Potential of Point-of-Care and At-Home Assessment of Immune Status via Rapid Cytokine Detection and Questionnaire-Based Anamnesis
Abstract
1. Introduction
- : What recent technologies are developed for the rapid determination of cytokines?
- : Which of these rapid technologies can measure multiple cytokines simultaneously?
- : Which of these rapid technologies are suitable for Point-of-Care (PoC) testing?
- : Which of these rapid technologies are suitable for At-Home testing?
- : What health questionnaires exist for health status assessment?
- : How can we link the collected health questionnaires with the identified rapid technologies?
- : How can we employ machine learning strategies to automatically derive the definition of the immune system’s fitness from literature?
- : How can we build an integrated system that assesses the person’s immune system fitness?
2. Materials and Methods
2.1. Literature Retrieval Methodology
- ‘rapid’: an immunoassay must deliver results faster than standard ELISA.
- ‘multiplex’: an immunoassay must measure rapidly multiple cytokines, which is necessary to evaluate the overall immune system status.
- ‘real-time’: immunoassays with superior feature to the rapid detection is the real-time continuous monitoring of the cytokine detection.
- ‘lateral flow assay’: LFAs are rapid tools known for their ease of application.
- SQ_1: “immunoassay for the rapid cytokine detection”,
- SQ_2: “multiplexed immunoassay for the rapid cytokine detection”,
- SQ_3: “immunoassay for real-time cytokine detection”, and
- SQ_4: “LFAs cytokine detection”.
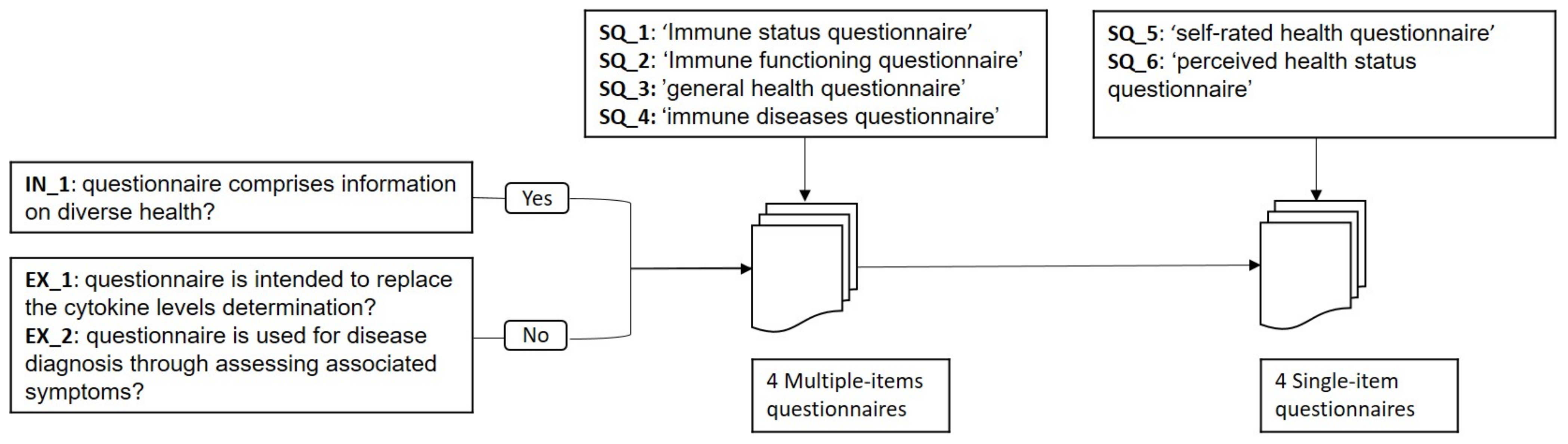
- ‘Immune status/functioning’: the questionnaire must aim at evaluating the immune system.
- ‘general health’: the questionnaire must consider several health aspects for the general assessment.
- ‘perceived health stats/self-rated health’: This type of questionnaires comprise a single item which asks: “How would you rate your health status?”
- ‘immune disease questionnaire’: This questionnaire includes items that collects information on immune diseases.
- SQ_1: “Immune status questionnaire”,
- SQ_2: “Immune functioning questionnaire”,
- SQ_3: “general health questionnaire”,
- SQ_4: “self-rated health questionnaire”,
- SQ_5: “perceived health status questionnaire”, and
- SQ_6: “immune diseases questionnaire”.
2.2. Literature Selection Methodology
- For studies retrieved with the search query “immunoassay for the rapid cytokine detection”,
- –
- IN_1: the study must include the term ‘rapid’ in the title or in the abstract.
- –
- IN_2: and must report the total assay time in the abstract as an indicator of how important this feature is for the immunoassay.
- For studies retrieved with the search query “multiplexed immunoassay for the rapid cytokine detection”,
- –
- IN_3: the study must include the term ‘multiplex’ in the title.
- –
- IN_4: and the term ‘rapid’ in the abstract.
- For studies retrieved with the search query “immunoassay for real-time cytokine detection”,
- –
- IN_5: the study must include the term ‘real-time’ in the title.
- In addition to four inclusion criteria that must be fulfilled by all the papers retained.
- –
- IN_6: The total assay time should be less than 1 hour.
- –
- IN_7: The study should be cited at least 5 times.
- –
- IN_8: The study must propose a mature technology. This was assessed by reading the abstract of the study.
- EX_1: Studies older than 2015 are not included. This review gathers most recent advanced immunoassays for the rapid cytokine detection.
- EX_2: Immunoassays that were tested on biological fluids other than serum, plasma, or whole blood sample, such as amniotic fluid are also excluded.
- IN_1: the questionnaire must comprise information on as diverse health aspects as possible.
- EX_1: are intended to replace the cytokine levels determination.
- EX_2: are used for disease diagnosis through assessing associated symptoms.
2.3. Feature Selection for the Identified Questionnaires
2.4. Categorization and Feature Selection for the Identified Detection Methods
- Sample: sample volume (mostly L) and type (e.g., serum),
- Cytokines: set of simultaneously detected cytokines,
- Dynamic Range (DR),
- Limit of Detection (LoD),
- Validity of measurements: it is used to assess the reliability of the method,
- Assay time,
- Signal reader: signal processing method, and reading equipment, e.g., scanner, special type of camera, smartphone (camera),
- Specific disease, marking cases where the technique is intended for specific disease(s) only
- Dimensions of the different parts of the immunoassay, and
- Cost of fabrication and materials used.
- Step 1: Preliminary literature collection and inspection
- Step 2: Collection of recent literature
- Three hits from 2020 onwards [42,43,44]. One hit [42] has already been identified in the preliminary literature collection), another article [43] appears twice, once under the name of the author (Andryukov) and once under the forename of the author (Boris), and another hit [44] is excluded because it does not refer to self-testing.
- One hit in 2019 [45].
- Step 3: Literature selection
- Step 4: Extraction of characteristics that indicate potential for self-testing
2.5. Combination of Sources and Features for Cytokine-Based Immune System Status Assessment
3. Results
3.1. Optical Detection Techniques
3.2. Electrochemical Detection Techniques
3.3. Colorimetric Detection Techniques
3.4. Questionnaire-Based Methods as Supportive Health-Assessment Tools
3.4.1. Multiple-Item Questionnaires
3.4.2. Single-Item Self-Rated Health Questionnaires
4. Discussion of the Findings on Rapid Technologies and Health Questionnaires
- multiple cytokine detection,
- small volume samples,
- smartphones-based readers,
- rapid assay time, and
- whole blood processing.
4.1. Multiple Cytokine Detection
- One-Strip xLFIA (Spatial Separation of Detection Sites) through increasing the number of test lines or dots on the same strip. Up to 32 measurements are feasible.
- Array of Strips by placing multiple test strips separately on one holder.
- Multiplexing LFA Based on the Probe by developing the detection element to capture multiple analytes at a time.
4.2. Small Volume Samples
4.3. Smartphone-Based Readers
4.4. Rapid Assay Time
4.5. Whole Blood Processing
5. Combination of Rapid Technologies with Health Questionnaires
6. Data Mining
6.1. Definition of Immune Fitness
6.2. Derivation of the Immune Fitness Definition
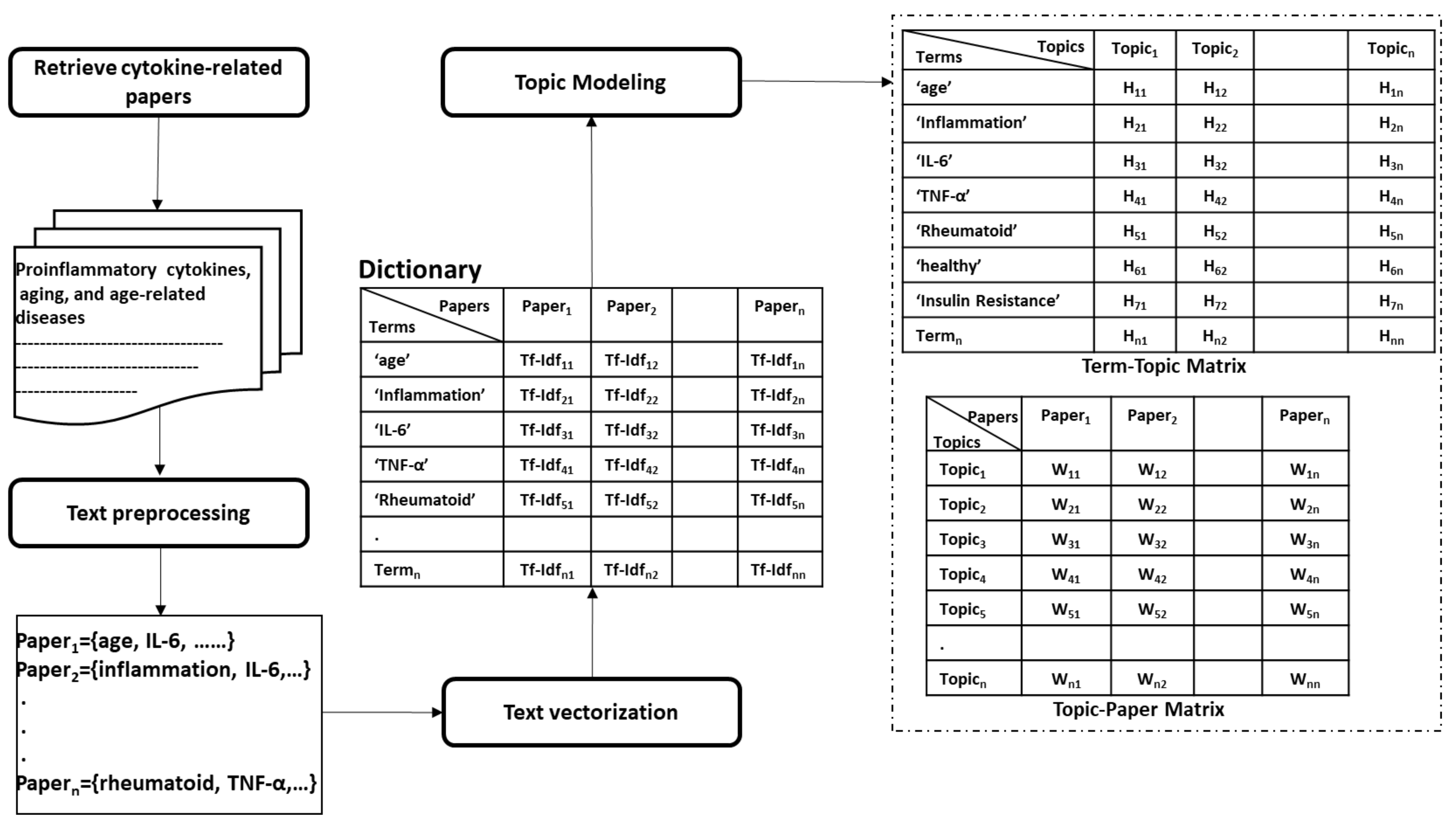
- Collecting papers. We start with retrieving scientific papers that contain the term ’cytokine’ from digital libraries.
