Biochemical Methanol Gas Sensor (MeOH Bio-Sniffer) for Non-Invasive Assessment of Intestinal Flora from Breath Methanol
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Reagents
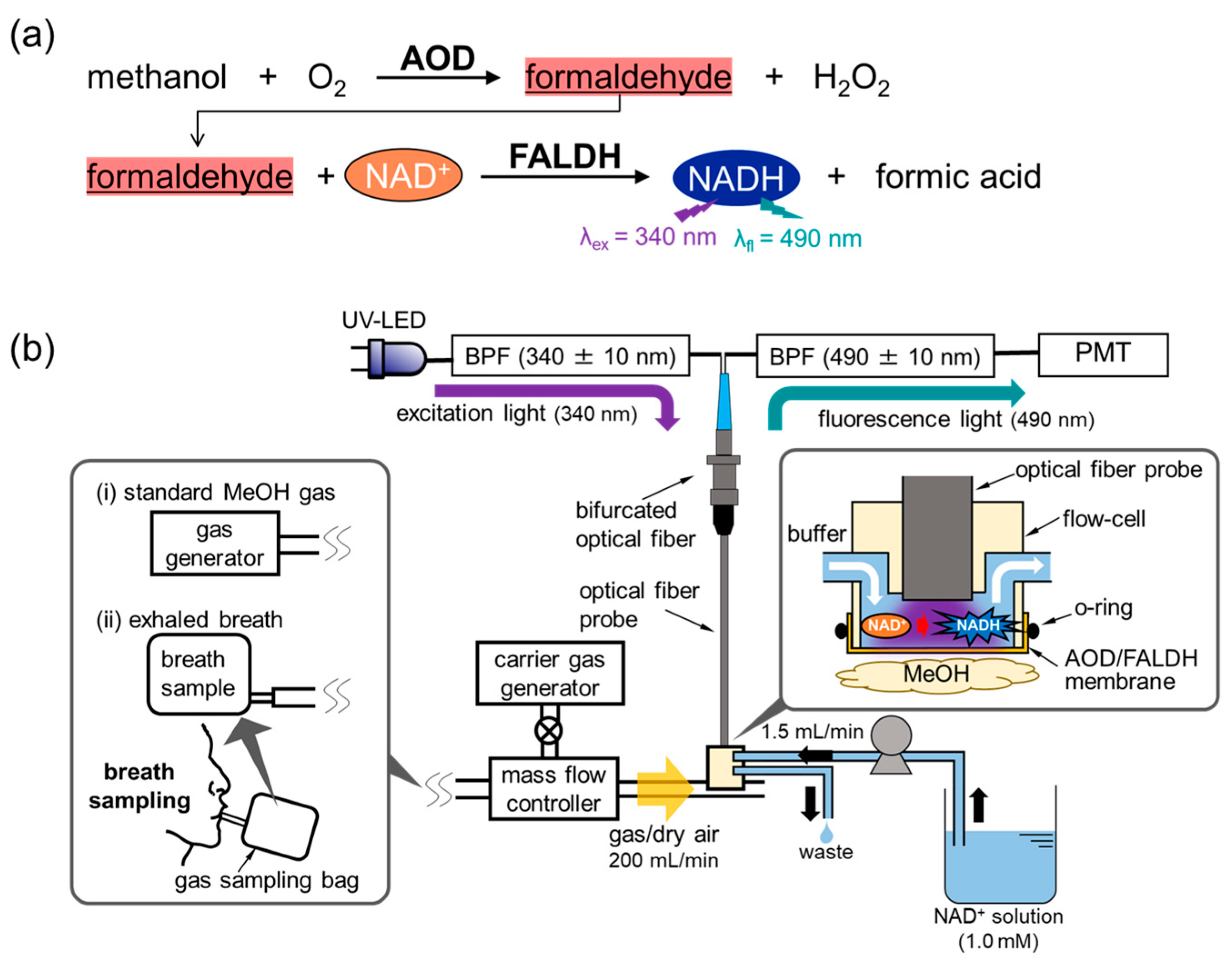
2.2. Construction of MeOH Bio-Sniffer
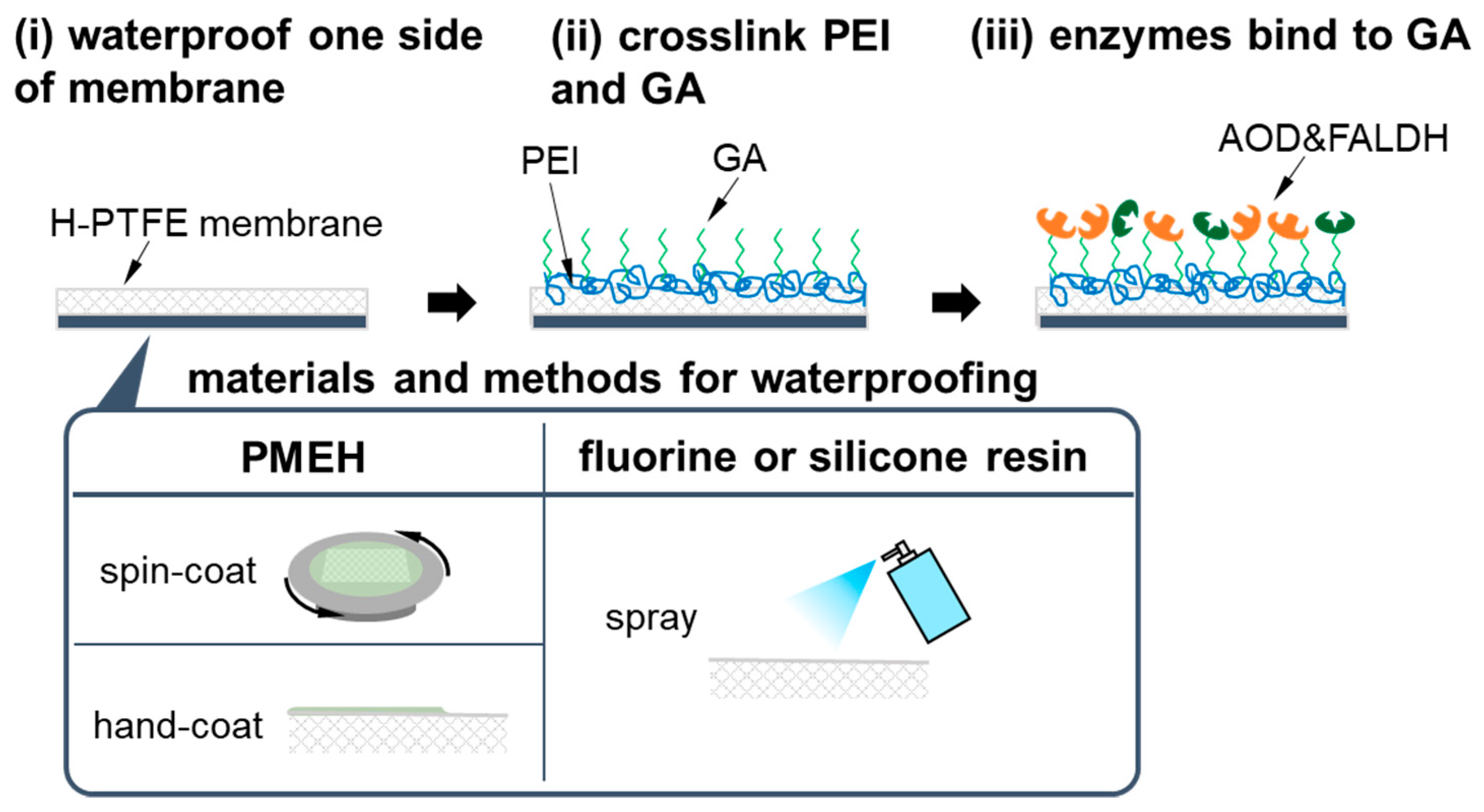
2.3. Preparation of AOD-FALDH Membrane
2.4. Measurement of Standard MeOH Vapor and Exhaled Breath MeOH
3. Results and Discussion
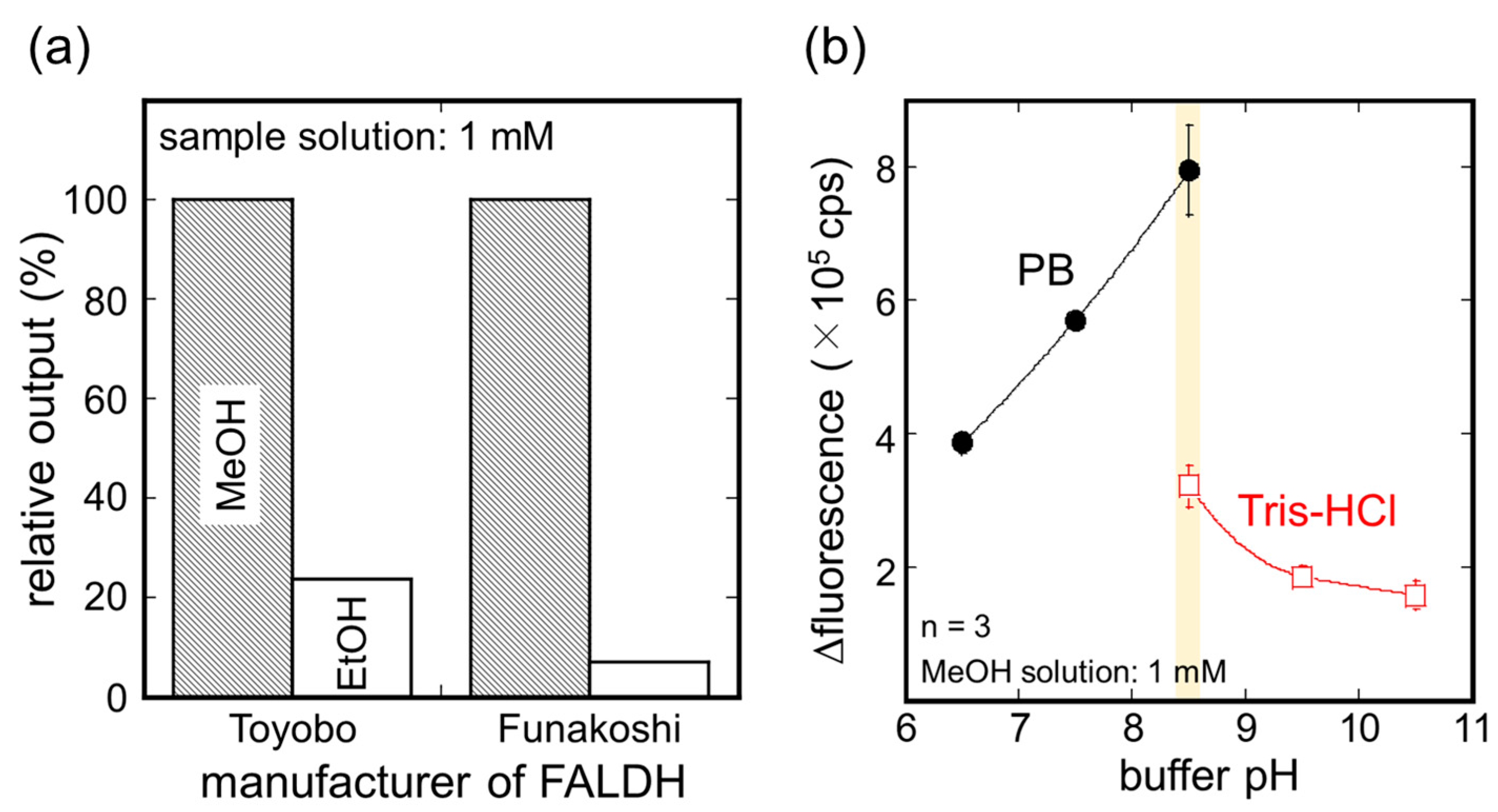
3.1. Optimization of AOD-FALDH Membrane
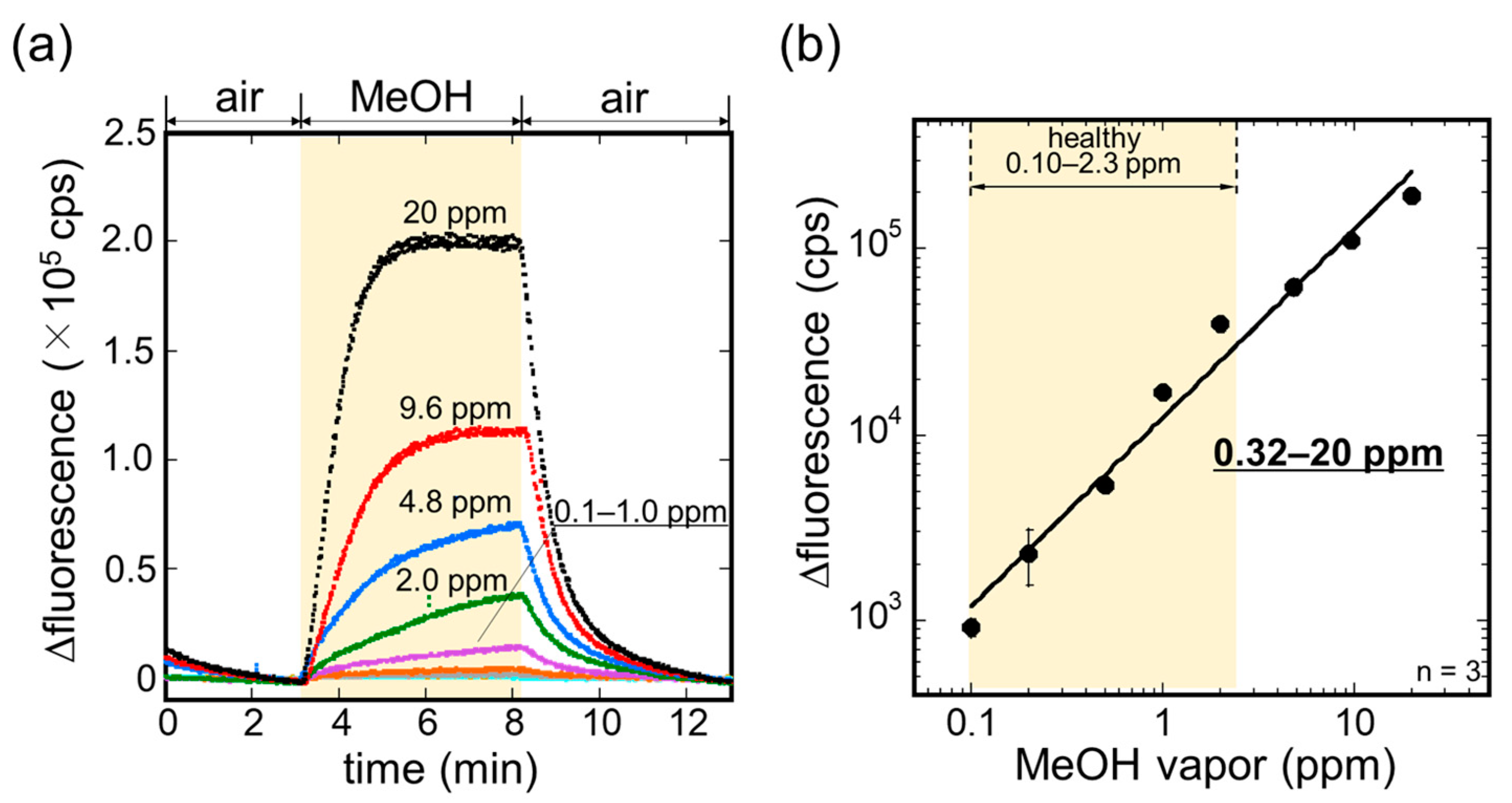
3.2. Sensitivity to MeOH Vapor
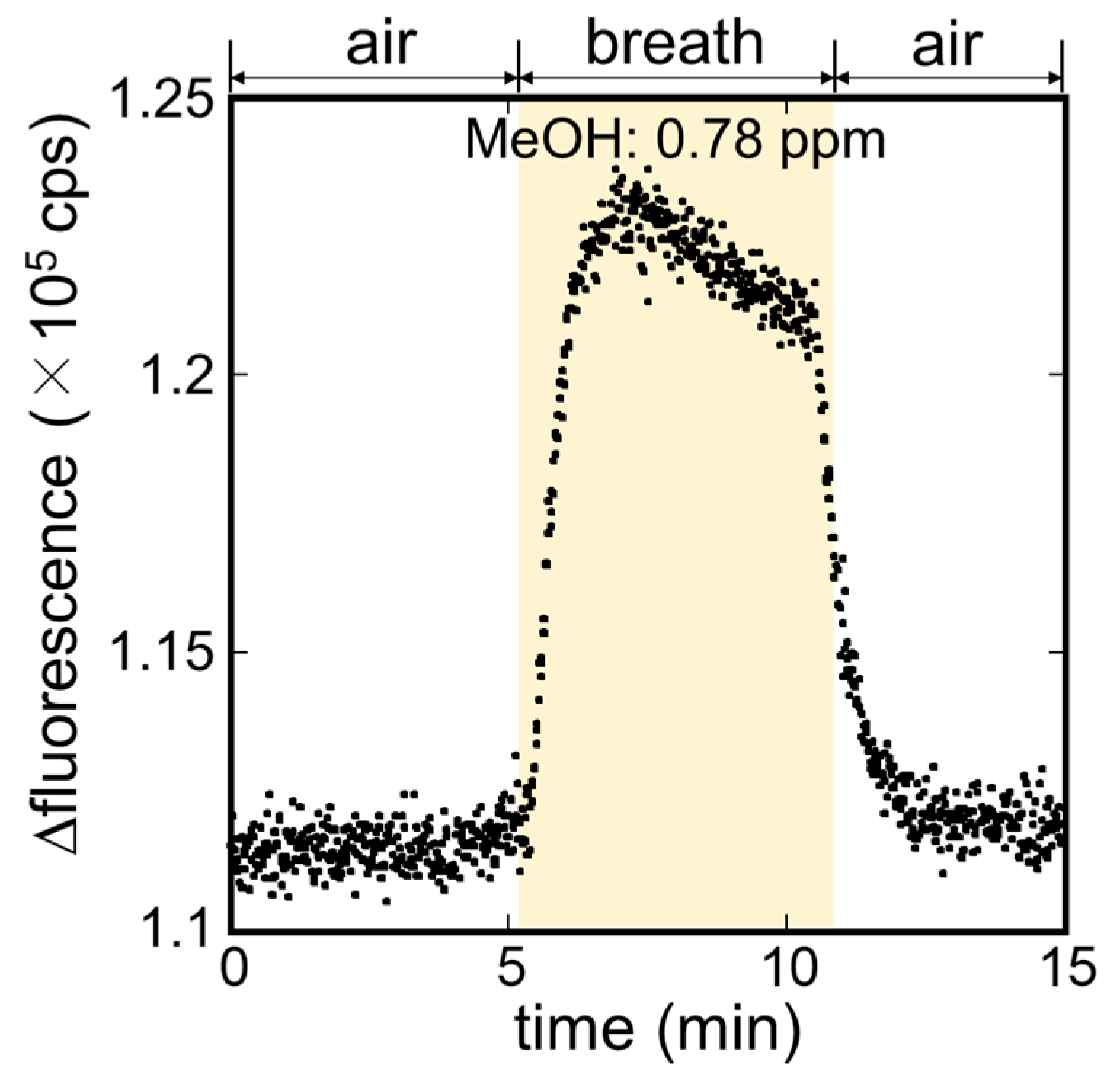
3.3. Measurement of MeOH in Exhaled Breath
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Larsen, N.; Vogensen, F.K.; Van Den Berg, F.W.J.; Nielsen, D.S.; Andreasen, A.S.; Pedersen, B.K.; Al-Soud, W.A.; Sørensen, S.J.; Hansen, L.H.; Jakobsen, M. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS ONE 2010, 5, e9085. [Google Scholar] [CrossRef] [PubMed]
- Ravcheev, D.A.; Godzik, A.; Osterman, A.L.; Rodionov, D.A. Polysaccharides utilization in human gut bacterium Bacteroides thetaiotaomicron: Comparative genomics reconstruction of metabolic and regulatory networks. BMC Genom. 2013, 14, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Ley, R.E.; Peterson, D.A.; Gordon, J.I. Ecological and Evolutionary Forces Shaping Microbial Diversity in the Human Intestine. Cell 2006, 124, 837–848. [Google Scholar] [CrossRef]
- Savage, D.C. Microbial Ecology of the Gastrointestinal Tract. Annu. Rev. Microbiol. 1977, 31, 107–133. [Google Scholar] [CrossRef] [PubMed]
- Whitman, W.B.; Coleman, D.C.; Wiebe, W.J. Prokaryotes: The unseen majority. Proc. Natl. Acad. Sci. USA 1998, 95, 6578–6583. [Google Scholar] [CrossRef]
