Review on Comparison of Different Energy Storage Technologies Used in Micro-Energy Harvesting, WSNs, Low-Cost Microelectronic Devices: Challenges and Recommendations
Abstract
1. Introduction
2. Energy Storage Importance
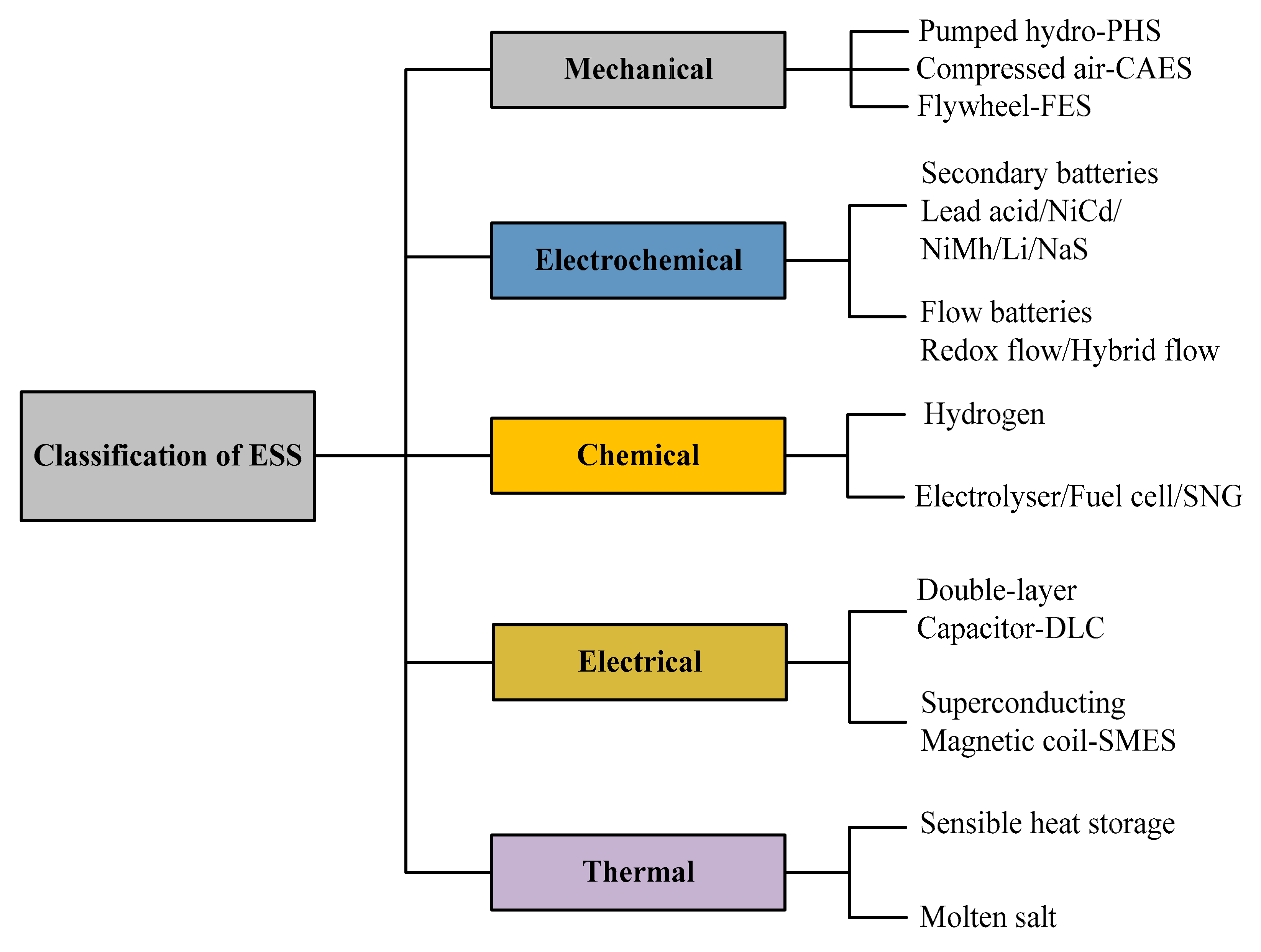
3. Electrical Energy Storage Classification
3.1. Batteries
3.2. Rechargeable Batteries
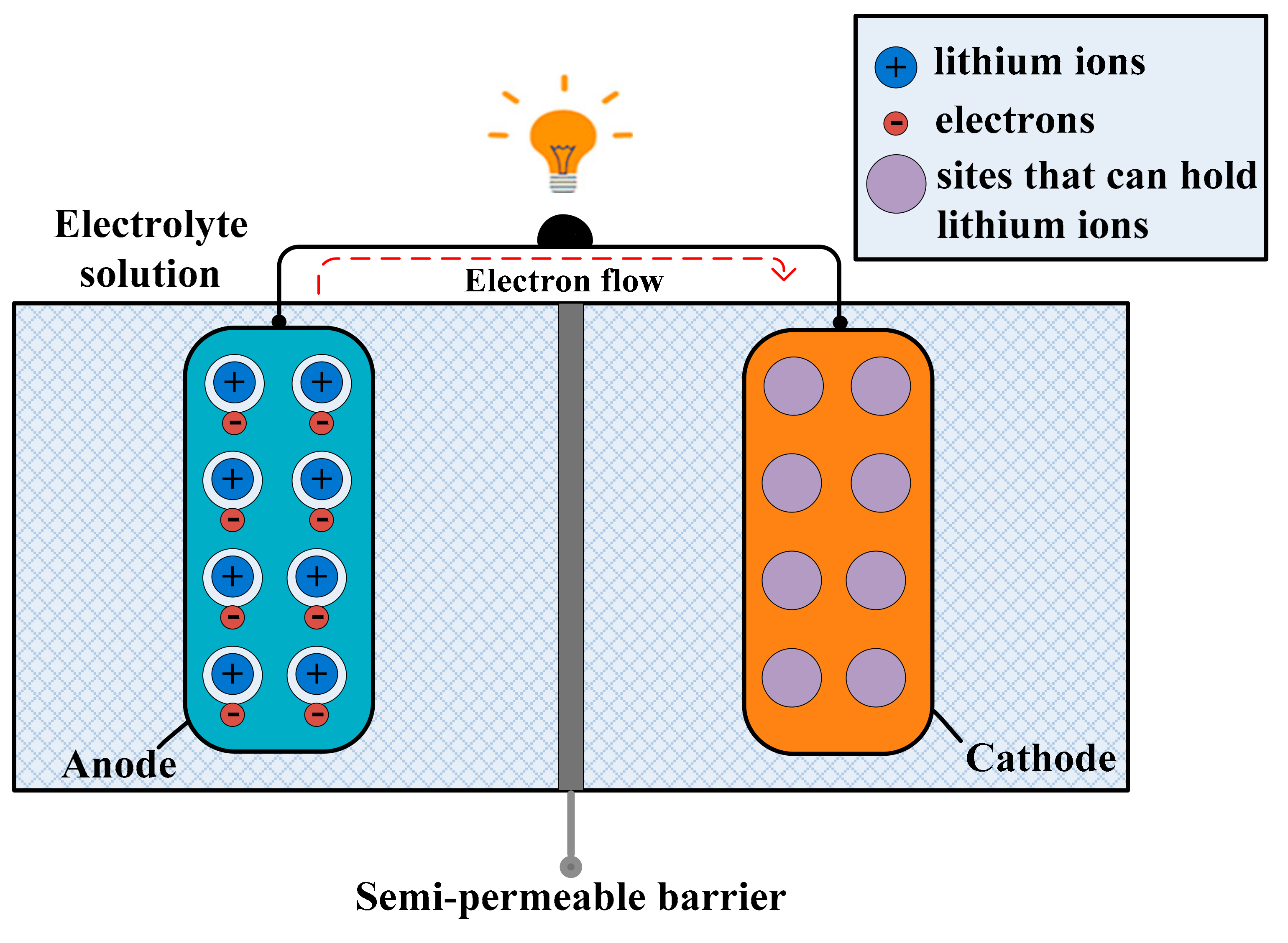
3.3. Lithium-Ion Batteries
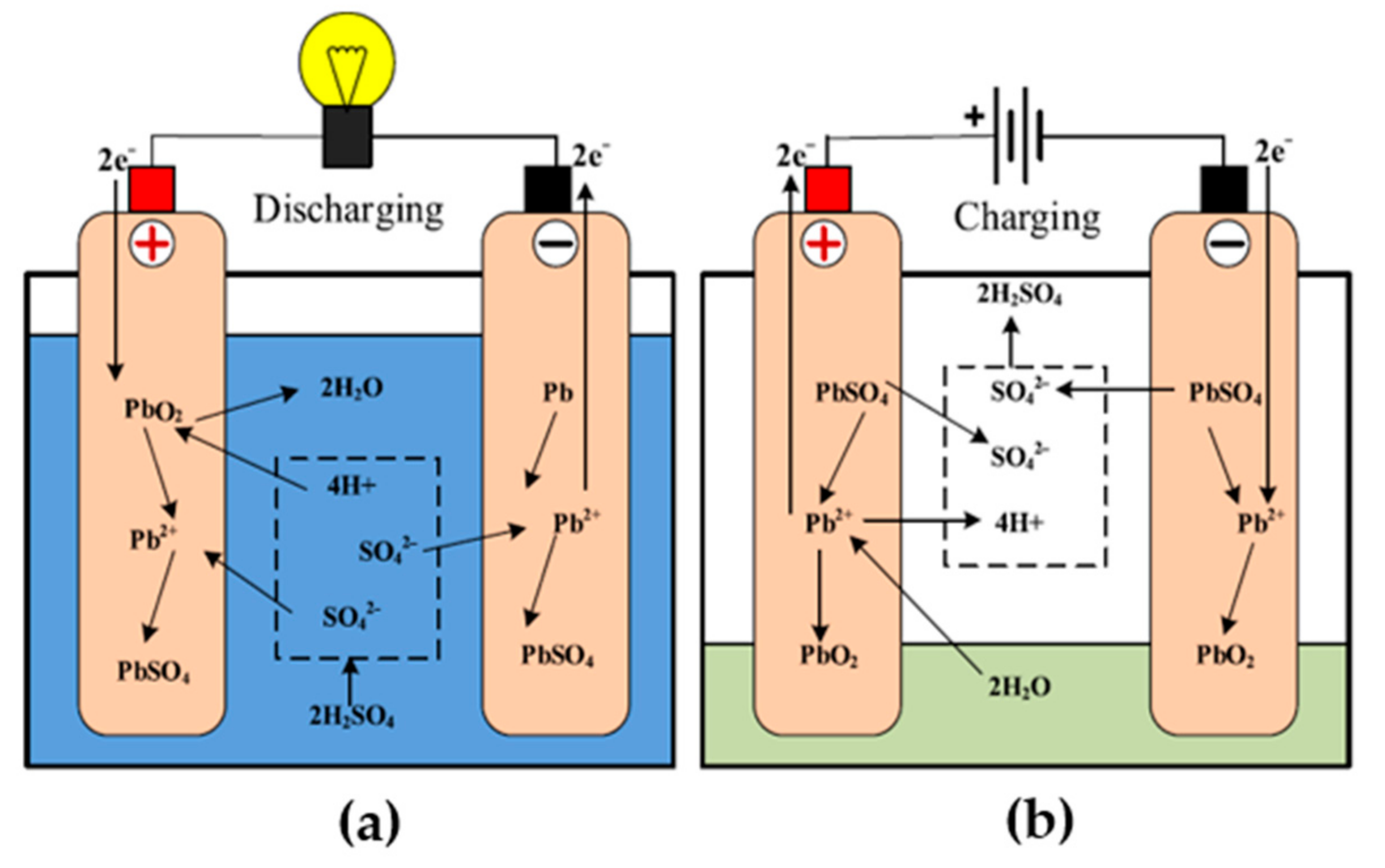
3.4. Lead Acid Battery
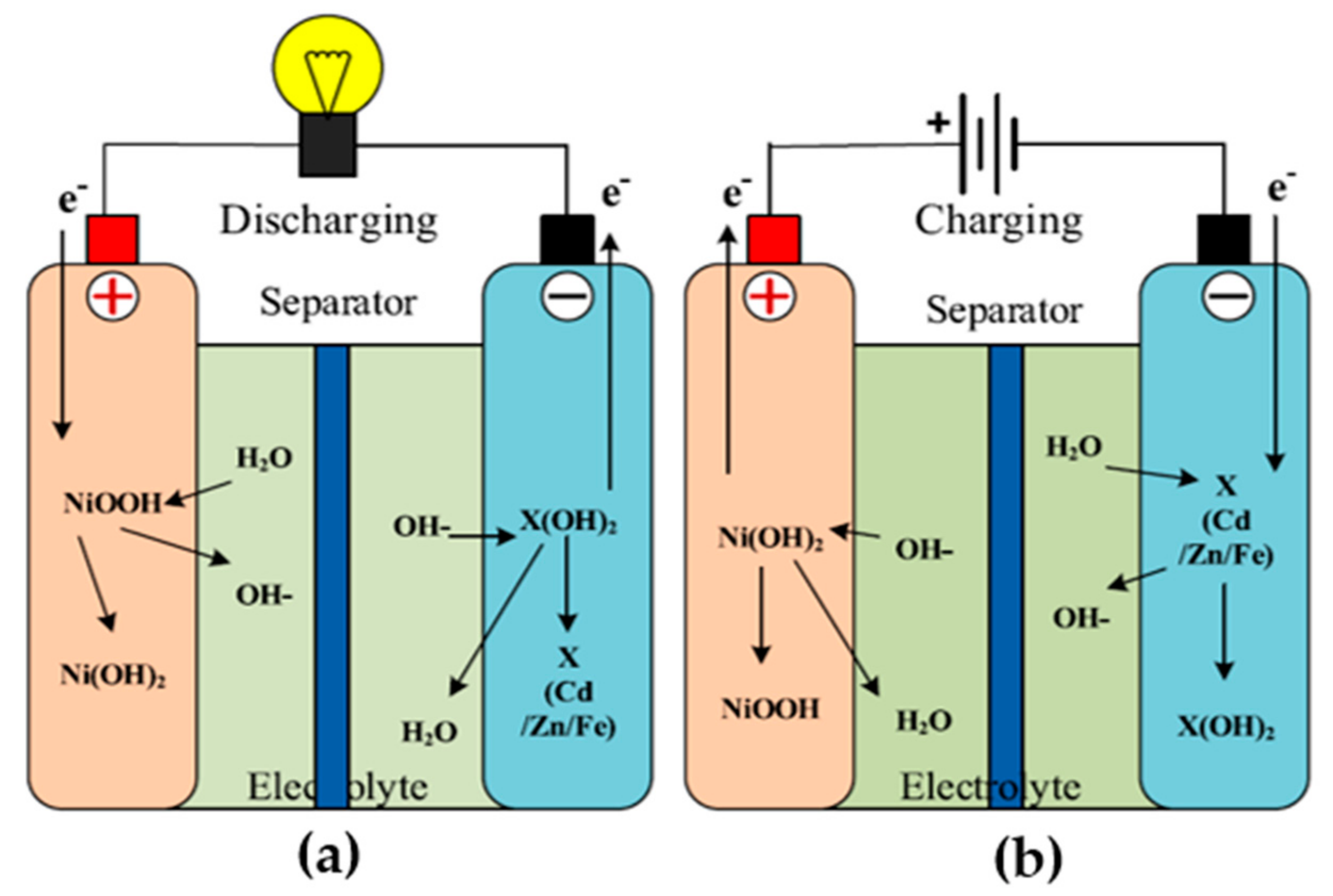
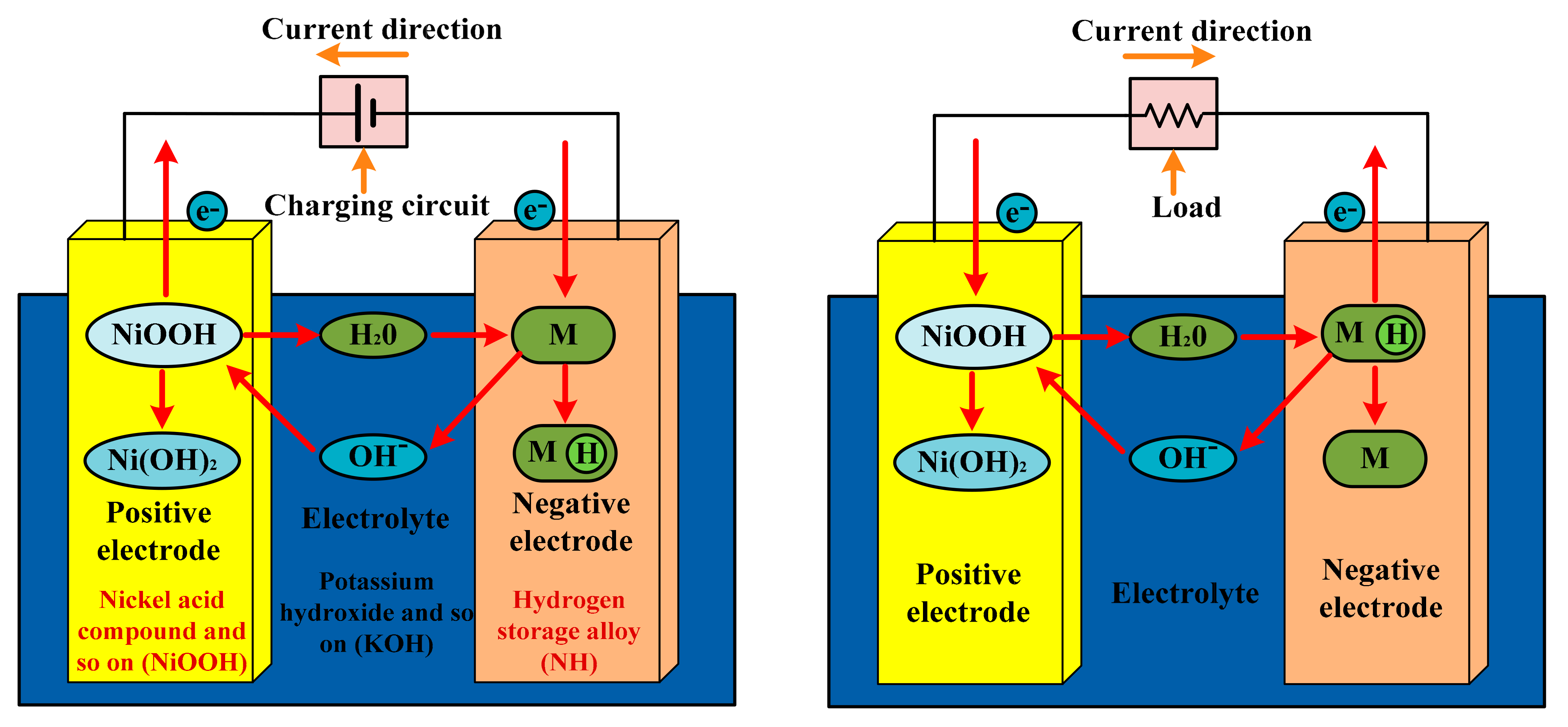
3.5. Nickel–Cadmium Battery
3.6. Sodium–Sulphur Battery
3.7. Electrical Storage System
3.7.1. Capacitor as Energy Storage Device
3.7.2. Ultra-Capacitors
3.8. Chemical Energy Storage Systems
3.9. Thermal Storage Systems
3.10. Hybrid Storage Systems
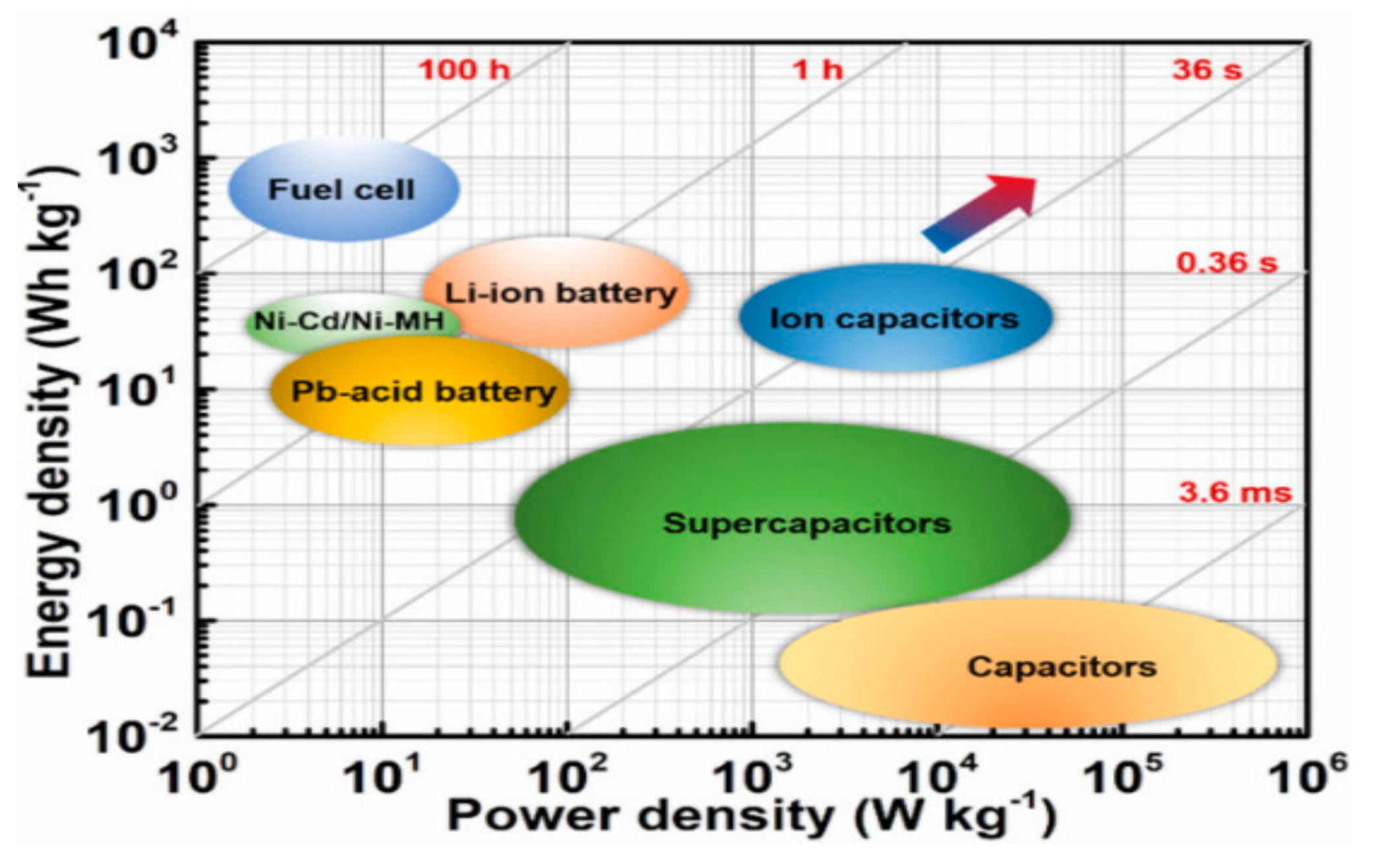
4. Comparisons of Energy Storage Technology
Supercapacitor vs. Battery
| Battery Unfavaourable Condition | Supercapacitor Unfavaourable Condition | Improvement | Analysis | Reference |
|---|---|---|---|---|
| Battery is stressed during peak demand conditions in electronic equipments. | Not stressed during peak demand conditions. | Supercapacitor should be connected in parallel with battery in microelectronic devices, WSNs to enhance the battery life. | The hybrid combination of supercapacitor, battery and inverter has improved efficiency and performance of the energy storage unit. | [116] |
| Battery has a finite lifespan; replacement on discharging in hilly areas is difficult. | Supercapacitor is a good option. | Hybrid combination increases the life of ESS. | Piezoelectric energy harvester is a good replacement for battery in remote locations. | [166] |
| i. Lead acid batteries are capable of long cycles and cylic life. ii.Li-ion batteries have advantages of energy density and specific energy, less important for static installations. | Supercapacitor in hybrid construction improves shallow cycle performance. | Some types of lead-acid batteries have hybrid construction with a supercapacitor element with conventional negative plate. | Only lead-acid batteries are good storage options among batteries and capable of complete recycling. | [167] |
| Conventional lead-acid batteries are the weakest link in photovoltaic installations. | Supercapacitors are a good option. | Hybrid valve regulated lead-acid batteries have (i) Lower internal-resistance (ii) High thermal stability. | Hybrid valve-regulated lead-acid battery performance is better. | [168] |
| In lead-acid batteriesmechanical stress on the charging/discharging cycle weakens the active material and causes softened corrosion and cracking of grids. The irreversible sulfation of the plates and internal short circuit damages the battery. | Important for micro electronic devices. Supercapacitors have high energy density, quick charge/discharge process. | The design can be modified by including glass fiber mats around the positive plates and using thicker positive grids. Flexible solid state supercapacitors have high power density. | Deep cycling ability is achieved. | [169] |
| The fabrication of capacitors with Na+. Advantages of energy-density along with stability. | [169] | |||
| Replacement of batteries is problematic. | Deliver high-power (10 k Wkg−1) release in a very short time. | Nano engineering improves the capacitance of the supercapacitors and attains high energy density. | Improve the energy density by increasing the working voltage window by using a stable electronic electrolyte. | [102] |
| Lithium ion batteries cannot discharge at large currents. lithium–sulfur batteries have low stability. Sodium ion batteries have worse electrochemical performance. | (i) Most efficient storage device (ii) High power density (iii) High energy density (iii) Long cycle life (iv) Fast charging discharging (v) Instant high current discharge (vi) Low cost (vii) Easy maintenance and (viii) No pollution. | Wise choice of electrode materials can improve the performance and reduce the cost. | Supercapacitors are green-devices. They are efficient. | [170] |
| Volume and long charging time is problematic. Advantages still lack. | In piezo energy harvester (PEH) if a regulator is used in a charging capacitor, the charging time is increased. | If in PEH a voltage regulator used for constant voltage to charge a supercapacitor, must be avoided as it increase charging time. | The main disadvantage of supercapacitors is low energy-density. | [171] |
| Reduced life. | Small voltage requirement for capacitor. Fast charge/discharge. | If the energy density of supercapacitors is improved they are ideal as energy storage devices. | Capacitors ideal for storage of energy for short duration. | [172] |
| Battery technology has several disadvantages (i) weight, (ii) volume, (iii) large internal resistance, (iv) poor power-density and (iv) transient response. | Supercapacitor most-reliable | With hybrid models better results can be achieved. | Batteries facing the limitation, reduce the power levels. However, supercapacitor deals with different power-levels. | [173] |
| Battery life is small. | Supercapacitors had resolved the limitations of lead-acid batteries and proved excellent power performance. | The energy density and voltage of the supercapacitor must be increased. | Rapid change in the power such as acceleration, regenerative braking and efficiency at low temperature all these problems can be solved by supercapacitors. | [174] |
5. Challenges and Issues of Supercapacitors and Batteries
5.1. Technical Problems
5.2. Establishment of Electrical Parameters Model
5.3. Consistency Detection
5.4. Industrial Standards
6. Conclusions and Recommendations
- The supercapacitors store a small energy of power. To overcome this problem, the hybrid combination of supercapacitors with the lithium-ion battery is ideal, which not only improves the power capacity, but also provides a high power density, energy density, and efficiency of the ESS;
- The ESS performance is also affected by substandard terminals and electrolytes. The use of new materials in the manufacturing of electrodes and electrolytes shows better results in batteries and supercapacitors. For the construction of new electrodes, the combination of different materials, such as polymers, metal oxides, and carbon materials, is suggested. For supercapacitor electrolytes, new electrolytes are introduced, which are a combination of aqueous and non-aqueous electrolytes. There is also an active way to increase the voltage level in aqueous electrolytes, with the design of an asymmetric supercapacitor. The voltage level will increase above the thermodynamic limit of 1.2 V, and, as a result, the energy density will also increase;