- Text pre-processing: We extract keywords from papers by passing them into multiple pre-processing steps (tokenization, removing stop words, lemmatization, and stemming).
- Text vectorization. Then we derive the term frequency-inverse document frequency (TF-IDF) features from collected papers. Afterwards, we build the paper-term matrix using the derived TF-IDF features.
- Topic modeling. This is the main step of this procedure where the paper-term matrix comprising the TF-IDF features is passed to the topic modeling algorithm to reveal the underlying topics. One of the commonly used topic modeling techniques is the non-negative matrix factorization (NMF) [117]. NMF yields two matrices, the paper-topic matrix W and the topic-term matrix H. The number of topics to be derived is a crucial parameter that needs to be tuned prior to the topic modeling process. One way to find the optimal number of topics is to monitor the number of topics derived across several testing runs on randomly sub-sampled documents where the optimal number of topics should remain consistent across these testing runs. Moreover, one can evaluate the stability of the topics derived through tracking the fraction of documents pairs that are assigned to the same topic across the multiple testing runs [118].
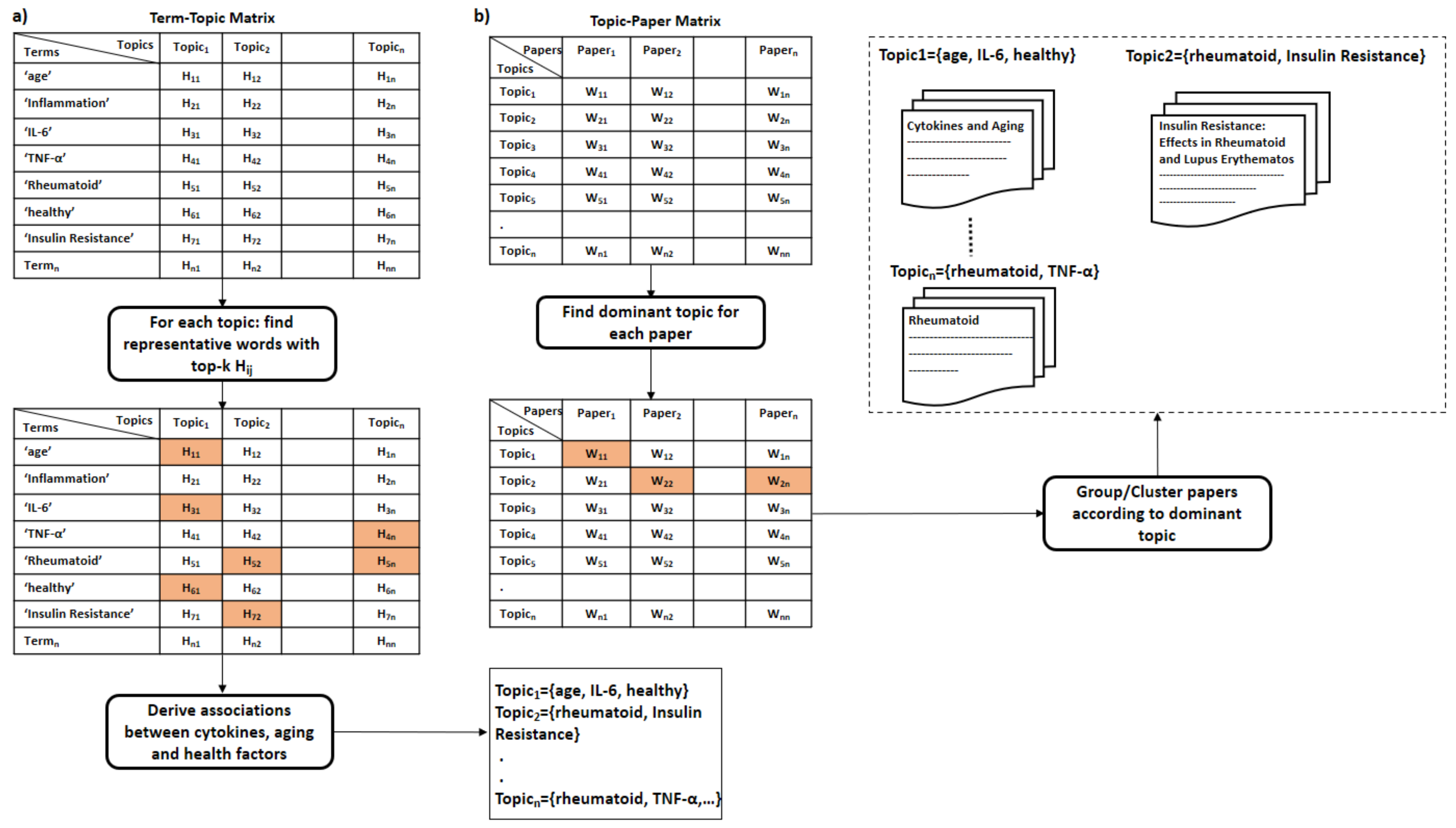
- Interpreting latent topics. This step implicitly derives associations between cytokines and other health factors/diseases. Topic modeling algorithms do not provide a straightforward interpretation of the derived topics, but we expect each topic to be presented by cytokines and their associated health factors. We can conclude the latent topics via finding the corresponding representative words for each topic. We consider the words with the top-k highest coefficients as the representative words for a topic, as shown in Figure 5a. The derived associations are stored in the system to be used later in the assessment procedure, as depicted in Figure 6. Even though we have attempted to derive these associations manually in Section 5, as shown in the 4th column in Table 9 and Table 10, this automatic method is more effective and practical.
- Clustering of papers according to topic. We apply unsupervised clustering on topic-paper matrix, as illustrated in Figure 5b. Alternatively, we can assign each paper to the topic with the highest relevance score in W. Then, we group papers by the dominant topic. Semantic validation of the topics modeled should be performed by experts.Steps 5 and 6 are equivalent to the post-processing step proposed by Asmussen et al. [112]. As an output of this step, we expect that more than one disease to belong to the same topic in case of co-morbidities, such as Rheumatoid and insulin resistance.Various evaluation strategies can be followed to validate the topics modeled. One way is to validate them by experts semantically. Another way would be through evaluating the topics at the term level. Terms belonging to the same topic are supposed to be more similar to each other than to term in a different topic [113]. Therefore, the similarity scores between the two representative terms within the same topic should be smaller than the similarity scores to terms of a different topic. As each term is represented as a vector of coefficients in the term-topic matrix, we can use any similarity measure, such as cosine similarity or euclidean distance.Zhang et al. validated the derived disease-gene associations by topic modeling with annotated disease-gene associations by the Online Mendelian Inheritance in Man (OMIM) knowledge base [113]. Similarly, we can validate the proposed method by computing the overlapping proportion between the extracted disease/aging-cytokine associations and the manually derived associations in Section 5.
