-photon microfabrication of biocompatible and biodegradable hydrogel constructs. Polym. Chem. 2014, 5, 6523–6533. [Google Scholar] [CrossRef] [Green Version]
- Ma, H.; Caldwell, A.S.; Azagarsamy, M.A.; Gonzalez Rodriguez, A.; Anseth, K.S. Bioorthogonal click chemistries enable simultaneous spatial patterning of multiple proteins to probe synergistic protein effects on fibroblast function. Biomaterials 2020, 255, 120205. [Google Scholar] [CrossRef]
- Basu, S.; Wolgemuth, C.W.; Campagnola, P.J. Measurement of normal and anomalous diffusion of dyes within protein structures fabricated via multiphoton excited cross-linking. Biomacromolecules 2004, 5, 2347–2357. [Google Scholar] [CrossRef]
- Basu, S.; Rodionov, V.; Terasaki, M.; Campagnola, P.J. Multiphoton-excited microfabrication in live cells via Rose Bengal cross-linking of cytoplasmic proteins. Opt. Lett. 2005, 30, 159–161. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-L.; Dong, W.-F.; Niu, L.-G.; Jiang, T.; Liu, D.-X.; Zhang, L.; Wang, Y.-S.; Chen, Q.-D.; Kim, D.-P.; Sun, H.-B. Protein-based soft micro-optics fabricated by femtosecond laser direct writing. Light Sci. Appl. 2014, 3, e129. [Google Scholar] [CrossRef]
- Zeynali, A.; Marini, M.; Collini, M.; Pallavicini, P.; Chirico, G. Multiphoton fabrication of flexible photo-thermally active proteinaceous microstructures. Chem. Eng. Trans. 2021, 84, 235–240. [Google Scholar] [CrossRef]
- Teh, W.H.; Durig, U.; Drechsler, U.; Smith, C.G.; Guentherodt, J. Effect of low numerical-aperture femtosecond two-photon absorption on (SU-8) resist for ultrahigh-aspect-ratio microstereolithography. J. Appl. Phys. 2005, 97, 054907. [Google Scholar] [CrossRef]
- Maruoa, H.; Inoue, S. Optically driven micropump produced by three-dimensional two-photon microfabrication. Appl. Phys. Lett. 2006, 89, 144101. [Google Scholar] [CrossRef] [Green Version]
- Seet, K.; Juodkazis, S.; Jarutis, V.; Misawa, H. Feature-size reduction of photopolymerized structures by femtosecond optical curing of SU-8. Appl. Phys. Lett. 2006, 89, 024106. [Google Scholar] [CrossRef]
- Yu, T.; Ober, C.; Kuebler, S.; Zhou, W.; Marder, S.; Perry, J. Chemically amplified positive resists for two-photon three-dimensional microfabrication. Adv. Mater. 2003, 15, 517–521. [Google Scholar] [CrossRef]
- Kuznetsova, N.; Malkov, G.; Gribov, B. Photoacid generators. Application and current state of development. Russ. Chem. Rev. 2020, 89, 173–190. [Google Scholar] [CrossRef]
- Yalcin, H.C.; Shekhar, A.; Nishimura, N.; Rane, A.A.; Schaffer, C.B.; Butcher, J.T. Two-photon microscopy-guided femtosecond-laser photoablation of avian cardiogenesis: Noninvasive creation of localized heart defects. Am. J. Physiol. Heart Circ. Physiol. 2010, 299, 1728–1735. [Google Scholar] [CrossRef] [Green Version]
- Qian, Z.; Mordovanakis, A.; Schoenly, J.E.; Covarrubias, A.; Feng, Y.; Lilge, L.; Marjoribanks, R.S. Pulsetrain-burst mode, ultrafast-laser interactions with 3D viable cell cultures as a model for soft biological tissues. Biomed. Opt. Express 2014, 5, 208–222. [Google Scholar] [CrossRef] [Green Version]
- Yalcin, H.C. Femtosecond laser photodisruption of vitelline vessels of avian embryos as a technique to study embryonic vascular remodeling. Exp. Biol. Med. 2014, 39, 1644–1652. [Google Scholar] [CrossRef]
- Siegel, J.; Šuláková, P.; Kaimlová, M.; Švorčík, V.; Hubáček, T. Underwater laser treatment of PET: Effect of processing parameters on surface morphology and chemistry. Appl. Sci. 2018, 8, 2389. [Google Scholar] [CrossRef] [Green Version]
