Qualitative Recognition of Primary Taste Sensation Based on Surface Electromyography
Abstract
:1. Introduction
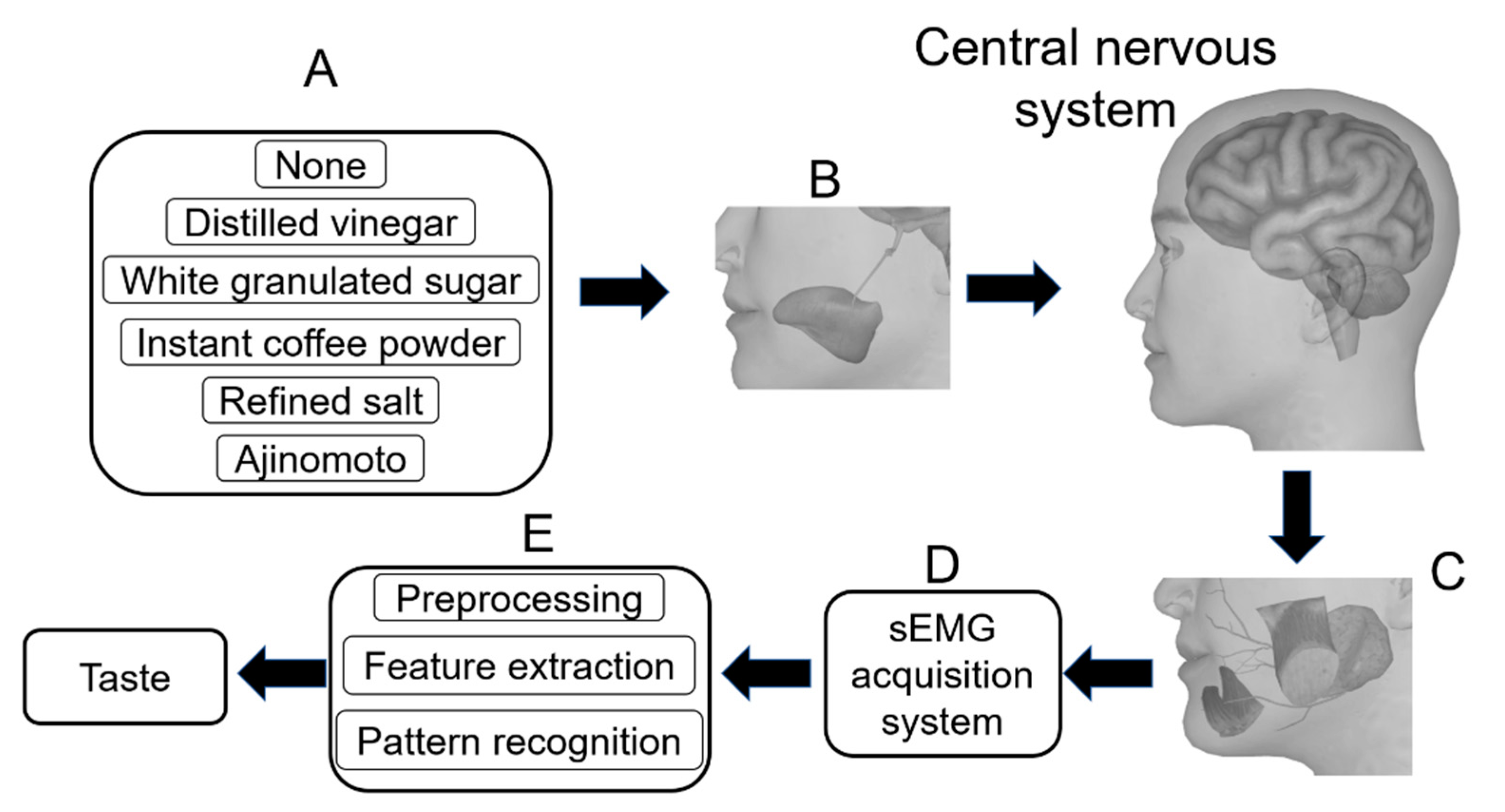
2. Materials and Methods
2.1. Data Acquisition
- (1)
- An experiment assistant checked the sEMG acquisition system.
- (2)
- A subject with stable consciousness and a cleaned face was asked to relax and the assistant stuck electrodes on the face of the subject according to Figure 2.
- (3)
- The subject was asked to close his/her eyes, think about nothing, and not control their facial expressions on purpose. Then the assistant started a device to capture the sEMG of the experiment category “No taste” for 12 s.
- (4)
- The subject gargled five times, taking 20 mL purified water each time.
- (5)
- The subject relaxed for 2 min.
