Biosensors for European Zoonotic Agents: A Current Portuguese Perspective
Abstract
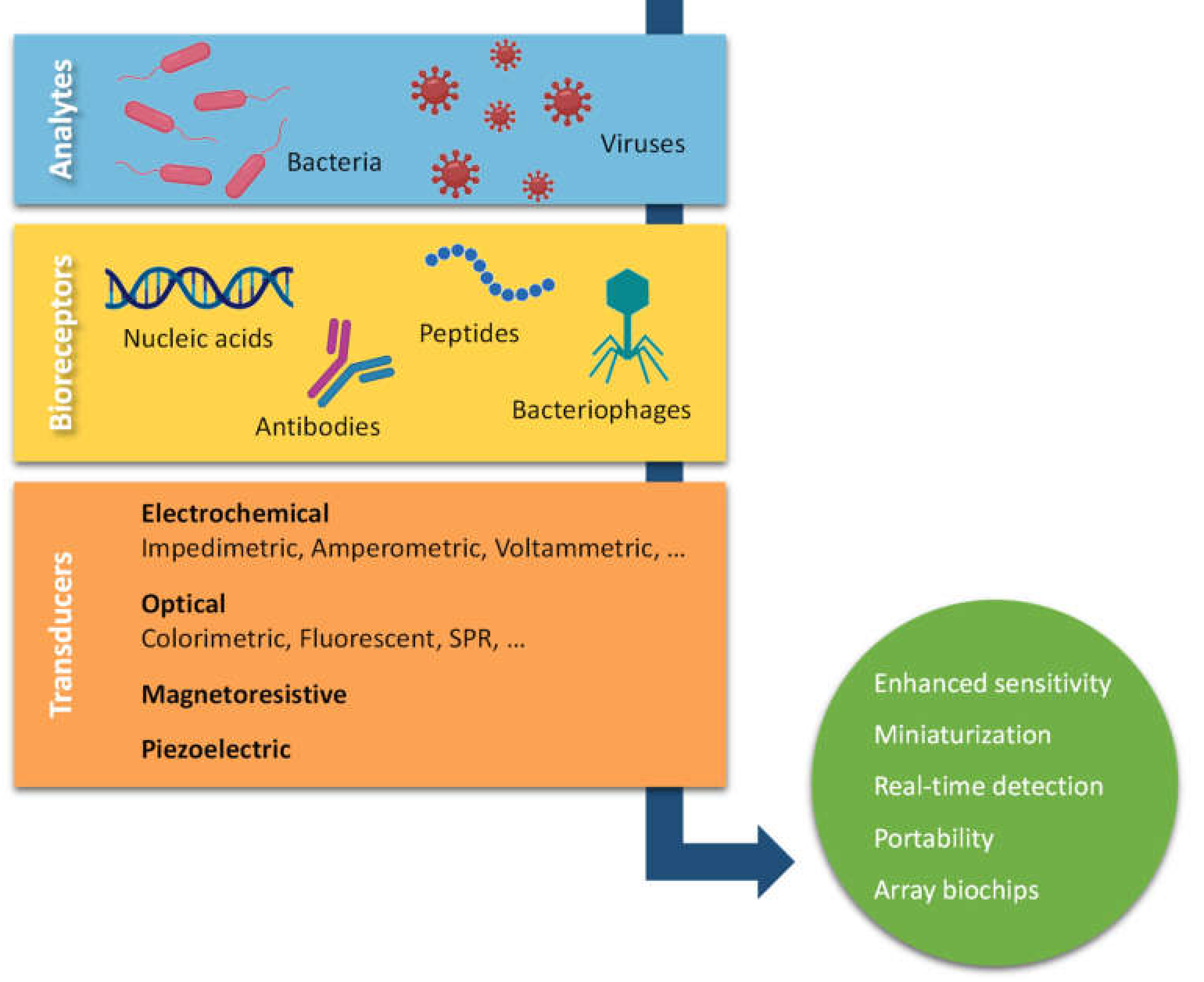
1. Introduction
2. Sensors for Commonly Reported Pathogenic Bacteria
2.1. Salmonella
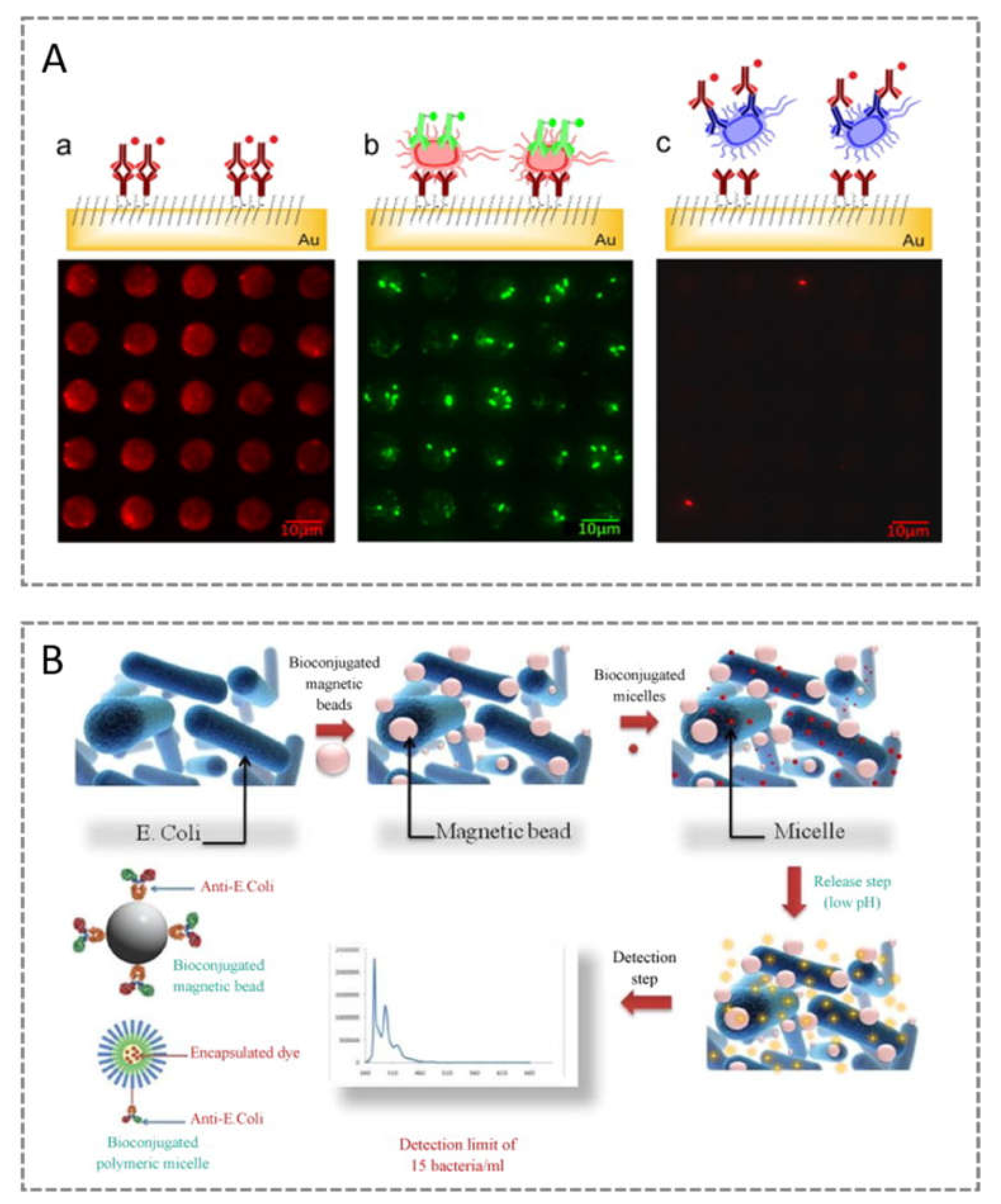
2.2. Escherichia coli
2.3. Listeria Monocytogenes
2.4. Staphylococcus spp.
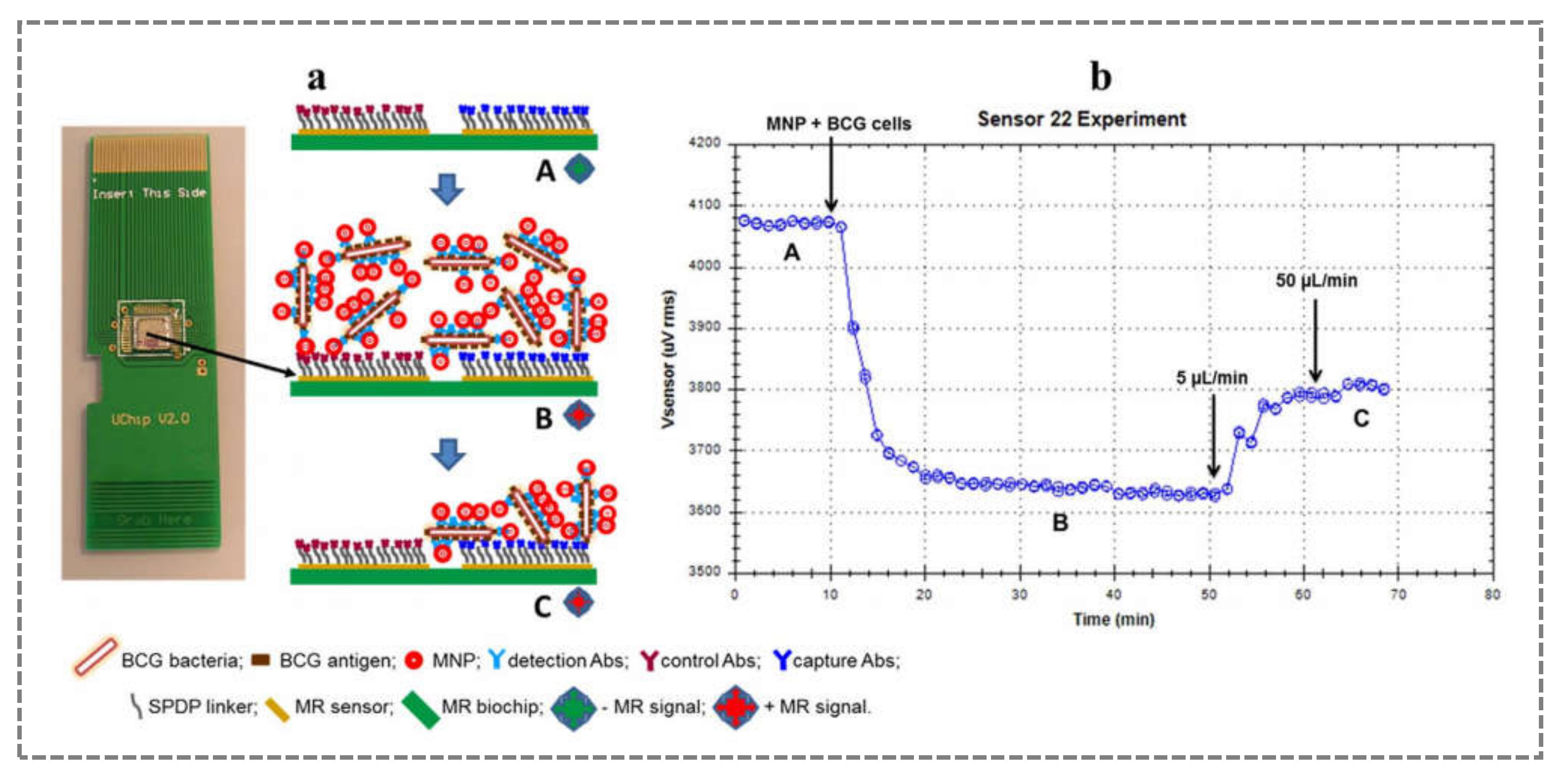
2.5. Mycobacterium spp.
2.6. Other Zoonotic Agents
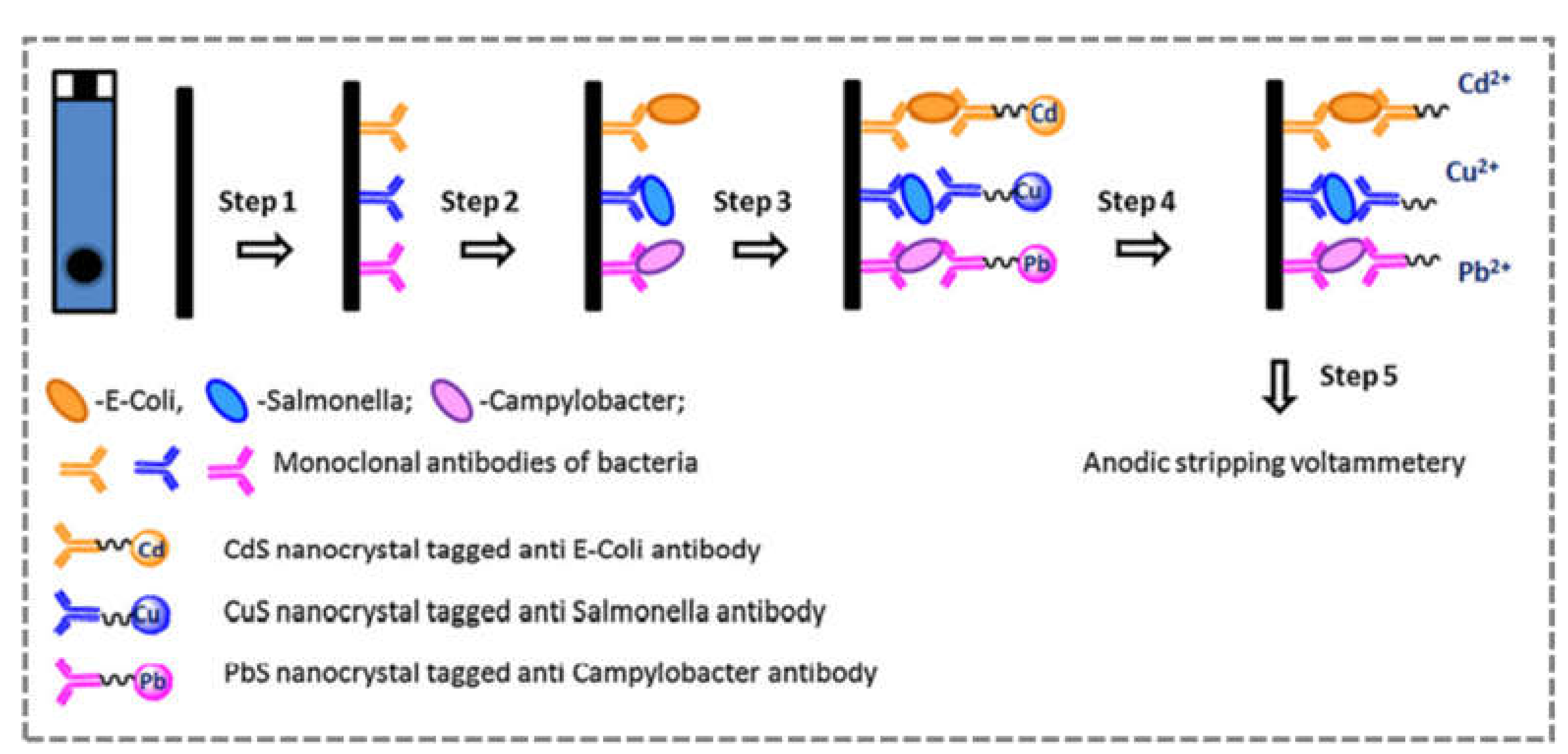
2.7. Multiplex Biosensors
3. Conclusions and Future Perspectives
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dincer, C.; Bruch, R.; Costa-Rama, E.; Fernández-Abedul, M.T.; Merkoçi, A.; Manz, A.; Urban, G.A.; Güder, F. Disposable Sensors in Diagnostics, Food, and Environmental Monitoring. Adv. Mater. 2019, 31, e1806739. [Google Scholar] [CrossRef]