- Rossier, J.S.; Bercier, P.; Schwarz, A.; Loridant, S.; Girault, H.H. Topography, crystallinity and wettability of photoablated PET surfaces. Langmuir 1999, 15, 5173–5178. [Google Scholar] [CrossRef]
- Roth, G.L.; Esen, C.; Hellmann, R. Vertical microchannels for microfluidic multilayer interconnections in PMMA. J. Laser Micro Nanoeng. 2018, 13, 155–159. [Google Scholar] [CrossRef]
- Ravi-Kumar, S.; Lies, B.; Zhang, X.; Lyu, H.; Qin, H. Laser ablation of polymers: A review. Polym. Int. 2019, 68, 1391–1401. [Google Scholar] [CrossRef]
- Yong, J.; Chen, F.; Yang, Q.; Du, G.; Bian, H.; Zhang, D.; Si, J.; Yun, F.; Hou, X. Rapid fabrication of large-area concave microlens arrays on PDMS by a femtosecond laser. ACS Appl. Mater. Interfaces 2013, 5, 9382–9385. [Google Scholar] [CrossRef] [PubMed]
- Tibbitt, M.W.; Kloxin, A.M.; Dyamenahalli, K.U.; Anseth, K.S. Controlled two-photon photodegradation of PEG hydrogels to study and manipulate subcellular interactions on soft materials. Soft Matter 2010, 6, 5100–5108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lunzer, M.; Shi, L.; Andriotis, O.G.; Gruber, P.; Markovic, M.; Thurner, P.J.; Ossipov, D.; Liska, R.; Ovsianikov, A. A modular approach to sensitized two-photon patterning of photodegradable hydrogels. Angew. Chem. Int. 2018, 57, 15122–15127. [Google Scholar] [CrossRef] [Green Version]
- Heintz, K.A.; Bregenzer, M.E.; Mantle, J.L.; Lee, K.H.; West, J.L.; Slater, J.H. Fabrication of 3D biomimetic microfluidic networks in hydrogels. Adv. Healthc. Mater. 2016, 5, 2153–2160. [Google Scholar] [CrossRef] [Green Version]
- Brandenberg, N.; Lutolf, M.P. In situ patterning of microfluidic networks in 3D cell-laden hydrogels. Adv. Mater. 2016, 28, 7450–7456. [Google Scholar] [CrossRef]
- Ilina, O.; Bakker, G.J.; Vasaturo, A.; Hoffman, R.M.; Friedl, P. Two-photon laser-generated microtracks in 3D collagen lattices: Principles of MMP-dependent and -independent collective cancer cell invasion. Phys. Biol. 2011, 8, 015010. [Google Scholar] [CrossRef] [PubMed]
- Doraiswamy, A.; Patz, T.; Narayan, R.; Dinescu, M.; Modi, R.; Auyeung, R.; Chrisey, D. Two-dimensional differential adherence of neuroblasts in laser micromachined CAD/CAM agarose channels. Appl. Surf. Sci. 2006, 252, 4748–4753. [Google Scholar] [CrossRef]
- Maximova, K.; Wang, X.; Balčytis, A.; Fan, L.; Li, J.; Juodkazis, S. Silk patterns made by direct femtosecond laser writing. Biomicrofluidics 2016, 10, 054101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qiao, M.; Wang, H.; Lu, H.; Li, S.; Yan, J.; Qu, L.; Zhang, Y.; Jiang, L.; Lu, Y. Micro/nano processing of natural silk fibers with near-field enhanced ultrafast laser. Sci. China Mater. 2020, 63, 1300–1309. [Google Scholar] [CrossRef]
- Xie, R.; Zheng, W.; Guan, L.; Ai, Y.; Liang, Q. Engineering of hydrogel materials with perfusable microchannels for building vascularized tissues. Small 2020, 16, 1902838. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Mironov, V.; Stampfl, J.; Liska, R. Engineering 3D cell-culture matrices: Multiphoton processing technologies for biological and tissue engineering applications. Expert Rev. Med. Devices 2012, 9, 613–633. [Google Scholar] [CrossRef]
- Schaffer, C.B.; Brodeur, A.; Mazur, E. Laser-induced breakdown and damage in bulk transparent materials induced by tightly focused femtosecond laser pulses. Meas. Sci. Technol. 2001, 12, 1784–1794. [Google Scholar] [CrossRef]
- Vogel, A.; Noack, J.; Hüttman, G.; Paltauf, G. Mechanisms of femtosecond laser nanosurgery of cells and tissues. Appl. Phys. B Laser 2005, 81, 1015–1047. [Google Scholar] [CrossRef]
