Smartphone-Enabled Quantification of Potassium in Blood Plasma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Stock Solutions
2.2. Sample Preparation for Generating a Calibration Curve
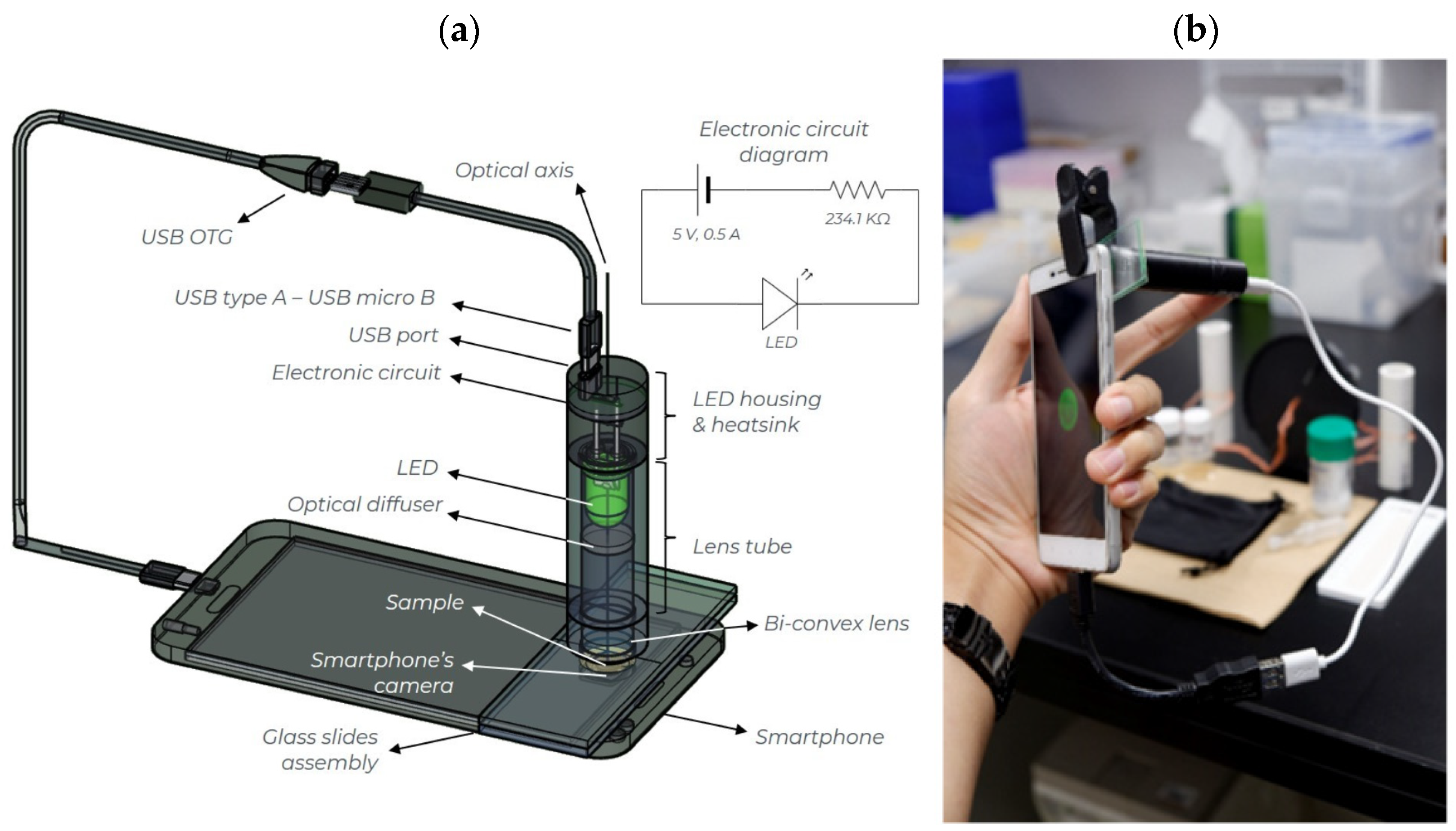
2.3. Smartphone-Based Platform for Turbidity Measurements
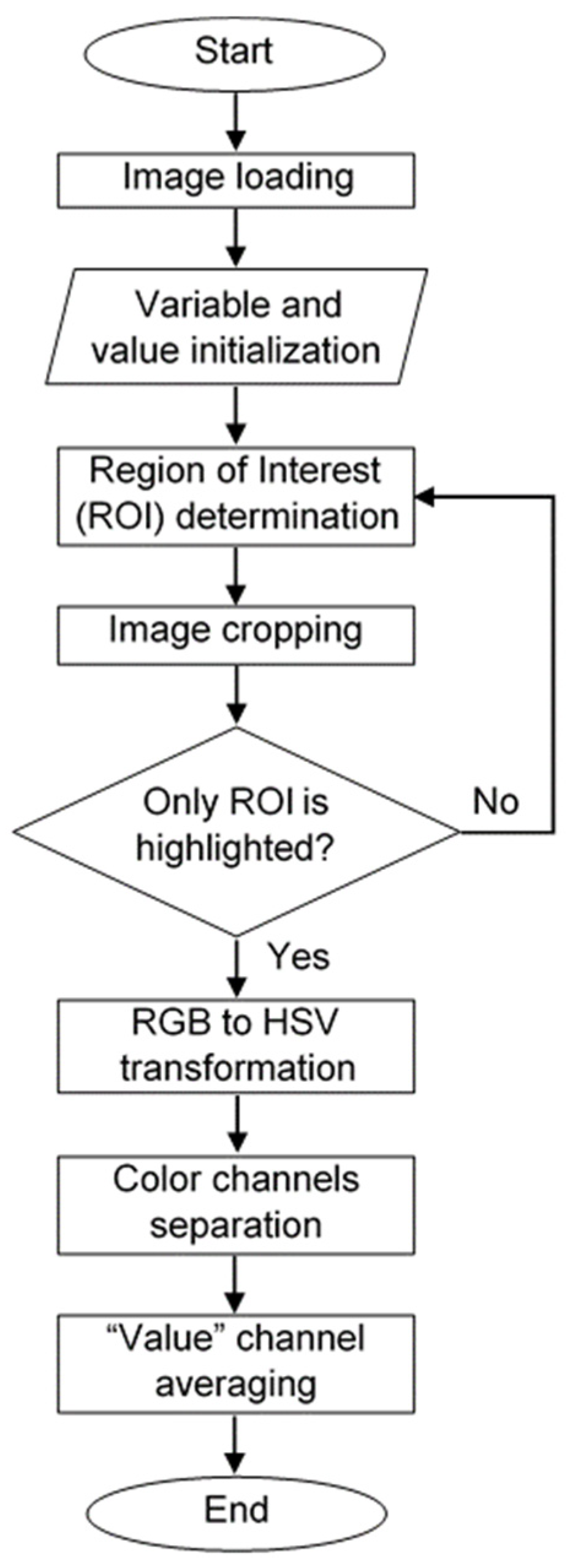
2.4. Image Acquisition and Processing Algorithm
2.5. Details of ISE Device and AAS Instrument
3. Results and Discussions
3.1. Turbidity Measurement Using a UV-VIS Spectrophotometer
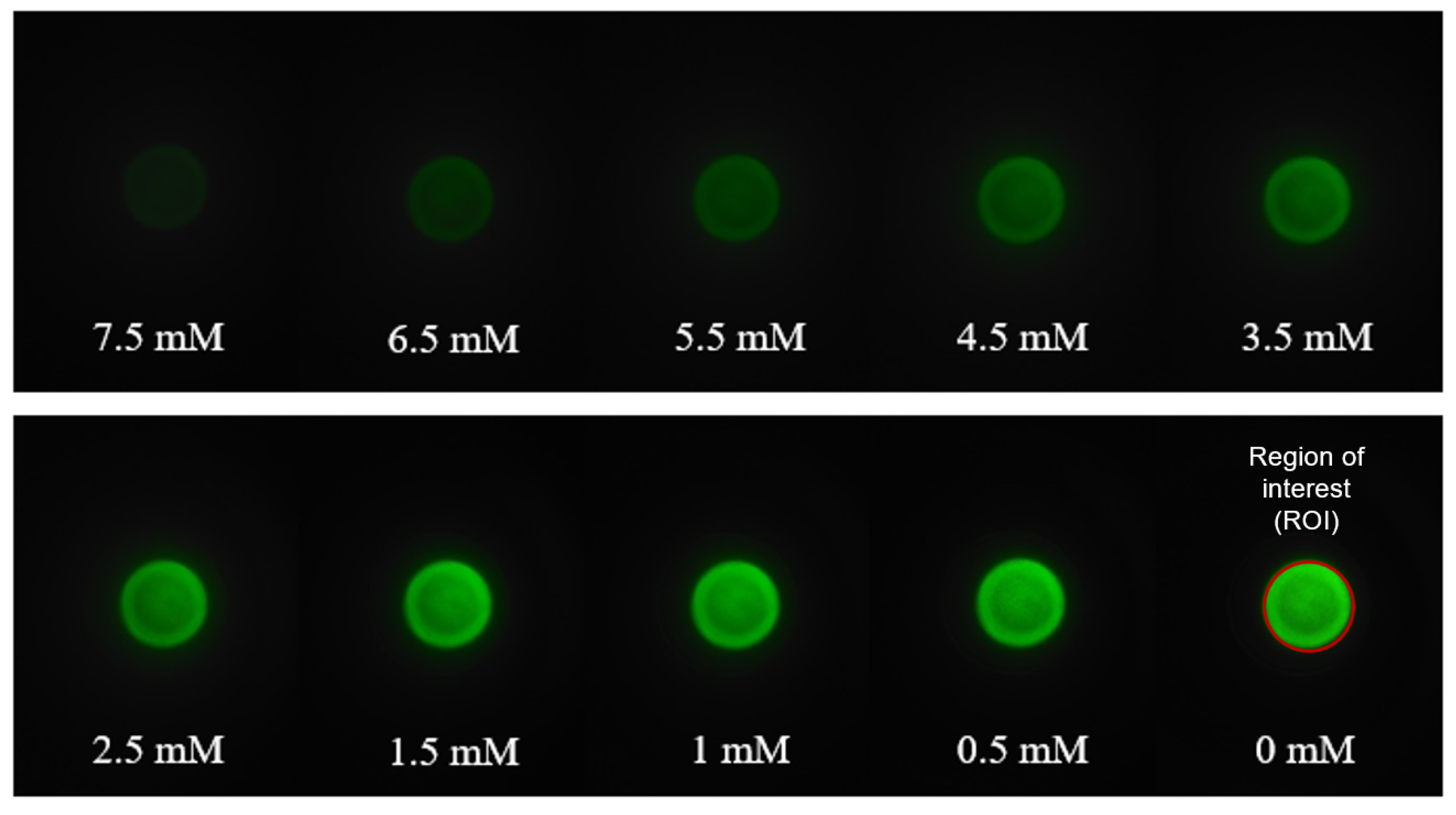
3.2. Turbidity Measurements Using the Smartphone-Based Platform
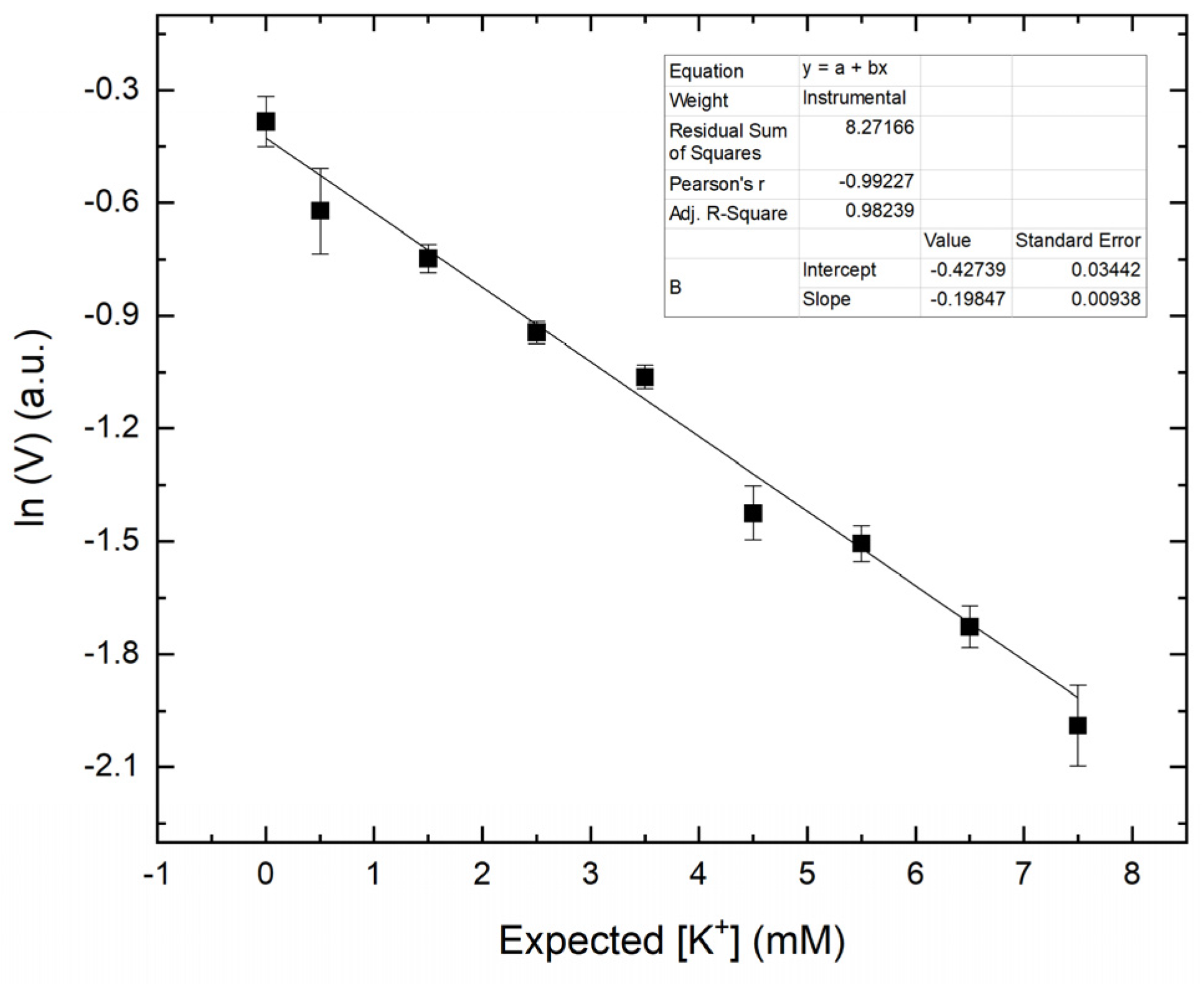
3.3. Generating a Calibration Curve for the Smartphone-Based Method
3.4. Accuracy, Precision, and Selectivity of the Smartphone-Based Method
3.5. Comparison with ISE Device
3.6. Comparison with AAS
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
| Script | Comments |
|---|---|
| close all; | % closing all window |
| clear; | % clearing workspace |
| clc; | % clearing command window |
| I = imread (‘image’s file name’); | % loading image |
| imageSize = size (I); | % initializing image’s size |
| ci = [300,1534,2039]; | % determining region of interest |
| [xx,yy] = ndgrid ((1:imageSize (1))-ci(1), (1:imageSize(2))-ci(2)); | % generating image’s mask |
| mask = uint8((xx.^2 + yy.^2)<ci(3)^2); | |
| croppedImage = uint8(zeros(size(I))); | |
| croppedImage(:,:,1) = I(:,:,1).*mask; | % cropping Red-image with mask |
| croppedImage(:,:,2) = I(:,:,2).*mask; | % cropping Green-image with mask |
| croppedImage(:,:,3) = I(:,:,3).*mask; | % cropping Blue-image with mask |
