Ultraviolet Radiation Sensor Based on ZnO Nanorods/La3Ga5SiO14 Microbalance
Abstract
1. Introduction
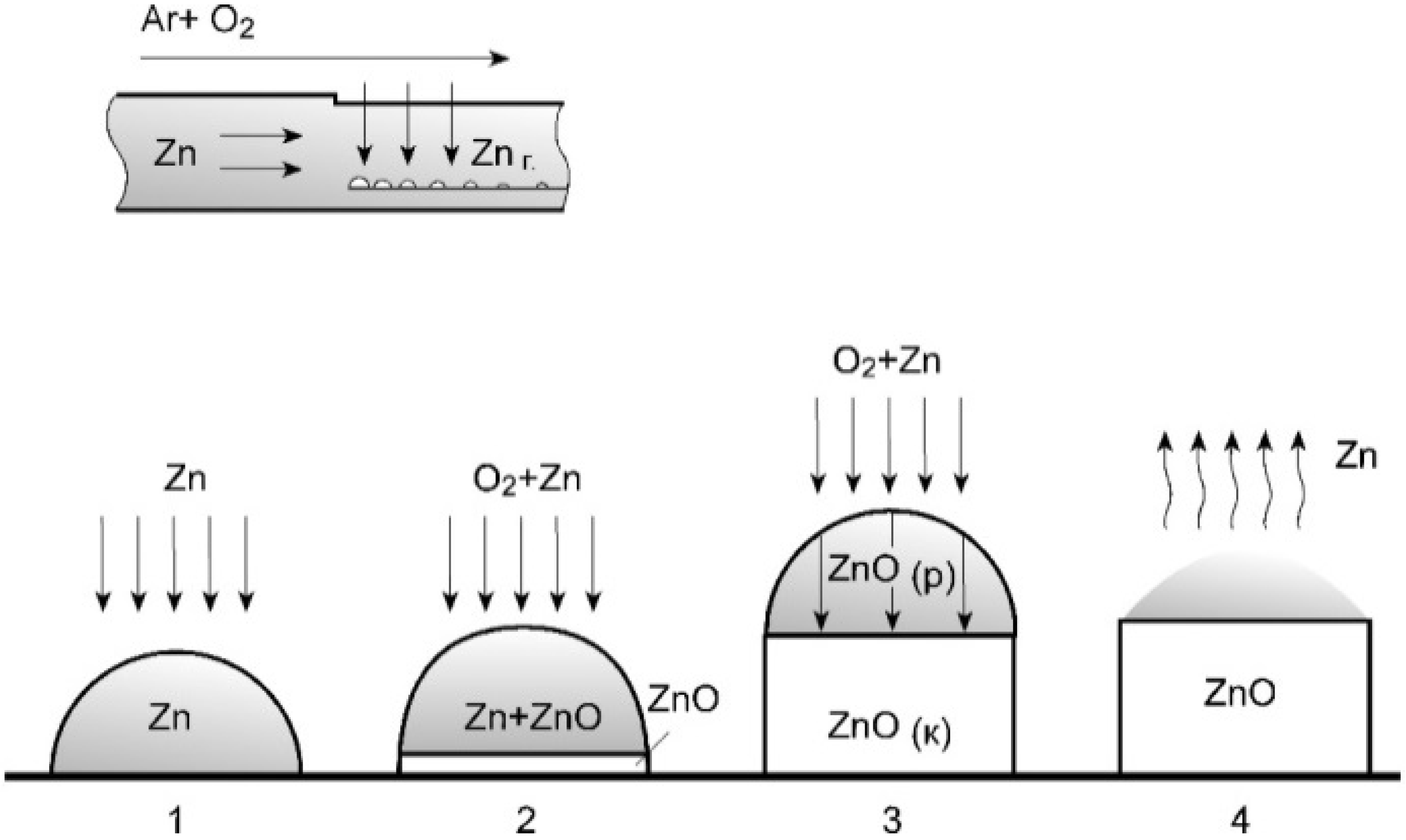
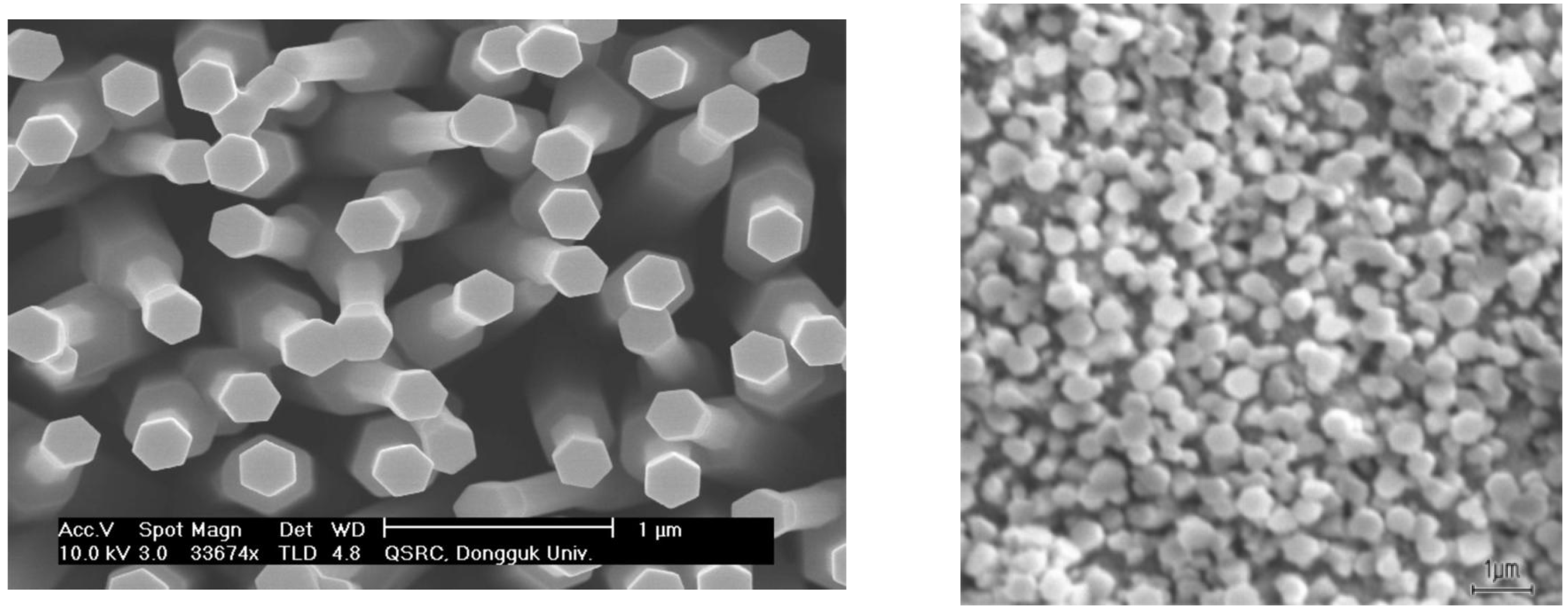
2. Fabrication of ZnO Nanorods/LCM Structure
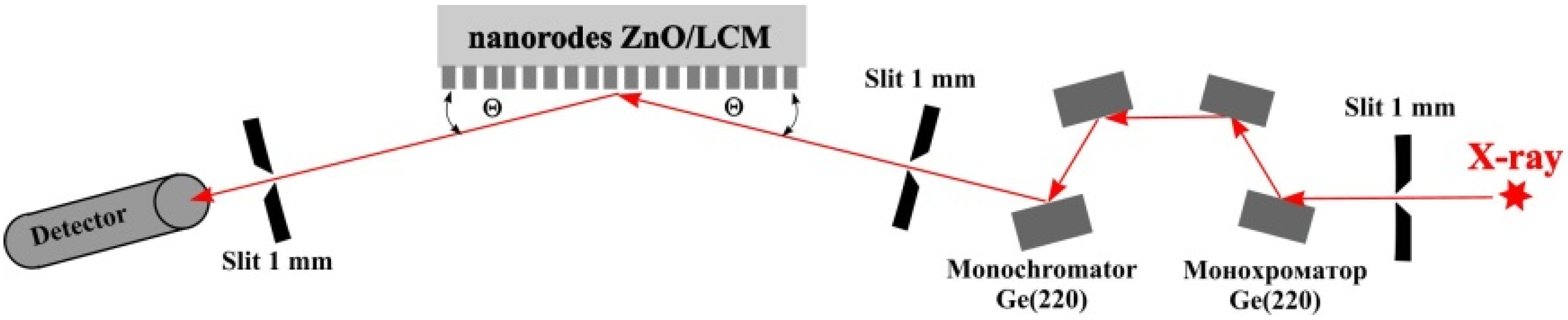
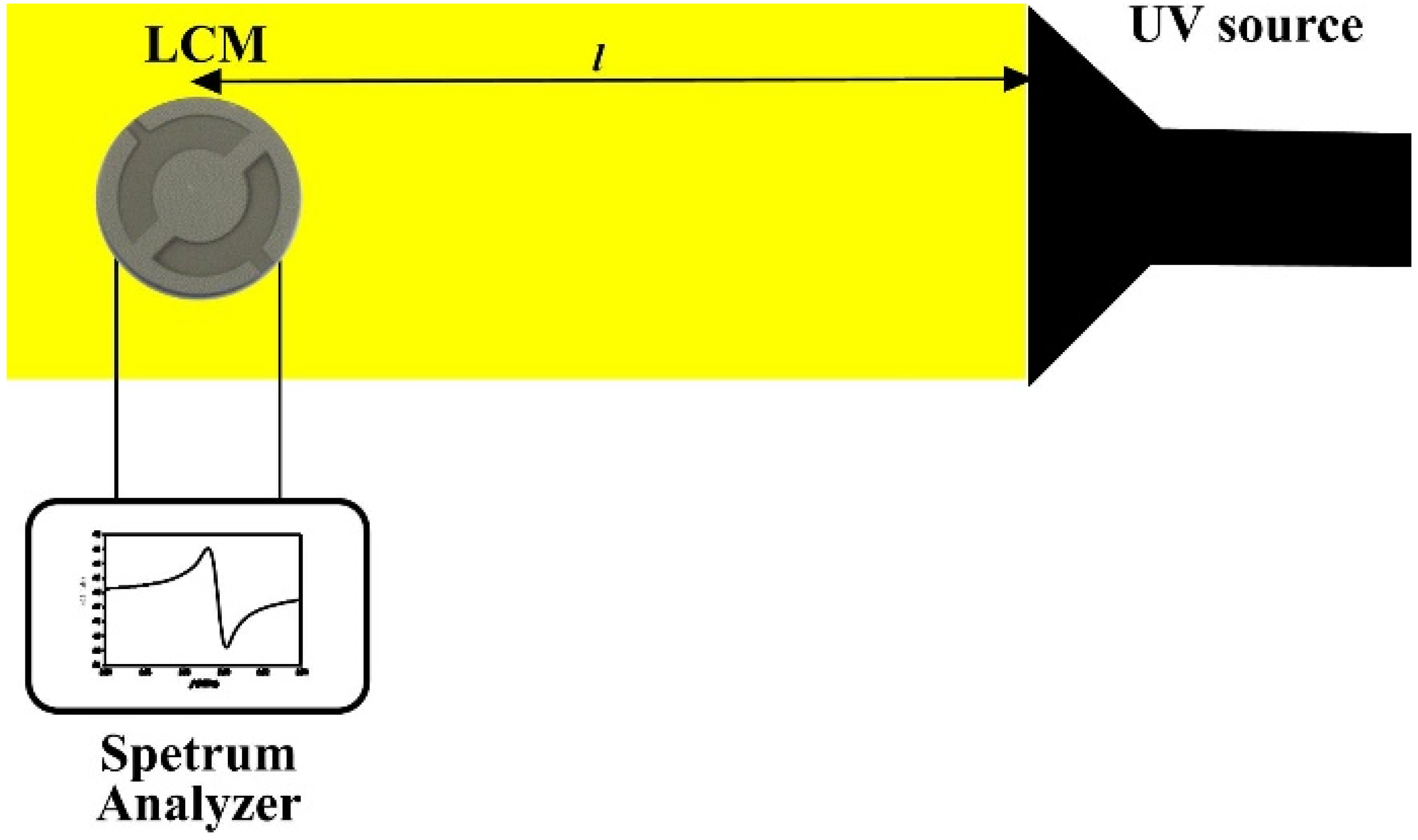
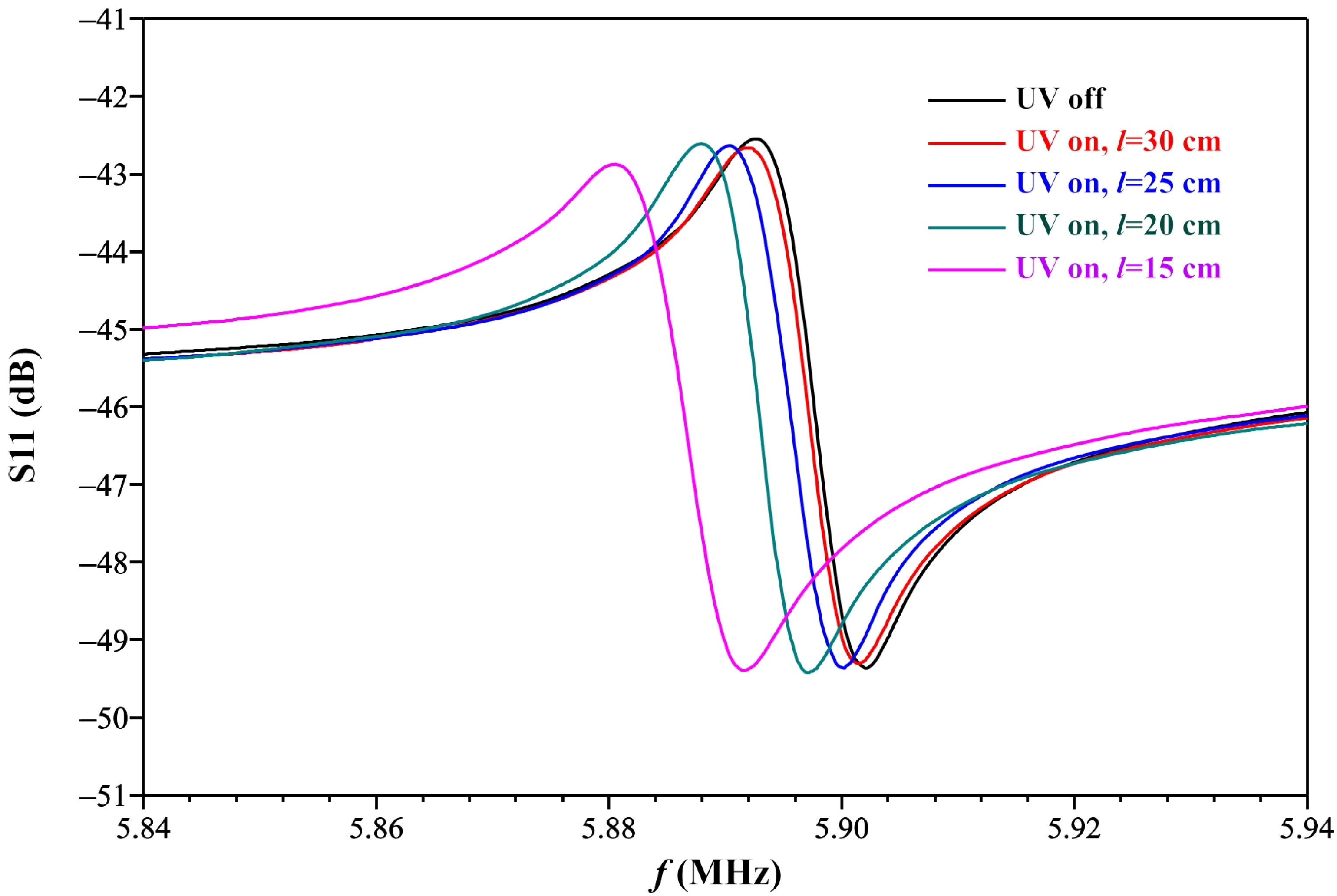
3. Results and Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Georgiou, P.; Kolokotronis, K.; Simitzis, J. Synthesis of ZnO nanostructures by hydrothermal method. J. Nano Res. 2009, 6, 157–168. [Google Scholar] [CrossRef]
- Shinde, S.D.; Patil, G.E.; Kajale, D.D.; Ahire, D.V.; Gaikwad, V.B.; Jain, G.H. Syntesis of ZnO nanorodes by hydrothermal method for gas sensor applications. Int. J. Smart Sens. Intell. Syst. 2012, 5, 57–70. [Google Scholar]
- Yang, W.; Wan, F.; Chen, S.; Jiang, C. Hydrothermal growth and application of ZnO nanowire films with ZnO and TiO2 buffer layers in dye-sensitized solar cells. Nanoscale Res. Lett. 2009, 4, 1486–1492. [Google Scholar] [CrossRef] [PubMed]
- Gridchin, V.O.; Kotlyar, K.P.; Vershinin, A.V.; Kryzhanovskaya, N.V.; Pirogov, E.V.; Semenov, A.A.; Belyavskiy, P.Y.; Nashchekin, A.V.; Cirlin, G.E.; Soshnikov, I.P. ZnO thin films synthesis by RF magnetron sputtering deposition. J. Phys. Conf. Ser. 2019, 1410, 012054. [Google Scholar] [CrossRef]