- The batteries and supercapacitors are affected by environmental conditions, such as temperature and humidity. Corrosion is a big problem for the terminals. The emission of carbon dioxide affects the environment;
- For the low-cost microelectronic devices, the size of ESS is an important feature. In these systems, nano-electrodes are suggested. These electrodes have the additional advantages of a faster transient response, increased mass transport, and reduced destructive probes. In supercapacitors, carbon nano-materials and silicon nano-wires are used as electrodes, but PANI (polyaniline) is the best electrode material. The combination of PANI with graphene oxide is best for future applications;
- Electrochemical behavior and flexibility are the main problems in supercapacitors. The carbon electrodes 3D and 4D supercapacitor structures are suggested for portable and wearable electronic devices;
- The future of supercapacitor electrodes is the fabrication with the waste materials, but a lot of research is required to gain the best results;
- The batteries face stress during peak demand conditions in electronic equipment. The best solution to this is the hybrid ESS, containing supercapacitors and batteries.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xu, Y.; Zhang, H.; Yang, F.; Tong, L.; Yang, Y.; Yan, D.; Wang, C.; Ren, J.; Wu, Y. Experimental study on small power generation energy storage device based on pneumatic motor and compressed air. Energy Convers. Manag. 2021, 234, 113949. [Google Scholar] [CrossRef]
- Sreenilayam, S.P.; Ul Ahad, I.; Nicolosi, V.; Brabazon, D. MXene materials based printed flexible devices for healthcare, biomedical and energy storage applications. Mater. Today 2021, 43, 99–131. [Google Scholar] [CrossRef]
- Moreno, C.; González, A.; Olazagoitia, J.L.; Vinolas, J. The Acquisition Rate and Soundness of a Low-Cost Data Acquisition System (LC-DAQ) for High Frequency Applications. Sensors 2020, 20, 524. [Google Scholar] [CrossRef] [PubMed]
- Costa, A.; Costa, D.; Morgado, J.; Santosa, H.; Ferreira, C. Autonomous wireless sensor with a low cost TEG for application in automobile vehicles. Procedia Eng. 2014, 87, 1226–1229. [Google Scholar] [CrossRef][Green Version]
- Moreno-Ramírez, C.; Iniesta, C.; González, A.; Olazagoitia, J.L. Development and characterization of a low-cost sensors system for an acoustic test bench. Sensors 2020, 20, 6663. [Google Scholar] [CrossRef]
- Circuits, E.H.; Applications, S. Review of Power Converter Impact of Electromagnetic Energy Harvesting Circuits and Devices for Autonomous Sensor Applications. Electronics 2021, 10, 1108. [Google Scholar]
- Han, H.; Wang, T.; Zhang, Y.; Nurpeissova, A.; Bakenov, Z. Three-dimensionally ordered macroporous zno framework as dual-functional sulfur host for high-efficiency lithium–sulfur batteries. Nanomaterials 2020, 10, 2267. [Google Scholar] [CrossRef]
- Zhang, H.; Wang, H.; Zhang, J.; Zhang, Z.; Yu, Y.; Luo, J.; Dong, S. A novel rhombic-shaped paper-based triboelectric nanogenerator for harvesting energy from environmental vibration. Sens. Actuators A: Phys. 2020, 302, 111806. [Google Scholar] [CrossRef]
- Halim, M.A.; Cho, H.; Salauddin, M.; Park, J.Y. A miniaturized electromagnetic vibration energy harvester using flux-guided magnet stacks for human-body-induced motion. Sens. Actuators A Phys. 2016, 249, 23–31. [Google Scholar] [CrossRef]
- Avtar, R.; Sahu, N.; Aggarwal, A.K.; Chakraborty, S.; Kharrazi, A.; Yunus, A.P.; Dou, J.; Kurniawan, T.A. Exploring Renewable Energy Resources Using Remote Sensing and GIS-A Review. Resources 2019, 8, 149. [Google Scholar] [CrossRef]
- Lallart, M.; Yan, L.; Miki, H.; Sebald, G.; Diguet, G.; Ohtsuka, M.; Kohl, M. Heusler alloy-based heat engine using pyroelectric conversion for small-scale thermal energy harvesting. Appl. Energy 2021, 288, 116617. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, M.; Long, R.; Liu, Z.; Liu, W. Dynamic modeling and analysis of an advanced adsorption-based osmotic heat engines to harvest solar energy. Renew. Energy 2021, 175, 638–649. [Google Scholar] [CrossRef]
- Rajavel, K.; Luo, S.; Wan, Y.; Yu, X.; Hu, Y.; Zhu, P.; Sun, R.; Wong, C. 2D Ti3C2Tx MXene/polyvinylidene fluoride (PVDF) nanocomposites for attenuation of electromagnetic radiation with excellent heat dissipation. Compos. Part A Appl. Sci. Manuf. 2020, 129, 105693. [Google Scholar] [CrossRef]
- Mann, B.P.; Sims, N.D. Energy harvesting from the nonlinear oscillations of magnetic levitation. J. Sound Vib. 2009, 319, 515–530. [Google Scholar] [CrossRef]
- Mann, B.P.; Sims, N.D. On the performance and resonant frequency of electromagnetic induction energy harvesters. J. Sound Vib. 2010, 329, 1348–1361. [Google Scholar] [CrossRef]
- Reddy, V.M.; Sudheer, S.; Prabhu, S.V.; Kumar, S. Design and calibration of a new compact radiative heat-flux gauge (RHFG) for combustion applications. Sens. Actuators A Phys. 2013, 203, 62–68. [Google Scholar] [CrossRef]
- Zergoune, Z.; Kacem, N.; Bouhaddi, N. On the energy localization in weakly coupled oscillators for electromagnetic vibration energy harvesting. Smart Mater. Struct. 2019, 28, 07LT02. [Google Scholar] [CrossRef]
- Pan, H.; Qi, L.; Zhang, Z.; Yan, J. Kinetic energy harvesting technologies for applications in land transportation: A comprehensive review. Appl. Energy 2021, 286, 116518. [Google Scholar] [CrossRef]
- Wu, X.; Qi, L.; Zhang, T.; Zhang, Z.; Yuan, Y.; Liu, Y. A novel kinetic energy harvester using vibration rectification mechanism for self-powered applications in railway. Energy Convers. Manag. 2021, 228, 113720. [Google Scholar] [CrossRef]
- Aouali, K.; Kacem, N.; Bouhaddi, N.; Mrabet, E.; Haddar, M. Efficient broadband vibration energy harvesting based on tuned non-linearity and energy localization. Smart Mater. Struct. 2020, 29, 10LT01. [Google Scholar] [CrossRef]
- Ahmed, S.; Kakkar, V. An Electret-Based Angular Electrostatic Energy Harvester for Battery-Less Cardiac and Neural Implants. IEEE Access 2017, 5, 19631–19643. [Google Scholar] [CrossRef]
- Gudavalli, G.S.; Dhakal, T.P. Simple parallel-plate capacitors to high-energy density future supercapacitors: A materials review. In Emerging Materials for Energy Conversion and Storage; Cheong, K.Y., Impellizzeri, G., Fraga, M.A., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 247–301. ISBN 9780128137949. [Google Scholar]
- Zhao, J.; Burke, A.F. Review on supercapacitors: Technologies and performance evaluation. J. Energy Chem. 2021, 59, 276–291. [Google Scholar] [CrossRef]
- Sarker, M.R.; Mohamed, R.; Saad, M.H.M.; Tahir, M.; Hussain, A.; Mohamed, A. A Hybrid Optimization Approach for the Enhancement of Efficiency of a Piezoelectric Energy Harvesting System. Electronics 2021, 10, 75. [Google Scholar] [CrossRef]