| imshow(croppedImage); | % generating cropped image |
| hsvcroppedImage = rgb2hsv(croppedImage); | % transforming RGB to HSV image |
| h = hsvcroppedImage(:,:,1); | % Separating Hue-image |
| s = hsvcroppedImage(:,:,2); | % Separating Saturation-image |
| v = hsvcroppedImage(:,:,3); | % Separating Value-image |
| counted=sum(v(:)>0); | % Counting total spatial pixels |
| summedh_h=sum(h); | % Counting horizontal pixel value of Hue-image |
| summedh_s=sum(s); | % Counting horizontal pixel value of Saturation-image |
| summedh_v=sum(v); | % Counting horizontal pixel value of Value-image |
| summedv_h=sum(summedh_h,2); | % Counting vertical pixel value of Hue-image |
| summedv_s=sum(summedh_s,2); | % Counting vertical pixel value of Saturation-image |
| summedv_v=sum(summedh_v,2); | % Counting vertical pixel value of Value-image |
| avg_h=summedv_h/counted; | % Averaging “Hue” value across image |
| avg_s=summedv_s/counted; | % Averaging “Saturation” value across image |
| avg_v=summedv_v/counted; | % Averaging “Value” value across image |
References
- Pohl, H.R.; Wheeler, J.S.; Murray, H.E. Sodium and Potassium in Health and Disease. Met. Ions Life Sci. 2013, 13, 29–47. [Google Scholar] [CrossRef] [PubMed]
- Rastegar, A. Serum Potassium. In Clinical Methods: The History, Physical, and Laboratory Examinations, 3rd ed.; Walker, H.K., Hall, W.D., Hurst, J.W., Eds.; Butterworths: Boston, MA, USA, 1990. [Google Scholar]
- Gumz, M.L.; Rabinowitz, L.; Wingo, C.S. An Integrated View of Potassium Homeostasis. N. Engl. J. Med. 2015, 373, 60–72. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mandal, A.K. Hypokalemia and Hyperkalemia. Med. Clin. N. Am. 1997, 81, 611–639. [Google Scholar] [CrossRef]