- Clark, L.C., Jr.; Lyons, C. Electrode systems for continuous monitoring in cardiovascular surgery. Ann. N. Y. Acad. Sci. 1962, 102, 29–45. [Google Scholar] [CrossRef]
- Tothill, I.E.; Turner, A.P.F. Biosensors. In Encyclopedia of Food Sciences and Nutrition, 2nd ed.; Caballero, B., Ed.; Academic Press: Oxford, UK, 2003; pp. 489–499. [Google Scholar] [CrossRef]
- Dargaville, T.R.; Farrugia, B.L.; Broadbent, J.A.; Pace, S.; Upton, Z.; Voelcker, N.H. Sensors and imaging for wound healing: A review. Biosens. Bioelectron. 2013, 41, 30–42. [Google Scholar] [CrossRef]
- Dang, Y.T.H.; Gangadoo, S.; Rajapaksha, P.; Truong, V.K.; Cozzolino, D.; Chapman, J. Biosensors in food traceability and quality. In Comprehensive Foodomics; Cifuentes, A., Ed.; Elsevier: Oxford, UK, 2021; pp. 308–321. [Google Scholar] [CrossRef]
- Marko, P.B.; Lee, S.C.; Rice, A.; Gramling, J.M.; Fitzhenry, T.M.; McAlister, J.S.; Harper, G.; Moran, A.L. Mislabelling of a depleted reef fish. Nature 2004, 430, 309–310. [Google Scholar] [CrossRef] [PubMed]
- Miller, D.; Jessel, A.; Mariani, S. Seafood mislabelling: Comparisons of two Western European case studies assist in defining influencing factors, mechanisms and motives. Fish Fish. 2011, 13, 345–358. [Google Scholar] [CrossRef]
- Mystery Fish: Seafood Fraud in Canada and How to Stop It. Oceana. Available online: http://www.oceana.ca/en/publications/reports/mystery-fish-seafood-fraud-canada-and-how-stop-it (accessed on 8 April 2021).
- Fish Substitution, 2015. European Commision Website. Available online: https://ec.europa.eu/food/food/agri-food-fraud/eu-coordinated-actions/coordinated-control-plans/fish-substitution-2015_en (accessed on 14 May 2021).