- Miorin, E.; Montagner, F.; Battiston, S.; Fiameni, S.; Fabrizio, M. ZnO:Al thin films deposited by RF-magnetron sputtering with tunable and uniform properties. J. Nanosci. Nanotechnol. 2011, 11, 2191–2195. [Google Scholar] [CrossRef]
- Kim, H.R.; Jin, S.B.; Wen, L.; Choi, Y.S.; Choi, I.S.; Han, J.G. Structural and electrical properties of ZnO films deposited with low-temperature facing targets magnetron sputtering (FTS) system with changes in H2 and O2 flow rate. J. Nanosci. Nanotechnol. 2013, 13, 7745–7750. [Google Scholar] [CrossRef]
- Tucoulou, R.; Pascal, R.; Brunel, M.; Mathon, O.; Roshchupkin, D.V.; Schelokov, I.A.; Cattan, E.; Remiens, D. X-ray diffraction from perfect silicon crystals distorted by surface acoustic waves. J. Appl. Crystallogr. 2000, 33, 1019–1022. [Google Scholar] [CrossRef]
- Redkin, A.N.; Ryzhova, M.V.; Yakimov, E.E.; Gruzintsev, A.N. Aligned Arrays of Zinc Oxide Nanorods on Silicon Substrates. Semiconductors 2013, 47, 252–258. [Google Scholar] [CrossRef]
- Dalal, S.; Baptista, D.; Teo, K.; Lacerda, R.; Jefferson, D.; Milne, W. Controllable growth of vertically aligned zinc oxide nanowires using vapour deposition. Nanotechnology 2006, 17, 4811–4818. [Google Scholar] [CrossRef]