- Schwartz, A.B. Potassium-related Cardiac Arrhythmias and Their Treatment. Angiology 1978, 29, 194–205. [Google Scholar] [CrossRef]
- Sica, D.A.; Struthers, A.D.; Cushman, W.C.; Wood, M.; Banas, J.S., Jr.; Epstein, M. Importance of Potassium in Cardiovascular Disease. J. Clin. Hypertens. 2002, 4, 198–206. [Google Scholar] [CrossRef]
- Karnik, J.A.; Young, B.S.; Lew, N.L.; Herget, M.; Dubinsky, C.; Lazarus, J.M.; Chertow, G.M. Cardiac Arrest and Sudden Death in Dialysis Units. Kidney Int. 2001, 60, 350–357. [Google Scholar] [CrossRef] [Green Version]
- Weaver, C.M. Potassium and Health. Adv. Nutr. 2013, 4, 368S–377S. [Google Scholar] [CrossRef]
- Pitt, B.; Rossignol, P. The Association Between Serum Potassium and Mortality in Patients with Hypertension: ‘A wake-up Call’. Eur. Heart J. 2016, 38, 113–115. [Google Scholar] [CrossRef] [Green Version]
- Ben Salem, C.; Badreddine, A.; Fathallah, N.; Slim, R.; Hmouda, H. Drug-induced Hyperkalemia. Drug Saf. 2014, 37, 677–692. [Google Scholar] [CrossRef]
- Sun, H.; Weaver, C.M. Rise in Potassium Deficiency in the US Population Linked to Agriculture Practices and Dietary Potassium Deficits. J. Agric. Food Chem. 2020, 68, 11121–11127. [Google Scholar] [CrossRef]
- Buzanovskii, V.A. Chronology of the Research on Methods for Determining the Potassium Concentration in Human Blood. Rev. J. Chem. 2015, 5, 1–20. [Google Scholar] [CrossRef]
- Breh, F.; Gaebler, O.H. The Determination of Potassium in Blood Serum. J. Biol. Chem. 1930, 87, 81–89. [Google Scholar] [CrossRef]