- Havelaar, A.H.; Kirk, M.D.; Torgerson, P.R.; Gibb, H.J.; Hald, T.; Lake, R.J.; Praet, N.; Bellinger, D.C.; de Silva, N.R.; Gargouri, N.; et al. World Health Organization Global estimates and regional comparisons of the burden of foodborne disease in 2010. PLoS Med. 2015, 12, e1001923. [Google Scholar] [CrossRef]
- Tavares, A.P.M.; Truta, L.A.; Moreira, F.T.C.; Carneiro, L.P.; Sales, M.G.F. Self-powered and self-signalled autonomous electrochemical biosensor applied to cancinoembryonic antigen determination. Biosens. Bioelectron. 2019, 140, 111320. [Google Scholar] [CrossRef] [PubMed]
- Resende, S.; Frasco, M.; Sales, M.G.F. A biomimetic photonic crystal sensor for label-free detection of urinary venous thromboembolism biomarker. Sens. Actuators B Chem. 2020, 312, 127947. [Google Scholar] [CrossRef]
- European Food Safety Authority; European Centre for Disease Prevention and Control. The European Union one health 2019 zoonoses report. EFSA J. 2021, 19, e06406. [Google Scholar] [CrossRef]
- Ellermeier, C.D.; Slauch, J.M. The genus Salmonella. In The Procaryotes—A Handbook on the Biology of Bacteria, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, J.-H., Stanckebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 6, pp. 123–158. [Google Scholar]
- Bailey, S.; Richardson, L.J.; Cox, N.A.; Cosby, D.E. Chapter 7: Salmonella. In Pathogens and Toxins in foods: Challenges and Interventions; Juneja, V.K., Sofos, J.N., Eds.; ASM Press: Washingtons, DC, USA, 2010; pp. 108–130. [Google Scholar]
- Sant’Ana, A.S.; Franco, B.D.G.M.; Schaffner, D.W. Risk of infection with Salmonella and Listeria monocytogenes due to consumption of ready-to-eat leafy vegetables in Brazil. Food Control 2014, 42, 1–8. [Google Scholar] [CrossRef]
- Freitas, M.; Viswanathan, S.; Nouws, H.P.; Oliveira, M.B.; Delerue-Matos, C. Iron oxide/gold core/shell nanomagnetic probes and CdS biolabels for amplified electrochemical immunosensing of Salmonella typhimurium. Biosens. Bioelectron. 2014, 51, 195–200. [Google Scholar] [CrossRef]
- Fernandes, E.; Martins, V.; Nóbrega, C.; Carvalho, C.; Cardoso, F.; Cardoso, S.; Dias, J.; Deng, D.; Kluskens, L.; Freitas, P.; et al. A bacteriophage detection tool for viability assessment of Salmonella cells. Biosens. Bioelectron. 2014, 52, 239–246. [Google Scholar] [CrossRef] [PubMed]
- Silva, N.F.; Magalhães, J.M.; Freire, C.; Delerue-Matos, C. Electrochemical biosensors for Salmonella: State of the art and challenges in food safety assessment. Biosens. Bioelectron. 2018, 99, 667–682. [Google Scholar] [CrossRef]
- Shen, Y.; Xu, L.; Li, Y. Biosensors for rapid detection of Salmonella in food: A review. Compr. Rev. Food Sci. Food Saf. 2021, 20, 149–197. [Google Scholar] [CrossRef] [PubMed]
- Serres, M.H.; Riley, M. Genomics and metabolism in Escherichia coli. In The Procaryotes—A Handbook on the Biology of Bacteria, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, J.-H., Stanckebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 1, pp. 261–274. [Google Scholar]
- Buchanan, R.; Edelson, S. Effect of pH-dependent, stationary phase acid resistance on the thermal tolerance of Escherichia coli O157:H7. Food Microbiol. 1999, 16, 447–458. [Google Scholar] [CrossRef]
- Johnson, J.R.; Oswald, E.; O’Bryan, T.T.; Kuskowski, M.A.; Spanjaard, L. Phylogenetic distribution of virulence-associated genes among Escherichia coli isolates associated with neonatal bacterial meningitis in The Netherlands. J. Infect. Dis. 2002, 185, 774–784. [Google Scholar] [CrossRef] [PubMed]
- Begue, R.E.; Mehta, D.I.; Blecker, U. Escherichia coli and the Hemolytic-uremic syndrome. South. Med. J. 1998, 91, 798–805. [Google Scholar] [CrossRef]
- Bacon, R.T.; Ransom, J.R.; Sofos, J.N.; Kendall, P.A.; Belk, K.E.; Smith, G.C. Thermal inactivation of susceptible and multiantimicrobial-resistant Salmonella strains grown in the absence or presence of glucose. Appl. Environ. Microbiol. 2003, 69, 4123–4128. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Buchanan, R.L.; Doyle, M.P. Foodborne disease significance of Escherichia coli O157:H7 and other enterohemorrhagic E. coli. Food Technol. 1997, 51, 69–76. [Google Scholar]
- Guion, C.E.; Ochoa, T.J.; Walker, C.M.; Barletta, F.; Cleary, T.G. Detection of diarrheagenic Escherichia coli by Use of melting-curve analysis and real-time multiplex PCR. J. Clin. Microbiol. 2008, 46, 1752–1757. [Google Scholar] [CrossRef]
- Nataro, J.P.; Martinez, J.; Woodford, N.; Johnson, A. Diagnosis and investigation of diarrheagenic Escherichia coli. Mol. Bacteriol. 2003, 15, 387–406. [Google Scholar] [CrossRef]
- Viveiros, S.; Rodrigues, M.; Albuquerque, D.; Martins, S.A.M.; Cardoso, S.; Martins, V.C. Multiple bacteria identification in the point-of-care: An old method serving a new approach. Sensors 2020, 20, 3351. [Google Scholar] [CrossRef]