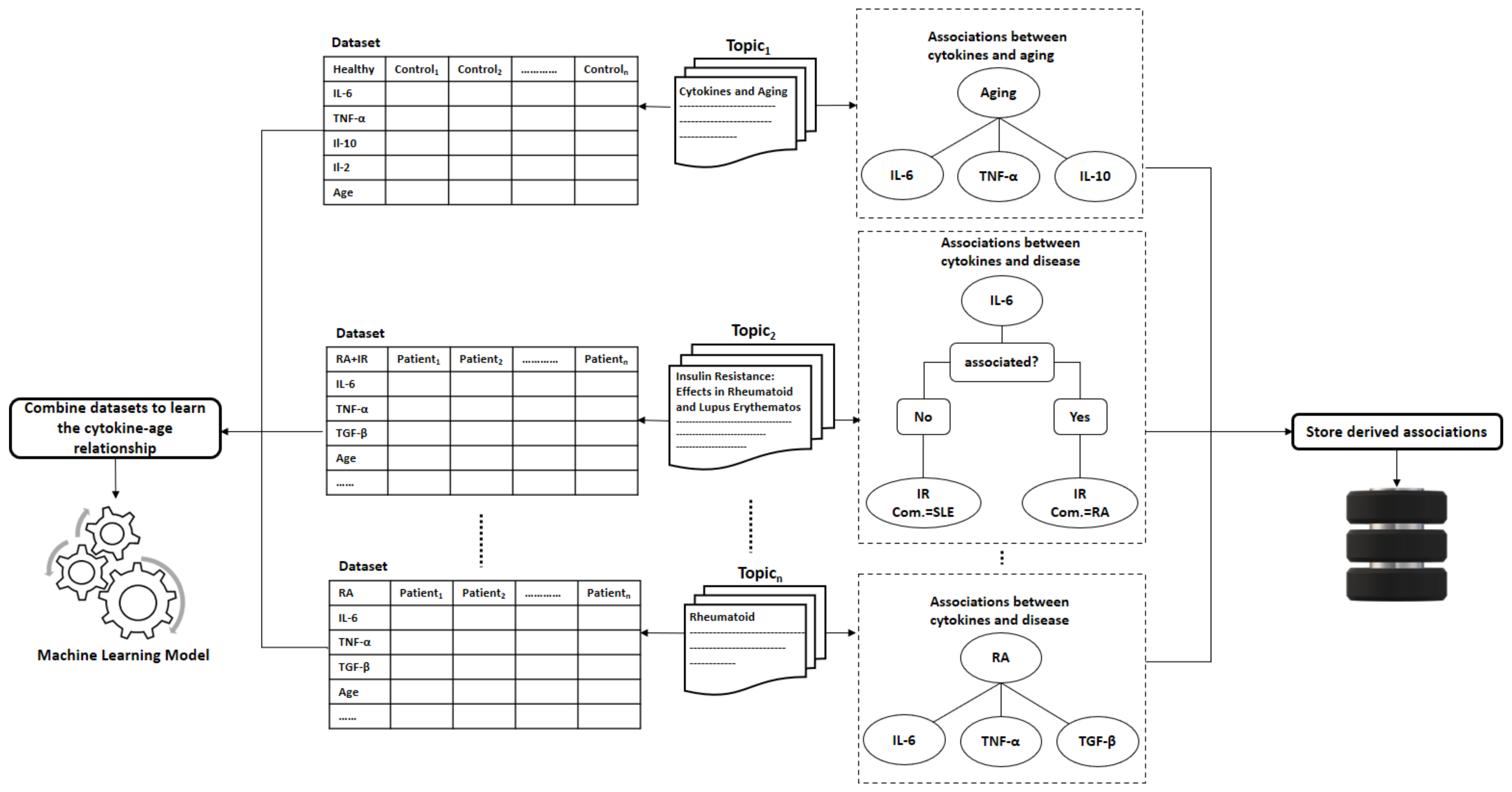
- Collecting datasets. Now, for each group of scientific papers that belong to the same topic, we would collect, if available, datasets that contain the cytokine measurements for patients/controls.
- Learning the Cytokines-Age relationship: Assuming terms that belong to the same topic are correlated, we can learn the correlations between diseases and cytokines. These correlations vary across different age slices. Therefore, researchers typically study these correlations with adjusting to age. To model the correlations between the cytokine levels and age, we can fit a machine learning model to estimate the age of the immune system on datasets containing cytokine measurements collected from the scientific papers, as shown in Figure 6. The fitted machine learning models can be evaluated with cross-validation on the collected datasets.
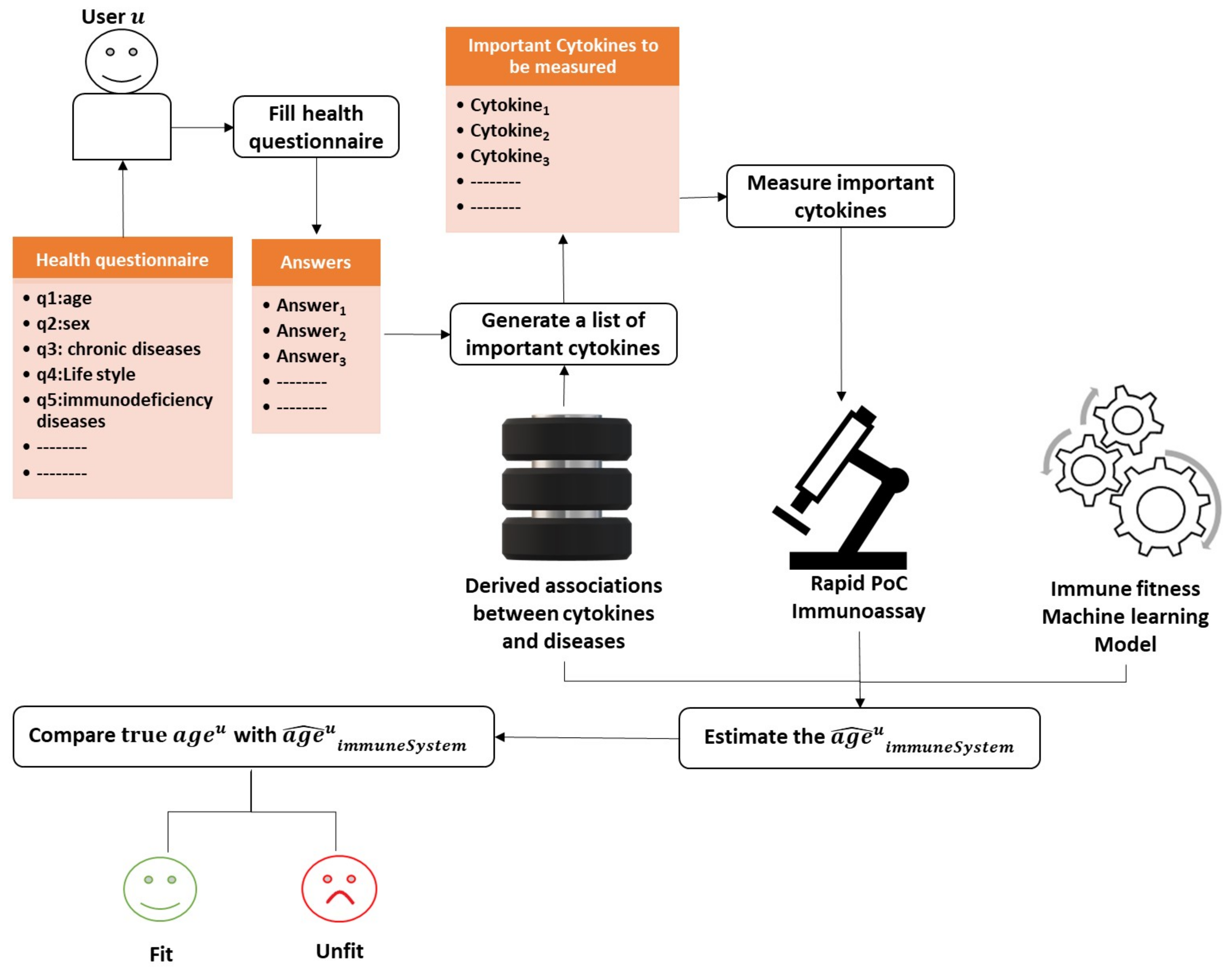
6.3. Assessing the Person’s Immune System Fitness
- The user u starts with filling the health questionnaire.
- As the disturbed cytokines change according to the diseases and the co-morbidities a user/person suffers from. The system is supposed to produce a customized list of important cytokines based on the user’s answers given to the health questionnaire and the previously derived associations from the method described in Section 6.2.
- Then, the system measures only the cytokines identified as the most critical in the immune system with one of the powerful technologies mentioned previously in this study that requires less than a drop of blood (less than 50 L). Reducing the number of measured cytokines is essential to promote efficiency and minimize the cost of the assessment procedure.
- The age of the immune system is estimated by injecting the cytokine measurements into the learned machine learning model.
- Let be the estimated age of the immune system of user u. We can compare with the real age of user u to decide whether the user’s immune system is fit or unfit.