- (6)
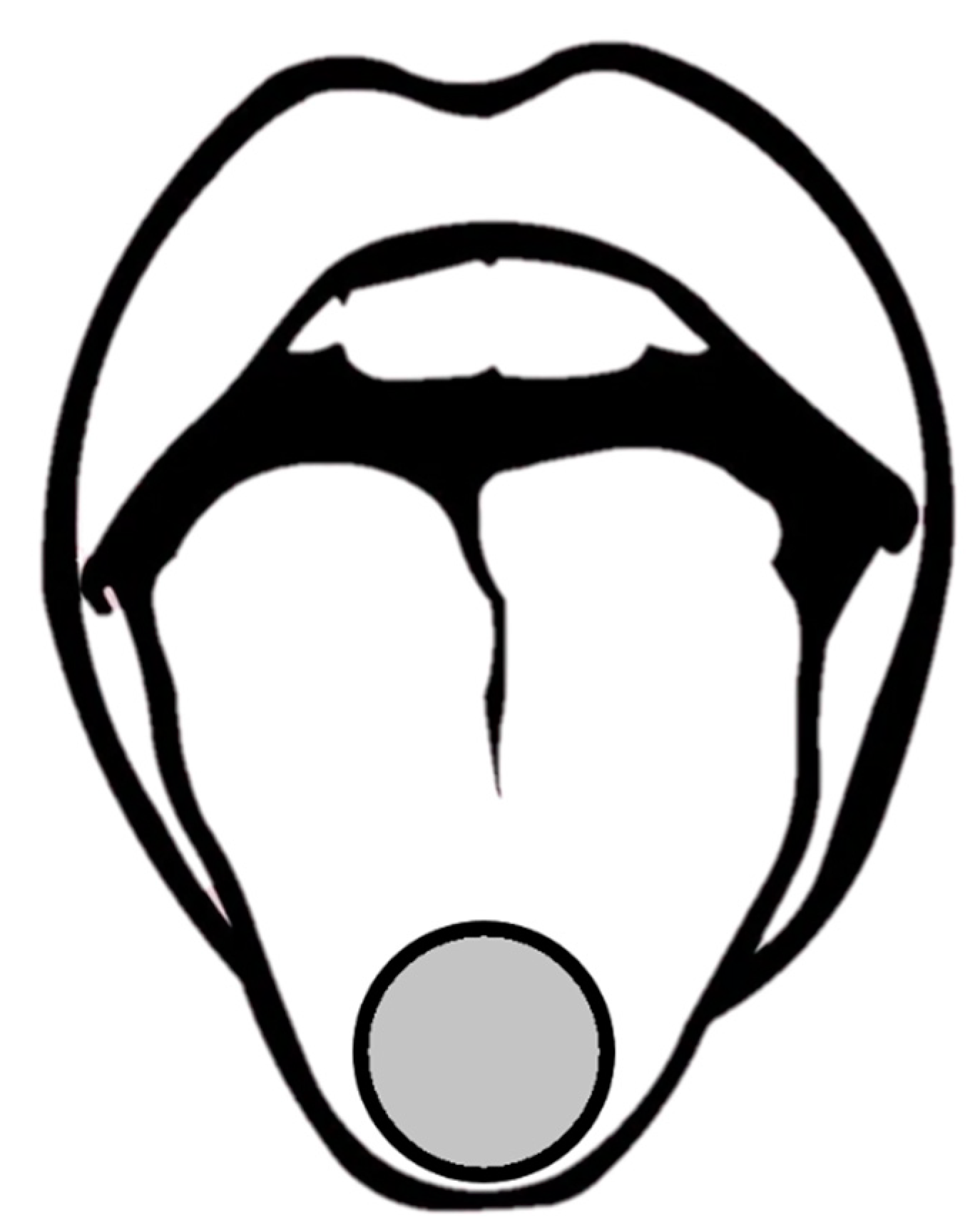
- The subject closed his/her eyes, then the assistant prepared one level spoon of instant coffee powder (UCC117) and inverted it on the tip of the subject’s tongue. The edge of the spoon was a circle with a radius of 11 cm and the volume of the spoon was 2 mL. The position of stimulus coverage is shown in Figure 3.
- (7)
- The subject backed his/her tongue, closed his/her mouth, and kept the tongue still. The subject was forbidden to control their facial expressions on purpose or think about anything but the taste experience. Then the assistant started the device to get an sEMG of the bitter taste for 12 s.
- (8)
- Repeat steps 4 and 5.
- (9)
- Repeat the steps from 6 to 8 and replace the instant coffee powder with white granulated sugar (Taigu, sucrose ratio was not less than 99.6%), refined salt (Zhongyan, sodium chloride ratio was not less than 98.5%), Ajinomoto (Xihu, sodium glutamate ratio was not less than 99.9%), and distilled vinegar (Hengshun, the total acid content was not less than 5 g per 100 mL) for sweet, salty, umami, and sour taste, respectively.