- Sarker, M.R.; Mohamed, A.; Mohamed, R. Vibration Based Piezoelectric Energy Harvesting Utilizing Bridgeless Rectifier Circuit. J. Kejuruter. 2016, 28, 87–94. [Google Scholar] [CrossRef]
- Sarker, M.R.; Julai, S.; Sabri, M.F.M.; Said, S.M.; Islam, M.M.; Tahir, M. Review of piezoelectric energy harvesting system and application of optimization techniques to enhance the performance of the harvesting system. Sens. Actuators A Phys. 2019, 300, 111634. [Google Scholar] [CrossRef]
- Sarker, M.R.; Mohamed, R.; Saad, M.H.M.; Tahir, M.; Hussain, A. Vibration based energy harvesting system for mobile device charging. Int. J. Appl. Electromagn. Mech. 2020, 65, 149–169. [Google Scholar] [CrossRef]
- Altin, S.; Bulut, F.; Yasar, S. The production of a low cost printing device for energy storage systems and the application for supercapacitors. J. Energy Storage 2019, 25, 100882. [Google Scholar] [CrossRef]
- Zhixiong Hing, W.W. A hybrid compression-assisted absorption thermal battery with high energy storage density/efficiency and low charging temperature. Appl. Energy 2021, 282, 116068. [Google Scholar]
- Roy, P.; He, J.; Liao, Y. Cost Minimization of Battery-Supercapacitor Hybrid Energy Storage for Hourly Dispatching Wind-Solar Hybrid Power System. IEEE Access 2020, 8, 210099–210115. [Google Scholar] [CrossRef]
- Vukajlović, N.; Milićević, D.; Dumnić, B.; Popadić, B. Comparative analysis of the supercapacitor influence on lithium battery cycle life in electric vehicle energy storage. J. Energy Storage 2020, 31, 101603. [Google Scholar] [CrossRef]
- Zhao, X.; Li, Z.; Guo, Q.; Yang, X.; Nie, G. High performance organic-inorganic hybrid material with multi-color change and high energy storage capacity for intelligent supercapacitor application. J. Alloys Compd. 2021, 855, 157480. [Google Scholar] [CrossRef]
- Yun, J.; Song, C.; Lee, H.; Park, H.; Jeong, Y.R.; Kim, J.W.; Jin, S.W.; Oh, S.Y.; Sun, L.; Zi, G.; et al. Stretchable array of high-performance micro-supercapacitors charged with solar cells for wireless powering of an integrated strain sensor. Nano Energy 2018, 49, 644–654. [Google Scholar] [CrossRef]
- Abdelkader, A.; Rabeh, A.; Mohamed Ali, D.; Mohamed, J. Multi-objective genetic algorithm based sizing optimization of a stand-alone wind/PV power supply system with enhanced battery/supercapacitor hybrid energy storage. Energy 2018, 163, 351–363. [Google Scholar] [CrossRef]
- Gu, Y.; Du, W.; Liu, X.; Gao, R.; Liu, Y.; Ma, H.; Xu, J.; Wei, S. Matching design of high-performance electrode materials with different energy-storage mechanism suitable for flexible hybrid supercapacitors. J. Alloys Compd. 2020, 844, 156196. [Google Scholar] [CrossRef]
- Soltani, M.; Ronsmans, J.; Kakihara, S.; Jaguemont, J.; Van den Bossche, P.; van Mierlo, J.; Omar, N. Hybrid battery/lithium-ion capacitor energy storage system for a pure electric bus for an urban transportation application. Appl. Sci. 2018, 8, 1176. [Google Scholar] [CrossRef]
- Tuna, G.; Gungor, V.C. Energy harvesting and battery technologies for powering wireless sensor networks. In Industrial Wireless Sensor Networks: Monitoring, Control and Automation; Budampati, R., Kolavennu, S., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2016; pp. 25–38. [Google Scholar]
- Zhang, Y.; Yang, H. Modeling and characterization of supercapacitors for wireless sensor network applications. J. Power Sources 2011, 196, 4128–4135. [Google Scholar] [CrossRef]
- Hosseinkhani, A.; Younesian, D.; Eghbali, P.; Moayedizadeh, A.; Fassih, A. Sound and vibration energy harvesting for railway applications: A review on linear and nonlinear techniques. Energy Rep. 2021, 7, 852–874. [Google Scholar] [CrossRef]
- Swain, N.; Mitra, A.; Saravanakumar, B.; Balasingam, S.K.; Mohanty, S.; Nayak, S.K.; Ramadoss, A. Construction of three-dimensional MnO2/Ni network as an efficient electrode material for high performance supercapacitors. Electrochim. Acta 2020, 342, 136041. [Google Scholar] [CrossRef]
- Harshavarthini, S.; Divya, M.; Bongarla, R.; Priya, C.H.; Balaji, R. A critical investigation on regenerative braking energy recovering system on HEV based on electric and natural extracted fuel. Materialstoday 2021, 11, 1029. [Google Scholar]
- Bu, F.; Zhou, W.; Xu, Y.; Du, Y.; Guan, C.; Huang, W. Recent developments of advanced micro-supercapacitors: Design, fabrication and applications. NPJ Flex. Electron. 2020, 4, 1–16. [Google Scholar] [CrossRef]
- Ferris, A.; Pech, D.; Garbarino, S.; Guay, D. Potentialities of micro-supercapacitors as energy storage buffers in embedded micro-systems. In Proceedings of the Symposium on Design, Test, Integration and Packaging of MEMS/MOEMS, DTIP 2016, Budapest, Hungary, 30 May–2 June 2016; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2016. [Google Scholar]
- Wang, H.; Hu, W.; Zhang, G.; Tang, Y.; Jing, S.; Chen, Z. Small-signal modelling of AC/MTDC hybrid power systems using Multi-Layer Component Connection Method. Energy Rep. 2020, 6, 1033–1040. [Google Scholar] [CrossRef]
- Satpathy, S.; Das, S.; Bhattacharyya, B.K. How and where to use super-capacitors effectively, an integration of review of past and new characterization works on super-capacitors. J. Energy Storage 2020, 27, 101044. [Google Scholar] [CrossRef]
- Teso-Fz-betoño, D.; Aramendia, I.; Martinez-Rico, J.; Fernandez-Gamiz, U.; Zulueta, E. Piezoelectric energy harvesting controlled with an IGBT H-bridge and bidirectional buck–boost for low-cost 4G devices. Sensors 2020, 20, 7039. [Google Scholar] [CrossRef] [PubMed]
- Sarker, M.R.; Mohamed, R.; Saad, M.H.M.; Mohamed, A. dSPACE Controller-Based Enhanced Piezoelectric Energy Harvesting System Using PI-Lightning Search Algorithm. IEEE Access 2019, 7, 3610–3626. [Google Scholar] [CrossRef]
- Zhou, P.; Wang, C.; Yang, Y. Self-sustainable Sensor Networks with Multi-source Energy Harvesting and Wireless Charging. In Proceedings of the Proceedings-IEEE INFOCOM, Paris, France, 29 April–2 May 2019; pp. 1828–1836. [Google Scholar]
- Liang, Y.; Zhao, C.; Yuan, H.; Chen, Y.; Zhang, W.; Huang, J.; Yu, D.; Liu, Y.; Titirici, M.; Chueh, Y.; et al. A review of rechargeable batteries for portable electronic devices. InfoMat 2019, 1, 6–32. [Google Scholar] [CrossRef]
- Yoo, H.D.; Markevich, E.; Salitra, G.; Sharon, D.; Aurbach, D. On the challenge of developing advanced technologies for electrochemical energy storage and conversion. Mater. Today 2014, 17, 110–121. [Google Scholar] [CrossRef]
- Ngui, M.H.; Lee, W.K. Low power wearable device with GPS and indoor positioning system. In Proceedings of the 2019 7th International Conference on Green and Human Information Technology, ICGHIT 2019, Kuala Lumpur, Malaysia, 15–17 January 2019; pp. 125–127. [Google Scholar]
- Zhang, Z.; Fang, Z.; Xiang, Y.; Liu, D.; Xie, Z.; Qu, D.; Sun, M.; Tang, H.; Li, J. Cellulose-based material in lithium-sulfur batteries: A review. Carbohydr. Polym. 2021, 255, 117469. [Google Scholar] [CrossRef]