- Wang, X.H.; Li, R.B.; Fan, D.H. Control growth of catalyst-free high-quality ZnO nanowire arrays on transparent quartz glass substrate by chemical vapor deposition. Appl. Surf. Sci. 2011, 257, 2960–2964. [Google Scholar] [CrossRef]
- Djurisic, A.B.; Ng, A.M.C.; Chen, X.Y. ZnO nanostructures for optoelectronics: Material properties and device applications. Prog. Quantum Electron. 2010, 34, 191–259. [Google Scholar] [CrossRef]
- Kumar, B.; Kim, S.-W. Energy harvesting based on semiconducting piezoelectric ZnO nanostructures. Nano Energy 2012, 1, 342–355. [Google Scholar] [CrossRef]
- Li, L.; Zhai, T.; Bando, Y.; Golberg, D. Recent progress of one-dimensional ZnO nanostructured solar cells. Nano Energy 2012, 1, 91–106. [Google Scholar] [CrossRef]
- Cui, F.; Chen, W.; Jin, L.; Zhang, H.; Jiang, Z.; Son, Z. Fabrication of ZIF-8 encapsulated ZnO microrods with enhanced sensing properties for H2 detection. J. Mater. Sci. Mater. Electron. 2018, 291, 19697–19709. [Google Scholar] [CrossRef]
- Galstyann, V.; Comini, E.; Baratto, C.; Faglia, G.; Sberveglieri, G. Nanostructured ZnO chemical gas sensors. Ceram. Int. 2015, 41, 14239–14244. [Google Scholar] [CrossRef]