- (10)
- The subject took a break for 10 min.
- (11)
- If there was another session, repeat steps from 3 to 10.
2.2. Preprocessing
2.3. Signals in Samples Were Filtered
2.4. Classification
3. Results and Discussion
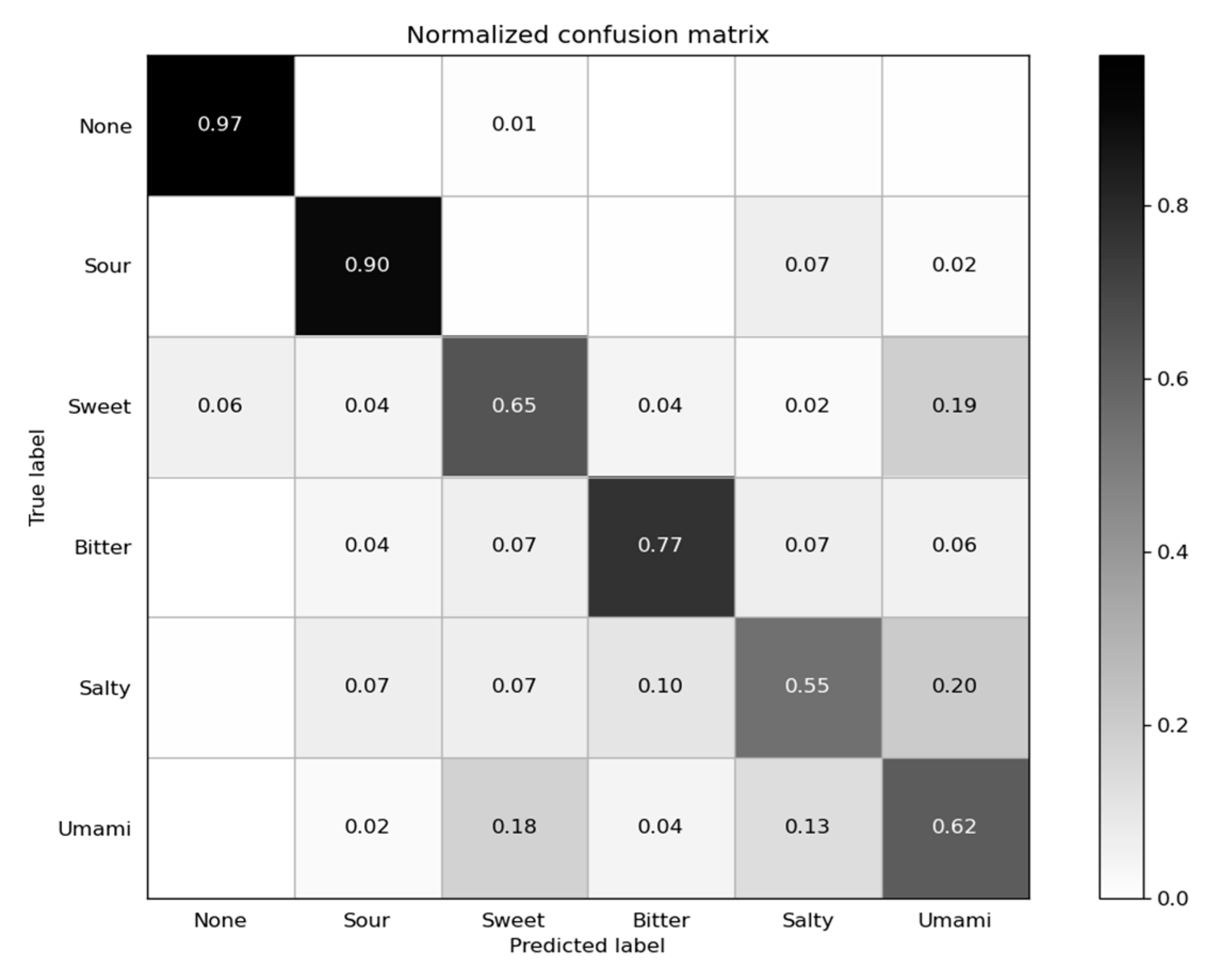
3.1. Classification Result
3.2. Feature Selection
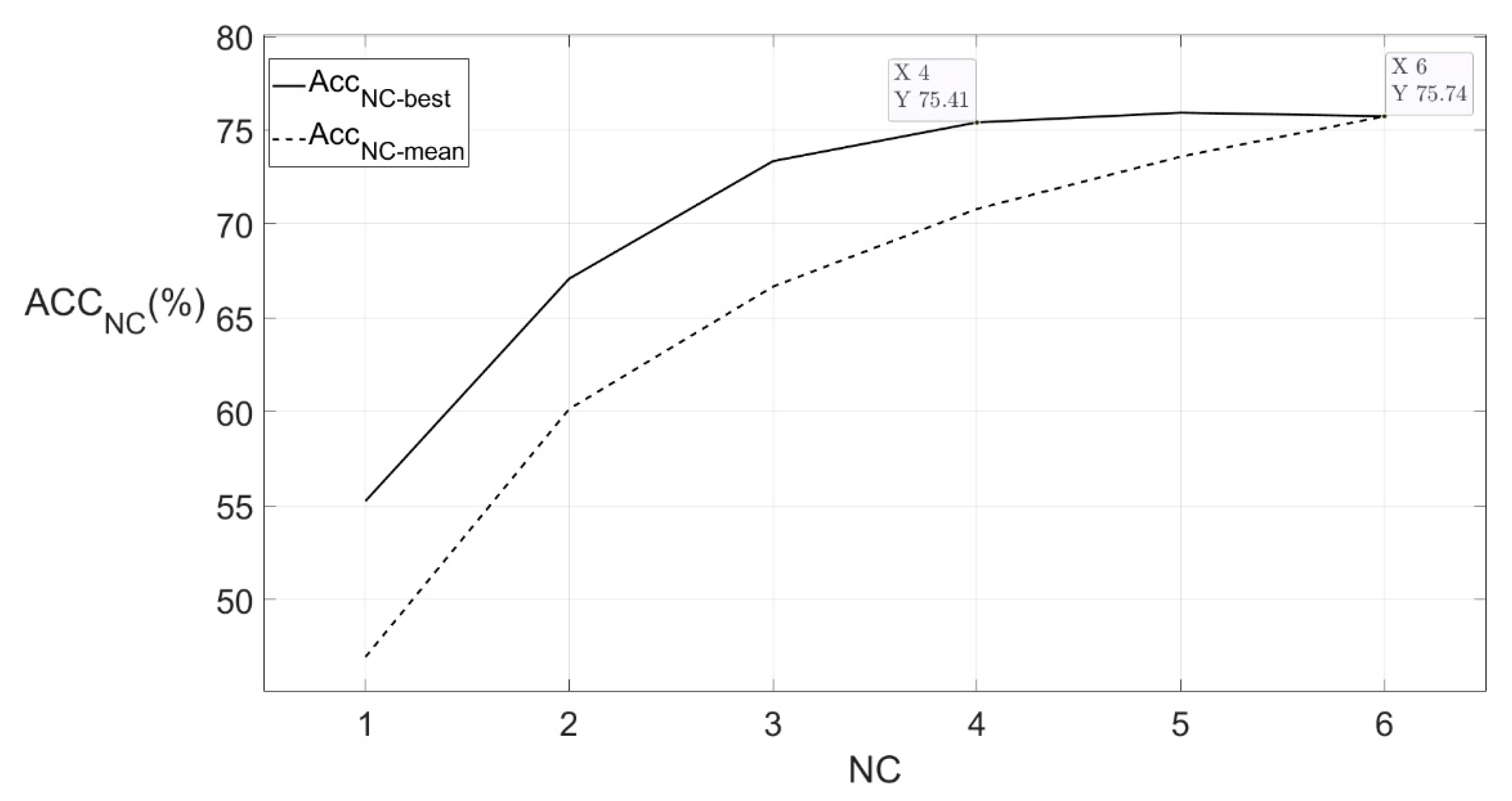
3.3. Channel Combination Selection
3.4. Model Performance on Different Subjects
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yarmolinsky, D.A.; Zuker, C.S.; Ryba, N.J.P. Common sense about taste: From mammals to insects. Cell 2009, 139, 234–244. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheok, A.D.; Karunanayaka, K. Virtual Taste and Smell Technologies for Multisensory Internet and Virtual Reality; Spring International Publishing AG: Cham, Switzerland, 2018; pp. 119–122. [Google Scholar]
- Ranasinghe, N.; Nguyen, T.N.T.; Liangkun, Y.; Lin, L.-Y.; Tolley, D. Vocktail: A virtual cocktail for pairing digital taste, smell, and color sensations. In Proceedings of the 2017 ACM Multimedia Conference, Mountain View, CA, USA, 23–27 October 2017. [Google Scholar]
- Karunanayaka, K.; Johari, N.; Hariri, S.; Camelia, H.; Bielawski, K.S.; Cheok, A.D. New thermal taste actuation technology for future multisensory virtual reality and internet. IEEE Trans. Vis. Comput. Graph. 2018, 24, 1496–1505. [Google Scholar] [CrossRef] [PubMed]
- Ciui, B.; Martin, A.; Mishra, R.K.; Nakagawa, T.; Dawkins, T.J.; Lyu, M.; Cristea, C.; Sandulescu, R.; Wang, J. Chemical sensing at the robot fingertips: Toward automated taste discrimination in food samples. ACS Sens. 2018, 3, 2375–2384. [Google Scholar] [CrossRef]