- Zhang, Z.; Tang, X.; Liu, Y.; Xu, Z.; Ye, H.; Tian, F.; Liu, K.; Yuan, Z.; Chen, W. Application of liquid scintillators as energy conversion materials in nuclear batteries. Sens. Actuators A Phys. 2019, 290, 162–171. [Google Scholar] [CrossRef]
- Schumm, B. Advances and trends in primary and small secondary batteries with zinc anodes and manganese dioxide and/or air cathodes. In Proceedings of the Proceedings of the Annual Battery Conference on Applications and Advances, Long Beach, CA, USA, 11–14 January 2000; pp. 89–94. [Google Scholar]
- Faria, R.; Marques, P.; Garcia, R.; Moura, P.; Freire, F.; Delgado, J.; De Almeida, A.T. Primary and secondary use of electric mobility batteries from a life cycle perspective. J. Power Sources 2014, 262, 169–177. [Google Scholar] [CrossRef]
- Hu, X.; Robles, A.; Vikström, T.; Väänänen, P.; Zackrisson, M.; Ye, G. A novel process on the recovery of zinc and manganese from spent alkaline and zinc-carbon batteries. J. Hazard. Mater. 2021, 411, 124928. [Google Scholar] [CrossRef]
- Hannan, M.A.; Hoque, M.M.; Mohamed, A.; Ayob, A. Review of energy storage systems for electric vehicle applications: Issues and challenges. Renew. Sustain. Energy Rev. 2017, 69, 771–789. [Google Scholar] [CrossRef]
- Massé, R.C.; Liu, C.; Li, Y.; Mai, L.; Cao, G. Energy storage through intercalation reactions: Electrodes for rechargeable batteries. Natl. Sci. Rev. 2017, 4, 26–53. [Google Scholar] [CrossRef]
- James, F.R.; Maksudul, H.; Sanjay, P.; Declan, P.C.; Tomás, M.C. Energy Storage: Battery Materials and Architectures at the Nanoscale. In ICT-Energy-Concepts Towards Zero-Power Information and Communication Technology; Gammaitoni, L., Paul, D., Abadal, G., Eds.; InTech: London, UK, 2014. [Google Scholar]
- Kim, J.; Krüger, L.; Kowal, J. On-line state-of-health estimation of Lithium-ion battery cells using frequency excitation. J. Energy Storage 2020, 32, 101841. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, X.; Peng, Y.; Li, L.; Cao, J.; Yang, L.; Cao, B. Quantitative study on the thermal failure features of lithium iron phosphate batteries under varied heating powers. Appl. Eng. 2021, 185, 116346. [Google Scholar]
- Guo, R.; Lu, L.; Ouyang, M.; Feng, X. Mechanism of the entire overdischarge process and overdischarge-induced internal short circuit in lithium-ion batteries. Sci. Rep. 2016, 6, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Xu, Y.; Cao, H.; Lin, X.; Ning, P.; Zhang, Y.; Garcia, Y.G.; Sun, Z. Internal failure of anode materials for lithium batteries—A critical review. Green Energy Environ. 2020, 5, 22–36. [Google Scholar] [CrossRef]
- Fras, T.; Pawlowski, P.; Li, W.; Wierzbicki, T. Performance of Li-ion pouch batteryunder a high-velocity impact: Experiment and numerical simulation. Int. J. Impact Eng. 2021, 155, 103915. [Google Scholar] [CrossRef]
- Fedeli, E.; Garcia-Calvo, O.; Thieu, T.; Phan, T.N.T.; Gigmes, D.; Urdampilleta, I.; Kvasha, A. Nanocomposite solid polymer electrolytes based on semi-interpenetrating hybrid polymer networks for high performance lithium metal batteries. Electrochim. Acta 2020, 353, 136481. [Google Scholar] [CrossRef]
- Gu, J.; Zhong, J.; Zhu, K.-D.; Wang, X.-R.; Wang, S.-L. In-situ synthesis of novel nanostructured Pb@C composites for improving the performance of lead-acid batteries under high-rate partial-state-of-charge operation. J. Energy Storage 2021, 33, 102082. [Google Scholar] [CrossRef]
- Yiding, L.; Wenwei, W.; Cheng, L.; Fenghao, Z. High-efficiency multiphysics coupling framework for cylindrical lithium-ion battery under mechanical abuse. J. Clean. Prod. 2021, 286, 125451. [Google Scholar] [CrossRef]
- Darcovich, K.; Henquin, E.R.; Kenney, B.; Davidson, I.J.; Saldanha, N.; Beausoleil-Morrison, I. Higher-capacity lithium ion battery chemistries for improved residential energy storage with micro-cogeneration. Appl. Energy 2013, 111, 853–861. [Google Scholar] [CrossRef]
- Kuchak, S.V. Autonomic power supply system based on Diesel generator set and storage of electrical energy from Li-ion battery. In Proceedings of the International Conference of Young Specialists on Micro/Nanotechnologies and Electron Devices, EDM, Novosibirsk, Russia, 30 June–4 July 2014; pp. 408–410. [Google Scholar]
- Yu, K.; Wang, J.; Wang, X.; Li, Y.; Liang, C. Zinc–cobalt bimetallic sulfide anchored on the surface of reduced graphene oxide used as anode for lithium ion battery. J. Solid State Chem. 2020, 290, 121619. [Google Scholar] [CrossRef]
- Cui, F.; Zhao, J.; Zhang, D.; Fang, Y.; Hu, F.; Zhu, K. VO2(B) nanobelts and reduced graphene oxides composites as cathode materials for low-cost rechargeable aqueous zinc ion batteries. Chem. Eng. J. 2020, 390, 124118. [Google Scholar] [CrossRef]
- Kashem, M.A.; Fattah, A.; Farhan, S.M.S.; Nafis, N. Fast Formation of Tubular Plate Deep Cycle Lead Acid Battery by Acid Recirculation System (ACS). In Proceedings of the 2nd International Conference on Electrical, Computer and Communication Engineering, ECCE 2019, Cox’sBazar, Bangladesh, 7–9 February 2019; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2019. [Google Scholar]
- Schnell, J.; Günther, T.; Knoche, T.; Vieider, C.; Köhler, L.; Just, A.; Keller, M.; Passerini, S.; Reinhart, G. All-solid-state lithium-ion and lithium metal batteries—Paving the way to large-scale production. J. Power Sources 2018, 382, 160–175. [Google Scholar] [CrossRef]
- Feng, X.; Ouyang, M.; Liu, X.; Lu, L.; Xia, Y.; He, X. Thermal runaway mechanism of lithium ion battery for electric vehicles: A review. Energy Storage Mater. 2018, 10, 246–267. [Google Scholar] [CrossRef]
- Ananda, S.; Lakshminarasamma, N.; Radhakrishna, V.; Srinivasan, M.S.; Satyanarayana, P.; Sankaran, M. Generic Lithium ion battery model for energy balance estimation in spacecraft. In Proceedings of the 2018 IEEE International Conference on Power Electronics, Drives and Energy Systems, PEDES 2018, Chennai, India, 18 June 2019; Institute of Electrical and Electronics Engineers Inc.: Piscataway, NJ, USA, 2018. [Google Scholar]
- Li, N.; Liu, X.; Yu, B.; Li, L.; Xu, J.; Tan, Q. Study on the environmental adaptability of lithium-ion battery powered UAV under extreme temperature conditions. Energy 2021, 219, 119481. [Google Scholar] [CrossRef]
- Keles, O.; Karahan, B.D.; Eryilmaz, L.; Amine, R.; Abouimrane, A.; Chen, Z.; Zuo, X.; Zhu, Z.; Al-Hallaj, S.; Amine, K. Superlattice-structured films by magnetron sputtering as new era electrodes for advanced lithium-ion batteries. Nano Energy 2020, 76, 105094. [Google Scholar] [CrossRef]
- Lithium-Ion Batteries. Available online: https://www.science.org.au/curious/technology-future/lithium-ion-batteries (accessed on 4 June 2021).