- Stowers, R.S. Advances in extracellular matrix-mimetic hydrogels to guide stem cell fate. Cells Tissues Organs 2021. [Google Scholar] [CrossRef]
- Torgersen, J.; Qin, X.H.; Li, Z.; Ovsianikov, A.; Liska, R.; Stampfl, J. Hydrogels for two-photon polymerization: A toolbox for mimicking the extracellular matrix. Adv. Funct. Mater. 2013, 23, 4542–4554. [Google Scholar] [CrossRef] [Green Version]
- Ruskowitz, E.R.; Deforest, C.A. Photoresponsive biomaterials for targeted drug delivery and 4D cell culture. Nat. Rev. Mater. 2018, 3, 17087. [Google Scholar] [CrossRef]
- Stowers, R.S.; Allen, S.C.; Suggs, L.J.; Anseth, K.S. Dynamic phototuning of 3D hydrogel stiffness. Proc. Natl. Acad. Sci. USA 2015, 112, 1953–1958. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kloxin, A.M.; Kasko, A.M.; Salinas, C.N.; Anseth, K.S. Photodegradable hydrogels for dynamic tuning of physical and chemical properties. Science 2009, 324, 59–63. [Google Scholar] [CrossRef] [Green Version]
- Hahn, M.S.; Miller, J.S.; West, J.L. Three-dimensional biochemical and biomechanical patterning of hydrogels for guiding cell behavior. Adv. Mater. 2006, 18, 2679–2684. [Google Scholar] [CrossRef]
- Mehta, S.M.; Jin, T.; Stanciulescu, I.; Grande-Allen, K.J. Engineering biologically extensible hydrogels using photolithographic printing. Acta Biomater. 2018, 75, 52–62. [Google Scholar] [CrossRef] [PubMed]
- Primo, G.A.; Mata, A. 3D patterning within hydrogels for the recreation of functional biological environments. Adv. Funct. Mater. 2021, 31, 2009574. [Google Scholar] [CrossRef]
- Hribar, K.C.; Choi, Y.S.; Ondeck, M.; Engler, A.J.; Chen, S. Digital plasmonic patterning for localized tuning of hydrogel stiffness. Adv. Funct. Mater. 2014, 24, 4922–4926. [Google Scholar] [CrossRef] [Green Version]
- Nishiguchi, A.; Zhang, H.; Schweizerhof, S.; Schulte, M.F.; Mourran, A.; Möller, M. 4D printing of a light-driven soft actuator with programmed printing density. ACS Appl. Mater. Interfaces 2020, 12, 12176–12185. [Google Scholar] [CrossRef] [Green Version]
- Chandorkar, Y.; Nava, A.C.; Schweizerhof, S.; Van Dongen, M.; Haraszti, T.; Köhler, J.; Zhang, H.; Windoffer, R.; Mourran, A.; Möller, M.; et al. Cellular responses to beating hydrogels to investigate mechanotransduction. Nat. Commun. 2019, 10, 4027. [Google Scholar] [CrossRef] [Green Version]
- Valentin, T.M.; Leggett, S.E.; Chen, P.-Y.; Sodhi, J.K.; Stephens, L.H.; McClintock, H.D.; Sim, J.Y.; Wong, I.Y. Stereolithographic printing of ionically-crosslinked alginate hydrogels for degradable biomaterials and microfluidics. Lab Chip 2017, 17, 3474–3488. [Google Scholar] [CrossRef]
- Kloxin, A.M.; Tibbitt, M.W.; Anseth, K.S. Synthesis of photodegradable hydrogels as dynamically tunable cell culture platforms. Nat. Prot. 2010, 5, 1867–1887. [Google Scholar] [CrossRef] [Green Version]
- Norris, S.C.P.; Tseng, P.; Kasko, A.M. Direct gradient photolithography of photodegradable hydrogels with patterned stiffness control with submicrometer resolution. ACS Biomater. Sci. Eng. 2016, 2, 1309–1318. [Google Scholar] [CrossRef]
- Applegate, M.B.; Coburn, J.M.; Partlow, B.P.; Moreau, J.E.; Mondia, J.P.; Marelli, B.; Kaplan, D.L.; Omenetto, F.G. Laser-based three-dimensional multiscale micropatterning of biocompatible hydrogels for customized tissue engineering scaffolds. Proc. Natl. Acad. Sci. USA 2015, 112, 12052–12057. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.S.; Aleman, J.; Shin, S.R.; Kilic, T.; Kim, D.; Shaegh, S.A.M.; Massa, S.; Riahi, R.; Chae, S.; Hu, N.; et al. Multisensor-integrated organs-on-chips platform for automated and continual in situ monitoring of organoid behaviors. Proc. Natl. Acad. Sci. USA 2017, 114, E2293–E2302. [Google Scholar] [CrossRef] [Green Version]