- Kumar, R.; Al-Dossary, O.; Kumar, G.; Umar, A. Zinc Oxide Nanostructures for NO2 Gas–Sensor Applications: A Review. Nano Micro Lett. 2015, 7, 97–120. [Google Scholar] [CrossRef]
- Zeng, Y.; Zhang, T.; Yuan, M.; Kang, M.; Lu, G.; Wang, R.; Fan, H.; He, Y.; Yang, H. Growth and selective acetone detection based on ZnO nanorod arrays. Sens. Actuators B 2009, 143, 93–98. [Google Scholar] [CrossRef]
- Constantinoiu, I.; Viespe, C. ZnO metal oxide semiconductor in surface acoustic wave sensors: A review. Sensors 2020, 20, 5118. [Google Scholar] [CrossRef] [PubMed]
- Shen, T.; Dai, X.; Zhang, D.; Wang, W.; Feng, Y. ZnO composite graphene coating micro-fiber interferometer for ultraviolet detection. Sensors 2020, 20, 1478. [Google Scholar] [CrossRef] [PubMed]
- Lupan, O.; Ursaki, V.V.; Chai, G.; Chow, L.; Emelchenko, G.A.; Tiginyanu, I.M.; Gruzintsev, A.N.; Redkin, A.N. Selective hydrogen gas nanosensor using individual ZnO nanowire with fast response at room temperature. Sens. Actuators B 2010, 144, 56–66. [Google Scholar] [CrossRef]
- Wang, L.; Kang, Y.; Liu, X.; Zhang, S.; Huang, W.; Wang, S. ZnO nanorod gas sensor for ethanol detection. Sens. Actuators B 2012, 162, 237–243. [Google Scholar] [CrossRef]
- Lang, Y.; Gao, H.; Jiang, W.; Xu, L.; Hou, H. Photoresponse and decay mechanism of an individual ZnO nanowire UV sensor. Sens. Actuators A 2012, 174, 43–46. [Google Scholar] [CrossRef]