- Rand, D.A.J.; Moseley, P.T. Energy Storage with Lead-Acid Batteries. In Electrochemical Energy Storage for Renewable Sources and Grid Balancing; Moseley, P.T., Garche, J., Eds.; Elsevier Inc.: Amsterdam, The Netherlands, 2015; pp. 201–222. [Google Scholar]
- Wu, Z.; Liu, Y.; Deng, C.; Zhao, H.; Zhao, R.; Chen, H. The critical role of boric acid as electrolyte additive on the electrochemical performance of lead-acid battery. J. Energy Storage 2020, 27, 101076. [Google Scholar] [CrossRef]
- Mithin Kumar, S.; Arun, S.; Mayavan, S. Effect of carbon nanotubes with varying dimensions and properties on the performance of lead acid batteries operating under high rate partial state of charge conditions. J. Energy Storage 2019, 24, 100806. [Google Scholar] [CrossRef]
- Shen, Y. Hybrid unscented particle filter based state-of-charge determination for lead-acid batteries. Energy 2014, 74, 795–803. [Google Scholar] [CrossRef]
- Singh, A.; Karandikar, P.B.; Kulkarni, N.R. Mitigation of sulfation in lead acid battery towards life time extension using ultra capacitor in hybrid electric vehicle. J. Energy Storage 2021, 34, 102219. [Google Scholar] [CrossRef]
- Boden, D.P. Comparison of methods for adding expander to lead-acid battery plates—Advantages and disadvantages. J. Power Sources 2004, 133, 47–51. [Google Scholar] [CrossRef]
- Zhang, Y.; Jiang, J.; An, Y.; Wu, L.; Dou, H.; Zhang, J.; Zhang, Y.; Wu, S.; Dong, M.; Zhang, X.; et al. Sodium-ion capacitors: Materials, Mechanism, and Challenges. ChemSusChem 2020, 13, 2522–2539. [Google Scholar] [CrossRef]
- Ericson, B.; Duong, T.T.; Keith, J.; Nguyen, T.C.; Havens, D.; Daniell, W.; Karr, C.J.; Ngoc Hai, D.; Van Tung, L.; Thi Nhi Ha, T.; et al. Improving human health outcomes with a low-cost intervention to reduce exposures from lead acid battery recycling: Dong Mai, Vietnam. Env. Res. 2018, 161, 181–187. [Google Scholar] [CrossRef]
- Paul, S.; Shakya, A.K.; Ghosh, P.K. Bacterially-assisted recovery of cadmium and nickel as their metal sulfide nanoparticles from spent Ni–Cd battery via hydrometallurgical route. J. Environ. Manag. 2020, 261, 110113. [Google Scholar] [CrossRef] [PubMed]
- What Happens inside the Rechargeable Battery during Charging and Discharging? Available online: https://www.matsusada.com/column/secondary-battery.html (accessed on 5 June 2021).
- Oghabi, H.; Haghshenas, D.F.; Firoozi, S. Selective separation of Cd from spent Ni-Cd battery using glycine as an eco-friendly leachant and its recovery as CdS nanoparticles. Sep. Purif. Technol. 2020, 242, 116832. [Google Scholar] [CrossRef]
- Moreira, T.F.M.; Santana, I.L.; Moura, M.N.; Ferreira, S.A.D.; Lelis, M.F.F.; Freitas, M.B.J.G. Recycling of negative electrodes from spent Ni-Cd batteries as CdO with nanoparticle sizes and its application in remediation of azo dye. Mater. Chem. Phys. 2017, 195, 19–27. [Google Scholar] [CrossRef]
- Marins, A.A.L.; Banhos, S.G.; Muri, E.J.B.; Rodrigues, R.V.; Cruz, P.C.M.; Freitas, M.B.J.G. Synthesis by coprecipitation with oxalic acid of rare earth and nickel oxides from the anode of spent Ni–MH batteries and its electrochemical properties. Mater. Chem. Phys. 2020, 242, 122440. [Google Scholar] [CrossRef]
- García-Plaza, M.; Serrano-Jiménez, D.; Eloy-García Carrasco, J.; Alonso-Martínez, J. A Ni-Cd battery model considering state of charge and hysteresis effects. J. Power Sources 2015, 275, 595–604. [Google Scholar] [CrossRef]
- Wang, H.; Deng, C.; Li, X.; Yan, D.; Xie, M.; Zhang, S.; Huang, B. Designing dual-defending system based on catalytic and kinetic iron Pyrite@C hybrid fibers for long-life room-temperature sodium-sulfur batteries. Chem. Eng. J. 2021, 420, 129681. [Google Scholar] [CrossRef]
- Ma, Q.; Du, G.; Zhong, W.; Du, W.; Bao, S.-J.; Xu, M.; Li, C. Template method for fabricating Co and Ni nanoparticles/porous channels carbon for solid-state sodium-sulfur battery. J. Colloid Interface Sci. 2020, 578, 710–716. [Google Scholar] [CrossRef]
- Xiao, R.; Chen, K.; Zhang, X.; Yang, Z.; Hu, G.; Sun, Z.; Cheng, H.M.; Li, F. Single-atom catalysts for metal-sulfur batteries: Current progress and future perspectives. J. Energy Chem. 2021, 54, 452–466. [Google Scholar] [CrossRef]
- He, L.; Sun, Y.R.; Wang, C.L.; Guo, H.Y.; Guo, Y.Q.; Li, C.; Zhou, Y. High performance sulphur-doped pitch-based carbon materials as anode materials for sodium-ion batteries. Xinxing Tan Cailiao New Carbon Mater. 2020, 35, 420–427. [Google Scholar] [CrossRef]
- Eizenberg, M. Introduction: Interlayer dielectrics in microelectronic devices. In Interlayer Dielectrics for Semiconductor Technologies; Muraka, S.P., Eizenberg, M., Sinha, A.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; pp. 1–6. [Google Scholar]
- Uesato, H.; Miyaoka, H.; Ichikawa, T.; Kojima, Y. Hybrid nickel-metal hydride/hydrogen battery. Int. J. Hydrogen Energy 2019, 44, 4263–4270. [Google Scholar] [CrossRef]
- Sanli, A.E.; Yilmaz, E.S.; Ozden, S.K.; Gordesel, M.; Gunlu, G. A direct borohydride–peroxide fuel cell–LiPO battery hybrid motorcycle prototype-II. Int. J. Hydrogen Energy 2018, 43, 992–1005. [Google Scholar] [CrossRef]
- Liu, J.; Auciello, O.; de Obaldia, E.; Da, B.; Koide, Y. Science and Technology of Integrated Super-High Dielectric Constant AlOx/TiOy Nanolaminates/Diamond for MOS Capacitors and MOSFETs. Carbon 2021, 172, 112–121. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Sun, X.; An, Y.; Zhang, X.; Wang, K.; Dong, C.; Huo, Q.; Wei, T.; Ma, Y. Experimental study of thermal charge–discharge behaviors of pouch lithium-ion capacitors. J. Energy Storage 2019, 25, 100902. [Google Scholar] [CrossRef]
- Kalaiarasi, S.; Kavitha, M.; Karpagavinayagam, P.; Vedhi, C.; Muthuchudarkodi, R.R. Tungsten oxide decorated graphene oxide nanocomposite: Chemical synthesis, characterization and application in super capacitors. Mater. Today Proc. 2020. (In Press) [CrossRef]
- Yang, F.; Pan, Z.; Ling, Z.; Hu, D.; Ding, J.; Li, P.; Liu, J.; Zhai, J. Realizing high comprehensive energy storage performances of BNT-based ceramics for application in pulse power capacitors. J. Eur. Ceram. Soc. 2021, 41, 2548–2558. [Google Scholar] [CrossRef]
- Sarker, M.R.; Ali, S.H.M.; Othman, M.; Islam, M.S. Designing a low voltage energy harvesting circuits for rectified storage voltage using vibrating piezoelectric. In Proceedings of the 2011 IEEE Student Conference on Research and Development, Cyberjaya, Malaysia, 19–20 December 2011; pp. 343–346. [Google Scholar]
- Cheng, Z.; Qiu, Y.; Tan, G.; Chang, X.; Luo, Q.; Cui, L. Synthesis of a Novel Mn (II)-porphyrins polycondensation polymer and its application as pseudo-capacitor electrode material. J. Organomet. Chem. 2019, 900, 120940. [Google Scholar] [CrossRef]
- Xiang, A.; Xie, S.; Pan, F.; Jin, H.; Zhai, Y.; Zhu, Y.; Kong, X.; Ji, H. Cobalt and nitrogen atoms co-doped porous carbon for advanced electrical double-layer capacitors. Chin. Chem. Lett. 2019, 32, 830–833. [Google Scholar] [CrossRef]
- Daraghmeh, A.; Hussain, S.; Haq, A.U.; Saadeddin, I.; Servera, L.; Ruiz, J.M. Carbon nanocomposite electrodes for electrical double layer capacitor. J. Energy Storage 2020, 32, 101798. [Google Scholar] [CrossRef]
- Rezzak, D.; Boudjerda, N. Robust energy management strategy based on non-linear cascade control of fuel cells-super capacitors hybrid power system. Int. J. Hydrogen Energy 2020, 45, 23254–23274. [Google Scholar] [CrossRef]
- Sridhar, V.; Park, H. Manganese nitride stabilized on reduced graphene oxide substrate for high performance sodium ion batteries, super-capacitors and EMI shielding. J. Alloys Compd. 2019, 808, 151748. [Google Scholar] [CrossRef]
