Inkjet-Printed and Electroplated 3D Electrodes for Recording Extracellular Signals in Cell Culture
Abstract
1. Introduction
2. Materials and Methods
2.1. Printing 3D Microelectrode Arrays
2.2. Electroplating Procedure
2.3. Printing the Passivation
2.4. Focused Ion Beam and Scanning Electron Microscopy
2.5. Electrochemical Characterisation and Data Analysis of 3D Electrodes
2.6. Laser Profilometry and Data Analysis
2.7. HL-1 Cell Culture and Measurement of Extracellular Signals
2.8. Data Analysis of Extracellular Potential Signals from HL-1 Cells
3. Results
3.1. Formation of 3D Microelectrodes
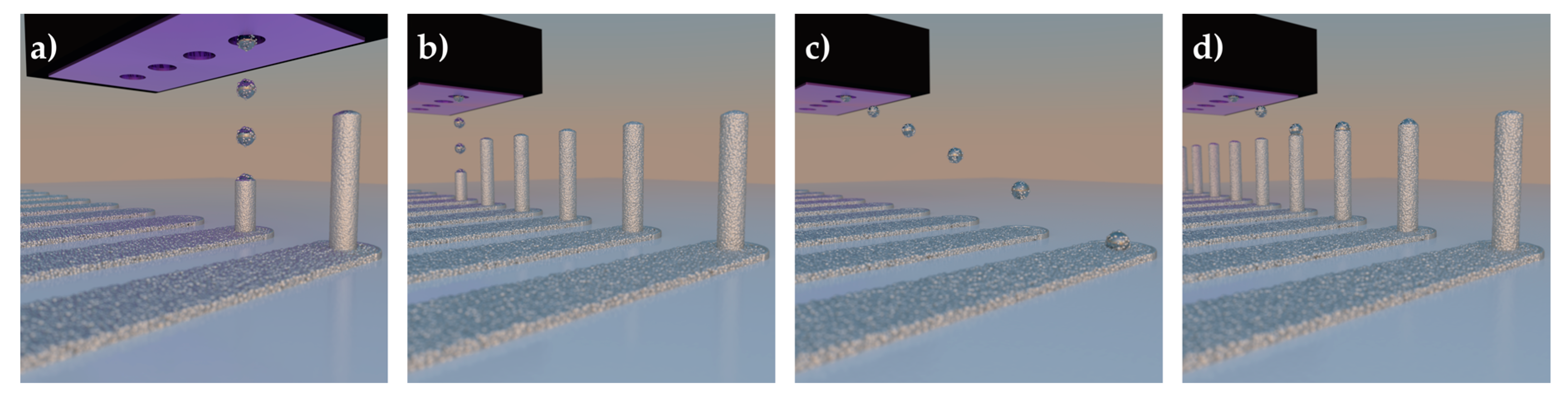
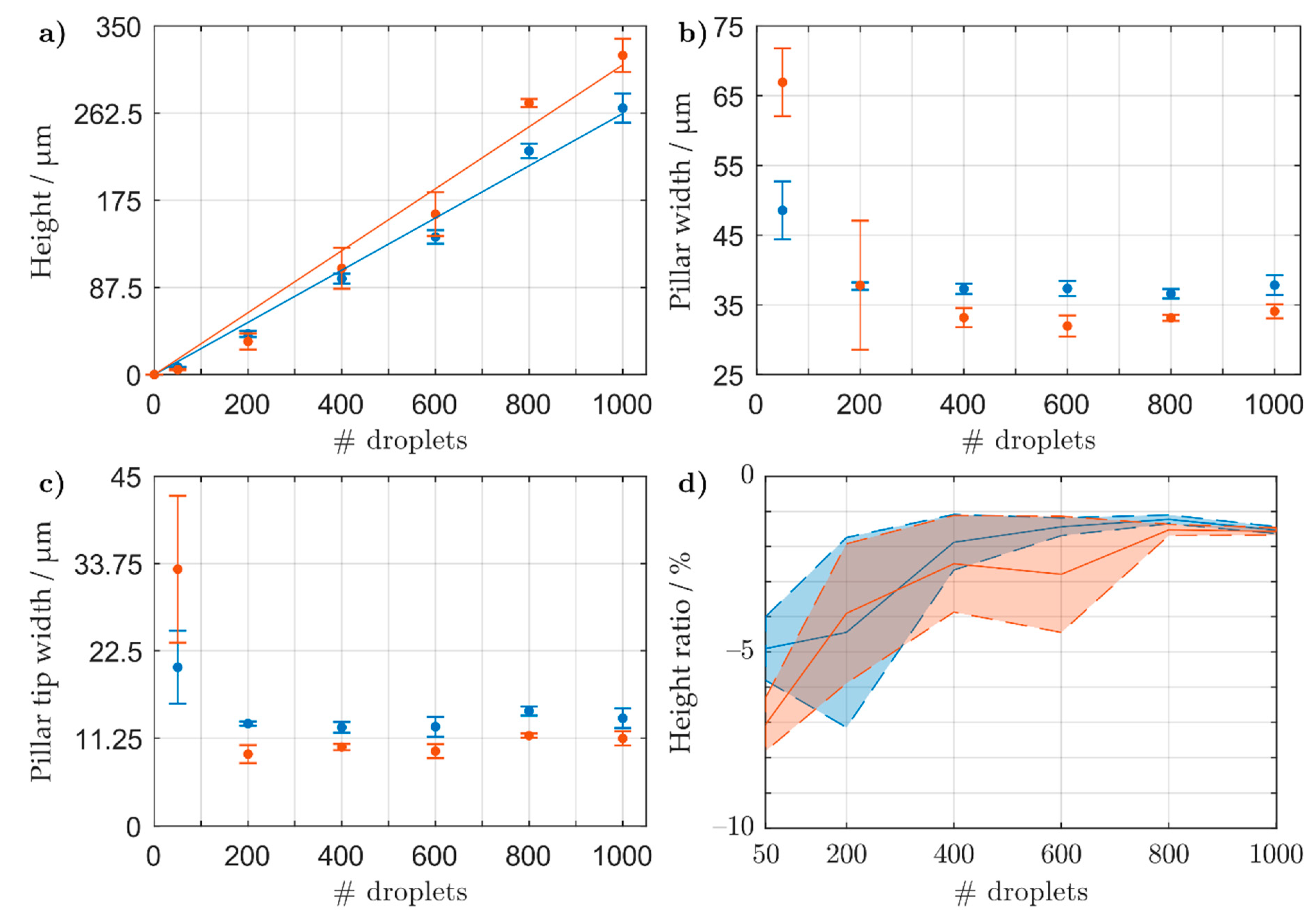
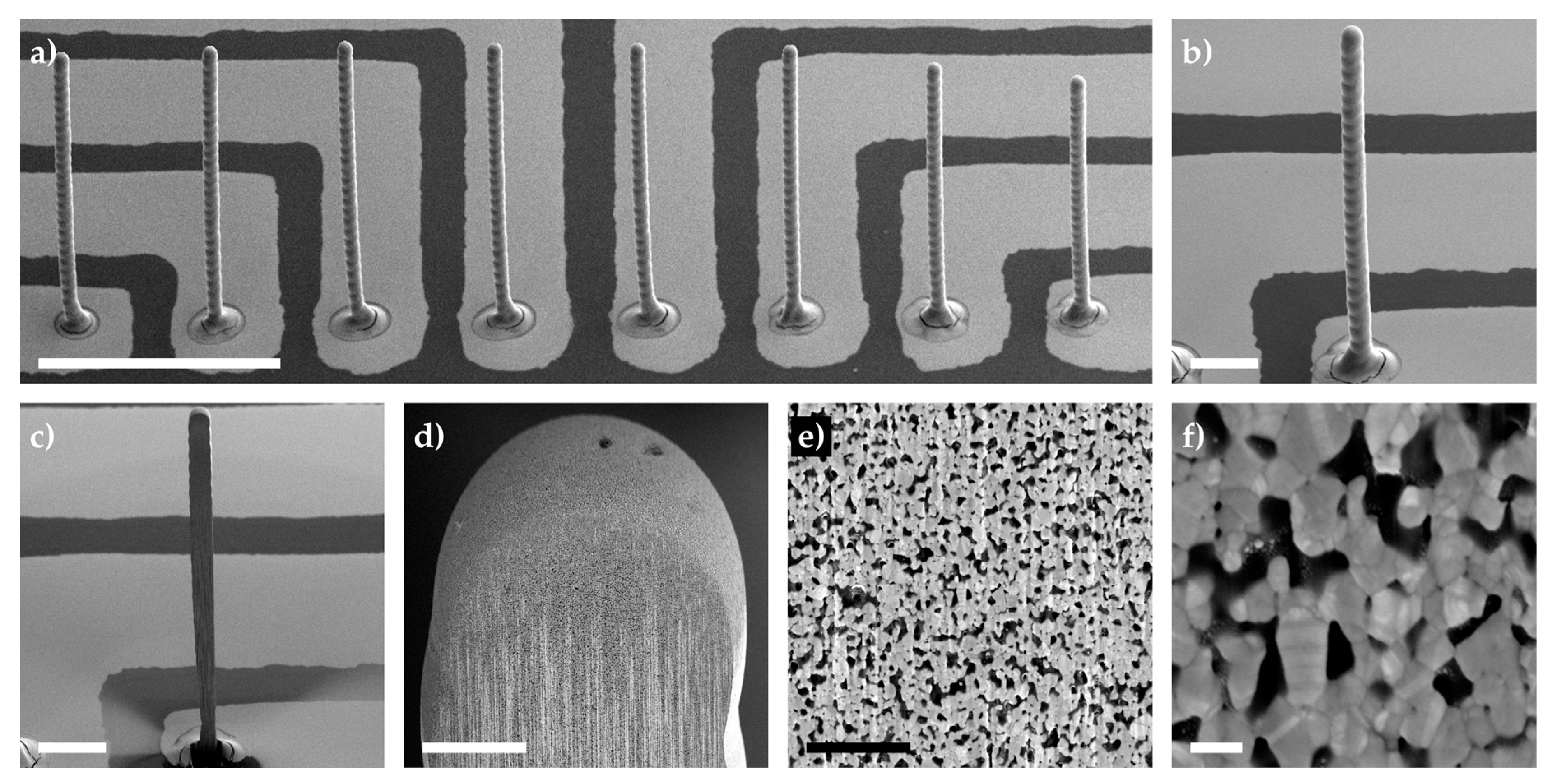
3.1.1. Pillar Growth Using a Continuous Printing Method
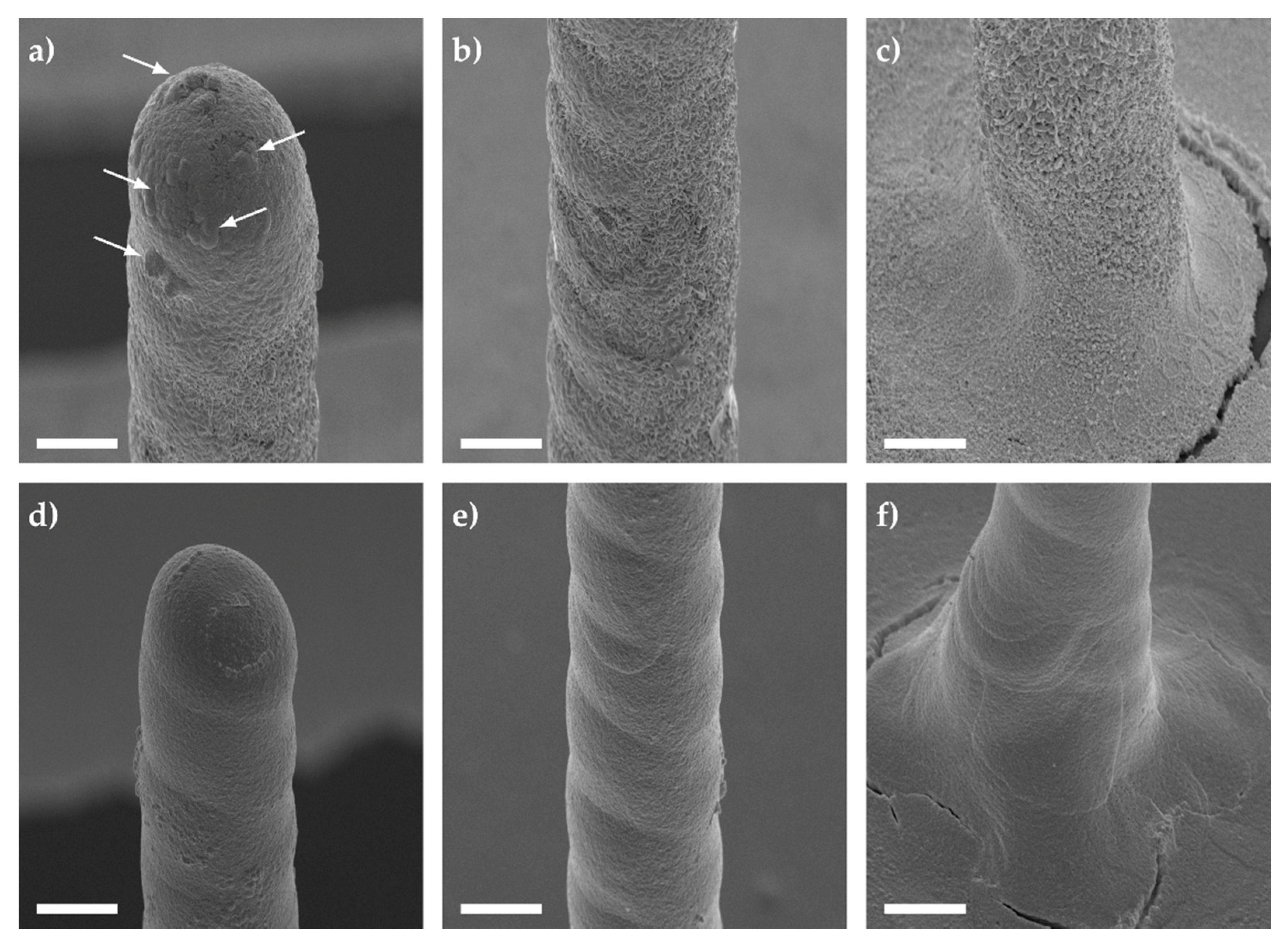
3.1.2. Thermal Sintering of 3D Microelectrodes
3.2. Electrochemically Stable 3D Microelectrodes: An Electroplating Approach
3.2.1. Constant vs. Pulsed Electrodeposition
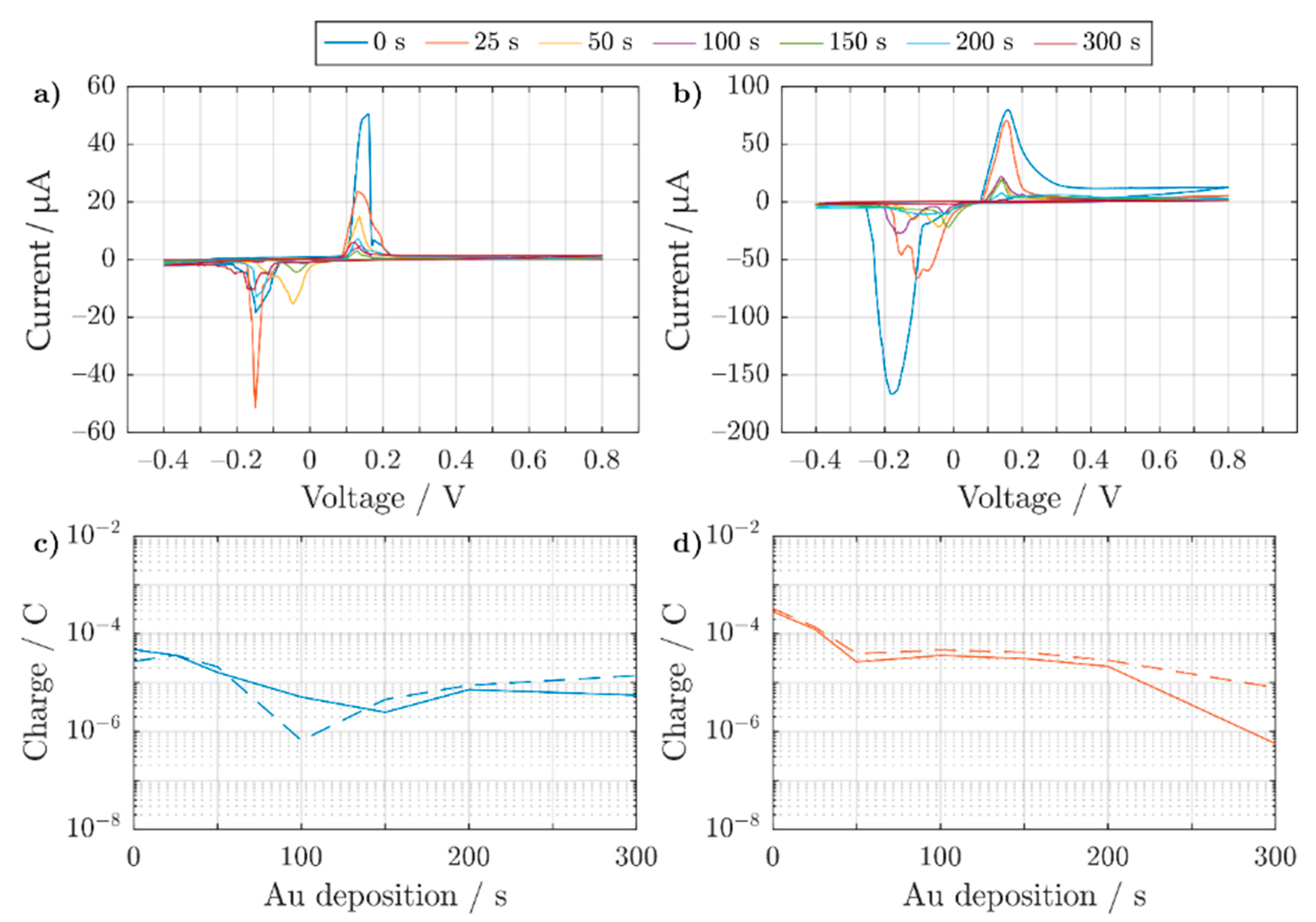
3.2.2. Electrochemical Characterization of Au Electroplated 3D Electrodes
3.2.3. Electrochemical Characterization of Pt Electroplated 3D Electrodes
3.3. 3D Electrodes for Cell Culture
3.4. Extracellular Potential Recording of HL-1 Cells Using Inkjet-Printed 3D Electrodes
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Blau, A.; Ziegler, C.; Heyer, M.; Endres, F.; Schwitzgebel, G.; Matthies, T.; Stieglitz, T.; Meyer, J.-U.; Göpel, W. Characterization and Optimization of Microelectrode Arrays for In Vivo Nerve Signal Recording and Stimulation. Biosens. Bioelectron. 1997, 12, 883–892. [Google Scholar] [CrossRef]
- Viventi, J.; Kim, D.-H.; Vigeland, L.; Frechette, E.S.; Blanco, J.A.; Kim, Y.-S.; Avrin, A.E.; Tiruvadi, V.R.; Hwang, S.-W.; Vanleer, A.C.; et al. Flexible, Foldable, Actively Multiplexed, High-Density Electrode Array for Mapping Brain Activity In Vivo. Nat. Neurosci. 2011, 14, 1599–1605. [Google Scholar] [CrossRef]
- Lee, W.; Kim, D.; Matsuhisa, N.; Nagase, M.; Sekino, M.; Malliaras, G.G.; Yokota, T.; Someya, T. Transparent, Conformable, Active Multielectrode Array Using Organic Electrochemical Transistors. Proc. Natl. Acad. Sci. USA 2017, 114, 10554–10559. [Google Scholar] [CrossRef]