- Nag, A.; Mukhopadhyay, S.C. Fabrication and implementation of printed sensors for taste sensing applications. Sens. Actuators. A. Phys. 2018, 269, 53–61. [Google Scholar] [CrossRef]
- Sehra, G.; Cole, M.; Gardner, J.W. Miniature taste sensing system based on dual SH-SAW sensor device: An electronic tongue. Sens. Actuators. B Chem. 2004, 103, 233–239. [Google Scholar] [CrossRef]
- Hartmann, C.; Siegrist, M. Development and validation of the Food Disgust Scale. Food Qual. Prefer. 2018, 63, 38–50. [Google Scholar] [CrossRef]
- Vidal, J.J. Toward direct brain-computer communication. Annu. Rev. Biophys. Bioeng. 1973, 2, 157–180. [Google Scholar] [CrossRef]
- Yadav, D.; Yadav, S.; Veer, K. A comprehensive assessment of Brain Computer Interfaces: Recent trends and challenges. J. Neurosci. Methods 2020, 346, 108918. [Google Scholar] [CrossRef]
- Kaneko, D.; Hogervorst, M.; Toet, A.; Van Erp, J.B.F.; Kallen, V.; Brouwer, A.-M. Explicit and implicit responses to tasting drinks associated with different tasting experiences. Sensors 2019, 19, 4397. [Google Scholar] [CrossRef] [Green Version]
- Wallroth, R.; Höchenberger, R.; Ohla, K. Delta activity encodes taste information in the human brain. NeuroImage 2018, 181, 471–479. [Google Scholar] [CrossRef]
- Mizoguchi, C.; Kobayakawa, T.; Saito, S.; Ogawa, H. Gustatory evoked cortical activity in humans studied by simultaneous EEG and MEG recording. Chem. Senses 2002, 27, 629–634. [Google Scholar] [CrossRef] [Green Version]