- Hald, P.M. The Flame Photometer for the Measurement of Sodium and Potassium in Biological Materials. J. Biol. Chem. 1947, 167, 499–510. [Google Scholar] [CrossRef]
- Willis, J.B. The Determination of Metals in Blood Serum by Atomic Absorption Spectroscopy—III: Sodium and Potassium. Spectrochim. Acta 1960, 16, 551–558. [Google Scholar] [CrossRef]
- Pioda, L.A.R.; Stankova, V.; Simon, W. Highly Selective Potassium Ion Responsive Liquid-membrane Electrode. Anal. Lett. 1969, 2, 665–674. [Google Scholar] [CrossRef]
- Annan, W.; Kirwan, N.A.; Robertson, W.S.; Teasdale, P.R.; Ager, B.P. An Evaluation of the NOVA I Ion Selective Electrode Analyser for Sodium and Potassium Determination. J. Autom. Chem. 1980, 2, 212–218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, J.; Hahn, Y.K.; Park, J.-Y.; Seo, H.; Jung, J.; Cho, E.; Choi, Y.-S.; Lee, S.S. An Integrated Ion-selective Optode Sensor Cartridge for Directly Detecting Electrolyte Ions in Blood Plasma Without Pretreatment to Adjust pH. Sens. Actuators B Chem. 2019, 280, 256–262. [Google Scholar] [CrossRef]
- de Oliveira Torres, J.R.; Tubino, M. Turbidimetric Determination of Potassium by Flow Injection Analysis. Anal. Lett. 1994, 27, 1625–1636. [Google Scholar] [CrossRef]
- Xiao, J.; Chen, J.; Ren, F.; Chen, Y.; Xu, M. Highly Sensitive Determination of Trace Potassium Ion in Serum Using the Resonance Light Scattering Technique with Sodium Tetraphenylboron. Microchim. Acta 2007, 159, 287–292. [Google Scholar] [CrossRef]
- Tubino, M.; Souza, R.L.d.; Hoehr, N.F. Rapid Quantitative Turbidimetric Spot Test Analysis of Potassium in Blood Serum. J. Braz. Chem. Soc. 2004, 15, 635–639. [Google Scholar] [CrossRef]