- He, C.; Tao, M.; Zhang, C.; He, Y.; Xu, W.; Liu, Y.; Zhu, W. Microelectrode-Based Electrochemical Sensing Technology for in Vivo Detection of Dopamine: Recent Developments and Future Prospects. Crit. Rev. Anal. Chem. 2020, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Gross, G.W.; Rieske, E.; Kreutzberg, G.W.; Meyer, A. A New Fixed-Array Multi-Microelectrode System Designed for Long-Term Monitoring of Extracellular Single Unit Neuronal Activity In Vitro. Neurosci. Lett. 1977, 6, 101–105. [Google Scholar] [CrossRef]
- Berdondini, L.; Chiappalone, M.; van der Wal, P.D.; Imfeld, K.; de Rooij, N.F.; Koudelka-Hep, M.; Tedesco, M.; Martinoia, S.; van Pelt, J.; Le Masson, G.; et al. A Microelectrode Array (MEA) Integrated with Clustering Structures for Investigating in Vitro Neurodynamics in Confined Interconnected Sub-Populations of Neurons. Sens. Actuators B Chem. 2006, 114, 530–541. [Google Scholar] [CrossRef]
- Cho, K.W.; Lee, W.H.; Kim, B.-S.; Kim, D.-H. Sensors in Heart-on-a-Chip: A Review on Recent Progress. Talanta 2020, 219, 121269. [Google Scholar] [CrossRef]
- Didier, C.M.; Kundu, A.; DeRoo, D.; Rajaraman, S. Development of in Vitro 2D and 3D Microelectrode Arrays and Their Role in Advancing Biomedical Research. J. Micromech. Microeng. 2020, 30, 103001. [Google Scholar] [CrossRef]
- Brüggemann, D.; Wolfrum, B.; Maybeck, V.; Mourzina, Y.; Jansen, M.; Offenhäusser, A. Nanostructured Gold Microelectrodes for Extracellular Recording from Electrogenic Cells. Nanotechnology 2011, 22, 265104. [Google Scholar] [CrossRef]
- Santoro, F.; Schnitker, J.; Panaitov, G.; Offenhäusser, A. On Chip Guidance and Recording of Cardiomyocytes with 3D Mushroom-Shaped Electrodes. Nano Lett. 2013, 13, 5379–5384. [Google Scholar] [CrossRef]
- Ballini, M.; Müller, J.; Livi, P.; Chen, Y.; Frey, U.; Stettler, A.; Shadmani, A.; Viswam, V.; Jones, I.L.; Jäckel, D.; et al. A 1024-Channel CMOS Microelectrode Array With 26,400 Electrodes for Recording and Stimulation of Electrogenic Cells In Vitro. IEEE J. Solid State Circuits 2014, 49, 2705–2719. [Google Scholar] [CrossRef]
- Maybeck, V.; Edgington, R.; Bongrain, A.; Welch, J.O.; Scorsone, E.; Bergonzo, P.; Jackman, R.B.; Offenhäusser, A. Boron-Doped Nanocrystalline Diamond Microelectrode Arrays Monitor Cardiac Action Potentials. Adv. Healthc. Mater. 2014, 3, 283–289. [Google Scholar] [CrossRef]
- Czeschik, A.; Rinklin, P.; Derra, U.; Ullmann, S.; Holik, P.; Steltenkamp, S.; Offenhäusser, A.; Wolfrum, B. Nanostructured Cavity Devices for Extracellular Stimulation of HL-1 Cells. Nanoscale 2015, 7, 9275–9281. [Google Scholar] [CrossRef] [PubMed]