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Sell, S. How the immune system works. Med. Times 1981, 108, 67. [Google Scholar]
- Arango Duque, G.; Descoteaux, A. Macrophage Cytokines: Involvement in Immunity and Infectious Diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef]
- Van der Meide, P.; Schellekens, H. Cytokines and the immune response. Biotherapy 1996, 8, 243–249. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, T.; Narazaki, M.; Kishimoto, T. IL-6 in Inflammation, Immunity, and Disease. Cold Spring Harbor Perspect. Biol. 2014, 6. [Google Scholar] [CrossRef]
- Eiras, C. A Point of Care Lateral Flow Assay for Rapid and Colorimetric Detection of Interleukin 6 and Perspectives in Bedside Diagnostics. J. Clin. Med. Res. 2020. [Google Scholar] [CrossRef]
- Vogt, R.; Schulte, P. Evaluation of immune responses. IARC Sci. Publ. 2011, 163, 215–239. [Google Scholar]
- Bloos, F.; Reinhart, K. Rapid diagnosis of sepsis. Virulence 2013, 5. [Google Scholar] [CrossRef] [PubMed]
- Hughes, L.E.; Whitehead, R.H. The Assessment of Immune Status. In Immunology for Surgeons; Castro, J.E., Ed.; Springer: Dordrecht, The Netherlands, 1976; pp. 90–134. [Google Scholar] [CrossRef]
- Liu, G.; Qi, M.; Hutchinson, M.; Yang, G.; Goldys, E. Recent advances in cytokine detection by immunosensing. Biosens. Bioelectron. 2016, 79. [Google Scholar] [CrossRef]
- Khan, M.; Mujahid, M. Recent Advances in Electrochemical and Optical Biosensors Designed for Detection of Interleukin 6. Sensors 2020, 20, 646. [Google Scholar] [CrossRef]
- Kitchenham, B. Procedures for Performing Systematic Reviews; Keele University: Keele, UK, 2004; Volume 33. [Google Scholar]
- Fairbank, J.; Pynsent, P. The Oswestry Disability Index. Spine 2000, 25, 2940–2952. [Google Scholar] [CrossRef]
- Ren, G.; Lutz, I.; Railton, P.; Wiley, J.; McAllister, J.; Powell, J.; Krawetz, R. Serum and synovial fluid cytokine profiling in hip osteoarthritis: Distinct from knee osteoarthritis and correlated with pain. BMC Musculoskelet. Disord. 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Lekander, M.; Elofsson, S.; Neve, I.M.; Hansson, L.O.; Undén, A.L. Self-rated Health Is Related to Levels of Circulating Cytokines. Psychosom. Med. 2004, 66, 559–563. [Google Scholar] [CrossRef] [PubMed]
- Andreasson, A.; Wicksell, R.K.; Lodin, K.; Karshikoff, B.; Axelsson, J.; Lekander, M. A global measure of sickness behaviour: Development of the Sickness Questionnaire. J. Health Psychol. 2018, 23, 1452–1463. [Google Scholar] [CrossRef] [PubMed]
- Jehangir, A.; Parkman, H. Rome IV Diagnostic Questionnaire Complements Patient Assessment of Gastrointestinal Symptoms for Patients with Gastroparesis Symptoms. Dig. Dis. Sci. 2018, 63. [Google Scholar] [CrossRef] [PubMed]
- Peter, H.H.; Goldacker, S.; Haraldseide, J.; Großmann, K.; Gross, W.; Warnatz, K.; Grimbacher, B.; Rusch, S.; Vach, W. Construction and Clinical Validation of a Questionnaire-based Risk Score to Identify Patients Suffering from Immunodeficiency or Systemic Autoimmunity. Br. J. Med. Med. Res. 2014, 4, 4751–4769. [Google Scholar] [CrossRef]
- Wilod Versprille, L.J.F.; van de Loo, A.J.A.E.; Mackus, M.; Arnoldy, L.; Sulzer, T.A.L.; Vermeulen, S.A.; Abdulahad, S.; Huls, H.; Baars, T.; Scholey, A.; et al. Development and Validation of the Immune Status Questionnaire (ISQ). Int. J. Environ. Res. Public Health 2019, 16. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, D.P.; Hillier, V.F. A scaled version of the General Health Questionnaire. Psychol. Med. 1979, 9, 139–145. [Google Scholar] [CrossRef] [PubMed]
- Reed, P.; Vile, R.; Osborne, L.; Romano, M.; Truzoli, R. Problematic Internet Usage and Immune Function. PLoS ONE 2015, 10, e0134538. [Google Scholar] [CrossRef]
- Eriksson, I.; Undén, A.; Elofsson, S. Self-rated health. Comparisons between three different measures. Results from a population study. Int. J. Epidemiol. 2001, 30, 326–333. [Google Scholar] [CrossRef]
- Cislaghi, B.; Cislaghi, C. Self-rated health as a valid indicator for health-equity analyses: Evidence from the Italian health interview survey. BMC Public Health 2019, 19. [Google Scholar] [CrossRef]
- Meng, Q.; Xie, Z.; Zhang, T. A Single-Item Self-Rated Health Measure Correlates with Objective Health Status in the Elderly: A Survey in Suburban Beijing. Front. Public Health 2014, 2, 27. [Google Scholar] [CrossRef]
- Lantman, M.; Mackus, M.; Otten, L.S.; Kruijff, D.; Loo, A.; Kraneveld, A.; Garssen, J.; Verster, J. Mental resilience, perceived immune functioning, and health. J. Multidiscip. Healthc. 2017, 10, 107–112. [Google Scholar] [CrossRef]
- Pashchenko, O.; Shelby, T.; Banerjee, T.; Santra, S. A Comparison of Optical, Electrochemical, Magnetic, and Colorimetric Point-of-Care Biosensors for Infectious Disease Diagnosis. ACS Infect. Dis. 2018, 4. [Google Scholar] [CrossRef]
- Chen, P.; Chung, M.T.; Mchugh, W.; Nidetz, R.; Liu, Y.; Jianping, f.; Cornell, T.; Shanley, T.; Kurabayashi, K. Multiplex Serum Cytokine Immunoassay Using Nanoplasmonic Biosensor Microarrays. ACS Nano 2015, 9, 4173–4181. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Zhu, J.; He, J.; Yang, W.; Ma, C.; Xiong, F.; Li, F.; Chen, W.; Chen, P. Magnet Patterned Superparamagnetic Fe3O4/Au Core-Shell Nanoplasmonic Sensing Array for Label-Free High Throughput Cytokine Immunoassay. Adv. Healthc. Mater. 2019, 8, 1801478. [Google Scholar] [CrossRef]
- Usuba, R.; Yokokawa, M.; Ackermann, T.N.; Llobera, A.; Fukunaga, K.; Murata, S.; Ohkohchi, N.; Suzuki, H. Photonic Lab-on-a-Chip for Rapid Cytokine Detection. ACS Sens. 2016, 1, 979–986. [Google Scholar] [CrossRef]
- Park, Y.; Ryu, B.; Oh, B.R.; Song, Y.; Liang, X.; Kurabayashi, K. Bio-Tunable Nanoplasmonic Filter on Few-Layer MoS 2 for Rapid and Highly Sensitive Optoelectronic Cytokine Immunosensing. ACS Nano 2017, 11. [Google Scholar] [CrossRef]
- Castanheira, A.P.; Barbosa, A.I.; Edwards, A.D.; Reis, N.M. Multiplexed femtomolar quantitation of human cytokines in a fluoropolymer microcapillary film. Analyst 2015, 140, 5609–5618. [Google Scholar] [CrossRef][Green Version]
- Cao, C.; Jin, R.; Wei, H.; Liu, Z.; Ni, S.; Liu, G.J.; Young, H.A.; Chen, X.; Liu, G. Adaptive in vivo device for theranostics of inflammation: Real-time monitoring of interferon-γ and aspirin. Acta Biomater. 2020, 101, 372–383. [Google Scholar] [CrossRef]
- Tang, C.K.; Vaze, A.; Shen, M.; Rusling, J.F. High-Throughput Electrochemical Microfluidic Immunoarray for Multiplexed Detection of Cancer Biomarker Proteins. ACS Sens. 2016, 1, 1036–1043. [Google Scholar] [CrossRef]
- Wu, D.; Ríos Aguirre, D.; Chounlakone, M.; Camacho-Leon, S.; Voldman, J. Sequentially Multiplexed Amperometry for Electrochemical Biosensors. Biosens. Bioelectron. 2018, 117. [Google Scholar] [CrossRef] [PubMed]
- Fischer, S.K.; Williams, K.; Wang, L.; Capio, E.; Briman, M. Development of an IL-6 point-of-care assay: Utility for real-time monitoring and management of cytokine release syndrome and sepsis. Bioanalysis 2019, 11, 1777–1785. [Google Scholar] [CrossRef] [PubMed]
- Min, J.; Nothing, M.; Coble, B.; Zheng, H.; Park, J.; Im, H.; Weber, G.; Castro, C.; Swirski, F.; Weissleder, R.; et al. Integrated Biosensor for Rapid and Point-Of-Care Sepsis Diagnosis. ACS Nano 2018, 12. [Google Scholar] [CrossRef]
- Alba-Patiño, A.; Russell, S.M.; Borges, M.; Pazos-Pérez, N.; Álvarez Puebla, R.A.; de la Rica, R. Nanoparticle-based mobile biosensors for the rapid detection of sepsis biomarkers in whole blood. Nanoscale Adv. 2020, 2, 1253–1260. [Google Scholar] [CrossRef]
- Borse, V.; Srivastava, R. Fluorescence lateral flow immunoassay based point-of-care nanodiagnostics for orthopedic implant-associated infection. Sens. Act. B Chem. 2018, 280. [Google Scholar] [CrossRef]
- McNaught, A.; Wilkinson, A. IUPAC Compendium of Chemical Terminology; Blackwell Science: Oxford, UK, 1997; Volume 2. [Google Scholar] [CrossRef]
- USFDA. Guidance for Industry: Bioanalytical Method Validation; U.S. Department of Health and Human Services, Food and Drug Administration: Washington, DC, USA, 2001; Volume 66. [Google Scholar]
- Christodouleas, D.C.; Kaur, B.; Chorti, P. From point-of-care testing to eHealth diagnostic devices (eDiagnostics). ACS Central Sci. 2018, 4, 1600–1616. [Google Scholar] [CrossRef]
- Alawsi, T.; Al-Bawi, Z. A review of smartphone point-of-care adapter design. Eng. Rep. 2019, 1, e12039. [Google Scholar] [CrossRef]
- Shrivastava, S.; Trung, T.Q.; Lee, N.E. Recent progress, challenges, and prospects of fully integrated mobile and wearable point-of-care testing systems for self-testing. Chem. Soc. Rev. 2020, 49, 1812–1866. [Google Scholar] [CrossRef] [PubMed]
- Andryukov, B.G. Six decades of lateral flow immunoassay: From determining metabolic markers to diagnosing COVID-19. AIMS Microbiol. 2020, 6, 280. [Google Scholar] [CrossRef]
- Xiao, Q.; Xu, C. Research progress on chemiluminescence immunoassay combined with novel technologies. TrAC Trends Anal. Chem. 2020, 124, 115780. [Google Scholar] [CrossRef]
- Poschenrieder, A.; Thaler, M.; Junker, R.; Luppa, P.B. Recent advances in immunodiagnostics based on biosensor technologies—From central laboratory to the point of care. Anal. Bioanal. Chem. 2019, 411, 7607–7621. [Google Scholar] [CrossRef]
- Luppa, P.B.; Junker, R.; Schimke, I.; Stürenburg, E. Immunological methods. In Point-of-Care Testing; Springer: Berlin/Heidelberg, Germany, 2018; pp. 69–79. [Google Scholar]
- Lu, J. Immunoassay Design of a Fiber Optic Biosensor for Medical and Agro-Food Applications. 2018. Available online: https://lirias.kuleuven.be/2001821?limo=0 (accessed on 1 July 2021).