- Luo, Y.; Wei, X.; Wan, Y.; Lin, X.; Wang, Z.; Huang, P. 3D printing of hydrogel scaffolds for future application in photothermal therapy of breast cancer and tissue repair. Acta Biomater. 2019, 92, 37–47. [Google Scholar] [CrossRef]
- Batalov, I.; Stevens, K.R.; Deforest, C.A.; Blau, H.M. Photopatterned biomolecule immobilization to guide three-dimensional cell fate in natural protein-based hydrogels. Proc. Natl. Acad. Sci. USA 2021, 118, e20141941. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.-H.; Labuda, K.; Chen, J.; Hruschka, V.; Khadem, A.; Liska, R.; Redl, H.; Slezak, P. Development of synthetic platelet-activating hydrogel matrices to induce local hemostasis. Adv. Funct. Mater. 2015, 25, 6606–6617. [Google Scholar] [CrossRef]
- Zhang, W.; Lohman, A.W.; Zhuravlova, Y.; Lu, X.; Wiens, M.; Hoi, H.; Yaganoglu, S.; Mohr, M.A.; Kitova, E.N.; Klassen, J.S.; et al. Optogenetic control with a photocleavable protein, PhoCl. Nat. Methods 2017, 14, 391–394. [Google Scholar] [CrossRef] [PubMed]
- Shadish, J.A.; Strange, A.C.; Deforest, C.A. Genetically encoded photocleavable linkers for patterned protein release from biomaterials. J. Am. Chem. Soc. 2019, 141, 15619–15625. [Google Scholar] [CrossRef] [PubMed]
- Qin, X.H.; Wang, X.; Rottmar, M.; Nelson, B.J.; Maniura-Weber, K. Near-infrared light-sensitive polyvinyl alcohol hydrogel photoresist for spatiotemporal control of cell-instructive 3D microenvironments. Adv. Mater. 2018, 30, 1705564. [Google Scholar] [CrossRef]
- Baudis, S.; Bomze, D.; Markovic, M.; Gruber, P.; Ovsianikov, A.; Liska, R. Modular material system for the microfabrication of biocompatible hydrogels based on thiol-ene-modified poly(vinyl alcohol). J. Polym. Sci. A Polym. Chem. 2016, 54, 2060–2070. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Q.; Dakin, K.; Xu, K.; Martinez, M.L.; Li, W.H. New caged coumarin fluorophores with extraordinary uncaging cross sections suitable for biological imaging applications. J. Am. Chem. Soc. 2004, 126, 4653–4663. [Google Scholar] [CrossRef]
- Álvarez, M.; Best, A.; Pradhan-Kadam, S.; Koynov, K.; Jonas, U.; Kreiter, M. Single-photon and two-photon induced photocleavage for monolayers of an alkyltriethoxysilane with a photoprotected carboxylic ester. Adv. Mater. 2008, 20, 4563–4567. [Google Scholar] [CrossRef]
- Wylie, R.G.; Ahsan, S.; Aizawa, Y.; Maxwell, K.L.; Morshead, C.M.; Shoichet, M.S. Spatially controlled simultaneous patterning of multiple growth factors in three-dimensional hydrogels. Nat. Mater. 2011, 10, 799–806. [Google Scholar] [CrossRef]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef] [PubMed]
- Nelson, C.M.; VanDuijn, M.; Inman, J.; Fletcher, D.; Bissell, M. Tissue geometry determines sites of mammary branching morphogenesis in organotypic cultures. Science 2006, 314, 298–300. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Gunasekara, D.B.; Reed, M.I.; DiSalvo, M.; Bultman, S.J.; Sims, C.E.; Magness, S.T.; Allbritton, N.L. A microengineered collagen scaffold for generating a polarized crypt-villus architecture of human small intestinal epithelium. Biomaterials 2017, 128, 44–55. [Google Scholar] [CrossRef] [PubMed]
- Turunen, S.; Haaparanta, A.M.; Äänismaa, R.; Kellomäki, M. Chemical and topographical patterning of hydrogels for neural cell guidance in vitro. J. Tissue Eng. Regen. Med. 2013, 7, 253–270. [Google Scholar] [CrossRef]