- Kireev, D.; Seyock, S.; Ernst, M.; Maybeck, V.; Wolfrum, B.; Offenhäusser, A. Versatile Flexible Graphene Multielectrode Arrays. Biosensors 2016, 7, 1. [Google Scholar] [CrossRef]
- Yakushenko, A.; Schnitker, J.; Wolfrum, B. Printed Carbon Microelectrodes for Electrochemical Detection of Single Vesicle Release from PC12 Cells. Anal. Chem. 2012, 84, 4613–4617. [Google Scholar] [CrossRef]
- Wang, J.; Trouillon, R.; Lin, Y.; Svensson, M.I.; Ewing, A.G. Individually Addressable Thin-Film Ultramicroelectrode Array for Spatial Measurements of Single Vesicle Release. Anal. Chem. 2013, 85, 5600–5608. [Google Scholar] [CrossRef]
- Yakushenko, A.; Kätelhön, E.; Wolfrum, B. Parallel On-Chip Analysis of Single Vesicle Neurotransmitter Release. Anal. Chem. 2013, 85, 5483–5490. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, B.; Kätelhön, E.; Yakushenko, A.; Krause, K.J.; Adly, N.; Hüske, M.; Rinklin, P. Nanoscale Electrochemical Sensor Arrays: Redox Cycling Amplification in Dual-Electrode Systems. Acc. Chem. Res. 2016. [Google Scholar] [CrossRef] [PubMed]
- Rinklin, P.; Mayer, D.; Wolfrum, B. Electrochemical Nanocavity Devices. In Label-Free Biosensing: Advanced Materials, Devices and Applications; Schöning, M.J., Poghossian, A., Eds.; Springer Series on Chemical Sensors and Biosensors; Springer International Publishing: Cham, Switzerland, 2018; pp. 199–214. ISBN 978-3-319-75220-4. [Google Scholar]
- Morefield, S.I.; Keefer, E.W.; Chapman, K.D.; Gross, G.W. Drug Evaluations Using Neuronal Networks Cultured on Microelectrode Arrays. Biosens. Bioelectron. 2000, 15, 383–396. [Google Scholar] [CrossRef]
- Stett, A.; Egert, U.; Guenther, E.; Hofmann, F.; Meyer, T.; Nisch, W.; Haemmerle, H. Biological Application of Microelectrode Arrays in Drug Discovery and Basic Research. Anal. Bioanal. Chem. 2003, 377, 486–495. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Molnar, P.; Sieverdes, K.; Jamshidi, A.; Hickman, J.J. Microelectrode Array Recordings of Cardiac Action Potentials as a High Throughput Method to Evaluate Pesticide Toxicity. Toxicology 2006, 20, 375–381. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, A.; Stancescu, M.; Dhir, V.; Armstrong, C.; Sommerhage, F.; Hickman, J.J.; Molnar, P. Patterned Cardiomyocytes on Microelectrode Arrays as a Functional, High Information Content Drug Screening Platform. Biomaterials 2011, 32, 4267–4274. [Google Scholar] [CrossRef] [PubMed]