- Barreiros dos Santos, M.; Agusil, J.; Prieto-Simon, B.; Sporer, C.; Teixeira, V.; Samitier, J. Highly sensitive detection of pathogen Escherichia coli O157:H7 by electrochemical impedance spectroscopy. Biosens. Bioelectron. 2013, 45, 174–180. [Google Scholar] [CrossRef] [PubMed]
- Couto, R.A.S.; Chen, L.; Kuss, S.; Compton, R.G. Detection of Escherichia coli bacteria by impact electrochemistry. Analyst 2018, 143, 4840–4843. [Google Scholar] [CrossRef]
- Kuss, S.; Couto, R.A.D.S.; Evans, R.; Lavender, H.; Tang, C.C.; Compton, R.G. Versatile electrochemical sensing platform for bacteria. Anal. Chem. 2019, 91, 4317–4322. [Google Scholar] [CrossRef]
- Queirós, R.B.; De-Los-Santos-Álvarez, N.; Noronha, J.P.; Sales, M.G.F. A label-free DNA aptamer-based impedance biosensor for the detection of E. coli outer membrane proteins. Sens. Actuators B Chem. 2013, 181, 766–772. [Google Scholar] [CrossRef]
- Barreiros dos Santos, M.; Azevedo, S.; Agusil, J.; Prieto-Simon, B.; Sporer, C.; Torrents, E.; Juárez, A.; Teixeira, V.; Samitier, J. Label-free ITO-based immunosensor for the detection of very low concentrations of pathogenic bacteria. Bioelectrochemistry 2015, 101, 146–152. [Google Scholar] [CrossRef]
- Martins, S.A.M.; Prazeres, D.M.F.; Fonseca, L.P.; Monteiro, G.A. Quantitation of non-amplified genomic DNA by bead-based hybridization and template mediated extension coupled to alkaline phosphatase signal amplification. Biotechnol. Lett. 2009, 32, 229–234. [Google Scholar] [CrossRef]
- Mouffouk, F.; da Costa, A.R.; Martins, J.; Zourob, M.; Abu-Salah, K.M.; Alrokayan, S. Development of a highly sensitive bacteria detection assay using fluorescent pH-responsive polymeric micelles. Biosens. Bioelectron. 2011, 26, 3517–3523. [Google Scholar] [CrossRef] [PubMed]
- Queirós, R.; Gouveia, C.; Fernandes, J.R.A.; Jorge, P. Evanescent wave DNA-aptamer biosensor based on long period gratings for the specific recognition of E. coli outer membrane proteins. Biosens. Bioelectron. 2014, 62, 227–233. [Google Scholar] [CrossRef]
- Kokkinis, G.; Cardoso, S.F.; Cardoso, F.A.; Giouroudi, I. Microfluidics for the rapid detection of pathogens using giant magnetoresistance sensors. IEEE Trans. Magn. 2014, 50, 1–4. [Google Scholar] [CrossRef]
- Bastos, A.R.; Vicente, C.M.S.; Oliveira-Silva, R.; Silva, N.J.O.; Tacão, M.; Da Costa, J.P.; Lima, M.J.S.; André, P.S.; Ferreira, R.A.S. Integrated optical mach-zehnder interferometer based on organic-inorganic hybrids for photonics-on-a-chip biosensing applications. Sensors 2018, 18, 840. [Google Scholar] [CrossRef]
- Rocourt, J.; Buchrieser, C. Chapter 1 The Genus Listeria and Listeria monocytogenes: Phylogenetic Position, Taxonomy, and Identification. In Literia, Listeriosis, and Food Safety, 3rd ed.; Ryser, E.T., Marth, A.H., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Khelef, N.; Lecuit, M.; Buchrieser, C.; Cabanes, D.; Dussurget, O.; Cossart, P. Listeria monocytogenes and the Genus Listeria. In The Procaryotes—A Handbook on the Biology of Bacteria, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, J.-H., Stanckebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 4. [Google Scholar]
- Ben Embarek, P.K. Presence, detection and growth of Listeria monocytogenes in seafoods: A review. Int. J. Food Microbiol. 1994, 23, 17–34. [Google Scholar] [CrossRef]
- Painter, J.; Slutsker, L. Chapter 4: Listeriosis in humans. In Listeria, Listeriosis, and Food Safety, 3rd ed.; Ryser, E.T., Marth, E.H., Eds.; CRC Press: Boca Raton, FL, USA, 2007. [Google Scholar]
- Hitchins, A.D.; Whiting, R.C. Food-borne Listeria monocytogenes risk assessment. Food Addit. Contam. 2001, 18, 1108–1117. [Google Scholar] [CrossRef] [PubMed]
- Tompkin, R.B. Control of Listeria monocytogenes in the food-processing environment. J. Food Prot. 2002, 65, 709–725. [Google Scholar] [CrossRef] [PubMed]
- McLauchlin, J. The relationship between Listeria and listeriosis. Food Control 1996, 7, 187–193. [Google Scholar] [CrossRef]
- Braga, V.; Vázquez, S.; Vico, V.; Pastorino, V.; Mota, M.I.; Legnani, M.; Schelotto, F.; Lancibidad, G.; Varela, G. Prevalence and serotype distribution of Listeria monocytogenes isolated from foods in Montevideo-Uruguay. Braz. J. Microbiol. 2017, 48, 689–694. [Google Scholar] [CrossRef]
- Schlech, W.F., 3rd; Lavigne, P.M.; Bortolussi, R.A.; Allen, A.C.; Haldane, E.V.; Wort, A.J.; Hightower, A.W.; Johnson, S.E.; King, S.H.; Nicholls, E.S.; et al. Epidemic Listeriosis—Evidence for transmission by food. N. Engl. J. Med. 1983, 308, 203–206. [Google Scholar] [CrossRef]
- Federighi, M.; Declerq, E.; Jugiau, F.; Cappelier, J.-M. Environmental and physico-chemical factors induce VBNC state in Listeria monocytogenes. Veter. Res. 2002, 33, 359–370. [Google Scholar] [CrossRef]