- Lupan, O.; Chai, G.; Chow, L.; Emelchenko, G.A.; Heinrich, H.; Ursaki, V.V.; Gruzintsev, A.N.; Tiginyanu, I.M.; Redkin, A.N. Ultraviolet photoconductive sensor based on single ZnO nanowire. Phys. Stat. Sol. (A) 2010, 207, 1735–1740. [Google Scholar] [CrossRef]
- Abe, T.; Suzuki, Y.; Nakagawa, A.; Chiba, T.; Nakagawa, M.; Kashiwaba, Y.; Niikura, I.; Kashiwaba, Y.; Osada, H. Application of a ZnO UV sensor for a scintillation-type radiation detector. J. Mater. Sci. Mater. Electron. 2019, 30, 16873–16877. [Google Scholar] [CrossRef]
- Sharma, P.; Kumar, S.; Sreenivas, K. Interaction of surface acoustic waves and ultraviolet light in ZnO films. J. Mater. Res. 2003, 18, 545–548. [Google Scholar] [CrossRef]
- Saha, T.; Guo, N.; Ramakrishnan, N. A novel langasite crystal microbalance instrumentation for UV sensing application. Sens. Actuators A Phys. 2016, 252, 16–25. [Google Scholar] [CrossRef]
- Bahadur, H.; Parshad, R. Scanning electron microscopy of vibrating quartz crystals. Scanning Electron. Microsc. 1980, 1, 509–522. [Google Scholar] [CrossRef]