- Wu, W. Inorganic Nanomaterials for Printed Electronics: A Review. Nanoscale 2017, 9, 7342–7372. [Google Scholar] [CrossRef]
- Kamyshny, A.; Steinke, J.; Magdassi, S. Metal-Based Inkjet Inks for Printed Electronics. Open Appl. Phys. J. 2011, 4, 19–36. [Google Scholar] [CrossRef]
- Cummins, G.; Desmulliez, M.P.Y. Inkjet Printing of Conductive Materials: A Review. Circuit World 2012, 38, 193–213. [Google Scholar] [CrossRef]
- de Gans, B.-J.; Duineveld, P.C.; Schubert, U.S. Inkjet Printing of Polymers: State of the Art and Future Developments. Adv. Mater. 2004, 16, 203–213. [Google Scholar] [CrossRef]
- Komuro, N.; Takaki, S.; Suzuki, K.; Citterio, D. Inkjet Printed (Bio)Chemical Sensing Devices. Anal. Bioanal. Chem. 2013, 405, 5785–5805. [Google Scholar] [CrossRef]
- Hamad, E.M.; Bilatto, S.E.R.; Adly, N.Y.; Correa, D.S.; Wolfrum, B.; Schöning, M.J.; Offenhäusser, A.; Yakushenko, A. Inkjet Printing of UV-Curable Adhesive and Dielectric Inks for Microfluidic Devices. Lab Chip 2015, 16, 70–74. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Huang, Y.; Liang, J.; Wan, X.; Chen, Y. Graphene-Based Conducting Inks for Direct Inkjet Printing of Flexible Conductive Patterns and Their Applications in Electric Circuits and Chemical Sensors. Nano Res. 2011, 4, 675–684. [Google Scholar] [CrossRef]
- Hu, G.; Kang, J.; Ng, L.W.T.; Zhu, X.; Howe, R.C.T.; Jones, C.G.; Hersam, M.C.; Hasan, T. Functional Inks and Printing of Two-Dimensional Materials. Chem. Soc. Rev. 2018, 47, 3265–3300. [Google Scholar] [CrossRef] [PubMed]
- Finn, D.J.; Lotya, M.; Coleman, J.N. Inkjet Printing of Silver Nanowire Networks. ACS Appl. Mater. Interfaces 2015, 7, 9254–9261. [Google Scholar] [CrossRef]
- Al-Milaji, K.N.; Huang, Q.; Li, Z.; Ng, T.N.; Zhao, H. Direct Embedment and Alignment of Silver Nanowires by Inkjet Printing for Stretchable Conductors. ACS Appl. Electron. Mater. 2020, 2, 3289–3298. [Google Scholar] [CrossRef]
- Patil, P.; Patil, S.; Kate, P.; Kulkarni, A.A. Inkjet Printing of Silver Nanowires on Flexible Surfaces and Methodologies to Improve the Conductivity and Stability of the Printed Patterns. Nanoscale Adv. 2021, 3, 240–248. [Google Scholar] [CrossRef]
- Calvert, P. Inkjet Printing for Materials and Devices. Chem. Mater. 2001, 13, 3299–3305. [Google Scholar] [CrossRef]
- Derby, B. Bioprinting: Inkjet Printing Proteins and Hybrid Cell-Containing Materials and Structures. J. Mater. Chem. 2008, 18, 5717–5721. [Google Scholar] [CrossRef]