- Hernández-Neuta, I.; Neumann, F.; Brightmeyer, J.; Ba Tis, T.; Madaboosi, N.; Wei, Q.; Ozcan, A.; Nilsson, M. Smartphone-based Clinical Diagnostics: Towards Democratization of Evidence-based Health Care. J. Intern. Med. 2019, 285, 19–39. [Google Scholar] [CrossRef] [Green Version]
- Gamal, A.E. Trends in CMOS Image Sensor Technology and Design. In Proceedings of the Digest. International Electron Devices Meeting, San Francisco, CA, USA, 8–11 December 2002; pp. 805–808. [Google Scholar]
- Bigas, M.; Cabruja, E.; Forest, J.; Salvi, J. Review of CMOS Image Sensors. Microelectron. J. 2006, 37, 433–451. [Google Scholar] [CrossRef] [Green Version]
- Boulos, M.N.K.; Wheeler, S.; Tavares, C.; Jones, R. How Smartphones are Changing the Face of Mobile and Participatory Healthcare: An overview, with example from eCAALYX. BioMed. Eng. OnLine 2011, 10, 24. [Google Scholar] [CrossRef] [Green Version]
- Yetisen, A.K.; Jiang, N.; Tamayol, A.; Ruiz-Esparza, G.U.; Zhang, Y.S.; Medina-Pando, S.; Gupta, A.; Wolffsohn, J.S.; Butt, H.; Khademhosseini, A.; et al. Paper-based Microfluidic System for Tear Electrolyte Analysis. Lab Chip 2017, 17, 1137–1148. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Liu, Q.; Chen, S.; Cheng, F.; Wang, H.; Peng, W. Surface Plasmon Resonance Biosensor Based on Smart phone Platforms. Sci. Rep. 2015, 5, 12864. [Google Scholar] [CrossRef] [Green Version]
- Zangheri, M.; Cevenini, L.; Anfossi, L.; Baggiani, C.; Simoni, P.; Di Nardo, F.; Roda, A. A Simple and Compact Smartphone Accessory for Quantitative Chemiluminescence-based Lateral Flow Immunoassay for Salivary Cortisol Detection. Biosens. Bioelectron. 2015, 64, 63–68. [Google Scholar] [CrossRef]