- Sekirov, I.; Russell, S.L.; Antunes, L.C.M.; Finlay, B.B. Gut Microbiota in Health and Disease Gut Microbiota in Health and Disease. Physiol. Rev. 2016, 859–904. [Google Scholar] [CrossRef]
- Hooper, L.V.; Stappenbeck, T.S.; Hong, C.V.; Gordon, J.I. Angiogenins: A new class of microbicidal proteins involved in innate immunity. Nat. Immunol. 2003, 4, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Achilefu, A.; Joshi, K.; Meier, M.; Mccarthy, L.H.; Medicine, F.; Program, R.; City, O. The influence of gut microbiota on drug metabolism and toxicity. Expert Opin. Drug Metab. Toxicol. 2017, 110, 14–16. [Google Scholar] [CrossRef]
- Turnbaugh, P.J.; Ley, R.E.; Mahowald, M.A.; Magrini, V.; Mardis, E.R.; Gordon, J.I. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature 2006, 444, 1027–1031. [Google Scholar] [CrossRef]
- Drasar, B.S. Cultivation of anaerobic intestinal bacteria. Pathology 1967, 94, 417–427. [Google Scholar] [CrossRef]
- Mata, L.J.; Carrillo, C.; Villatoro, E. Fecal Microflora in Healthy Persons in a Preindustrial Region. Appl. Microbiol. 1969, 17, 596–602. [Google Scholar] [CrossRef] [PubMed]
- Ignyś, I.; Szachta, P.; Gałęcka, M.; Schmidt, M.; Pazgrat-Patan, M.; Pazgrat-Patan, M. Methods of analysis of gut microorganism—Actual state of knowledge. Ann. Agric. Environ. Med. 2014, 21, 799–803. [Google Scholar] [CrossRef]
- Bäckhed, F.; Ley, R.E.; Sonnenburg, J.L.; Peterson, D.A.; Gordon, J.I. Host-bacterial mutualism in the human intestine. Science 2005, 307, 1915–1920. [Google Scholar] [CrossRef]
- Abubucker, S.; Segata, N.; Goll, J.; Schubert, A.M.; Izard, J.; Cantarel, B.L.; Rodriguez-Mueller, B.; Zucker, J.; Thiagarajan, M.; Henrissat, B.; et al. Metabolic reconstruction for metagenomic data and its application to the human microbiome. PLoS Comput. Biol. 2012, 8. [Google Scholar] [CrossRef]