- Delaney, J.T.; Smith, P.J.; Schubert, U.S. Inkjet Printing of Proteins. Soft Matter 2009, 5, 4866–4877. [Google Scholar] [CrossRef]
- Donderwinkel, I.; van Hest, J.C.M.; Cameron, N.R. Bio-Inks for 3D Bioprinting: Recent Advances and Future Prospects. Polym. Chem. 2017, 8, 4451–4471. [Google Scholar] [CrossRef]
- Bachmann, B.; Adly, N.Y.; Schnitker, J.; Yakushenko, A.; Rinklin, P.; Offenhäusser, A.; Wolfrum, B. All-Inkjet-Printed Gold Microelectrode Arrays for Extracellular Recording of Action Potentials. Flex. Print. Electron. 2017, 2, 035003. [Google Scholar] [CrossRef]
- Adly, N.; Weidlich, S.; Seyock, S.; Brings, F.; Yakushenko, A.; Offenhäusser, A.; Wolfrum, B. Printed Microelectrode Arrays on Soft Materials: From PDMS to Hydrogels. NPJ Flex. Electron. 2018, 2, 15. [Google Scholar] [CrossRef]
- Schnitker, J.; Adly, N.; Seyock, S.; Bachmann, B.; Yakushenko, A.; Wolfrum, B.; Offenhäusser, A. Rapid Prototyping of Ultralow-Cost, Inkjet-Printed Carbon Microelectrodes for Flexible Bioelectronic Devices. Adv. Biosyst. 2018, 2, 1700136. [Google Scholar] [CrossRef]
- Garma, L.D.; Ferrari, L.M.; Scognamiglio, P.; Greco, F.; Santoro, F. Inkjet-Printed PEDOT:PSS Multi-Electrode Arrays for Low-Cost in Vitro Electrophysiology. Lab Chip 2019, 19, 3776–3786. [Google Scholar] [CrossRef]
- Roberts, T.; Graaf, J.B.D.; Nicol, C.; Hervé, T.; Fiocchi, M.; Sanaur, S. Flexible Inkjet-Printed Multielectrode Arrays for Neuromuscular Cartography. Adv. Healthc. Mater. 2016, 5, 1462–1470. [Google Scholar] [CrossRef]
- Borda, E.; Ferlauto, L.; Schleuniger, J.; Mustaccio, A.; Lütolf, F.; Lücke, A.; Fricke, S.; Marjanović, N.; Ghezzi, D. All-Printed Electrocorticography Array for In Vivo Neural Recordings. Adv. Eng. Mater. 2020, 22, 1901403. [Google Scholar] [CrossRef]
- Skardal, A.; Shupe, T.; Atala, A. Organoid-on-a-Chip and Body-on-a-Chip Systems for Drug Screening and Disease Modeling. Drug Discov. Today 2016, 21, 1399–1411. [Google Scholar] [CrossRef] [PubMed]
- Choi, J.S.; Lee, H.J.; Rajaraman, S.; Kim, D.-H. Recent Advances in Three-Dimensional Microelectrode Array Technologies for in Vitro and in Vivo Cardiac and Neuronal Interfaces. Biosens. Bioelectron. 2021, 171, 112687. [Google Scholar] [CrossRef]
- Liu, H.; Bolonduro, O.A.; Hu, N.; Ju, J.; Rao, A.A.; Duffy, B.M.; Huang, Z.; Black, L.D.; Timko, B.P. Heart-on-a-Chip Model with Integrated Extra- and Intracellular Bioelectronics for Monitoring Cardiac Electrophysiology under Acute Hypoxia. Nano Lett. 2020, 20, 2585–2593. [Google Scholar] [CrossRef]