- Itoh, Y.; Hattori, M.; Wazawa, T.; Arai, Y.; Nagai, T. Ratiometric Bioluminescent Indicator for Simple and Rapid Diagnosis of Bilirubin. ACS Sens. 2021. [Google Scholar] [CrossRef]
- Tomimuro, K.; Tenda, K.; Ni, Y.; Hiruta, Y.; Merkx, M.; Citterio, D. Thread-Based Bioluminescent Sensor for Detecting Multiple Antibodies in a Single Drop of Whole Blood. ACS Sens. 2020, 5, 1786–1794. [Google Scholar] [CrossRef]
- Tian, Y.; Zhang, L.; Wang, H.; Ji, W.; Zhang, Z.; Zhang, Y.; Yang, Z.; Cao, Z.; Zhang, S.; Chang, J. Intelligent Detection Platform for Simultaneous Detection of Multiple MiRNAs Based on Smartphone. ACS Sens. 2019, 4, 1873–1880. [Google Scholar] [CrossRef]
- Ming, K.; Kim, J.; Biondi, M.J.; Syed, A.; Chen, K.; Lam, A.; Ostrowski, M.; Rebbapragada, A.; Feld, J.J.; Chan, W.C.W. Integrated Quantum Dot Barcode Smartphone Optical Device for Wireless Multiplexed Diagnosis of Infected Patients. ACS Nano 2015, 9, 3060–3074. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ghaderinezhad, F.; Ceylan Koydemir, H.; Tseng, D.; Karinca, D.; Liang, K.; Ozcan, A.; Tasoglu, S. Sensing of Electrolytes in Urine using a Miniaturized Paper-based Device. Sci. Rep. 2020, 10, 13620. [Google Scholar] [CrossRef]
- Luo, W.; Deng, J.; He, J.; Han, Z.; Huang, C.; Li, Y.; Fu, Q.; Chen, H. A Smartphone-based Multi-wavelength Photometer for on-site Detection of the Liquid Colorimetric Assays for Clinical Biochemical Analyses. Sens. Actuators B Chem. 2021, 329, 129266. [Google Scholar] [CrossRef]
- Pflaum, R.T.; Howick, L.C. Spectrophotometric Determination of Potassium with Sodium Tetraphenylborate. Anal. Chem. 1956, 28, 1542–1544. [Google Scholar] [CrossRef]