- Canna, A.; Prinster, A.; Cantone, E.; Ponticorvo, S.; Russo, A.G.; Di Salle, F.; Esposito, F. Intensity-related distribution of sweet and bitter taste fMRI responses in the insular cortex. Hum. Brain Mapp. 2019, 40, 3631–3646. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ramadan, R.A.; Vasilakos, A.V. Brain computer interface: Control signals review. Neurocomputing 2017, 223, 26–44. [Google Scholar] [CrossRef]
- Aydemir, O. Olfactory recognition based on EEG gamma-band activity. Neural Comput. 2017, 29, 1667–1680. [Google Scholar] [CrossRef]
- Hou, H.; Zhang, X.; Meng, Q. Olfactory EEG signal classification using a trapezoid difference-based electrode sequence hashing approach. Int. J. Neural Syst. 2020, 30, 2050011. [Google Scholar] [CrossRef]
- Sridhar, S.; Manian, V. EEG and deep learning based brain cognitive function classification. Computers 2020, 9, 104. [Google Scholar] [CrossRef]
- Di Flumeri, G.; Arico, P.; Borghini, G.; Sciaraffa, N.; Maglione, A.G.; Rossi, D.; Modica, E.; Trettel, A.; Babiloni, F.; Colosimo, A.; et al. EEG-based Approach-Withdrawal index for the pleasantness evaluation during taste experience in realistic settings. In Proceedings of the 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju Island, Korea, 11–15 July 2017. [Google Scholar]
- Tavares Filho, E.R.; Esmerino, E.A.; Santos-Junior, V.d.A.; Tavares, E.J.S.; de Castro, R.O.M.; Bolini, H.M.A. Electroencephalography and acceptance test to assess sodium reduction in tomato sauce: An exploratory research. Emir. J. Food Agric. 2020, 32, 417–425. [Google Scholar]
- Pagan, K.M.; Giraldi, J.d.M.E.; Maheshwari, V.; de Paula, A.L.D.; de Oliveira, J.H.C. Evaluating cognitive processing and preferences through brain responses towards country of origin for wines: The role of gender and involvement. Int. J. Wine Bus. Res. ahead-of-print.