- Cho, Y.H.; Park, Y.-G.; Kim, S.; Park, J.-U. 3D Electrodes for Bioelectronics. Adv. Mater. 2005. [Google Scholar] [CrossRef]
- Campbell, P.K.; Jones, K.E.; Huber, R.J.; Horch, K.W.; Normann, R.A. A Silicon-Based, Three-Dimensional Neural Interface: Manufacturing Processes for an Intracortical Electrode Array. IEEE Trans. Biomed. Eng. 1991, 38, 758–768. [Google Scholar] [CrossRef] [PubMed]
- Boretius, T.; Badia, J.; Pascual-Font, A.; Schuettler, M.; Navarro, X.; Yoshida, K.; Stieglitz, T. A Transverse Intrafascicular Multichannel Electrode (TIME) to Interface with the Peripheral Nerve. Biosens. Bioelectron. 2010, 26, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Simeral, J.D.; Kim, S.-P.; Black, M.J.; Donoghue, J.P.; Hochberg, L.R. Neural Control of Cursor Trajectory and Click by a Human with Tetraplegia 1000 Days after Implant of an Intracortical Microelectrode Array. J. Neural Eng. 2011, 8, 025027. [Google Scholar] [CrossRef] [PubMed]
- Fekete, Z. Recent Advances in Silicon-Based Neural Microelectrodes and Microsystems: A Review. Sens. Actuators B Chem. 2015, 215, 300–315. [Google Scholar] [CrossRef]
- Leber, M.; Körner, J.; Reiche, C.F.; Yin, M.; Bhandari, R.; Franklin, R.; Negi, S.; Solzbacher, F. Advances in Penetrating Multichannel Microelectrodes Based on the Utah Array Platform. In Neural Interface: Frontiers and Applications; Zheng, X., Ed.; Advances in Experimental Medicine and Biology; Springer: Singapore, 2019; pp. 1–40. ISBN 9789811320507. [Google Scholar]
- Heuschkel, M.O.; Fejtl, M.; Raggenbass, M.; Bertrand, D.; Renaud, P. A Three-Dimensional Multi-Electrode Array for Multi-Site Stimulation and Recording in Acute Brain Slices. J. Neurosci. Methods 2002, 114, 135–148. [Google Scholar] [CrossRef]
- Nam, Y.; Wheeler, B.C.; Heuschkel, M.O. Neural Recording and Stimulation of Dissociated Hippocampal Cultures Using Microfabricated Three-Dimensional Tip Electrode Array. J. Neurosci. Methods 2006, 155, 296–299. [Google Scholar] [CrossRef]
- Liu, Y.; McGuire, A.F.; Lou, H.-Y.; Li, T.L.; Tok, J.B.-H.; Cui, B.; Bao, Z. Soft Conductive Micropillar Electrode Arrays for Biologically Relevant Electrophysiological Recording. Proc. Natl. Acad. Sci. USA 2018, 115, 11718–11723. [Google Scholar] [CrossRef] [PubMed]
- Patil, A.C.; Thakor, N.V. Implantable Neurotechnologies: A Review of Micro- and Nanoelectrodes for Neural Recording. Med. Biol. Eng. Comput. 2016, 54, 23–44. [Google Scholar] [CrossRef] [PubMed]