- Kirgintsev, A.N.; Kozitskii, V.P. Solubility of Tetraphenylborates of the Alkali Metals and Ammonium in Acetone-water Mixtures at 25°. Bull. Acad. Sci. USSR Div. Chem. Sci. 1968, 17, 1116–1118. [Google Scholar] [CrossRef]
- Ibraheem, N.; Hasan, M.; Khan, R.Z.; Mishra, P. Understanding Color Models: A Review. ARPN J. Sci. Tech. 2012, 2, 265–275. [Google Scholar]
- Plöntzke, J.; Berg, M.; Olany, A.; Leonhard-Marek, S.; Müller, K.E.; Röblitz, S. Modeling Potassium Balance in Dairy Cows. ZIB Rep. 2013, 3–17. [Google Scholar]
- Fibbioli, M.; Morf, W.E.; Badertscher, M.; de Rooij, N.F.; Pretsch, E. Potential Drifts of Solid-Contacted Ion-Selective Electrodes Due to Zero-Current Ion Fluxes Through the Sensor Membrane. Electroanalysis 2000, 12, 1286–1292. [Google Scholar] [CrossRef]
- Delves, H.T. Atomic Absorption Spectroscopy in Clinical Analysis. Annals Clin. Biochem. 1987, 24, 529–551. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kastelic, M.; Kalyuzhnyi, Y.V.; Hribar-Lee, B.; Dill, K.A.; Vlachy, V. Protein Aggregation in Salt Solutions. Proc. Natl. Acad. Sci. USA 2015, 201507303. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curtis, R.A.; Ulrich, J.; Montaser, A.; Prausnitz, J.M.; Blanch, H.W. Protein–protein Interactions in Concentrated Electrolyte Solutions. Biotech. Bioeng. 2002, 79, 367–380. [Google Scholar] [CrossRef]
- Fernandez-Moure, J.; Maisha, N.; Lavik, E.B.; Cannon, J.W. The Chemistry of Lyophilized Blood Products. Bioconjug Chem. 2018, 29, 2150–2160. [Google Scholar] [CrossRef]