- Kurihara, K. Glutamate: From discovery as a food flavor to role as a basic taste (umami). The Am. J. Clin. Nutr. 2009, 90, 719S–722S. [Google Scholar] [CrossRef]
- Abidi, I.; Farooq, O.; Beg, M.M.S. Sweet and sour taste classification using EEG based brain computer interface. In Proceedings of the 2015 Annual IEEE India Conference (INDICON), New Delhi, India, 17–20 December 2015. [Google Scholar]
- Andersen, C.A.; Kring, M.L.; Andersen, R.H.; Larsen, O.N.; Kjaer, T.W.; Kidmose, U.; Møller, S.; Kidmose, P. EEG discrimination of perceptually similar tastes. J. Neurosci. Res. 2019, 97, 241–252. [Google Scholar] [CrossRef]
- Steiner, J.E. Human facial expressions in response to taste and smell stimulation. Adv. Child Dev. Behav. 1979, 13, 257–295. [Google Scholar]
- Dawes, C.; Pedersen, A.M.L.; Villa, A.; Ekström, J.; Proctor, G.; Vissink, A.; Aframian, D.; McGowan, R.; Aliko, A.; Narayana, N.; et al. The functions of human saliva: A review sponsored by the World Workshop on Oral Medicine VI. Arch. Oral Biol. 2015, 60, 863–874. [Google Scholar] [CrossRef]
- Del Vecchio, A.; Negro, F.; Felici, F.; Farina, D. Associations between motor unit action potential parameters and surface EMG features. J. Appl. Physiol. 2017, 123, 835–843. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, M.; Wu, R.; Gao, H.; Yang, M.; Luo, Z.; Li, G. Silent speech decoding using spectrogram features based on neuromuscular activities. Brain Sci. 2020, 10, 442. [Google Scholar] [CrossRef] [PubMed]
- Nasri, N.; Orts-Escolano, S.; Gomez-Donoso, F.; Cazorla, M. Inferring static hand poses from a low-cost non-intrusive sEMG sensor. Sensors 2019, 19, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nasri, N.; Orts-Escolano, S.; Cazorla, M. An semg-controlled 3d game for rehabilitation therapies: Real-time time hand gesture recognition using deep learning techniques. Sensors 2020, 20, 6451. [Google Scholar] [CrossRef]
- Kaczmarek, P.; Mankowski, T.; Tomczynski, J. putEMG-A surface electromyography hand gesture recognition dataset. Sensors 2019, 19, 3548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, S.; Player, K.A.; Mcchesney, K.A.; Dalistan, M.D.; Tyner, C.A.; Scozzafava, J.E. Facial EMG as an indicator of palatability in humans. Physiol. Behav. 1999, 68, 31–35. [Google Scholar] [CrossRef]