- Bille, J. Epidemiology of Human Listeriosis in Europe, with Special Reference to the Swiss Outbreak; Elsevier: Amsterdam, The Netherlands, 1990; pp. 71–74. [Google Scholar]
- McIntyre, L.; Wilcott, L.; Naus, M. Listeriosis outbreaks in British Columbia, Canada, caused by soft ripened cheese contaminated from environmental sources. BioMed Res. Int. 2015, 2015, 131623. [Google Scholar] [CrossRef]
- Gilbert, R.J.; McLauchlin, J.; Velani, S.K. The contamination of paté byListeria monocytogenesin England and Wales in 1989 and 1990. Epidemiol. Infect. 1993, 110, 543–551. [Google Scholar] [CrossRef]
- Jacquet, C.; Catimel, B.; Brosch, R.; Buchrieser, C.; Dehaumont, P.; Goulet, V.; Lepoutre, A.; Veit, P.; Rocourt, J. Investigations related to the epidemic strain involved in the French listeriosis outbreak in 1992. Appl. Environ. Microbiol. 1995, 61, 2242–2246. [Google Scholar] [CrossRef] [PubMed]
- Dalton, C.B.; Austin, C.C.; Sobel, J.; Hayes, P.S.; Bibb, W.F.; Graves, L.M.; Swaminathan, B.; Proctor, M.E.; Griffin, P.M. An Outbreak of gastroenteritis and fever due to Listeria monocytogenesin milk. N. Engl. J. Med. 1997, 336, 100–106. [Google Scholar] [CrossRef] [PubMed]
- Salamina, G.; Donne, E.D.; Niccolini, A.; Poda, G.; Cesaroni, D.; Bucci, M.; Fini, R.; Maldini, M.; Schuchat, A.; Swaminathan, B.; et al. A foodborne outbreak of gastroenteritis involvingListeria monocytogenes. Epidemiol. Infect. 1996, 117, 429–436. [Google Scholar] [CrossRef] [PubMed]
- Farber, J.; Daley, E.; Mackie, M.; Limerick, B. A small outbreak of listeriosis potentially linked to the consumption of imitation crab meat. Lett. Appl. Microbiol. 2000, 31, 100–104. [Google Scholar] [CrossRef]
- Soni, D.K.; Ahmad, R.; Dubey, S.K. Biosensor for the detection of Listeria monocytogenes: Emerging trends. Crit. Rev. Microbiol. 2018, 44, 590–608. [Google Scholar] [CrossRef]
- Silva, N.F.D.; Neves, M.M.; Magalhães, J.M.; Freire, C.; Delerue-Matos, C. Electrochemical immunosensor towards invasion-associated protein p60: An alternative strategy for Listeria monocytogenes screening in food. Talanta 2020, 216, 120976. [Google Scholar] [CrossRef]
- Seo, K.S.; Bohach, G.A. Chapter 8: Staphylococcal food poisoning. In Pathogens and Toxins in Foods: Challenges and Interventions; Juneja, V.K., Sofos, J.N., Eds.; ASM Press: Washingtons, DC, USA, 2010; pp. 119–130. [Google Scholar]
- Ray, B.; Bhunia, A. Foodborne intoxication. In Fundamental Food Microbiology, 4th ed.; Ray, B., Bhunia, A., Eds.; CRC Press: New York, NY, USA, 2008; pp. 269–282. [Google Scholar]
- Gotz, F.; Bannerman, T.; Schleifer, J.-H. The genus staphylococcus and macrococcus. In The Procaryotes—A Handbook on the Biology of Bacteria, 3rd ed.; Dworkin, M., Falkow, S., Rosenberg, E., Schleifer, J.-H., Stanckebrandt, E., Eds.; Springer: New York, NY, USA, 2006; Volume 4, pp. 5–75. [Google Scholar]
- Kloos, W.E. Natural populations of the genus staphylococcus. Annu. Rev. Microbiol. 1980, 34, 559–592. [Google Scholar] [CrossRef]
- Lowy, F.D. Staphylococcus aureus infections. N. Engl. J. Med. 1998, 339, 520–532. [Google Scholar] [CrossRef]
- Ryu, S.; Song, P.I.; Seo, C.H.; Cheong, H.; Park, Y. Colonization and infection of the skin by S. aureus: Immune system evasion and the response to cationic antimicrobial peptides. Int. J. Mol. Sci. 2014, 15, 8753–8772. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control. Country Summaries-Antimicrobial Resistance in the EU/EEA (EARS-Net) Annual Epidemiological Report for 2019. Available online: https://www.ecdc.europa.eu/en/publications-data/surveillance-antimicrobial-resistance-europe-2019 (accessed on 1 May 2021).
- Gautam, S.; Kim, T.; Shoda, T.; Sen, S.; Deep, D.; Luthra, R.; Ferreira, M.T.; Pinho, M.G.; Spiegel, D.A. An activity-based probe for studying crosslinking in live bacteria. Angew. Chem. Int. Ed. 2015, 54, 10492–10496. [Google Scholar] [CrossRef] [PubMed]
- Duarte, C.M.; Carneiro, C.; Cardoso, S.; De Freitas, S.C.; Bexiga, R. Semi-quantitative method for Staphylococci magnetic detection in raw milk. J. Dairy Res. 2016, 84, 80–88. [Google Scholar] [CrossRef] [PubMed]
- Behr, M.A.; Gagneux, S. The rise and fall of the Mycobacterium tuberculosis complex. In Genetics and Evolution of Infectious Disease; Elsevier: Amsterdam, The Netherlands, 2011; pp. 651–667. [Google Scholar]
- Velayati, A.A.; Farnia, P. Atlas of Mycobacterium tuberculosis. In Atlas of Myobacterium Tuberculosis; Elsevier: Amsterdam, The Netherlands, 2017; pp. 1–16. [Google Scholar]
- World Health Organization. Global Tuberculosis Report; World Health Organization: Geneva, Switzerland, 2020; Available online: https://apps.who.int/iris/bitstream/handle/10665/336069/9789240013131-eng.pdf (accessed on 6 March 2021).