- Bhamla, M.S.; Benson, B.; Chai, C.; Katsikis, G.; Johri, A.; Prakash, M. Hand-powered Ultralow-cost Paper Centrifuge. Nat. Biomed. Eng. 2017, 1, 0009. [Google Scholar] [CrossRef]

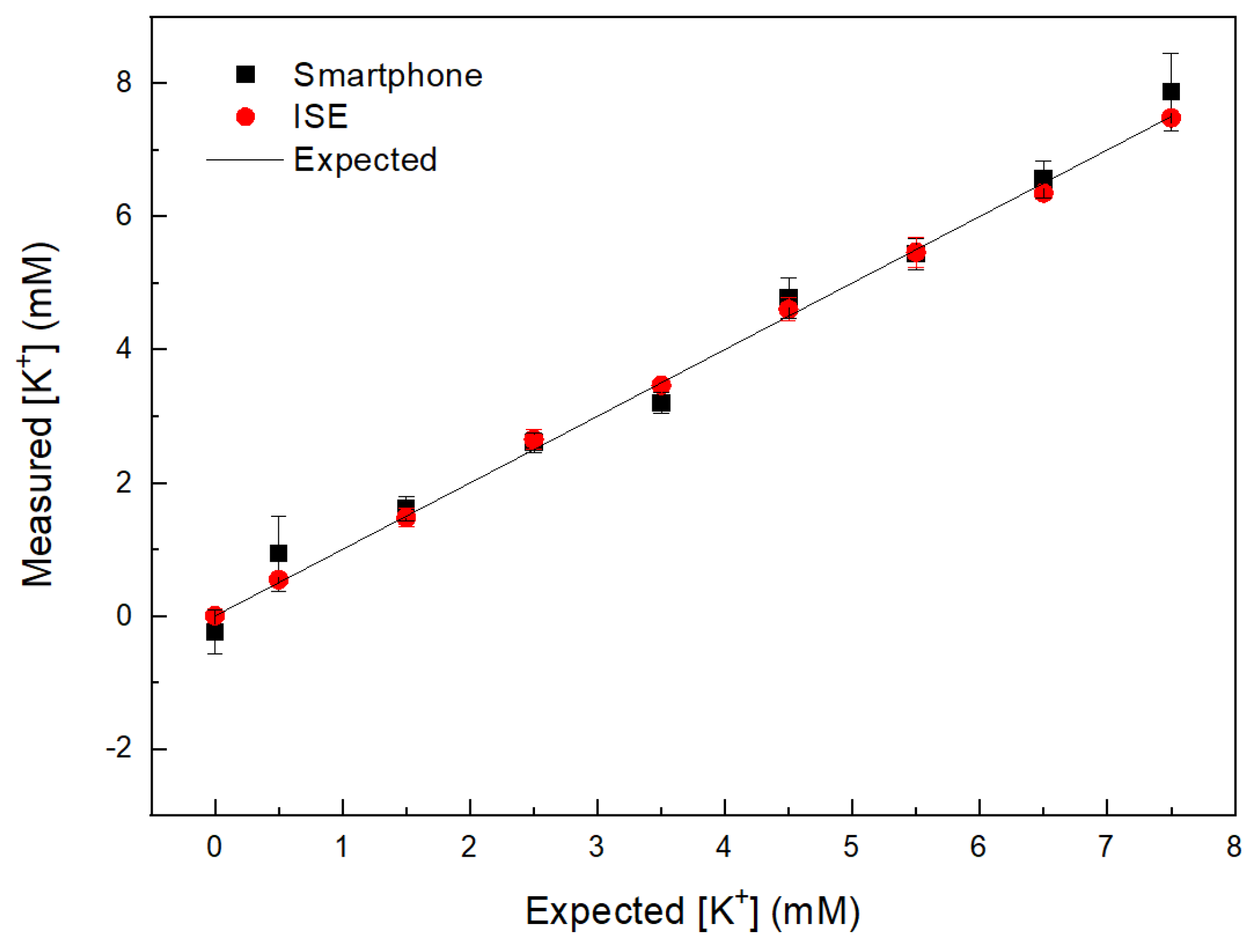

| [K+] (mM) Expected | [K+] (mM) Smartphone | [K+] (mM) ISE |
|---|---|---|
| 0.5 | 0.93 ± 0.56 | 0.54 ± 0.05 |
| 1.5 | 1.60 ± 0.18 | 1.47 ± 0.14 |
| 2.5 | 2.59 ± 0.15 | 2.65 ± 0.15 |
| 3.5 | 3.19 ± 0.16 | 3.46 ± 0.03 |
| 4.5 | 4.70 ± 0.30 | 4.60 ± 0.17 |
| 5.5 | 5.44 ± 0.24 | 5.46 ± 0.22 |
| 6.5 | 6.56 ± 0.27 | 6.35 ± 0.09 |
| 7.5 | 7.86 ± 0.55 | 7.48 ± 0.08 |
| Fresh [K+] plasma | 4.90 ± 0.11 | 5.22 ± 0.09 |
| Sample Number | [K+] (mM) Smartphone | [K+] (mM) AAS |
|---|---|---|
| 1 | 2.21 ± 0.24 | 2.74 ± 0.001 |
| 2 | 2.69 ± 0.42 | 2.59 ± 0.007 |
| 3 | 2.76 ± 0.37 | 2.62 ± 0.005 |
| 4 | 2.94 ± 0.55 | 2.73 ± 0.009 |
| 5 | 2.42 ± 0.35 | 2.73 ± 0.008 |
| 6 | 2.52 ± 0.12 | 2.69 ± 0.007 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hidayat, A.S.; Horino, H.; Rzeznicka, I.I. Smartphone-Enabled Quantification of Potassium in Blood Plasma. Sensors 2021, 21, 4751. https://doi.org/10.3390/s21144751
Hidayat AS, Horino H, Rzeznicka II. Smartphone-Enabled Quantification of Potassium in Blood Plasma. Sensors. 2021; 21(14):4751. https://doi.org/10.3390/s21144751
Chicago/Turabian StyleHidayat, Achmad Syarif, Hideyuki Horino, and Izabela I. Rzeznicka. 2021. "Smartphone-Enabled Quantification of Potassium in Blood Plasma" Sensors 21, no. 14: 4751. https://doi.org/10.3390/s21144751
APA StyleHidayat, A. S., Horino, H., & Rzeznicka, I. I. (2021). Smartphone-Enabled Quantification of Potassium in Blood Plasma. Sensors, 21(14), 4751. https://doi.org/10.3390/s21144751