- Miura, Y.; Morita, Y.; Koizumi, H.; Shingai, T. Effects of Taste Solutions, Carbonation, and Cold Stimulus on the Power Frequency Content of Swallowing Submental Surface Electromyography. Chem. Senses 2009, 34, 325–331. [Google Scholar] [CrossRef] [Green Version]
- Sato, W.; Minemoto, K.; Ikegami, A.; Nakauma, M.; Funami, T.; Fushiki, T. Facial EMG correlates of subjective hedonic responses during food consumption. Nutrients 2020, 12, 1174. [Google Scholar] [CrossRef] [Green Version]
- Armstrong, J.E.; Hutchinson, I.; Laing, D.G.; Jinks, A.L. Facial electromyography: Responses of children to odor and taste stimuli. Chem. Senses 2007, 32, 611–621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Horio, T. EMG activities of facial and chewing muscles of human adults in response to taste stimuli. Percept. Mot. Ski. 2003, 97, 289–298. [Google Scholar] [CrossRef]
- Guan, X.; Zhang, M.; Wu, R.; Gao, H.; Ai, Q.; Jin, S.; Wang, Y.; Li, G. Remote Control System of Spherical Robot based on Silent Speech Recognition. In Proceedings of the 8th International Winter Conference on Brain-Computer Interface (BCI), Gangwon, Korea, 26–28 February 2020. [Google Scholar]
- Zhang, M.; Zhang, W.; Zhang, B.; Wang, Y.; Li, G. Feature selection of mime speech recognition using surface electromyography data. In Proceedings of the 2019 Chinese Automation Congress (CAC), Hangzhou, China, 22–24 November 2019. [Google Scholar]
- Karthick, P.A.; Ghosh, D.M.; Ramakrishnan, S. Surface electromyography based muscle fatigue detection using high-resolution time-frequency methods and machine learning algorithms. Comput. Methods Programs Biomed. 2018, 154, 45–56. [Google Scholar] [CrossRef] [PubMed]
- Merletti, R.; Farina, D. Surface Electromyography: Physiology, Engineering and Applications; Wiley-IEEE Press: Piscataway, NJ, USA, 2016. [Google Scholar]
- Castroflorio, T.; Farina, D.; Bottin, A.; Piancino, M.G.; Bracco, P.; Merletti, R. Surface EMG of jaw elevator muscles: Effect of electrode location and inter-electrode distance. J. Oral Rehabil. 2005, 32, 411–417. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Merletti, R.; Muceli, S. Tutorial. Surface EMG detection in space and time: Best practices. J. Electromyogr. Kinesiol. 2019, 49, 102363. [Google Scholar] [CrossRef] [PubMed]



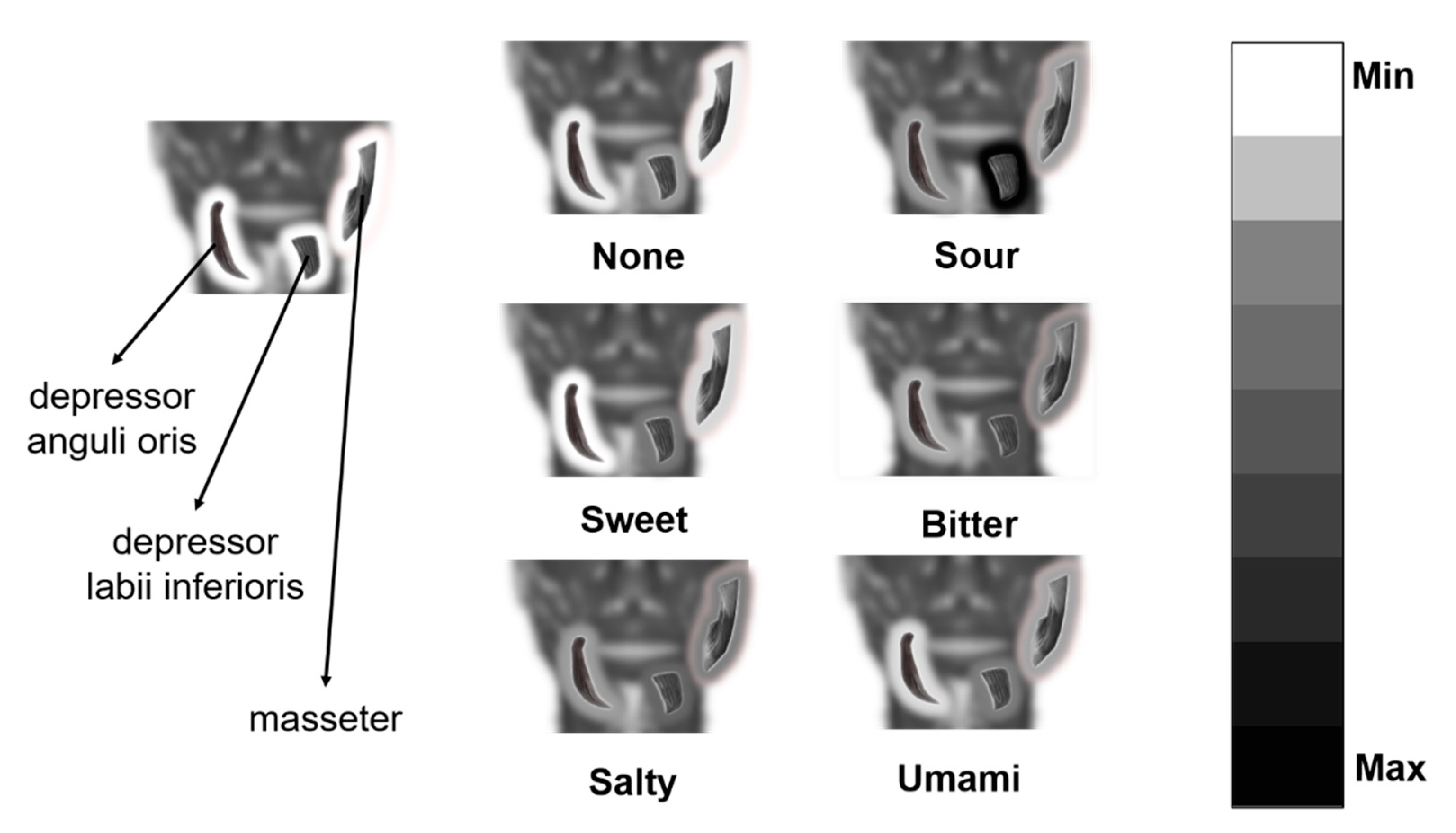

| Electrode Index | Electrode Type | Muscle | Detailed Position * |
|---|---|---|---|
| 1 | Differential electrode pairs | Left masseter | The lower end of the right ear cartilage forward 3 cm |