- Sennu, P.; Chua, R.; Dintakurti, S.S.H.; Hanna, J.V.; Ramabhadran, R.O.; Aravindan, V.; Madhavi, S. Supersaturated “water-in-salt” hybrid electrolyte towards building high voltage Na-ion capacitors with wide temperatures operation. J. Power Sources 2020, 472, 228558. [Google Scholar] [CrossRef]
- Chen, X.; Paul, R.; Dai, L. Carbon-based supercapacitors for efficient energy storage. Natl. Sci. Rev. 2017, 4, 453–489. [Google Scholar] [CrossRef]
- Alexe-Ionescu, A.L.; Zaccagnini, P.; Lamberti, A.; Pirri, C.F.; Barbero, G. Generalized Langmuir kinetic equation for ions adsorption model applied to electrical double layer capacitor. Electrochim. Acta 2019, 323, 134700. [Google Scholar] [CrossRef]
- Sellali, M.; Abdeddaim, S.; Betka, A.; Djerdir, A.; Drid, S.; Tiar, M. Fuzzy-Super twisting control implementation of battery/super capacitor for electric vehicles. Isa Trans. 2019, 95, 243–253. [Google Scholar] [CrossRef] [PubMed]
- Ajay, K.M.; Dinesh, M.N.; Vishnu Murthy, K.A.; Ravikumar, C.R.; Nagaswarupa, H.P. Deposition & Electrochemical characterization of Multilayer coated electrode material for super capacitor application. Mater. Today Proc. 2018, 5, 21452–21457. [Google Scholar]
- Veneri, O.; Capasso, C.; Patalano, S. Experimental investigation into the effectiveness of a super-capacitor based hybrid energy storage system for urban commercial vehicles. Appl. Energy 2018, 227, 312–323. [Google Scholar] [CrossRef]
- Abdul Wahab, Y.; Naseer, M.N.; Zaidi, A.A.; Umair, T.; Khan, H.; Siddiqi, M.M.; Javed, M.S. Super Capacitors in Various Dimensionalities: Applications and Recent Advancements. Ref. Modul. Earth Syst. Environ. Sci. 2020. [Google Scholar] [CrossRef]
- Zhang, L.; Wu, D.; Wang, G.; Xu, Y.; Li, H.; Yan, X. An aqueous zinc-ion hybrid super-capacitor for achieving ultrahigh-volumetric energy density. Chin. Chem. Lett. 2020, 32, 926–931. [Google Scholar] [CrossRef]
- Que, L.-F.; Yu, F.-D.; Sui, X.-L.; Zhao, L.; Zhou, J.-G.; Gu, D.-M.; Wang, Z.-B. Thermal-induced interlayer defect engineering toward super high-performance sodium ion capacitors. Nano Energy 2019, 59, 17–25. [Google Scholar] [CrossRef]
- Galek, P.; Frackowiak, E.; Fic, K. Interfacial aspects induced by saturated aqueous electrolytes in electrochemical capacitor applications. Electrochim. Acta 2020, 334, 135572. [Google Scholar] [CrossRef]
- Palneedi, H.; Peddigari, M.; Upadhyay, A.; Silva, J.P.B.; Hwang, G.-T.; Ryu, J. Lead-based and lead-free ferroelectric ceramic capacitors for electrical energy storage. In Ferroelectric Materials for Energy Harvesting and Storage; Maurya, D., Pramanick, A., Viehland, D., Eds.; Elsevier: Amsterdam, The Netherlands, 2021; pp. 279–356. [Google Scholar]
- Liu, Y.; Wu, Q.; Liu, L.; Manasa, P.; Kang, L.; Ran, F. Vanadium nitride for aqueous supercapacitors: A topic review. J. Mater. Chem. A 2020, 8, 8218–8233. [Google Scholar] [CrossRef]
- Chung-Parsons, R.; Bailey, J.M. The hierarchical (not fluid) nature of preservice secondary science teachers’ perceptions of their science teacher identity. Teach. Teach. Educ. 2019, 78, 39–48. [Google Scholar] [CrossRef]
- Heyd-Metzuyanim, E. Changing teaching practices towards explorative mathematics instruction—The interweaving of teacher identity and pedagogical discourse. Teach. Teach. Educ. 2019, 86, 102862. [Google Scholar] [CrossRef]
- Lindberg, S.; Ndiaye, N.M.; Manyala, N.; Johansson, P.; Matic, A. A VO2 based hybrid super-capacitor utilizing a highly concentrated aqueous electrolyte for increased potential window and capacity. Electrochim. Acta 2020, 345, 136225. [Google Scholar] [CrossRef]
- Park, E.; Chung, D.J.; Park, M.S.; Kim, H. Pre-lithiated carbon-coated Si/SiOx nanospheres as a negative electrode material for advanced lithium ion capacitors. J. Power Sources 2019, 440, 227094. [Google Scholar] [CrossRef]
- Yang, Y.; Zhang, Q.; Yu, H.; Feng, X. Tech-economic and environmental analysis of energy-efficient shale gas and flue gas coupling system for chemicals manufacture and carbon capture storage and utilization. Energy 2021, 217, 119348. [Google Scholar] [CrossRef]
- Chen, X.; Chu, A.; Li, D.; Yuan, Y.; Fan, X.; Deng, Y. Development of the cycling life model of Ni-MH power batteries for hybrid electric vehicles based on real-world operating conditions. J. Energy Storage 2021, 34, 101999. [Google Scholar] [CrossRef]
- Hosseini, S.E.; Butler, B. An overview of development and challenges in hydrogen powered vehicles. Int. J. Green Energy 2020, 17, 13–37. [Google Scholar] [CrossRef]
- Kumar, P.; Chaudhary, D.; Varshney, P.; Varshney, U.; Yahya, S.M.; Rafat, Y. Critical review on battery thermal management and role of nanomaterial in heat transfer enhancement for electrical vehicle application. J. Energy Storage 2020, 32, 102003. [Google Scholar] [CrossRef]
- Jidhesh, P.; Arjunan, T.V.; David Rathnaraj, J. Experimental investigation on heat transfer characteristics of phase change composite for thermal energy storage system. Mater. Today Proc. 2020, 42, 618–625. [Google Scholar] [CrossRef]
- Frazzica, A.; Brancato, V.; Caprì, A.; Cannilla, C.; Gordeeva, L.G.; Aristov, Y.I. Development of “salt in porous matrix” composites based on LiCl for sorption thermal energy storage. Energy 2020, 208, 118338. [Google Scholar] [CrossRef]
- Akba, T.; Baker, D.; Yazıcıoğlu, A.G. Modeling, transient simulations and parametric studies of parabolic trough collectors with thermal energy storage. Sol. Energy 2020, 199, 497–509. [Google Scholar] [CrossRef]
- Zhang, X.; Klein, R.; Subbaraman, A.; Chumakov, S.; Li, X.; Christensen, J.; Linder, C.; Kim, S.U. Evaluation of convective heat transfer coefficient and specific heat capacity of a lithium-ion battery using infrared camera and lumped capacitance method. J. Power Sources 2019, 412, 552–558. [Google Scholar] [CrossRef]
- Kumar, A.; Saha, S.K. Performance study of a novel funnel shaped shell and tube latent heat thermal energy storage system. Renew. Energy 2021, 165, 731–747. [Google Scholar] [CrossRef]
- Long, R.; Lai, X.; Liu, Z.; Liu, W. A continuous concentration gradient flow electrical energy storage system based on reverse osmosis and pressure retarded osmosis. Energy 2018, 152, 896–905. [Google Scholar] [CrossRef]
- Khomutov, M.; Chereshneva, A.; Petrovskiy, P.; Daubarayte, D.; Cheverikin, V.; Sova, A.; Travyanov, A.; Smurov, I. Microstructure of Al–Mg-Sc-Zr alloy cold spray deposits after heat treatment and hot isostatic pressing. J. Alloys Compd. 2021, 858, 157644. [Google Scholar] [CrossRef]
- Rathna, R.; Jeno, J.G.A.; Sivagami, N.; Bharathi, V.P.; Nakkeeran, E. Invisible membrane revolution: Shaping the future of air purification. In Nanomaterials for Air Remediation; Amrane, A., Assadi, A.A., Nguyen-Tri, P., Nguyen, T.A., Rtimi, S., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 343–358. [Google Scholar]
- Wu, M.; Cong, X.; Li, M.; Rao, S.; Liu, Y.; Guo, J.; Zhu, S.; Chen, S.; Xu, F.; Cheng, S.; et al. Effects of different exogenous selenium on Se accumulation, nutrition quality, elements uptake, and antioxidant response in the hyperaccumulation plant Cardamine violifolia. Ecotoxicol. Env. Saf. 2020, 204, 111045. [Google Scholar] [CrossRef]
- Gil–-González, W.; Montoya, O.D.; Garces, A. Direct power control of electrical energy storage systems: A passivity-based PI approach. Electr. Power Syst. Res. 2019, 175, 105885. [Google Scholar] [CrossRef]
- Walter, O.; Tremel, A.; Prenzel, M.; Becker, S.; Schaefer, J. Techno-economic analysis of hybrid energy storage concepts via flowsheet simulations, cost modeling and energy system design. Energy Convers. Manag. 2020, 218, 112955. [Google Scholar] [CrossRef]
- Arabi-Nowdeh, S.; Nasri, S.; Saftjani, P.B.; Naderipour, A.; Abdul-Malek, Z.; Kamyab, H.; Jafar-Nowdeh, A. Multi-criteria optimal design of hybrid clean energy system with battery storage considering off- and on-grid application. J. Clean. Prod. 2021, 290, 125808. [Google Scholar] [CrossRef]