- Szostak, K.M.; Grand, L.; Constandinou, T.G. Neural Interfaces for Intracortical Recording: Requirements, Fabrication Methods, and Characteristics. Front. Neurosci. 2017, 11, 665. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Liu, B.; Zhou, W.; Jiang, L. A Mini Review of Microneedle Array Electrode for Bio-Signal Recording: A Review. IEEE Sens. J. 2020, 20, 577–590. [Google Scholar] [CrossRef]
- Kundu, A.; Nattoo, C.; Fremgen, S.; Springer, S.; Ausaf, T.; Rajaraman, S. Optimization of Makerspace Microfabrication Techniques and Materials for the Realization of Planar, 3D Printed Microelectrode Arrays in under Four Days. RSC Adv. 2019, 9, 8949–8963. [Google Scholar] [CrossRef]
- Didier, C.M.; Kundu, A.; Rajaraman, S. Facile, Packaging Substrate-Agnostic, Microfabrication and Assembly of Scalable 3D Metal Microelectrode Arrays for in Vitro Organ-on-a-Chip and Cellular Disease Modeling. In Proceedings of the 2019 20th International Conference on Solid-State Sensors, Actuators and Microsystems Eurosensors XXXIII (TRANSDUCERS EUROSENSORS XXXIII), Berlin, Germany, 23–27 June 2019; pp. 1686–1689. [Google Scholar]
- Azim, N.; Kundu, A.; Royse, M.; Sip, Y.Y.L.; Young, M.; Santra, S.; Zhai, L.; Rajaraman, S. Fabrication and Characterization of a 3D Printed, MicroElectrodes Platform With Functionalized Electrospun Nano-Scaffolds and Spin Coated 3D Insulation Towards Multi- Functional Biosystems. J. Microelectromech. Syst. 2019, 28, 606–618. [Google Scholar] [CrossRef]
- Morales-Carvajal, P.M.; Kundu, A.; Didier, C.M.; Hart, C.; Sommerhage, F.; Rajaraman, S. Makerspace Microfabrication of a Stainless Steel 3D Microneedle Electrode Array (3D MEA) on a Glass Substrate for Simultaneous Optical and Electrical Probing of Electrogenic Cells. RSC Adv. 2020, 10, 41577–41587. [Google Scholar] [CrossRef]
- Kundu, A.; Rozman, A.; Rajaraman, S. Development of a 3D Printed, Self-Insulated, High-Throughput 3D Microelectrode Array (HT-3DMEA). J. Microelectromech. Syst. 2020, 29, 1091–1093. [Google Scholar] [CrossRef]
- Ko, S.H.; Chung, J.; Hotz, N.; Nam, K.H.; Grigoropoulos, C.P. Metal Nanoparticle Direct Inkjet Printing for Low-Temperature 3D Micro Metal Structure Fabrication. J. Micromech. Microeng. 2010, 20, 125010. [Google Scholar] [CrossRef]
- Kullmann, C.; Schirmer, N.C.; Lee, M.-T.; Ko, S.H.; Hotz, N.; Grigoropoulos, C.P.; Poulikakos, D. 3D Micro-Structures by Piezoelectric Inkjet Printing of Gold Nanofluids. J. Micromech. Microeng. 2012, 22, 055022. [Google Scholar] [CrossRef]