- Houben, R.M.G.J.; Dodd, P.J. The global burden of latent tuberculosis infection: A re-estimation using mathematical modelling. PLoS Med. 2016, 13, e1002152. [Google Scholar] [CrossRef] [PubMed]
- Barroso, T.G.; Martins, R.; Fernandes, E.; Cardoso, S.; Rivas, J.; Freitas, P. Detection of BCG bacteria using a magnetoresistive biosensor: A step towards a fully electronic platform for tuberculosis point-of-care detection. Biosens. Bioelectron. 2018, 100, 259–265. [Google Scholar] [CrossRef] [PubMed]
- Garberi, J.E.; Labrador, J.; Garberi, F.; Peneipil, J.; Garberi, M.; Scigliano, L.; Troncoso, A. Rapid and biosecure diagnostic test for tuberculosis. Cell Biophys. 2012, 65, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Barroso, T.R.; Martins, V.C.; Cardoso, F.; Cardoso, S.; Pedrosa, J.; Correia-Neves, M.; Rivas, J.; De Freitas, S.C. Detecting antibody-labeled BCG MNPs using a magnetoresistive biosensor and magnetic labeling technique. J. Nano Res. 2015, 34, 49–60. [Google Scholar] [CrossRef]
- Bernacka-Wojcik, I.; Senadeera, R.; Wojcik, P.J.; Silva, L.B.; Doria, G.; Baptista, P.; Águas, H.; Fortunato, E.; Martins, R. Inkjet printed and “doctor blade” TiO2 photodetectors for DNA biosensors. Biosens. Bioelectron. 2010, 25, 1229–1234. [Google Scholar] [CrossRef] [PubMed]
- Baptista, P.V.; Koziol-Montewka, M.; Paluch-Oles, J.; Doria, G.; Franco, R. Gold-nanoparticle-probe–based assay for rapid and direct detection of Mycobacterium tuberculosis DNA in clinical samples. Clin. Chem. 2006, 52, 1433–1434. [Google Scholar] [CrossRef]
- Doria, G.; Franco, R.; Baptista, P. Nanodiagnostics: Fast colorimetric method for single nucleotide polymorphism/mutation detection. IET Nanobiotechnol. 2007, 1, 53–57. [Google Scholar] [CrossRef]
- Silva, L.B.; Veigas, B.; Doria, G.; Costa, P.; Inácio, J.; Martins, R.; Fortunato, E.; Baptista, P.V. Portable optoelectronic biosensing platform for identification of mycobacteria from the Mycobacterium tuberculosis complex. Biosens. Bioelectron. 2011, 26, 2012–2017. [Google Scholar] [CrossRef]
- Prabowo, B.A.; Purwidyantri, A.; Liu, B.; Lai, H.-C.; Liu, K.-C. Gold nanoparticle-assisted plasmonic enhancement for DNA detection on a graphene-based portable surface plasmon resonance sensor. Nanotechnology 2021, 32, 095503. [Google Scholar] [CrossRef] [PubMed]
- Wagenaar, J.A.; Van Bergen, M.A.; Mueller, M.A.; Wassenaar, T.M.; Carlton, R.M. Phage therapy reduces Campylobacter jejuni colonization in broilers. Veter. Microbiol. 2005, 109, 275–283. [Google Scholar] [CrossRef]
- Meldrum, R.J.; Ribeiro, C.D. Campylobacter in ready-to-eat foods: The result of a 15-month survey. J. Food Prot. 2003, 66, 2135–2137. [Google Scholar] [CrossRef]
- Verhoeff-Bakkenes, L.; Beumer, R.R.; De Jonge, R.; Van Leusden, F.M.; De Jong, A.E.I. Quantification of Campylobacter jejuni cross-contamination via hands, cutlery, and cutting board during preparation of a chicken fruit salad. J. Food Prot. 2008, 71, 1018–1022. [Google Scholar] [CrossRef] [PubMed]
- Black, R.E.; Levine, M.M.; Clements, M.L.; Hughes, T.P.; Blaser, M.J. Experimental Campylobacter jejuni infection in humans. J. Infect. Dis. 1988, 157, 472–479. [Google Scholar] [CrossRef] [PubMed]
- Khurana, S.K.; Sehrawat, A.; Tiwari, R.; Prasad, M.; Gulati, B.; Shabbir, M.Z.; Chhabra, R.; Karthik, K.; Patel, S.K.; Pathak, M.; et al. Bovine brucellosis—A comprehensive review. Veter. Q. 2021, 41, 61–88. [Google Scholar] [CrossRef] [PubMed]
- Franco, M.P.; Mulder, M.; Gilman, R.H.; Smits, H.L. Human brucellosis. Lancet Infect. Dis. 2007, 7, 775–786. [Google Scholar] [CrossRef]
- De Glanville, W.A.; Conde-Álvarez, R.; Moriyon, I.; Njeru, J.; Díaz, R.; Cook, E.; Morin, M.; Bronsvoort, B.M.D.C.; Thomas, L.; Kariuki, S.; et al. Poor performance of the rapid test for human brucellosis in health facilities in Kenya. PLoS Negl. Trop. Dis. 2017, 11, e0005508. [Google Scholar] [CrossRef]