- Chikaoka, Y.; Iwama, E.; Seto, S.; Okuno, Y.; Shirane, T.; Ueda, T.; Naoi, W.; Reid, M.T.H.; Naoi, K. Dual-cation electrolytes for low H2 gas generation in Li4Ti5O12//AC hybrid capacitor system. Electrochim. Acta 2021, 368, 137619. [Google Scholar] [CrossRef]
- Li, J.; Liu, J.; Zeng, M.; He, Z.; Li, H.; Yuan, Y.; Zhang, S. High efficiency and power density relaxor ferroelectric Sr0.875Pb0.125TiO3-Bi(Mg0.5Zr0.5)O3 ceramics for pulsed power capacitors. J. Eur. Ceram. Soc. 2020, 40, 2907–2916. [Google Scholar] [CrossRef]
- Mitra, A.; Jena, S.; Majumder, S.B.; Das, S. Supercapacitor like behavior in nano-sized, amorphous mixed poly-anion cathode materials for high power density lithium and other alkali-metal ion batteries. Electrochim. Acta 2020, 338, 135899. [Google Scholar] [CrossRef]
- Balsamo, F.; Capasso, C.; Lauria, D.; Veneri, O. Optimal design and energy management of hybrid storage systems for marine propulsion applications. Appl. Energy 2020, 278, 115629. [Google Scholar] [CrossRef]
- Shen, D.; Wu, L.; Kang, G.; Guan, Y.; Peng, Z. A novel online method for predicting the remaining useful life of lithium-ion batteries considering random variable discharge current. Energy 2021, 218, 119490. [Google Scholar] [CrossRef]
- Wu, W.; Bai, Y.; Wang, X.; Wu, C. Sulfone-based high-voltage electrolytes for high energy density rechargeable lithium batteries: Progress and perspective. Chin. Chem. Lett. 2020, 32, 1309–1315. [Google Scholar] [CrossRef]
- Pidluzhna, A. TiO2 composite electrode materials for lithium batteries. Electrochim. Acta 2021, 367, 137569. [Google Scholar] [CrossRef]
- Zhu, X.; Pan, X.; Wu, Y.; Wan, W.; Chen, T.; Wang, Y.; Ji, X.; Lü, Z. Layered perovskite oxide PrBaCo2O5+δ as a potential cathode for lithium–oxygen batteries: High-performance bi-functional electrocatalysts. Mater. Lett. 2019, 237, 200–203. [Google Scholar] [CrossRef]
- Du, T.; Liu, Z.; Sun, X.; Geng, L.; Zhang, X.; An, Y.; Zhang, X.; Wang, K.; Ma, Y. Segmented bi-material cathodes to boost the lithium-ion battery-capacitors. J. Power Sources 2020, 478, 228994. [Google Scholar] [CrossRef]
- Yılmazoğlu, M.; Bayıroğlu, F.; Erdemi, H.; Abaci, U.; Guney, H.Y. Dielectric properties of sulfonated poly (ether ether ketone) (SPEEK) electrolytes with 1-ethyl-3-methylimidazolium tetrafluoroborate salt: Ionic liquid-based conduction pathways. Colloids Surf. A Phys. Eng. Asp. 2021, 611, 125825. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, L.; Hao, C.; Zheng, X.; Guo, Y.; Chen, L.; Lai, K.; Zhang, Y.; Ci, L. Potassium pre-inserted K1.04Mn8O16 as cathode materials for aqueous Li-ion and Na-ion hybrid capacitors. J. Energy Chem. 2020, 46, 53–61. [Google Scholar] [CrossRef]
- Liang, Z.; Nakamura, K.; Kobayashi, N. A multicolor electrochromic device having hybrid capacitor architecture with a porous carbon electrode. Sol. Energy Mater. Sol. Cells 2019, 200, 109914. [Google Scholar] [CrossRef]
- Cui, H.; Overend, M. A review of heat transfer characteristics of switchable insulation technologies for thermally adaptive building envelopes. Energy Build. 2019, 199, 427–444. [Google Scholar] [CrossRef]
- Ye, H.; Jiang, B. Adaptive switching control for hypersonic vehicle with uncertain control direction. J. Frankl. Inst. 2020, 357, 8851–8869. [Google Scholar] [CrossRef]
- Salehpour, M.J.; Tafreshi, S.M.M. Contract-based utilization of plug-in electric vehicle batteries for day-ahead optimal operation of a smart micro-grid. J. Energy Storage 2020, 27, 101157. [Google Scholar] [CrossRef]
- Wegener, M.; Villarroel Schneider, J.; Malmquist, A.; Isalgue, A.; Martin, A.; Martin, V. Techno-economic optimization model for polygeneration hybrid energy storage systems using biogas and batteries. Energy 2021, 218, 119544. [Google Scholar] [CrossRef]
- Hassan, M.; Paracha, Z.J.; Armghan, H.; Ali, N.; Said, H.A.; Farooq, U.; Afzal, A.; Hassan, M.A.S. Lyapunov based adaptive controller for power converters used in hybrid energy storage systems. Sustain. Energy Technol. Assess. 2020, 42, 100853. [Google Scholar]
- Chen, S.; Rahbari, H.R.; Arabkoohsar, A.; Zhu, T. Impacts of partial-load service on energy, exergy, environmental and economic performances of low-temperature compressed air energy storage system. J. Energy Storage 2020, 32, 101900. [Google Scholar] [CrossRef]
- Uchman, W.; Kotowicz, J.; Li, K.F. Evaluation of a micro-cogeneration unit with integrated electrical energy storage for residential application. Appl. Energy 2021, 282, 116196. [Google Scholar] [CrossRef]
- Wang, C.; Liang, W.; Tang, Z.; Jia, J.; Liu, F.; Yang, Y.; Sun, H.; Zhu, Z.; Li, A. Enhanced light-thermal conversion efficiency of mixed clay base phase change composites for thermal energy storage. Appl. Clay Sci. 2020, 189, 105535. [Google Scholar] [CrossRef]
- Abedi, H.; Migliorini, F.; Dondè, R.; De Iuliis, S.; Passaretti, F.; Fanciulli, C. Small size thermoelectric power supply for battery backup. Energy 2019, 188, 116061. [Google Scholar] [CrossRef]
- Sagaria, S.; Costa Neto, R.; Baptista, P. Assessing the performance of vehicles powered by battery, fuel cell and ultra-capacitor: Application to light-duty vehicles and buses. Energy Convers. Manag. 2021, 229, 113767. [Google Scholar] [CrossRef]
- Shellikeri, A.; Yturriaga, S.; Zheng, J.S.; Cao, W.; Hagen, M.; Read, J.A.; Jow, T.R.; Zheng, J.P. Hybrid lithium-ion capacitor with LiFePO4/AC composite cathode—Long term cycle life study, rate effect and charge sharing analysis. J. Power Sources 2018, 392, 285–295. [Google Scholar] [CrossRef]
- Delmonte, N.; Cabezuelo, D.; Kortabarria, I.; Santoro, D.; Toscani, A.; Cova, P. A method to extract lumped thermal networks of capacitors for reliability oriented design. Microelectron. Reliab. 2020, 114, 113737. [Google Scholar] [CrossRef]
- Sunithamani, S.; Rooban, S.; Nalinashini, G.; Rajasekhar, K. A review on mems based vibration energy harvester cantilever geometry (2015–2020). Mater. Today Proc. 2020, 1–3. (In Press)
- May, G.J.; Davidson, A.; Monahov, B. Lead batteries for utility energy storage: A review. J. Energy Storage 2018, 15, 145–157. [Google Scholar] [CrossRef]
- Yang, K.; Ying, Y.; Cui, L.; Sun, J.; Luo, H.; Hu, Y.; Zhao, J. Stable aqueous Zn–Ag and Zn–polyoxometalate hybrid battery driven by successive Ag+ cation and polyoxoanion redox reactions. Energy Storage Mater. 2021, 34, 203–210. [Google Scholar] [CrossRef]
- Raza, W.; Ali, F.; Raza, N.; Luo, Y.; Kim, K.H.; Yang, J.; Kumar, S.; Mehmood, A.; Kwon, E.E. Recent advancements in supercapacitor technology. Nano Energy 2018, 52, 441–473. [Google Scholar] [CrossRef]
- Huang, S.; Zhu, X.; Sarkar, S.; Zhao, Y. Challenges and opportunities for supercapacitors. Apl. Mater. 2019, 7, 100901. [Google Scholar] [CrossRef]
- Sarker, M.; Mohamed, A.; Mohamed, R.; Sarker, M.R.; Mohamed, A.; Mohamed, R. A New Method for a Piezoelectric Energy Harvesting System Using a Backtracking Search Algorithm-Based PI Voltage Controller. Micromachines 2016, 7, 171. [Google Scholar] [CrossRef]
- Jami, M.; Shafiee, Q.; Gholami, M.; Bevrani, H. Control of a super-capacitor energy storage system to mimic inertia and transient response improvement of a direct current micro-grid. J. Energy Storage 2020, 32, 101788. [Google Scholar] [CrossRef]
- Li, Z.; Luo, J.; Xie, S.; Xin, L.; Guo, H.; Pu, H.; Yin, P.; Xu, Z.; Zhang, D.; Peng, Y.; et al. Instantaneous peak 2.1 W-level hybrid energy harvesting from human motions for self-charging battery-powered electronics. Nano Energy 2021, 81, 105629. [Google Scholar] [CrossRef]