- Dorokhov, Y.L.; Shindyapina, A.V.; Sheshukova, E.V.; Komarova, T.V. Metabolic Methanol: Molecular Pathways and Physiological Roles. Physiol. Rev. 2015, 95, 603–644. [Google Scholar] [CrossRef] [PubMed]
- Martens, E.C.; Lowe, E.C.; Chiang, H.; Pudlo, N.A.; Wu, M.; McNulty, N.P.; Abbott, D.W.; Henrissat, B.; Gilbert, H.J.; Bolam, D.N.; et al. Recognition and degradation of plant cell wall polysaccharides by two human gut symbionts. PLoS Biol. 2011, 9. [Google Scholar] [CrossRef]
- Siragusa, R.J.; Cerda, J.J.; Baig, M.M.; Burgin, C.W.; Robbins, F.L. Methanol production from the degradation of pectin by human colonic bacteria. Am. J. Clin. Nutr. 1988, 47, 848–851. [Google Scholar] [CrossRef] [PubMed]
- Laakso, O.; Haapala, M.; Jaakkola, P.; Laaksonen, R.; Luomanmäki, K.; Nieminen, J.; Pettersson, M.; Päivä, H.; Räsänen, M.; Himberg, J.J. FT-IR breath test in the diagnosis and control of treatment of methanol intoxications. J. Anal. Toxicol. 2001, 25, 26–30. [Google Scholar] [CrossRef][Green Version]
- Dorokhov, Y.L.; Komarova, T.V.; Petrunia, I.V.; Kosorukov, V.S.; Zinovkin, R.A.; Shindyapina, A.V.; Frolova, O.Y.; Gleba, Y.Y. Methanol may function as a Cross-Kingdom signal. PLoS ONE 2012, 7, e36122. [Google Scholar] [CrossRef] [PubMed]
- Lindinger, W.; Taucher, J.; Jordan, A.; Hansel, A.; Vogel, W. Endogenous production of methanol after the consumption of fruit. Alcohol. Clin. Exp. Res. 1997, 21, 939–943. [Google Scholar] [CrossRef]
- Eriksen, S.P.; Kulkarni, A.B. Methanol in normal human breath. Science 1963, 141, 639–640. [Google Scholar] [CrossRef]
- Turner, C.; Španěl, P.; Smith, D. A longitudinal study of methanol in the exhaled breath of 30 healthy volunteers using selected ion flow tube mass spectrometry, SIFT-MS. Physiol. Meas. 2006, 27, 637–648. [Google Scholar] [CrossRef]