| 2 | Differential electrode pairs | Left masseter | Parallel to and below electrodes for channel 1 and close to the edge of the jaw |
| 3 | Single electrode | Left depressor anguli oris | The lower right of the right corner of the mouth |
| 4 | Single electrode | Left depressor labii inferioris | Below the lower lip with the whole electrode on the right of the middle line of the face |
| 5 | Single electrode | Right masseter | The lower end of the left ear cartilage forward 3 cm |
| 6 | Single electrode | Right masseter | Below the electrode for channel 5 and close to the edge of the jaw |
| B | Bias electrode | Left mastoid | The protuberance behind the left ear |
| R | Reference electrode | Right mastoid | The protuberance behind the right ear |
| Feature Name | Feature Type | Feature Dimension |
|---|---|---|
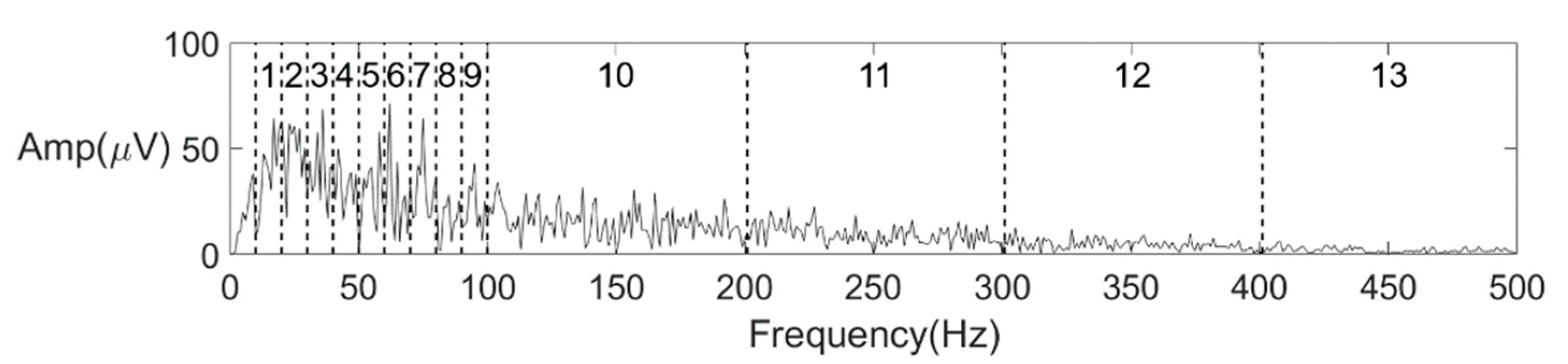
| Spectrum average amplitude | Frequency-domain | 13 |
| FC | Frequency-domain | 1 |
| RMSF | Frequency-domain | 1 |
| RVF | Frequency-domain | 1 |
| RMS | Time-domain | 1 |
| ZCR | Time-domain | 1 |
| MAV | Time-domain | 1 |
| Ku | Time-domain | 1 |
| Sk | Time-domain | 1 |
| Subject ID | Number of All Samples of the Dataset | Accuracy of Random Forest on the Dataset (%) |
|---|---|---|
| 1 | 3054 | 75.41 |
| 2 | 3085 | 61.49 |
| 3 | 2098 | 55.10 |
| 4 | 1474 | 48.78 |
| 5 | 2130 | 52.11 |
| 6 | 2912 | 57.07 |
| 7 | 1504 | 47.87 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wang, H.; Li, H.; Ullah, A.; Zhang, M.; Gao, H.; Hu, R.; Li, G. Qualitative Recognition of Primary Taste Sensation Based on Surface Electromyography. Sensors 2021, 21, 4994. https://doi.org/10.3390/s21154994
Wang Y, Wang H, Li H, Ullah A, Zhang M, Gao H, Hu R, Li G. Qualitative Recognition of Primary Taste Sensation Based on Surface Electromyography. Sensors. 2021; 21(15):4994. https://doi.org/10.3390/s21154994
Chicago/Turabian StyleWang, You, Hengyang Wang, Huiyan Li, Asif Ullah, Ming Zhang, Han Gao, Ruifen Hu, and Guang Li. 2021. "Qualitative Recognition of Primary Taste Sensation Based on Surface Electromyography" Sensors 21, no. 15: 4994. https://doi.org/10.3390/s21154994
APA StyleWang, Y., Wang, H., Li, H., Ullah, A., Zhang, M., Gao, H., Hu, R., & Li, G. (2021). Qualitative Recognition of Primary Taste Sensation Based on Surface Electromyography. Sensors, 21(15), 4994. https://doi.org/10.3390/s21154994