- Yamamoto, H.; Okano, K.; Demura, T.; Hosokawa, Y.; Masuhara, H.; Tanii, T.; Nakamura, S. In-situ guidance of individual neuronal processes by wet femtosecond-laser processing of self-assembled monolayers. Appl. Phys. Lett. 2011, 99, 163701. [Google Scholar] [CrossRef] [Green Version]
- Song, Y.M.; Xie, Y.; Malyarchuk, V.; Xiao, J.; Jung, I.; Choi, K.-J.; Liu, Z.; Park, H.; Lu, C.; Kim, R.-H.; et al. Digital cameras with designs inspired by the arthropod eye. Nature 2013, 497, 95–99. [Google Scholar] [CrossRef]
- Juodkazis, S. Manufacturing: 3D printed micro-optics. Nat. Phot. 2016, 10, 499–501. [Google Scholar] [CrossRef]
- Chen, Q.-D.; Wu, D.; Niu, L.-G.; Wang, J.; Lin, X.-F.; Xia, H.; Sun, H. Phase lenses and mirrors created by laser micronanofabrication via two-photon photopolymerization. Appl. Phys. Lett. 2007, 91, 171105. [Google Scholar] [CrossRef] [Green Version]
- Gissibl, T.; Thiele, S.; Herkommer, A.; Giessen, H. Two-photon direct laser writing of ultracompact multi-lens objectives. Nat. Phot. 2016, 10, 554–560. [Google Scholar] [CrossRef]
- Sun, X.; Tyagi, P.; Agate, S.; McCord, M.G.; Lucia, L.A.; Pal, L. Highly tunable bioadhesion and optics of 3D printable PNIPAm/cellulose nanofibrils hydrogels. Carbohydr. Polym. 2020, 234, 115898. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.-L.; Dong, W.; Yang, R.-Z.; Meng, X.; Zhang, L.; Chen, Q.; Sun, H. Dynamically tunable protein microlenses. Angew. Chem. Int. Ed. Engl. 2012, 51, 1558–1562. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.L.; Liu, D.X.; Dong, W.F.; Chen, Q.D.; Sun, H.B. Tunable protein harmonic diffractive micro-optical elements. Opt. Lett. 2012, 37, 2973–2975. [Google Scholar] [CrossRef]
- You, S.; Li, J.; Zhu, W.; Yu, C.; Mei, D.; Chen, S. Nanoscale 3D printing of hydrogels for cellular tissue engineering. J. Mater. Chem. B 2018, 6, 2187–2197. [Google Scholar] [CrossRef]
- Choi, N.; Cabodi, M.; Held, B.; Gleghorn, J.; Bonassar, L.J.; Stroock, A. Microfluidic scaffolds for tissue engineering. Nat. Mater. 2007, 6, 908–915. [Google Scholar] [CrossRef]
- Chrobak, K.M.; Potter, D.R.; Tien, J. Formation of perfused, functional microvascular tubes in vitro. Microvasc. Res. 2006, 71, 185–196. [Google Scholar] [CrossRef]
- Wong, K.H.K.; Truslow, J.G.; Tien, J. The role of cyclic AMP in normalizing the function of engineered human blood microvessels in microfluidic collagen gels. Biomaterials 2010, 31, 4706–4714. [Google Scholar] [CrossRef] [Green Version]
- Zheng, Y.; Chen, J.; Craven, M.; Choi, N.; Totorica, S.; Diaz-Santana, A.; Kermani, P.; Hempstead, B.; Fischbach, C.; Lopez, J.A.; et al. In vitro microvessels for the study of angiogenesis and thrombosis. Proc. Natl. Acad. Sci. USA 2012, 109, 9342–9347. [Google Scholar] [CrossRef] [Green Version]
- Sarig-Nadir, O.; Livnat, N.; Zajdman, R.; Shoham, S.; Seliktar, D. Laser photoablation of guidance microchannels into hydrogels directs cell growth in three dimensions. Biophys. J. 2009, 96, 4743–4752. [Google Scholar] [CrossRef] [Green Version]
- Rayner, S.G.; Howard, C.C.; Mandrycky, C.J.; Stamenkovic, S.; Himmelfarb, J.; Shih, A.Y.; Zheng, Y. Multiphoton-guided creation of complex organ-specific microvasculature. Adv. Healthc. Mater. 2021, 2, 100031. [Google Scholar] [CrossRef]
- Lee, S.H.; Moon, J.J.; West, J.L. Three-dimensional micropatterning of bioactive hydrogels via two-photon laser scanning photolithography for guided 3D cell migration. Biomaterials 2008, 29, 2962–2968. [Google Scholar] [CrossRef] [Green Version]