- Španěl, P.; Dryahina, K.; Vicherková, P.; Smith, D. Increase of methanol in exhaled breath quantified by SIFT-MS following aspartame ingestion. J. Breath Res. 2015, 9, 047104. [Google Scholar] [CrossRef] [PubMed]
- He, C.; Liu, L.; Korposh, S.; Correia, R.; Morgan, S.P. Volatile Organic Compound Vapour Measurements Using a Localised Surface Plasmon Resonance Optical Fibre Sensor Decorated with a Metal-Organic Framework. Sensors 2021, 21, 1420. [Google Scholar] [CrossRef] [PubMed]
- Kittle, J.; Fisher, B.; Kunselman, C.; Morey, A.; Abel, A. Vapor Selectivity of a Natural Photonic Crystal to Binary and Tertiary Mixtures Containing Chemical Warfare Agent Simulants. Sensors 2019, 20, 157. [Google Scholar] [CrossRef] [PubMed]
- Furuuchi, N.; Shrestha, R.; Yamashita, Y.; Hirao, T.; Ariga, K.; Shrestha, L. Self-Assembled Fullerene Crystals as Excellent Aromatic Vapor Sensors. Sensors 2019, 19, 267. [Google Scholar] [CrossRef] [PubMed]
- Slobodian, P.; Riha, P.; Olejnik, R.; Matyas, J.; Slobodian, R. Microstrip Resonant Sensor for Differentiation of Components in Vapor Mixtures. Sensors 2021, 21, 298. [Google Scholar] [CrossRef] [PubMed]
- Blank, A.; Guendelman, G.; Linzon, Y. Vapor Sensing with Polymer Coated Straight Optical Fiber Microtapers Based on Index Sensitive Interference Spectroscopy of Surface Stress Birefringence. Sensors 2020, 20, 2675. [Google Scholar] [CrossRef]
- Angulo Barrios, C. Scotch Tape Optical Vapor Sensor for Ethanol–Methanol Mixtures. Sensors 2019, 19, 5381. [Google Scholar] [CrossRef]