- Roshchupkin, D.V.; Fournier, T.; Brunel, M.; Plotitsyna, O.; Sorokin, N.G. Scanning electron microscopy observation of excitation of the surface acoustic waves by the regular domain structures in the LiNbO3 crystals. Appl. Phys. Lett. 1992, 60, 2330–2331. [Google Scholar] [CrossRef]
- Roshchupkin, D.V.; Brunel, M. Scanning electron microscopy observation of surface acoustic wave propagation in the LiNbO3 crystals with regular domain structures. IEEE Trans. Ultrason. Ferroelectr. Freq. Control 1994, 41, 512–517. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Roshchupkin, D.; Redkin, A.; Emelin, E.; Sakharov, S. Ultraviolet Radiation Sensor Based on ZnO Nanorods/La3Ga5SiO14 Microbalance. Sensors 2021, 21, 4170. https://doi.org/10.3390/s21124170
Roshchupkin D, Redkin A, Emelin E, Sakharov S. Ultraviolet Radiation Sensor Based on ZnO Nanorods/La3Ga5SiO14 Microbalance. Sensors. 2021; 21(12):4170. https://doi.org/10.3390/s21124170
Chicago/Turabian StyleRoshchupkin, Dmitry, Arkady Redkin, Eugenii Emelin, and Sergey Sakharov. 2021. "Ultraviolet Radiation Sensor Based on ZnO Nanorods/La3Ga5SiO14 Microbalance" Sensors 21, no. 12: 4170. https://doi.org/10.3390/s21124170
APA StyleRoshchupkin, D., Redkin, A., Emelin, E., & Sakharov, S. (2021). Ultraviolet Radiation Sensor Based on ZnO Nanorods/La3Ga5SiO14 Microbalance. Sensors, 21(12), 4170. https://doi.org/10.3390/s21124170