- Hasselmann, N.F.; Hackmann, M.J.; Horn, W. Two-photon fabrication of hydrogel microstructures for excitation and immobilization of cells. Biomed. Microdevices 2018, 20, 8. [Google Scholar] [CrossRef]
- Hasselmann, N.F.; Horn, W. Attachment of microstructures to single bacteria by two-photon patterning of a protein based hydrogel. Biomed. Phys. Eng. Express. 2018, 4, 035004. [Google Scholar] [CrossRef]
- Tromayer, M.; Dobos, A.; Gruber, P.; Ajami, A.; Dědic, R.; Ovsianikov, A.; Liska, R. A biocompatible diazosulfonate initiator for direct encapsulation of human stem cells: Via two-photon polymerization. Polym. Chem. 2018, 9, 3108–3117. [Google Scholar] [CrossRef]
- Li, Z.; Torgersen, J.; Ajami, A.; Mühleder, S.; Qin, X.-H.; Husinsky, W.; Holnthoner, W.; Ovsianikov, A.; Stampfl, J.; Liska, R. Initiation efficiency and cytotoxicity of novel water-soluble two-photon photoinitiators for direct 3D microfabrication of hydrogels. RCS Adv. 2013, 3, 15939. [Google Scholar] [CrossRef] [Green Version]
- Tytgat, L.; Dobos, A.; Markovic, M.; Van Damme, L.; Van Hoorick, J.; Bray, F.; Thienpont, H.; Ottevaere, H.; Dubruel, P.; Ovsianikov, A.; et al. High-resolution 3D bioprinting of photo-cross-linkable recombinant collagen to serve tissue engineering applications. Biomacromolecules 2020, 21, 3997–4007. [Google Scholar] [CrossRef] [PubMed]
- Urciuolo, A.; Poli, I.; Brandolino, L.; Raffa, P.; Scattolini, V.; Laterza, C.; Giobbe, G.G.; Zambaiti, E.; Selmin, G.; Magnussen, M.; et al. Intravital three-dimensional bioprinting. Nat. Biomed. Eng. 2020, 4, 901–915. [Google Scholar] [CrossRef] [PubMed]
- Kaehr, B.; Shear, J.B. Multiphoton fabrication of chemically responsive protein hydrogels for microactuation. Proc. Natl. Acad. Sci. USA 2008, 105, 8850–8854. [Google Scholar] [CrossRef] [Green Version]
- Torgersen, J.; Ovsianikov, A.; Mironov, V.; Pucher, N.; Qin, X.-H.; Li, Z.; Cicha, K.; Machacek, T.; Liska, R.; Jantsch, V.; et al. Photo-sensitive hydrogels for three-dimensional laser microfabrication in the presence of whole organisms. J. Biomed. Opt. 2012, 17, 105008. [Google Scholar] [CrossRef]
- Huang, Z.; Chi-Pong Tsui, G.; Deng, Y.; Tang, C.Y. Two-photon polymerization nanolithography technology for fabrication of stimulus-responsive micro/nano-structures for biomedical applications. Nanotechnol. Rev. 2020, 9, 1118–1136. [Google Scholar] [CrossRef]
- Kawamura, A. Design of nano- and micro-structured molecule-responsive hydrogels. Polym. J. 2017, 49, 751–757. [Google Scholar] [CrossRef]
- Mahinroosta, M.; Jomeh Farsangi, Z.; Allahverdi, A.; Shakoori, Z. Hydrogels as intelligent materials: A brief review of synthesis, properties and applications. Mater. Today Chem. 2018, 8, 42–55. [Google Scholar] [CrossRef]
- Lee, M.R.; Phang, I.Y.; Cui, Y.; Lee, Y.H.; Ling, X.Y. Shape-shifting 3D protein microstructures with programmable directionality via quantitative nanoscale stiffness modulation. Small 2015, 11, 740–748. [Google Scholar] [CrossRef] [PubMed]
- Lay, C.L.; Lee, M.R.; Lee, H.K.; Phang, I.Y.; Ling, X.Y. Transformative two-dimensional array configurations by geometrical shape-shifting protein microstructures. ACS Nano 2015, 9, 9708–9717. [Google Scholar] [CrossRef] [PubMed]
- Wei, S.; Liu, J.; Zhao, Y.; Zhang, T.; Zheng, M.-L.; Jin, F.; Dong, X.; Xing, J.; Duan, X. Protein-based 3D microstructures with controllable morphology and pH-responsive properties. ACS Appl. Mater. Interfaces 2017, 9, 42247–42257. [Google Scholar] [CrossRef]