- Li, Y.; Deng, D.; Chen, N.; Xing, X.; Xiao, X.; Wang, Y. Enhanced methanol sensing properties of SnO2 microspheres in a composite with Pt nanoparticles. RSC Adv. 2016, 6, 83870–83879. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, Z.; Xue, X.; Chen, S.; Natan, A.; Lv, Y.; Chen, C.; Yang, Y.Y.; Cen, W.; Yang, Y.Y. High-sensitivity and high-selectivity detection of methanol based on La-doped SnO2 sensor. Appl. Phys. A Mater. Sci. Process. 2020, 126. [Google Scholar] [CrossRef]
- Andrés, M.A.; Vijjapu, M.T.; Surya, S.G.; Shekhah, O.; Salama, K.N.; Serre, C.; Eddaoudi, M.; Roubeau, O.; Gascón, I. Methanol and Humidity Capacitive Sensors Based on Thin Films of MOF Nanoparticles. ACS Appl. Mater. Interfaces 2020, 12, 4155–4162. [Google Scholar] [CrossRef]
- van den Broek, J.; Abegg, S.; Pratsinis, S.E.; Güntner, A.T. Highly selective detection of methanol over ethanol by a handheld gas sensor. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Güntner, A.T.; Magro, L.; van den Broek, J.; Pratsinis, S.E. Detecting methanol in hand sanitizers. iScience 2021, 24, 102050. [Google Scholar] [CrossRef]
- Hayasaka, T.; Lin, A.; Copa, V.C.; Lopez, L.P.; Loberternos, R.A.; Ballesteros, L.I.M.; Kubota, Y.; Liu, Y.; Salvador, A.A.; Lin, L. An electronic nose using a single graphene FET and machine learning for water, methanol, and ethanol. Microsyst. Nanoeng. 2020, 6. [Google Scholar] [CrossRef]
- Toma, K.; Iwasaki, K.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. Sensitive and selective methanol biosensor using two-enzyme cascade reaction and fluorometry for non-invasive assessment of intestinal bacteria activity. Biosens. Bioelectron. 2021, 181, 113136. [Google Scholar] [CrossRef] [PubMed]
- Mansour, E.; Vishinkin, R.; Rihet, S.; Saliba, W.; Fish, F.; Sarfati, P.; Haick, H. Measurement of temperature and relative humidity in exhaled breath. Sens. Actuators B Chem. 2020, 304, 127371. [Google Scholar] [CrossRef]