- Mantecón, M.Á.; Gutiérrez, P.; Zarzosa, M.D.P.; Dueñas, A.I.; Solera, J.; Fernández-Lago, L.; Vizcaino, N.; Almaraz, A.; Bratos, M.A.; Torres, A.R.; et al. Utility of an immunocapture-agglutination test and an enzyme-linked immunosorbent assay test against cytosolic proteins from Brucella melitensis B115 in the diagnosis and follow-up of human acute brucellosis. Diagn. Microbiol. Infect. Dis. 2006, 55, 27–35. [Google Scholar] [CrossRef] [PubMed]
- Ariza, J.; Pellicer, T.; Pallares, R.; Foz, A.; Gudiol, F. Specific antibody profile in human brucellosis. Clin. Infect. Dis. 1992, 14, 131–140. [Google Scholar] [CrossRef]
- Silva, M.; Cruz, H.; Rossetti, O.; Arese, A.; Oliva, A. Development of an optical immunosensor based on the fluorescence of Cyanine-5 for veterinarian diagnostics. Biotechnol. Lett. 2004, 26, 993–997. [Google Scholar] [CrossRef]
- Gilmore, M.; Clewell, D.; Ike, Y.; Shankar, N. Enterococci: From Commensals to Leading Causes of Drug Resistant Infection [Internet]; Massachusetts Eye and Ear Infirmary: Boston, MA, USA, 2014. [Google Scholar]
- European Comission. Commission Directive (EU) 2015/1787 of 6 October 2015 Amending Annexes II and III to Council Directive 98/83/EC on the Quality of Water Intended for Human Consumption; Official Journal of the European Union; European Comission: Brussels, Belgium, 2015; pp. 6–17. [Google Scholar]
- Lei, K.-M.; Heidari, H.; Mak, P.-I.; Law, M.-K.; Maloberti, F.; Martins, R.P. A handheld high-sensitivity micro-NMR CMOS platform with B-field stabilization for multi-type biological/chemical assays. IEEE J. Solid State Circuits 2016, 52, 284–297. [Google Scholar] [CrossRef]
- Schaffer, J.N.; Pearson, M.M. Proteus mirabilis and urinary tract infections. Microbiol. Spectr. 2015, 3. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.A.R.; Cardoso, A.R.A.; Sales, M.G.F.; Merino, S.; Tomás, J.M.; Rius, F.X.; Riu, J. Artificial receptors for the electrochemical detection of bacterial flagellar filaments from Proteus mirabilis. Sens. Actuators B Chem. 2017, 244, 732–741. [Google Scholar] [CrossRef]
- Viswanathan, S.; Rani, C.; Ho, A. Electrochemical immunosensor for multiplexed detection of food-borne pathogens using nanocrystal bioconjugates and MWCNT screen-printed electrode. Talanta 2012, 94, 315–319. [Google Scholar] [CrossRef]
- Pereira, C.B.; Bocková, M.; Santos, R.F.; Santos, A.M.; De Araújo, M.M.; Oliveira, L.; Homola, J.; Carmo, A.M. The scavenger receptor SSc5D physically interacts with bacteria through the SRCR-containing N-terminal domain. Front. Immunol. 2016, 7, 416. [Google Scholar] [CrossRef]
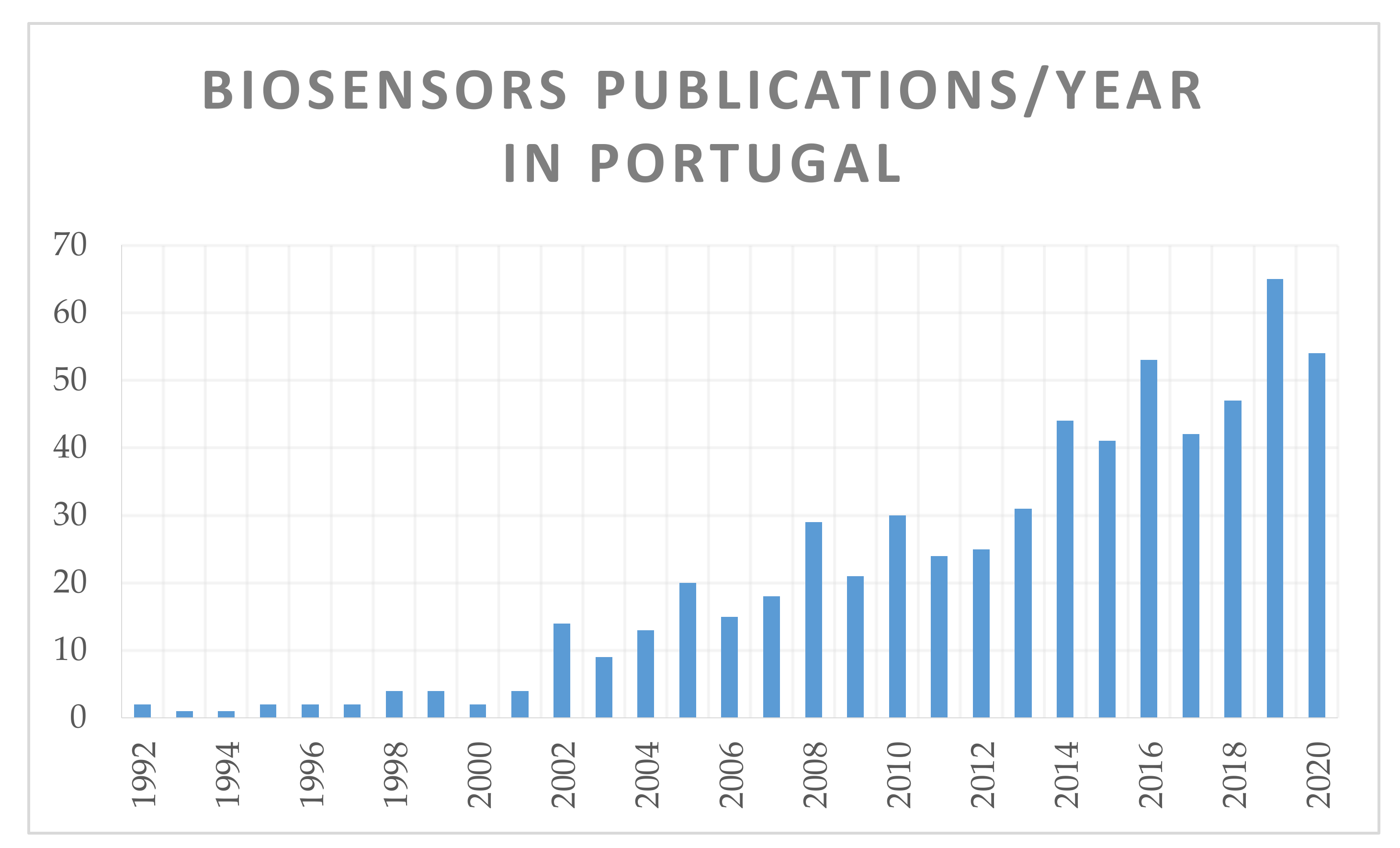



| Number of Publications with Biosensor | ||
|---|---|---|
| Area | Worldwide | Portuguese |
| Chemistry analytical | 27,585 | 299 |
| Electrochemistry | 15,750 | 199 |
| Nanoscience nanotechnology | 12,328 | 93 |
| Biotechnology applied microbiology | 9096 | 77 |
| Instrument instrumentation | 7972 | 75 |
| Biophysics | 7806 | 93 |
| Chemistry multidisciplinary | 7161 | 63 |
| Materials science multidisciplinary | 6793 | 40 |
| Physics applied | 5792 | 43 |
| Engineering, electrical and electronic | 5774 | 48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Miguéis, S.d.C.; Tavares, A.P.M.; Martins, G.V.; Frasco, M.F.; Sales, M.G.F. Biosensors for European Zoonotic Agents: A Current Portuguese Perspective. Sensors 2021, 21, 4547. https://doi.org/10.3390/s21134547
Miguéis SdC, Tavares APM, Martins GV, Frasco MF, Sales MGF. Biosensors for European Zoonotic Agents: A Current Portuguese Perspective. Sensors. 2021; 21(13):4547. https://doi.org/10.3390/s21134547
Chicago/Turabian StyleMiguéis, Samuel da Costa, Ana P. M. Tavares, Gabriela V. Martins, Manuela F. Frasco, and Maria Goreti Ferreira Sales. 2021. "Biosensors for European Zoonotic Agents: A Current Portuguese Perspective" Sensors 21, no. 13: 4547. https://doi.org/10.3390/s21134547
APA StyleMiguéis, S. d. C., Tavares, A. P. M., Martins, G. V., Frasco, M. F., & Sales, M. G. F. (2021). Biosensors for European Zoonotic Agents: A Current Portuguese Perspective. Sensors, 21(13), 4547. https://doi.org/10.3390/s21134547