- Scarpa, E.; Lemma, E.D.; Fiammengo, R.; Cipolla, M.P.; Pisanello, F.; Rizzi, F.; De Vittorio, M. Microfabrication of pH-responsive 3D hydrogel structures via two-photon polymerization of high-molecular-weight poly(ethylene glycol) diacrylates. Sens. Actuators B Chem. 2019, 279, 418–426. [Google Scholar] [CrossRef]
- Chirico, G.; Pallavicini, P.; Collini, M. Gold nanostars for superficial diseases: A promising tool for localized hyperthermia? Nanomedicine 2014, 9, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Jaque, D.; Martínez Maestro, L.; del Rosal, B.; Haro-Gonzalez, P.; Benayas, A.; Plaza, J.L.; Rodríguez, E.M.; García Solé, J. Nanoparticles for photothermal therapies. Nanoscale 2014, 6, 9494–9530. [Google Scholar] [CrossRef]
- Palermo, G.; Ritacco, T.; Aceti, D.M.; Pezzi, L.; Giocondo, M.; De Luca, A. Photo-thermal effects in 1D gratings of gold nanoparticles. Crystals 2017, 7, 14. [Google Scholar] [CrossRef]
- Bouzin, M.; Marini, M.; Zeynali, A.; Borzenkov, M.; Sironi, L.; D’Alfonso, L.; Mingozzi, F.; Granucci, F.; Pallavicini, P.; Chirico, G.; et al. Photo-activated raster scanning thermal imaging at sub-diffraction resolution. Nat. Commun. 2019, 10, 5523. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, L.; Zhang, X.; Li, L.; Liu, Y.; Wang, D.; Xu, L.; Bao, J.; Zhang, A. Fabrication of Photothermally responsive nanocomposite hydrogel through 3D printing. Macromol. Mater. Eng. 2020, 305, 1900718. [Google Scholar] [CrossRef]
- Ginger, D.S.; Zhang, H.; Mirkin, C.A. The evolution of dip-pen nanolithography. Angew. Chem. Int. 2004, 43, 30–45. [Google Scholar] [CrossRef] [Green Version]
- Lippert, T. Interaction of photons with polymers: From surface modification to ablation. Plasma Process. Polym. 2005, 2, 525–546. [Google Scholar] [CrossRef]
- Batchelor, R.; Messer, T.; Hippler, M.; Wegener, M.; Barner-Kowollik, C.; Blasco, E. Two in one: Light as a tool for 3D printing and erasing at the microscale. Adv. Mater. 2019, 31, 1904085. [Google Scholar] [CrossRef] [Green Version]
- Zieger, M.M.; Müller, P.; Blasco, E.; Petit, C.; Hahn, V.; Michalek, L.; Mutlu, H.; Wegener, M.; Barner-Kowollik, C. A Subtractive photoresist platform for micro- and macroscopic 3D printed structures. Adv. Funct. Mater. 2018, 28, 1801405. [Google Scholar] [CrossRef]
- Gattass, R.R.; Mazur, E. Femtosecond laser micromachining in transparent materials. Nat. Phot. 2008, 2, 219–225. [Google Scholar] [CrossRef]
- Marshall, G.; Ams, M.; Withford, M. Direct laser written waveguide-Bragg gratings inbulk fused silica. Opt. Lett. 2006, 31, 2690–2691. [Google Scholar] [CrossRef] [Green Version]
- Volpe, B.A.; Fotino, T.H.; Steiner, A.B. Confocal microscope-based laser ablation and regeneration assay in zebrafish interneuromast cells. J. Vis. Exp. 2020, 159, e60966. [Google Scholar] [CrossRef] [PubMed]
- Shen, N.; Datta, D.; Schaffer, C.B.; LeDuc, P.; Ingber, D.E.; Mazur, E. Ablation of cytoskeletal filaments and mitochondria in live cells using a femtosecond laser nanoscissor. Mech. Chem. Biosyst. 2005, 2, 17–26. [Google Scholar] [CrossRef]
- Xiong, W.; Zhou, Y.S.; He, X.N.; Gao, Y.; Mahjouri-Samani, M.; Jiang, L.; Baldacchini, T.; Lu, Y.F. Simultaneous additive and subtractive three-dimensional nanofabrication using integrated two-photon polymerization and multiphoton ablation. Light Sci. Appl. 2012, 1, e6. [Google Scholar] [CrossRef] [Green Version]