- Mahshid, S.S.; Ricci, F.; Kelley, S.O.; Vallée-Bélisle, A. Electrochemical DNA-based immunoassay that employs steric hindrance to detect small molecules directly in whole blood. ACS Sens. 2017, 2, 718–723. [Google Scholar] [CrossRef] [PubMed]
- Marks, H.; Schechinger, M.; Garza, J.; Locke, A.; Coté, G. Surface enhanced Raman spectroscopy (SERS) for in vitro diagnostic testing at the point of care. Nanophotonics 2017, 6, 681–701. [Google Scholar] [CrossRef]
- Nayak, S.; Blumenfeld, N.R.; Laksanasopin, T.; Sia, S.K. Point-of-care diagnostics: Recent developments in a connected age. Anal. Chem. 2017, 89, 102–123. [Google Scholar] [CrossRef]
- Romao, V.C.; Martins, S.A.; Germano, J.; Cardoso, F.A.; Cardoso, S.; Freitas, P.P. Lab-on-chip devices: Gaining ground losing size. ACS Nano 2017, 11, 10659–10664. [Google Scholar] [CrossRef] [PubMed]
- Zarei, M. Portable biosensing devices for point-of-care diagnostics: Recent developments and applications. TrAC Trends Anal. Chem. 2017, 91, 26–41. [Google Scholar] [CrossRef]
- Malekjahani, A.; Sindhwani, S.; Syed, A.M.; Chan, W.C. Engineering steps for mobile point-of-care diagnostic devices. Account. Chem. Res. 2019, 52, 2406–2414. [Google Scholar] [CrossRef]
- Sachdeva, S.; Davis, R.W.; Saha, A.K. Microfluidic Point-of-Care Testing: Commercial Landscape and Future Directions. Front. Bioeng. Biotechnol. 2021, 8, 1537. [Google Scholar] [CrossRef]
- Jothi, L.; Nageswaran, G. Chapter 15 - Plasma Modified Polymeric Materials for Biosensors/Biodevice Applications. In Non-Thermal Plasma Technology for Polymeric Materials; Thomas, S., Mozetič, M., Cvelbar, U., Špatenka, P., Praveen, K.M., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; pp. 409–437. [Google Scholar] [CrossRef]
- Kim, H.; Kim, H.S.; Youn, J.C.; Shin, E.C.; Park, S. Serum cytokine profiles in healthy young and elderly population assessed using multiplexed bead-based immunoassays. J. Transl. Med. 2011, 9, 113. [Google Scholar] [CrossRef] [PubMed]
- Nicolau, J.; Lequerré, T.; Bacquet, H.; Vittecoq, O. Rheumatoid arthritis, insulin resistance, and diabetes. Jt. Bone Spine 2017, 84, 411–416. [Google Scholar] [CrossRef]
- Chung, C.P.; Oeser, A.; Solus, J.F.; Gebretsadik, T.; Shintani, A.; Avalos, I.; Sokka, T.; Raggi, P.; Pincus, T.; Stein, C.M. Inflammation-associated insulin resistance: Differential effects in rheumatoid arthritis and systemic lupus erythematosus define potential mechanisms. Arthritis Rheum. 2008, 58, 2105–2112. [Google Scholar] [CrossRef] [PubMed]
- Trifonova, E.; Sazonova, O.; Zonova, E. AB0752 The Association between Clinical Symptoms and Serum Cytokines Levels in Patients with Knee Osteoarthritis and Comorbidity. Ann. Rheum. Dis. 2016, 75, 1162. [Google Scholar] [CrossRef]
- Ragino, Y.; Oblaukhova, V.; Polonskaya, Y.; Kuzminykh, N.; Shcherbakova, L.; Kashtanova, E. The Blood Cytokine Profile of Young People with Early Ischemic Heart Disease Comorbid with Abdominal Obesity. J. Personal. Med. 2020, 10, 87. [Google Scholar] [CrossRef] [PubMed]
- Duggal, N. Reversing the immune ageing clock: Lifestyle modifications and pharmacological interventions. Biogerontology 2018, 19. [Google Scholar] [CrossRef] [PubMed]
- Gunes, H.; Mete, R.; Aydin, M.; Topçu, B.; Oran, M.; Dogan, M.; Yildirim, O.; Erdem, I.; Eren, A.; Gürel, A. Relationship among MIF, MCP-1, viral loads, and HBs Ag levelsin chronic hepatitis B patients. Turk. J. Med. Sci. 2015, 45, 634–637. [Google Scholar] [CrossRef] [PubMed]
- Janssens, P.; Decuypere, J.P.; De Rechter, S.; Breysem, L.; Giel, D.; Billen, J.; Hindryckx, A.; De Catte, L.; Baldewijns, M.; Claes, K.; et al. Enhanced MCP-1 Release in Early Autosomal Dominant Polycystic Kidney Disease. Kidney Int. Rep. 2021, 6. [Google Scholar] [CrossRef]
- Smith, B.; Dalen, J.; Wiggins, K.; Tooley, E.; Christopher, P.; Bernard, J. The Brief Resilience Scale: Assessing the Ability to Bounce Back. Int. J. Behav. Med. 2008, 15, 194–200. [Google Scholar] [CrossRef]
- Anfossi, L.; Di Nardo, F.; Cavalera, S.; Giovannoli, C.; Baggiani, C. Multiplex Lateral Flow Immunoassay: An Overview of Strategies towards High-throughput Point-of-Need Testing. Biosensors 2018, 9. [Google Scholar] [CrossRef]
- Ruppert, C.; Phogat, N.; Laufer, S.; Kohl, M.; Deigner, P. A smartphone readout system for gold nanoparticle-based lateral flow assays: Application to monitoring of digoxigenin. Microchim. Acta 2019, 186. [Google Scholar] [CrossRef] [PubMed]
- Schneider, S.; Selig, M.; Keil, V.; Lehmann, M.; Foitzik, A.; Casalboni, M.; Richetta, M. Development of a Smartphone Based Reader for the Quantitative Analysis of Lateral Flow Assays. Mater. Sci. Forum 2018, 941, 2522–2527. [Google Scholar] [CrossRef]
- Foysal, K.; Seo, S.; Kim, M.; Kwon, O.S.; Chong, J. Analyte Quantity Detection from Lateral Flow Assay Using a Smartphone. Sensors 2019, 19, 4812. [Google Scholar] [CrossRef] [PubMed]
- Hauser, J.; Lenk, G.; Hansson, J.; Beck, O.; Stemme, G.; Roxhed, N. High-Yield Passive Plasma Filtration from Human Finger Prick Blood. Anal. Chem. 2018, 90, 13393–13399. [Google Scholar] [CrossRef]
- Madadi, H.; Casals-Terré, J.; Mohammadi, M. Self-driven filter-based blood plasma separator microfluidic chip for point-of-care testing. Biofabrication 2015, 7. [Google Scholar] [CrossRef]
- Homsy, A.; Wal, P.; Doll, W.; Schaller-Ammann, R.; Korsatko, S.; Ratzer, M.; Ellmerer, M.; Pieber, T.; Nicol, A.; Rooij, N. Development and validation of a low cost blood filtration element separating plasma from undiluted whole blood. Biomicrofluidics 2012, 6, 12804–128049. [Google Scholar] [CrossRef]
- Andersen, N.; Holub, M.; Lawrence, D.; Davidova, A.; Beran, O.; Maresova, V.; Chalupa, P. Cytokines and Chemokines as Biomarkers of Community-Acquired Bacterial Infection. Mediat. Inflamm. 2013, 2013, 190145. [Google Scholar] [CrossRef]
- Kedzierska, K.; Crowe, S. Cytokines and HIV-1: Interactions and Clinical Implications. Antivir. Chem. Chemother. 2001, 12, 133–150. [Google Scholar] [CrossRef]
- Röseler, S.; Holtappels, G.; Wagenmann, M.; Bachert, C. Elevated levels of interleukins IL-1 β, IL-6 and IL-8 in naturally acquired viral rhinitis. Eur. Arch. Oto-Rhino-Laryngol. 1995, 252 (Suppl. 1), S61–S63. [Google Scholar] [CrossRef] [PubMed]
- Noah, T.; Becker, S. Chemokines in Nasal Secretions of Normal Adults Experimentally Infected with Respiratory Syncytial Virus. Clin. Immunol. 2000, 97, 43–49. [Google Scholar] [CrossRef]
- Bénard, A.; Jacobsen, A.; Brunner, M.; Krautz, C.; Klösch, B.; Swierzy, I.; Naschberger, E.; Podolska, M.; Kouhestani, D.; David, P.; et al. Interleukin-3 is a predictive marker for severity and outcome during SARS-CoV-2 infections. Nat. Commun. 2021, 12, 1112. [Google Scholar] [CrossRef] [PubMed]
- Kunz, M.; Ibrahim, S. Cytokines and Cytokine Profiles in Human Autoimmune Diseases and Animal Models of Autoimmunity. Mediat. Inflamm. 2009, 2009, 979258. [Google Scholar] [CrossRef]
- Navarro, J.; Mora, C. The Role of Inflammatory Cytokines in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2008, 19, 433–442. [Google Scholar] [CrossRef]
- Volkmann, E.; Tashkin, D.; Roth, M.; Clements, P.; Khanna, D.; Furst, D.; Mayes, M.; Charles, J.; Tseng, C.; Elashoff, R.; et al. Changes in plasma CXCL4 levels are associated with improvements in lung function in patients receiving immunosuppressive therapy for systemic sclerosis-related interstitial lung disease. Arthritis Res. Ther. 2016, 18, 305. [Google Scholar] [CrossRef] [PubMed]
- Gee, K.; Guzzo, C.; Che Mat, N.F.; Ma, W.; Kumar, A. The IL-12 Family of Cytokines in Infection, Inflammation and Autoimmune Disorders. Inflamm. Allergy Drug Targets 2009, 8, 40–52. [Google Scholar] [CrossRef] [PubMed]