- Chien, P.-J.; Suzuki, T.; Tsujii, M.; Ye, M.; Toma, K.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. Bio-sniffer (gas-phase biosensor) with secondary alcohol dehydrogenase (S-ADH) for determination of isopropanol in exhaled air as a potential volatile biomarker. Biosens. Bioelectron. 2017, 91, 341–346. [Google Scholar] [CrossRef] [PubMed]
- Iitani, K.; Chien, P.-J.; Suzuki, T.; Toma, K.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. Fiber-Optic Bio-sniffer (Biochemical Gas Sensor) Using Reverse Reaction of Alcohol Dehydrogenase for Exhaled Acetaldehyde. ACS Sens. 2018, 3, 425–431. [Google Scholar] [CrossRef]
- Chien, P.-J.J.; Suzuki, T.; Tsujii, M.; Ye, M.; Minami, I.; Toda, K.; Otsuka, H.; Toma, K.; Arakawa, T.; Araki, K.; et al. Biochemical Gas Sensors (Biosniffers) Using Forward and Reverse Reactions of Secondary Alcohol Dehydrogenase for Breath Isopropanol and Acetone as Potential Volatile Biomarkers of Diabetes Mellitus. Anal. Chem. 2017, 89, 12261–12268. [Google Scholar] [CrossRef] [PubMed]
- Toma, K.; Suzuki, S.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. External ears for non-invasive and stable monitoring of volatile organic compounds in human blood. Sci. Rep. 2021, 11, 10415. [Google Scholar] [CrossRef]
- Kudo, H.; Yagi, T.; Chu, M.X.; Saito, H.; Morimoto, N.; Iwasaki, Y.; Akiyoshi, K.; Mitsubayashi, K. Glucose sensor using a phospholipid polymer-based enzyme immobilization method. Anal. Bioanal. Chem. 2008, 391, 1269–1274. [Google Scholar] [CrossRef]
- Toma, K.; Tsujii, M.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. Dual-target gas-phase biosensor (bio-sniffer) for assessment of lipid metabolism from breath acetone and isopropanol. Sens. Actuators B Chem. 2021, 329, 129260. [Google Scholar] [CrossRef]