- Malinauskas, M.; Rekštytė, S.; Lukoševičius, L.; Butkus, S.; Balčiūnas, E.; Pečiukaitytė, M.; Baltriukienė, D.; Bukelskienė, V.; Butkevičius, A.; Kucevičius, P.; et al. 3D microporous scaffolds manufactured via combination of fused filament fabrication and direct laser writing ablation. Micromachines 2014, 5, 839–858. [Google Scholar] [CrossRef] [Green Version]
- Izzo, L.; Tunesi, M.; Boeri, L.; Lagana, M.; Giordano, C.; Raimondi, M.T. Influence of the static magnetic field on cell response in a miniaturized optically accessible bioreactor for 3D cell culture. Biomed. Microdevices 2019, 21, 29. [Google Scholar] [CrossRef] [Green Version]
- Arbabian, M.E.; Wagner, M.R. The impact of 3D printing on manufacturer–retailer supply chains. Eur. J. Oper. Res. 2020, 285, 538–552. [Google Scholar] [CrossRef]
- Nasiri, S.; Khosravani, M.R. Machine learning in predicting mechanical behavior of additively manufactured parts. J. Mater. Res. Technol. 2021, 14, 1137–1153. [Google Scholar] [CrossRef]

| AFM | Atomic force microscopy | AuNR | Gold nanorods |
| BSA | Bovine serum albumin | CEA | 2-carboxyethyl acrylate |
| CNF | Cellulose nanofibril | CuAAC | Copper(I)-catalyzed azide-alkyne cycloaddition |
| DAS | Tetrapotassium 4,4′-(1,2-ethenediyl) bis[2-(3-sulfophenyl) diazenesulfonate] | ECM | Extracellular matrix |
| FFF | Filament extrusion fabrication | HA | Hyaluronic acid |
| HCC | 7-hydroxycoumarin-3-carboxylic acid | HDF | Human dermo-fibroblasts |
| HDL | Harmonic diffractive lenses | ITX | 2-/4-isopropylthioxanthone |
| LAP | Lithium phenyl-2,4,6-trimethyl benzoylphosphinate | LA | Laser ablation |
| LSL | Laser-scanning lithography | MAP | Multiphoton absorption polymerization |
| MPI | Multiphoton ionization | MPL | Multi-photon lithography |
| MSC | Mesenchymal stem cells | NIR | Near-infrared |
| NBE | Ortho-nitrobenzylether | PAG | Photoacid generator |
| PDMS | Polydimethylsiloxane | PEG | Poly-ethylene glycol |
| PEGDA | PEG diacrylate | PET | Poly(ethylene terephthalate) |
| PI | Photoinitiator | PLA | Polylactic acid |
| PMMA | Polymethyl methalcrylate | PS | Photosensitizer |
| PVA | Poly-vinyl-alchool | PNIPAm | Poly(N-isopropylacrylamide) |
| TI | tunneling ionization | TPA | Two-photon absorption |
| TPP | Two-photon polymerization | RGDS | Arginylglycylaspartic acid |
| SEM | Scanning electron microscopy | SPAAC | Strain-promoted azide-alkyne cycloaddition |
| I | Average intensity | Laser beam waist | |
| Scanning speed | P | Average power | |
| Two-photon cross-section | Laser repetition rate | ||
| Laser pulse width | Rate of volume fabrication | ||
| Voxel time ( |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bouzin, M.; Zeynali, A.; Marini, M.; Sironi, L.; Scodellaro, R.; D’Alfonso, L.; Collini, M.; Chirico, G. Multiphoton Laser Fabrication of Hybrid Photo-Activable Biomaterials. Sensors 2021, 21, 5891. https://doi.org/10.3390/s21175891
Bouzin M, Zeynali A, Marini M, Sironi L, Scodellaro R, D’Alfonso L, Collini M, Chirico G. Multiphoton Laser Fabrication of Hybrid Photo-Activable Biomaterials. Sensors. 2021; 21(17):5891. https://doi.org/10.3390/s21175891
Chicago/Turabian StyleBouzin, Margaux, Amirbahador Zeynali, Mario Marini, Laura Sironi, Riccardo Scodellaro, Laura D’Alfonso, Maddalena Collini, and Giuseppe Chirico. 2021. "Multiphoton Laser Fabrication of Hybrid Photo-Activable Biomaterials" Sensors 21, no. 17: 5891. https://doi.org/10.3390/s21175891
APA StyleBouzin, M., Zeynali, A., Marini, M., Sironi, L., Scodellaro, R., D’Alfonso, L., Collini, M., & Chirico, G. (2021). Multiphoton Laser Fabrication of Hybrid Photo-Activable Biomaterials. Sensors, 21(17), 5891. https://doi.org/10.3390/s21175891