- Moldoveanu, A.; Shephard, R.; Shek, P. The Cytokine Response to Physical Activity and Training. Sports Med. 2001, 31, 115–144. [Google Scholar] [CrossRef] [PubMed]
- Valle, D.; Kim-Schulze, S.; Huang, H.H.; Beckmann, N.; Nirenberg, S.; Wang, B.; Lavin, Y.; Swartz, T.; Madduri, D.; Stock, A.; et al. An inflammatory cytokine signature predicts COVID-19 severity and survival. Nat. Med. 2020, 26, 1–8. [Google Scholar] [CrossRef]
- Rana, S.; Sharma, S.; Sinha, S.; Parsad, K.; Malik, A.; Jass, H. Pro-inflammatory and anti-inflammatory cytokine response in diarrhoea-predominant Irritable bowel syndrome patients. Trop. Gastroenterol. 2011, 33, 251–256. [Google Scholar] [CrossRef]
- Vedova, C.; Cathcart, S.; Dohnalek, A.; Lee, V.; Hutchinson, M.; Immink, M.; Hayball, J. Peripheral Interleukin-1β Levels are Elevated in Chronic Tension-Type Headache Patients. Pain Res. Manag. 2013, 18. [Google Scholar] [CrossRef] [PubMed]
- Kunz, S.; Wolk, K.; Witte, E.; Witte, K.; Doecke, W.D.; Volk, H.D.; Sterry, W.; Asadullah, K.; Sabat, R. Interleukin (IL)-19, IL-20 and IL-24 are produced by and act on keratinocytes and are distinct from classical ILs. Exp. Dermatol. 2006, 15, 991–1004. [Google Scholar] [CrossRef] [PubMed]
- Wolk, K.; Kunz, S.; Witte, E.; Friedrich, M.; Asadullah, K.; Sabat, R. IL-22 Increases the Innate Immunity of Tissues. Immunity 2004, 21, 241–254. [Google Scholar] [CrossRef]
- Albanesi, C.; Scarponi, C.; Cavani, A.; Federici, M.; Nasorri, F.; Girolomoni, G. Interleukin-17 is Produced by Both Th1 and Th2 Lymphocytes, and Modulates Interferon- and Interleukin-4-Induced Activation of Human Keratinocytes. J. Investig. Dermatol. 2000, 115, 81–87. [Google Scholar] [CrossRef] [PubMed]
- Hänel, K.; Cornelissen, C.; Lüscher, B.; Baron, J. Cytokines and the Skin Barrier. Int. J. Mol. Sci. 2013, 14, 6720–6745. [Google Scholar] [CrossRef]
- Alfieri, F. Serum Levels of Proinflammatory Cytokines in Painful Knee Osteoarthritis and Sensitization. Int. J. Inflamm. 2015, 2015. [Google Scholar] [CrossRef]
- El-Kady, I.; El-Masry, S. Pro-inflammatory and anti-inflammatory cytokines profile in rheumatoid arthritis patients. Egypt. J. Immunol. 2008, 15, 109–114. [Google Scholar] [PubMed]
- Euteneuer, F.; Schwarz, M.; Hennings, A.; Riemer, S.; Stapf, T.; Selberdinger, V.; Rief, W. Psychobiological aspects of somatization syndromes: Contributions of inflammatory cytokines and neopterin. Psychiatry Res. 2011, 195, 60–65. [Google Scholar] [CrossRef] [PubMed]
- Montinaro, V.; Iaffaldano, G.; Granata, S.; Porcelli, P.; Todarello, O.; Schena, F.P.; Pertosa, G. Emotional symptoms, quality of life and cytokine profile in hemodialysis patients. Clin. Nephrol. 2010, 73, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Krueger, J. The Role of Cytokines in Sleep Regulation. Curr. Pharm. Design 2008, 14, 3408–3416. [Google Scholar] [CrossRef] [PubMed]
- Jenike, M. Depression and other psychiatric disorders. In Geriatric Neuropsychology; Albert, M.S., Moss, M.B., Eds.; The Guilford Press: New York, NY, USA, 1988; pp. 115–144. [Google Scholar]
- Paats, M.; Bergen, I.; Hanselaar, W.; Zoelen, E.; Hoogsteden, H.; Hendriks, R.; Eerden, M. Local and systemic cytokine profiles in nonsevere and severe community-acquired pneumonia. Eur. Respir. J. 2012, 41, 1378–1385. [Google Scholar] [CrossRef]
- Bozza, F.; Salluh, J.; Japiassú, A.; Soares, M.; Assis, E.; Gomes, R.; Bozza, M.; Castro-Faria-Neto, H.; Bozza, P. Cytokine profiles as makers of disease severity in sepsis: A multiplex analysis. Crit. Care 2007, 11, R49. [Google Scholar] [CrossRef]
- Lopez-Castejon, G.; Brough, D. Understanding the mechanism of IL-1β secretion. Cytokine Growth Factor Rev. 2011, 22, 189–195. [Google Scholar] [CrossRef]
- Lambrecht, B.; Hammad, H.; Fahy, J. The Cytokines of Asthma. Immunity 2019, 50, 975–991. [Google Scholar] [CrossRef]
- Tănase, D.; Gosav, E.; Radu, S.; Ouatu, A.; Rezuş, C.; Ciocoiu, M.; Costea, C.; Floria, M. Arterial Hypertension and Interleukins: Potential Therapeutic Target or Future Diagnostic Marker? Int. J. Hypertens. 2019, 2019, 3159283. [Google Scholar] [CrossRef] [PubMed]
- Daniilidis, A.; Koutsos, J.; Oikonomou, Z.; Nasioutziki, M.; Hatziparadisi, K.; Tantanasis, T. Cytokines of Cervical Mucosa and Human Papilloma Virus Infection of the Cervix: A Descriptive Study. Acta Cytol. 2016, 60. [Google Scholar] [CrossRef] [PubMed]
- Xie, T.; Du, G.H. Cytokines and Parkinson’s disease. Chin. Pharm. J. 2014, 49, 1773–1777. [Google Scholar] [CrossRef]
- Cuellar, J.; Golish, S.; Reuter, M.; Cuellar, V.; Angst, M.; Carragee, E.; Yeomans, D.; Scuderi, G. Cytokine evaluation in individuals with low back pain using discographic lavage. Spine J. 2010, 10, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Singh, S. Cytokines in chronic obstructive pulmonary disease. Int. J. Res. Dev. Pharm. Life Sci. 2016, 4, 1611–1619. [Google Scholar]
- Setrerrahmane, S.; Xu, H. Tumor-related interleukins: Old validated targets for new anti-cancer drug development. Mol. Cancer 2017, 16, 153. [Google Scholar] [CrossRef] [PubMed]
- Zheng, C.; Zhou, X.W.; Wang, J.Z. The dual roles of cytokines in Alzheimer’s disease: Update on interleukins, TNF-α, TGF-β and IFN-γ. Transl. Neurodegener. 2016, 5, 7. [Google Scholar] [CrossRef]
- Rajappa, M.; Sen, S.; Sharma, A. Role of Pro-/Anti-Inflammatory Cytokines and Their Correlation With Established Risk Factors in South Indians With Coronary Artery Disease. Angiology 2008, 60, 419–426. [Google Scholar] [CrossRef]
- Llorent, L.; Richaud-Patin, Y.; Alcocer-Castillejos, N.; Ruiz-Soto, R.; Mercado, M.; Orozco, H.; Gamboa-Dominguez, A.; Alcocer-Varela, J. Cytokine gene expression in cirrhotic and non-cirrhotic human liver. J. Hepatol. 1996, 24, 555–563. [Google Scholar] [CrossRef]
- Lindsey, M.; Jung, M.; Yabluchanskiy, A.; Cannon, P.; Iyer, R.; Flynn, E.; DeLeon-Pennell, K.; Valerio, F.; Harrison, C.; Ripplinger, C.; et al. Exogenous CXCL4 Infusion Inhibits Macrophage Phagocytosis by Limiting CD36 Signaling to Enhance Post-myocardial Infarction Cardiac Dilation and Mortality. Cardiovasc. Res. 2018, 115. [Google Scholar] [CrossRef]
- Lin, A.; Kenis, G.; Bignotti, S.; Tura, G.J.B.; Jong, R.; Bosmans, E.; Pioli, R.; Altamura, A.; Scharpé, S.; Maes, M. The inflammatory response system in treatment-resistant schizophrenia: Increased serum interleukin-6. Schizophr. Res. 1998, 32, 9–15. [Google Scholar] [CrossRef]
- Zhang, X.; Zhou, D.; Zhang, P.; Wu, G.; Cao, L.; Shen, Y. Corrigendum to “Elevated interleukin-2, interleukin-6 and interleukin-8 serum levels in neuroleptic-free schizophrenia: Association with psychopathology” [Schizophr. Res. 57 (2002) 247–258]. Schizophr. Res. 2003, 60, 101. [Google Scholar] [CrossRef]
- Loo, A.; Kerssemakers, N.; Scholey, A.; Garssen, J.; Kraneveld, A.; Verster, J. Perceived Immune Fitness, Individual Strength and Hangover Severity. Int. J. Environ. Res. Public Health 2020, 17, 4039. [Google Scholar] [CrossRef]
- Boye Asmussen, C.; Møller, C. Smart literature review: A practical topic modelling approach to exploratory literature review. J. Big Data 2019, 6. [Google Scholar] [CrossRef]
- Zhang, Y.; Shen, F.; Rastegar-Mojarad, M.; Li, D.; Liu, S.; Tao, C.; Yu, Y.; Liu, H. Systematic identification of latent disease-gene associations from PubMed articles. PLoS ONE 2018, 13, e0191568. [Google Scholar] [CrossRef]
- Blei, D.; Ng, A.; Jordan, M. Latent Dirichlet Allocation. J. Mach. Learn. Res. 2003, 3, 993–1022. [Google Scholar] [CrossRef]
- Trindade, F.; Perpétuo, L.; Ferreira, R.; Leite-Moreira, A.; Falcão-Pires, I.; Guedes, S.; Vitorino, R. Automatic text-mining as an unbiased approach to uncover molecular associations between periodontitis and coronary artery disease. Biomarkers 2021, 26, 385–394. [Google Scholar] [CrossRef]