- Arakawa, T.; Suzuki, T.; Tsujii, M.; Iitani, K.; Chien, P.-J.J.; Ye, M.; Toma, K.; Iwasaki, Y.; Mitsubayashi, K. Real-time monitoring of skin ethanol gas by a high-sensitivity gas phase biosensor (bio-sniffer) for the non-invasive evaluation of volatile blood compounds. Biosens. Bioelectron. 2019, 129, 245–253. [Google Scholar] [CrossRef] [PubMed]
- Hesse, C.; Schulz, F.; Bull, C.T.; Shaffer, B.T.; Yan, Q.; Shapiro, N.; Hassan, K.A.; Varghese, N.; Elbourne, L.D.H.; Paulsen, I.T.; et al. Genome-based evolutionary history of Pseudomonas spp. Environ. Microbiol. 2018, 20, 2142–2159. [Google Scholar] [CrossRef] [PubMed]
- Čáp, P.; Dryahina, K.; Pehal, F.; Španěl, P. Selected ion flow tube mass spectrometry of exhaled breath condensate headspace. Rapid Commun. Mass Spectrom. 2008, 22, 2844–2850. [Google Scholar] [CrossRef]
- Kumar, A.; Wang, C.; Meng, F.Y.; Jiang, C.P.; Yan, G.F.; Zhao, M.; Jing, C.Q.; Wang, L. Ultrafast Detection and Discrimination of Methanol Gas Using a Polyindole-Embedded Substrate Integrated Waveguide Microwave Sensor. ACS Sens. 2020, 5, 3939–3948. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.K.; Azad, P.; Akhtar, M.J.; Kar, K.K. Improved Methanol Detection Using Carbon Nanotube-Coated Carbon Fibers Integrated with a Split-Ring Resonator-Based Microwave Sensor. ACS Appl. Nano Mater. 2018, 1, 4746–4755. [Google Scholar] [CrossRef]
- Han, D.; Song, P.; Zhang, S.; Zhang, H.; Xu, Q.; Wang, Q. Enhanced methanol gas-sensing performance of Ce-doped In2O3 porous nanospheres prepared by hydrothermal method. Sens. Actuators B Chem. 2015, 216, 488–496. [Google Scholar] [CrossRef]
- Meng, D.; Zhang, S.; Thomas, T.; Zhao, R.; Shi, Y.; Qu, F.; Yang, M. Platinum decorated mesoporous titanium nitride for fuel-cell type methanol gas sensor. Sens. Actuators B Chem. 2020, 308, 127713. [Google Scholar] [CrossRef]