- Chen, H.; Pan, Z.; Wang, W.; Chen, Y.; Xing, S.; Cheng, Y.; Ding, X.; Liu, J.; Zhai, J.; Yu, J. Ultrahigh discharge efficiency and improved energy density in polymer-based nanocomposite for high-temperature capacitors application. Compos. Part A Appl. Sci. Manuf. 2021, 142, 106266. [Google Scholar] [CrossRef]
- Ma, Y.; Yin, J.; Liang, H.; Yao, D.; Xia, Y.; Zuo, K.; Zeng, Y.P. A two step approach for making super capacitors from waste wood. J. Clean. Prod. 2021, 279, 123786. [Google Scholar] [CrossRef]
- Mondal, M.; Goswami, D.K.; Bhattacharyya, T.K. Lignocellulose based Bio-waste Materials derived Activated Porous Carbon as Superior Electrode Materials for High-Performance Supercapacitor. J. Energy Storage 2021, 34, 102229. [Google Scholar] [CrossRef]
- Franco-Pérez, L.; Fernández-Anaya, G.; Quezada-Téllez, L.A. On stability of nonlinear nonautonomous discrete fractional Caputo systems. J. Math. Anal. Appl. 2020, 487, 124021. [Google Scholar] [CrossRef]
- Ebadi, R.; Sadeghi Yazdankhah, A.; Kazemzadeh, R.; Mohammadi-Ivatloo, B. Techno-economic evaluation of transportable battery energy storage in robust day-ahead scheduling of integrated power and railway transportation networks. Int. J. Electr. Power Energy Syst. 2021, 126, 106606. [Google Scholar] [CrossRef]


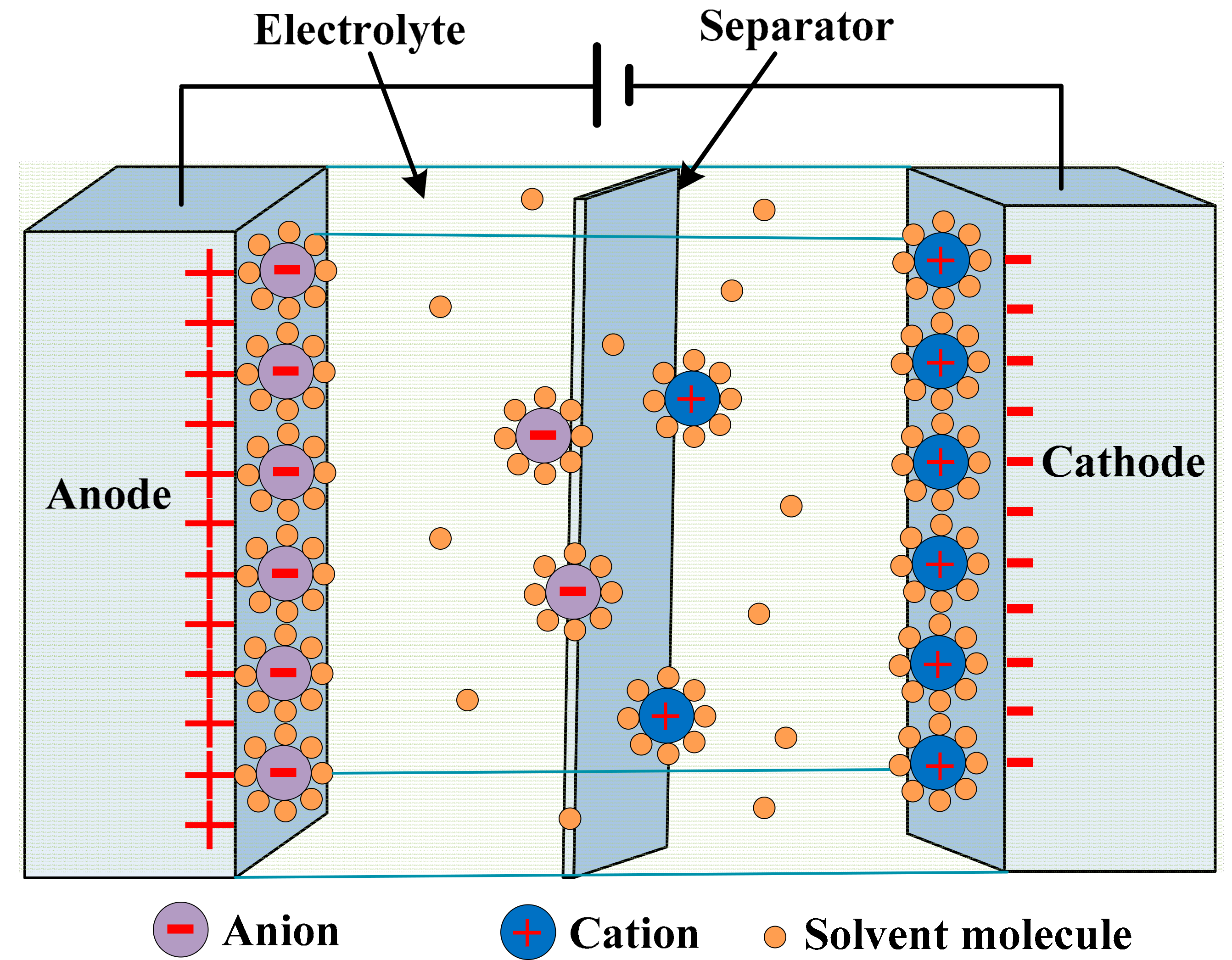

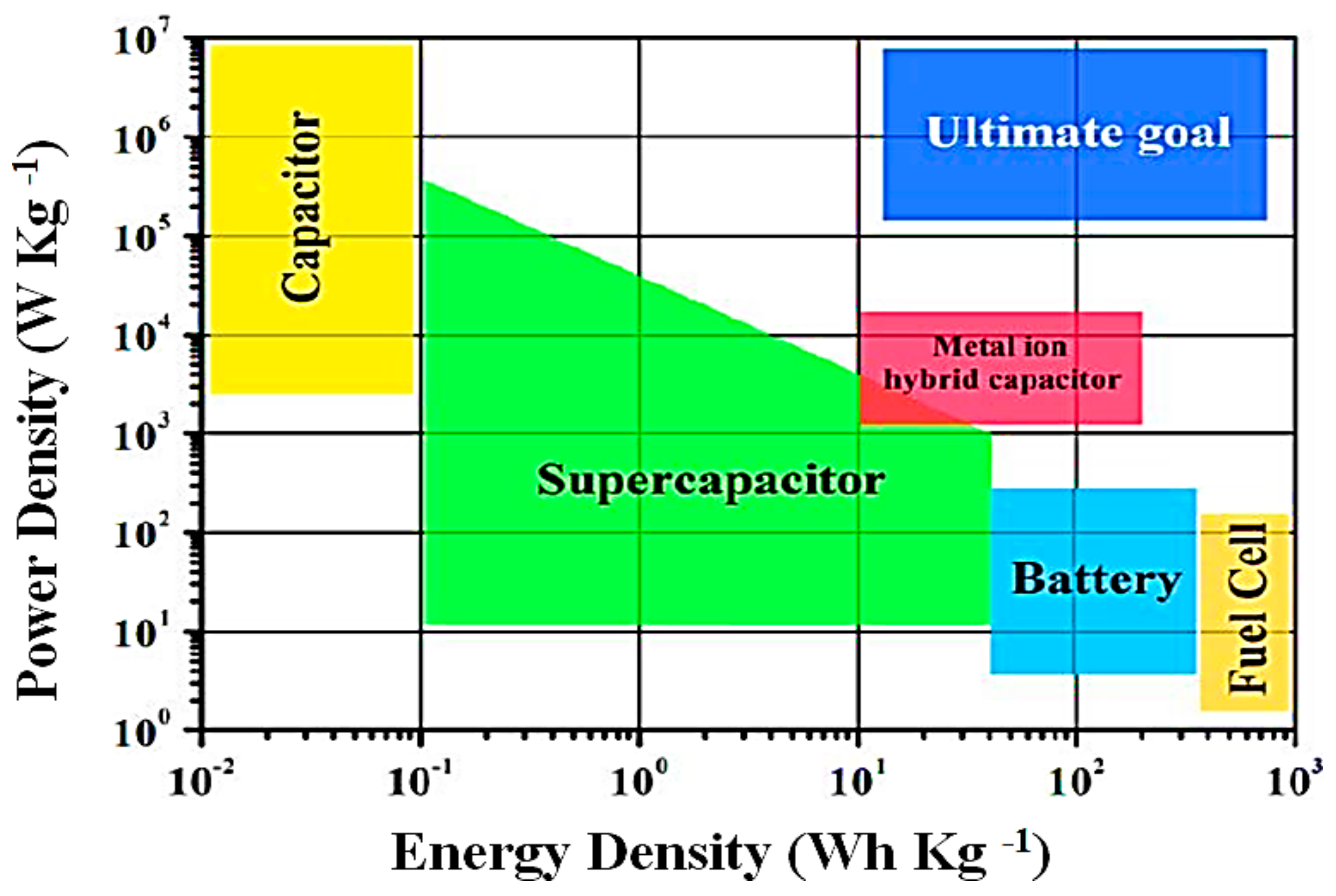

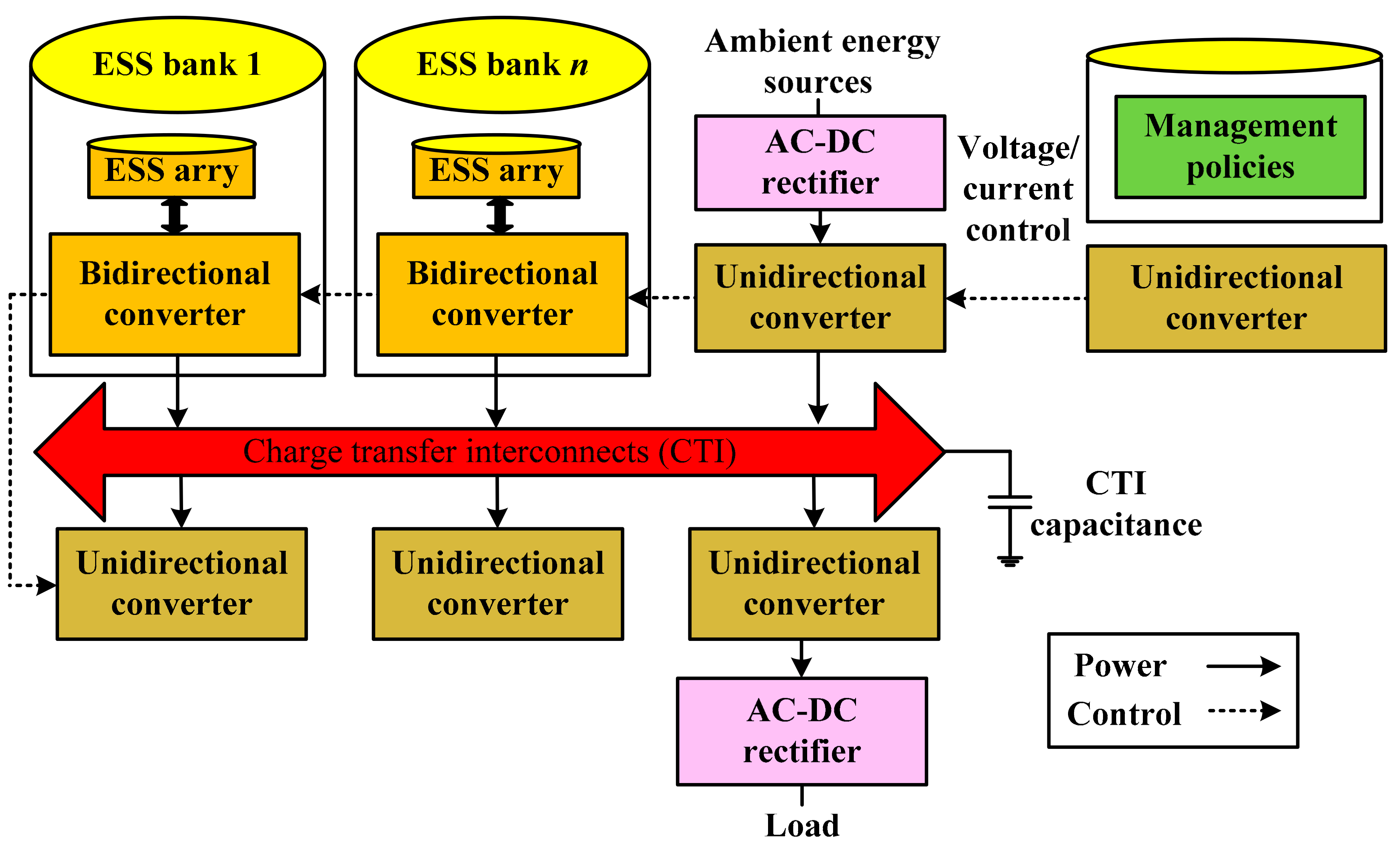
| Battery Deterioration and Failure Processes | Battery Features |
|---|---|
| Reduction in the availability of electrochemical reaction due to densification of active materials with loss of porosity. | Over a good depth discharge stable voltage. |
| Shedding and expansion of active material from the electrode materials. | High energy density and high specific density, made of easily available and inexpensive material. |
| At the negative electrode, the growth of metallic needles causes an internal short circuit. | High energy efficiency, maximum recycling efficiency. |
| Overcharging causes gassing of electrodes, affects battery performance. | Wide operating temperature, ability to work properly under overcharge/discharge. |
| Battery performance is affected by parasitic reactions. | On open circuit, maintain the charge, rugged, abuse-resistant, maintenance-free, safe in normal and abnormal conditions. |
| Advantages | Durable and has a large life cycles if properly maintained |
| Only battery with ultra-fast charging with little stress | |
| Good load performance; can bear rough handling | |
| Long shelf life | |
| Simple storage | |
| Low temperature does not affect the performance | |
| Low cost | |
| Availability in different sizes and performance options | |
| Disadvantages | The specific energy is low as compared to hybrid systems |
| Memory effect must be fully discharged before recharged | |
| Due to the toxic nature of cadmium metal cannot be disposed of in dumping grounds | |
| High self-discharging | |
| The cell voltage is very low, only 1.2 V, to achieve high voltages a large number of cells are required |
| Sodium Sulphur Battery | Lead-Acid Battery |
|---|---|
| Specific energy density 760 Wh/kg at 350 °C, three times greater than lead-acid battery. | Energy density is three times less than sodium sulfur battery. |
| Less than half the space required as compared to lead-acid batteries in commercial applications. | More space required in commercial applications. |
| No self-discharge. | The self-discharge rate is approximately 4% per week. |
| 100% coulombic efficiency (also called current efficiency, by which charges are transferred in the battery). | 90% coulombic efficiency. |
| No intermediate reaction, 85% average DC conversion efficiency. | 80% DC conversion efficiency. |
| No need for pumps or valves. | Can use a valve for gas blow off. |
| Maintenance is required after periodic inspection and cleaning. | Maintenance is required after periodic inspection and cleaning. |
| Environmental friendly, sealed properly, no risk of explosion during operation. | Not environmentally friendly. |
| 99% recycled. | Each part of the old batteries is recycled. |
| Capacitor | Battery |
|---|---|
| Electric field for storage | The chemical reaction for storage |
| Submissive component | Active component |
| Energy-density low | Energy-density is high |
| Charging/discharging fast | Charging/discharging slow |
| Provide unstable voltage | Provide constant voltage |
| Operating temperature range is −3 °C to +125 °C | 20 °C to 30 °C during charging and 15 °C to 25 °C during discharging |
| Higher cost | Low cost |
| Contrive of metal sheets | Contrive of metals, chemicals |
| Advantages | Disadvantages | Applications |
|---|---|---|
| Super-fast rate of charging and discharging | They store a smaller amount of energy than a battery does. | Energy harvesting |
| Life spans 500,000 plus charge/recharge cycles | Faster time to discharge. | Railways |
| Capacitance greater than 1000 F at 1.2 V | Highly trained persons required to operate. | Charging laptops |
| A good option for WSN’s sensors that need peak currents. | Cost is relatively higher than batteries. | WSN’s sensors |
| High energy density, specific energy and cyclic life. | Cost is relatively higher than batteries. | Micro-energy harvesting |
| Application | Required Features | Energy Storage Devices |
|---|---|---|
| Cell phone | There are two requirements of cell batteries, high specific energy and high specific power | Modern mobiles mostly use lithium ion batteries due to their specific power and energy |
| Laptops | Long cycle and shelf life, low discharging, withstand high temperature, rapid charging, low maintenance, should be sealed | Nickel–cadmium, nickel metal hydride and lithium-ion batteries |
| Digital SLR (single lens reflex) Cameras | They need lighter batteries with more power | Lithium-ion batteries are used because they can hold power more than 40% and these are very light |
| Toys, remotes, the game controllers and all gadgets | Toys need a lot of energy High-performance batteries are required, such as lithium and alkaline batteries. | Lithium-ion batteries are used due to their highest performance |
| WSNs sensors | Peak currents are needed during signal transmission and reception. WSNs need a low cost, small size, and portability. | lithium-ion batteries and supercapacitors. |
| Low cost microelectronics | Energy density, specific energy and cyclic life should be high. | Micro lithium-ion batteries, supercapacitors. |
| Low cost micro-energy harvesting | Energy density, specific energy and cyclic life should be high. | Lithium-ion battery and supercapacitors |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Riaz, A.; Sarker, M.R.; Saad, M.H.M.; Mohamed, R. Review on Comparison of Different Energy Storage Technologies Used in Micro-Energy Harvesting, WSNs, Low-Cost Microelectronic Devices: Challenges and Recommendations. Sensors 2021, 21, 5041. https://doi.org/10.3390/s21155041
Riaz A, Sarker MR, Saad MHM, Mohamed R. Review on Comparison of Different Energy Storage Technologies Used in Micro-Energy Harvesting, WSNs, Low-Cost Microelectronic Devices: Challenges and Recommendations. Sensors. 2021; 21(15):5041. https://doi.org/10.3390/s21155041
Chicago/Turabian StyleRiaz, Amna, Mahidur R. Sarker, Mohamad Hanif Md Saad, and Ramizi Mohamed. 2021. "Review on Comparison of Different Energy Storage Technologies Used in Micro-Energy Harvesting, WSNs, Low-Cost Microelectronic Devices: Challenges and Recommendations" Sensors 21, no. 15: 5041. https://doi.org/10.3390/s21155041
APA StyleRiaz, A., Sarker, M. R., Saad, M. H. M., & Mohamed, R. (2021). Review on Comparison of Different Energy Storage Technologies Used in Micro-Energy Harvesting, WSNs, Low-Cost Microelectronic Devices: Challenges and Recommendations. Sensors, 21(15), 5041. https://doi.org/10.3390/s21155041