- Kuang, D.; Choo, J.; Park, H. Nonnegative Matrix Factorization for Interactive Topic Modeling and Document Clustering; Springer: Berlin/Heidelberg, Germany, 2015; pp. 215–243. [Google Scholar] [CrossRef]
- Kim, H.; Park, H. Sparse Non-negative Matrix Factorizations via Alternating Non-negativity-constrained Least Squares for Microarray Data Analysis. Bioinformatics 2007, 23, 1495–1502. [Google Scholar] [CrossRef] [PubMed]
- Greene, D.; O’Callaghan, D.; Cunningham, P. How Many Topics? Stability Analysis for Topic Models; Springer: Berlin/Heidelberg, Germany, 2014. [Google Scholar] [CrossRef]


| Our Proposed Feature | Feature from Literature on Non-Cytokine Immunoassay Readers | Action |
|---|---|---|
| Assay time | Assessment speed | We used ‘Assay time’. |
| Sample volume and type | type and size of analyzed sample | We used ‘Sample’ as a composite feature. |
| Multiple simultaneous cytokine detection | n/a, since the reviews were not on cytokines | We used the feature ’Cytokines’. |
| Signal reader | support for smartphone/wearable, including readers that can be attached to smartphones or wearables | We stressed the use of smartphones whenever appropriate under the feature ‘Signal reader’. |
| Optical Signal Detection | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Technique | Sample | Cytokines | Dynamic Range (DR) | Limit of Detection (LoD) | Validity of Measurements | Assay Time | Signal Reader | Specific Disease | PoC ? |
| LSPR-AuNRs [26] | 1 L of blood serum | IL-2 IL-4 IL-6 IL-10 TNF- IFN- | Multiplex detection: 10–10,000 pg/mL | Multiplex detection: 6.46–20.56 pg/mL | Correlation with singleplex ELISA R= 0.9726 Recovery within a range of 85–115% | 40 min | dark-field imaging | Inflammatory responses after CBP surgery | × |
| LSPR-FACSNPs [27] | 1 L of biological sample | IL-6 MCP-1 TNF- TGF- | 50–1000 pg/mL | 18.96 pg/mL 14.57 pg/mL 32.62 pg/mL 22.08 pg/mL | Correlation with singleplex ELISA R = 0.9252 | real time | dark-field microscopy | – | √ |
| PhLoC [28] | 5 L of Lymphocytes separated from whole blood | IL-2 | 50–1000 pg/mL | 50 pg/mL | – | 15 min | UV-vis light, a microscope and a unit filter | – | √ |
| LSPR-MoS2 [29] | Blood serum and plasma | IL-1 | pg/mL | 250 fg/mL | – | 10 min | Few-Layer MoS2 Photodetector | – | √ |
| MCF [30] | 150 L of undiluted human serum | IL-6 IL-1, IL- 12p70, TNF- | 1.5–2.0 log units | singleplex detection: 2.0–15.0 pg/mL Multiplex detection: 60–150 pg/mL | intra- and inter-assay coefficient of variation (CV) within 10% | 20 min | HP ScanJet G4050 Scanner | – | √ |
| Optical Signal Detection-Supporting Information | |||
|---|---|---|---|
| Technique | Dimensions | Reaction on | Cost |
| LSPR-AuNRs [26] | An array of 480 stripe-shaped LSPR biosensing spots (25 m × 200 m) | AuNR microarray | – |
| LSPR-FACSNPs [27] | 6 × sample-flow microfluidic channels (500 m (W) × 2.5 cm (L) × 50 m (H)) | FACSNP microarray | – |
| PhLoC [28] | Measuring chamber (5 mm, 250 m, and 200 m) | the surface of the measuring chamber | – |
| LSPR-MoS [29] | SiO (300 nm thick) + few-layer MOS (0.65 nm thick) + SiO(170 m thick) | an SiO thin layer | – |
| MCF [30] | External dimensions of the MCF (4.5 ± 0.10 mm wide and 0.6 ± 0.05 mm thick) | the internal walls of the microcapillaries | – |
| Electrochemical Signal Detection | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Technique | Sample | Cytokines | Dynamic Range (DR) | Limit of Detection (LoD) | Validity of Measurements | Assay Time | Signal Reader | Specific Disease | PoC ? |
| aptamer-based biosensor [31] | in vitro 1 mL of WB | IFN- | 10–500 pg/mL | 10 pg/mL | – | vivo: real time vitro: 1 h | Shimadzu UV–Vis spectrophotometer model 2450 | Inflam-mation | × |
| 8-port manifold microfluidic biosensor [32] | 5 L of serum | IL-6 PF-4 | Multiplex detection: sub pg/mL to well above ng/mL | Multiplex detection: 0.05–2 pg/mL | IL-6: correlation with singleplex ELISA R = 0.97418PF-4: correlation with singleplex ELISA R = 0.984 | <1 h | CHI 1040A multi-potentiostat coupled with CHI 685 multiplexer | prostate cancer | × |
| sequentially-multiplexed biosensor [33] | 150 L of serum | IL-6 | 5–1000 pg/mL | 5.0 pg/mL | – | 40 min | ADC recordings via serial port communication | Inflam-mation | √ |
| PoC-Proxim biosensor [34] | 100 L of plasma or serum samples | IL-6 | 1– pg/mL | 0.6 pg/mL | (n = 2) CV of concentration < 14% correlation with singleplex ELISA R = 0.96172 | 20 min | smartphone | Sepsis & cytokine release syndrome | √ |
| hybrid magneto-based biosensor [35] | 100 L of WB, plasma, or serum samples | IL-3 | ∼10 pg/mL | ∼5 pg/mL | (n = 62) sensitivity = 91.3% specificity = 82.4% | ≤1 h | smartphone | Sepsis & organ failure | √ |
| Electrochemical Signal Detection-Supporting Information | |||
|---|---|---|---|
| Technique | Dimensions | Reaction on | Cost |
| aptamer-based biosensor [31] | GC electrode (3 mm disks) + spectrophotometer (660 × 275 ×570 mm) | a glassy carbon (GC) rod | – |
| 8-port manifold microfluidic biosensor [32] | Detection device (1 in. × 1 in. × 0.75 in.) | gold microelectrode | $0.50 in materials per 32-sensorcomplete system |
| sequentially-multiplexed biosensor [33] | single-chip potentiostat (1.23 × 8.1 × 8.1 mm) + N ECBs | working electrodes (WE) for the ECBs | single-chip potentiostat (<20 USD) |
| PoC-Proxim technology [34] | A handheld testing platform (11 × 4 × 3) cm | ECBs in disposable Profile cartridges | – |
| hybrid magneto-based biosensor [35] | Integrated device (10 × 1 × 2.5 cm) | portable biosensor consisting of a disposable kit for blood processing | cost of goods 50 for the device, and the reagent cost 5 per test |
| Colorimetric Signal Detection | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Technique | Sample | Cytokines | Dynamic Range (DR) | Limit of Detection (LoD) | Validity of Measurements | Assay Time | Signal Reader | Specific Disease | PoC ? |
| plasmonic-based mobile biosensor [36] | 2.5 L of WB | IL-6 | variations in the basal concentration as small as 12.5 pg/mL | 0.1 pg/mL | 99% confidence | 17 min | smartphone | sepsis | × |
| LFA-AuNPs [5] | 150 L of plasma | IL-6 | 1.25–9000 ng/mL | 0.38 ng/mL | No statistical difference with cytometric bead array (CBA)n = 5, t-test (p < 0.05) | 20 min | Canon T61 camera with 18–55 lens | visceral leishmania | √ |
| LFA-CdTe QDs [37] | 50 L of serum | IL-6 | 1–1000 pg/mL | 0.9 pg/mL | (n = 7) CV of concentration < 7% Errors < 14% correlation with singleplex ELISA R = 0.9994 | 30 min | portable fluorescence reader (PorFloR) | orthopedic implant-associated infections | √ |
| LFA (Milenia Biotec GmbH, Germany) | 100 L of serum, plasma, cell culture supernatant, amniotic fluid | IL-6 | 10–10,000 pg/mL | 10 pg/mL | – | 20 min | densitometry (PicoScan) reader & chip reading card | – | √ |
| LFA (hIL-8 XpressCard) | 80 L of biological fluids | IL-8 | 0.7–140 ng/mL | 0.7 ng/mL | – | 5 min | Naked eye | primary & secondary infections | √ |
| Questionnaire | Score Range | #Items | Major Health Factors |
|---|---|---|---|
| Immunodeficiency disease questionnaire (ISAQ) (Peter et al., 2014) [17] | from 20 to 85 | 17 | infections, immune diseases, sport activities, vaccination, life style, surgically removed immune organs radiation exposure, use of antibiotics, and immunosuppressants or immunostimulants |
| Immune Status Questionnaire (ISQ) (Versprille et al., 2019) [18] | from 7 to 35 | 7 | sudden high fever, diarrhea, headache, skin problems, muscle and joint pain, and common cold and coughing |
| from 0 to 10 | 1-item question | perceived immune functioning | |
| from 0 to 10 | 1-item question | perceived general health | |