| Technology | Material | Dynamic Range | Operating Temp. | Ref. |
|---|---|---|---|---|
| Chemoresistive | Pd-SnO2 nanoparticles | 1–1000 ppm | 350 °C | [33] |
| Substrate integrated waveguide | Polyindole | 5–500 ppm | RT | [47] |
| Split ring resonator | Carbon nanotube coated carbon fiber | 10–300 ppm | RT | [48] |
| Chemoresistive | Ce-doped In2O3 porous nanospheres | 2–1000 ppm | 320 °C | [49] |
| Amperometric | Platinum decorated mesoporous TiN | 10–300 ppm | RT | [50] |
| Biofluorescence | AOD-FALDH | 0.32–20 ppm | RT | This work |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Toma, K.; Iwasaki, K.; Zhang, G.; Iitani, K.; Arakawa, T.; Iwasaki, Y.; Mitsubayashi, K. Biochemical Methanol Gas Sensor (MeOH Bio-Sniffer) for Non-Invasive Assessment of Intestinal Flora from Breath Methanol. Sensors 2021, 21, 4897. https://doi.org/10.3390/s21144897
Toma K, Iwasaki K, Zhang G, Iitani K, Arakawa T, Iwasaki Y, Mitsubayashi K. Biochemical Methanol Gas Sensor (MeOH Bio-Sniffer) for Non-Invasive Assessment of Intestinal Flora from Breath Methanol. Sensors. 2021; 21(14):4897. https://doi.org/10.3390/s21144897
Chicago/Turabian StyleToma, Koji, Kanako Iwasaki, Geng Zhang, Kenta Iitani, Takahiro Arakawa, Yasuhiko Iwasaki, and Kohji Mitsubayashi. 2021. "Biochemical Methanol Gas Sensor (MeOH Bio-Sniffer) for Non-Invasive Assessment of Intestinal Flora from Breath Methanol" Sensors 21, no. 14: 4897. https://doi.org/10.3390/s21144897
APA StyleToma, K., Iwasaki, K., Zhang, G., Iitani, K., Arakawa, T., Iwasaki, Y., & Mitsubayashi, K. (2021). Biochemical Methanol Gas Sensor (MeOH Bio-Sniffer) for Non-Invasive Assessment of Intestinal Flora from Breath Methanol. Sensors, 21(14), 4897. https://doi.org/10.3390/s21144897