| General health questionnaire (GHQ) (Goldberg et al., 1979) [19] | from 0 to 28 | 28 | somatic symptoms, anxiety, insomnia, social dysfunction, and severe depression |
| Immune functioning assessment questionnaire (IFQ) (Reed et al., 2015) [20] | from 0 to 79 | 19 | common cold, influenza, cold sores pneumonia, sepsis, and skin infections |
| Questionnaire | Question | Score Range | Found to be Strongly Correlated with |
|---|---|---|---|
| Self-rated health questionnaire (SRH)(Eriksson et al., 2001) [21] | “How would you rate your health status?” | from 1 to 5 | mental health, chronic diseases, |
| “How would you rate your health status?” | from 1 to 7 | health care visits, chronic functional limitations, | |
| “How would you rate your health status compared to that of others of your own age?” | from 1 to 5 | lifestyle factors, psycho-social factors, and quality of life. | |
| Self-rated health questionnaire (SRH) (Cislaghi et al., 2019) [22] | “How is your health in general?” | from 1 to 5 | chronic health conditions, chronic functional limitations, 28 diagnosed health conditions, demand of hospitalization, consultation of medical, or surgical specialist and medicine use |
| Self-rated health questionnaire (SRH) (Meng et al., 2014) [23] | “Which can best represent your health today?” | from 0 to 100 | diagnosed diseases, physical functional status, two-weeks status, mental health status, and the need for hospitalization |
| Perceived immune functioning and health questionnaire (Lantman et al., 2017) [24] | – | from 0 to 10 | mental resilience BRS and IFQ |
| Questionnaire | Major Health Factors | Found to be Linked To | |
|---|---|---|---|
| Cytokines | Immunoassays | ||
| Immunodeficiency disease questionnaire (ISAQ) (Peter et al., 2014) [17] | infections | bacterial infections: IL-2, IL-6, TNF- [72] HIV: IL-2, (IFN)-, IL-4, IL-10, IL-6, IL-8, TNF- [73] common cold: IL-1 [74], MCP-1 [75] SARS-CoV-2: IL-3 [76] | {IL-6}: [32,34,36] and LFA (Milenia Biotec) {IL-6, IL-1, IL-12p70, TNF-}: [30] {IL-3}: [35] |
| immune diseases | RA, SLE, Psoriasis: IL-2, IL-4, IL-8, MCP-1 [77] Diabetes: IL-6, IL-18, TNF- [78] Systemic Sclerosis: PF-4 [79] Crohn’s disease: IL-12p70 [80] | {IL-8}: LFA (hIL-8 XpressCard) {IL-1}: [29] {PF-4, IL-6}: [32] {MCP-1}: [27] {IL-2, IL-4, IL-6, TNF-, IFN-}: [26] | |
| sport activities | IL-6, TNF-, IFN-, IL-2 [81] | ||
| Immune Status Questionnaire (ISQ) (Versprille et al., 2019) [18] | sudden high fever | IL-6, IL-1 [82] | |
| diarrhea | IL-6, TNF- [83] | {IL-4, IL-6, IL-10, TNF- | |
| headache | IL-1 [84] | IFN}: [26] | |
| skin problems | AD, Psoriasis: IFN [85,86] IL-4 [87] IL-1 [88] | {IL-6, MCP-1, TNF-, TGF-}: [27] {IL-1}: [29] {IL-6}: [32,34,36], LFA (Milenia Biotec) | |
| muscle and joint pain | OA: IL-6, IL-10 [89] RA:TNF-, IL-6, TGF- [90] | {IL-8}: LFA (hIL-8 XpressCard) {IL-6, IL-1, TNF- }: [30] | |
| common cold and coughing | IL-1, IL-6, IL-8, TNF- [74] MCP-1 [75] | ||
| General health questionnaire (GHQ) (Goldberg et al., 1979) [19] | somatic symptoms | TNF-, IFN- [91] | {IL-2, IL-4, IL-6, IL-10, TNF-, IFN-}: [26] |
| anxiety | IL-6, TNF-, IL-10 [92] | {IL-6, TNF-, TGF-}: [27] | |
| insomnia | IL-2, IL-6, IL-8, TGF-, IL-4 [93] | {IL-8}: LFA (hIL-8 XpressCard) | |
| social dysfunction, and severe depression | IL-6, IL-10, TNF-, IFN- [94] | {IL-6}: [32,34,36], LFA (Milenia Biotec) {IL-6, TNF-}: [30] | |
| Immune functioning questionnaire (IFQ) (Reed et al., 2015) [20] | cold, influenza, and cold sores | IL-1, IL-6, TNF- [74], MCP-1 [75] | {IL-4, IL-6, IL-8, IL-10, TNF-, IFN-}: [26] |
| pneumonia | IL-6, IL-8 and IFN-, IL-10 [95] | {IL-1}: [29] | |
| sepsis | IL-1, IL-6, IL-7, IL-8, IL-10, IFN-, MCP-1 and TNF- [96] IL-3 [35] | {IL-6, IL-1, TNF-}: [30] {IL-6, MCP-1, TNF-}: [27] {IL-3}: [35] | |
| skin infections | IFN- [85,86] IL-4 [87] IL-1 [88] | {IL-8}: LFA (hIL-8 XpressCard) {IL-6}:[32,34,36] LFA (Milenia Biotec) | |
| Questionnaire | Major Health Factors of the | Found to be Linked To | |
|---|---|---|---|
| Validation Questionnaire | Cytokines | Immunoassays | |
| Diabetes: IL-6, TNF- [78] | {IL-2, IL-4, IL-6, IL-10, TNF-, | ||
| chronic diseases | Crohn’s disease: IL-12p70 [80] | IFN-}: [26] | |
| Self-rated health (SRH) | RA, SLE, Psoriasis: IL-2, IL-4, IL-8, | {IL-6}: [32,34,36], LFA (Milenia Biotec) | |
| (Eriksson et al., 2001) [21] | MCP-1 [77] | {IL-8}: LFA (hIL-8 XpressCard) | |
| psycho-social factors | Depression: IL-6, IL-10, TNF-, IFN- [94] | {IL-6, IL-12p70, TNF-}: [30] | |
| Anxiety: IL-6, TNF-, IL-10 [92] | {IL-6, MCP-1, TNF-}: [27] | ||
| Diabetes: IL-6, TNF- [78] | |||
| chronic health conditions | IL-1 [97] | ||
| RA, SLE, Psoriasis: IL-2, IL-4, IL-8, | |||
| MCP-1 [77] | |||
| chronic functional | OA: IL-6, IL-10 [89] | {IL-2, IL-4, IL-6, IL-10, TNF-, | |
| limitations | RA: TNF-, IL-6, TGF- [90] | IFN-}: [26] | |
| Asthma: IL-4 [98] | {IL-6}: [32,34,36], LFA (Milenia Biotec) | ||
| Hypertension: IL-6, IL-8, TGF-, | {IL-8}: LFA (hIL-8 XpressCard) | ||
| Self-rated health (SRH) | TNF- [99] | {IL-6, IL-1, TNF-}: [30] | |
| (Cislaghi et al., 2019) [22] | HPV: L-1, IL-2, IL-4, IL-8, IL-10, TNF-, | {IL-6, MCP-1, TNF-, TGF-}: [27] | |
| TGF- [100] | {IL-1}: [29] | ||
| 28 diagnosed health | Parkinson: TNF-, IL-1, IL-2, IL-4, IL-6, | ||
| conditions | TGF- [101] | ||
| Lumbar disc: IFN- [102] | |||
| chronic bronchitis: TNF-, IL-1, MCP-1, IL-6, IL-8, TGF- [103] | |||
| Tumor: IL-1, IL-4 and IL-6, IL-2 [104] | |||
| Alzheimer: TNF-, TGF-, IFN- [105] | |||
| Angina: IL-10 [106] | |||
| cirrhosis of liver: TGF-, IL-1, IL-10, | |||
| TNF-, IFN- [107] | |||
| Diabetes: IL-6, TNF- [78] | |||
| acute coronary syndromes: PF-4 [108] | {IL-2, IL-4, IL-6 | ||
| chronic diseases | RA, SLE, Psoriasis: IL-8, MCP-1 [77] | IL-10, TNF-, IFN-}: [26] | |
| Self-rated health (SRH) | HIV: IL-2, IFN-, IL-4, IL-10 | {IL-6}: [30,34,36], LFA (Milenia Biotec) | |
| (Meng et al., 2014) [23] | IL-6, IL-8, TNF- [73] | {IL-8}: LFA (hIL-8 XpressCard) | |
| physical functional status | OA: IL-6, IL-10 [89] | {IL-6, MCP-1, TNF-, TGF-}: [27] | |
| RA: TNF- and IL-6, TGF- [90] | {IL-6, PF-4}: [32] | ||
| mental health status | Schizophrenia: IL-2, IL-6, IL-8, | ||
| IL-10 [109,110] | |||
| Perceived immune | mental resilience | Schizophrenia: IL-2, IL-6, | {IL-2, IL-6, IL-10}: [26] |
| functioning and health | IL-8, IL-10 [109,110] | {IL-8}: LFA (hIL-8 XpressCard) | |
| (Lantman et al., 2017) [24] | {IL-6 }: [27,30,32,34,36], | ||
| LFA (Milenia Biotec) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jamaludeen, N.; Beyer, C.; Billing, U.; Vogel, K.; Brunner-Weinzierl, M.; Spiliopoulou, M. Potential of Point-of-Care and At-Home Assessment of Immune Status via Rapid Cytokine Detection and Questionnaire-Based Anamnesis. Sensors 2021, 21, 4960. https://doi.org/10.3390/s21154960
Jamaludeen N, Beyer C, Billing U, Vogel K, Brunner-Weinzierl M, Spiliopoulou M. Potential of Point-of-Care and At-Home Assessment of Immune Status via Rapid Cytokine Detection and Questionnaire-Based Anamnesis. Sensors. 2021; 21(15):4960. https://doi.org/10.3390/s21154960
Chicago/Turabian StyleJamaludeen, Noor, Christian Beyer, Ulrike Billing, Katrin Vogel, Monika Brunner-Weinzierl, and Myra Spiliopoulou. 2021. "Potential of Point-of-Care and At-Home Assessment of Immune Status via Rapid Cytokine Detection and Questionnaire-Based Anamnesis" Sensors 21, no. 15: 4960. https://doi.org/10.3390/s21154960
APA StyleJamaludeen, N., Beyer, C., Billing, U., Vogel, K., Brunner-Weinzierl, M., & Spiliopoulou, M. (2021). Potential of Point-of-Care and At-Home Assessment of Immune Status via Rapid Cytokine Detection and Questionnaire-Based Anamnesis. Sensors, 21(15), 4960. https://doi.org/10.3390/s21154960