- Nallan, H.C.; Sadie, J.A.; Kitsomboonloha, R.; Volkman, S.K.; Subramanian, V. Systematic Design of Jettable Nanoparticle-Based Inkjet Inks: Rheology, Acoustics, and Jettability. Langmuir 2014, 30, 13470–13477. [Google Scholar] [CrossRef] [PubMed]
- Layani, M.; Cooperstein, I.; Magdassi, S. UV Crosslinkable Emulsions with Silver Nanoparticles for Inkjet Printing of Conductive 3D Structures. J. Mater. Chem. C 2013, 1, 3244–3249. [Google Scholar] [CrossRef]
- Saleh, E.; Zhang, F.; He, Y.; Vaithilingam, J.; Fernandez, J.L.; Wildman, R.; Ashcroft, I.; Hague, R.; Dickens, P.; Tuck, C. 3D Inkjet Printing of Electronics Using UV Conversion. Adv. Mater. Technol. 2017, 2, 1700134. [Google Scholar] [CrossRef]
- Sadie, J.A.; Subramanian, V. Three-Dimensional Inkjet-Printed Interconnects Using Functional Metallic Nanoparticle Inks. Adv. Funct. Mater. 2014, 24, 6834–6842. [Google Scholar] [CrossRef]
- Tehrani, B.K.; Cook, B.S.; Tentzeris, M.M. Inkjet-Printed 3D Interconnects for Millimeter-Wave System-on-Package Solutions. In Proceedings of the 2016 IEEE MTT-S International Microwave Symposium (IMS), San Francisco, CA, USA, 22–27 May 2016; pp. 1–4. [Google Scholar]
- Khorramdel, B.; Kraft, T.M.; Mantysalo, M. Inkjet Printed Metallic Micropillars for Bare-Die Flip-Chip Bonding. Flex. Print. Electron. 2017. [Google Scholar] [CrossRef]
- Vaithilingam, J.; Saleh, E.; Körner, L.; Wildman, R.D.; Hague, R.J.M.; Leach, R.K.; Tuck, C.J. 3-Dimensional Inkjet Printing of Macro Structures from Silver Nanoparticles. Mater. Des. 2018, 139, 81–88. [Google Scholar] [CrossRef]
- Sowade, E.; Polomoshnov, M.; Willert, A.; Baumann, R.R. Toward 3D-Printed Electronics: Inkjet-Printed Vertical Metal Wire Interconnects and Screen-Printed Batteries. Adv. Eng. Mater. 2019, 21, 1900568. [Google Scholar] [CrossRef]
- Grob, L.; Yamamoto, H.; Zips, S.; Rinklin, P.; Hirano-Iwata, A.; Wolfrum, B. Printed 3D Electrode Arrays with Micrometer-Scale Lateral Resolution for Extracellular Recording of Action Potentials. Adv. Mater. Technol. 2020, 5, 1900517. [Google Scholar] [CrossRef]
- Elias, J.; Gizowska, M.; Brodard, P.; Widmer, R.; de Hazan, Y.; Graule, T.; Michler, J.; Philippe, L. Electrodeposition of Gold Thin Films with Controlled Morphologies and Their Applications in Electrocatalysis and SERS. Nanotechnology 2012, 23, 255705. [Google Scholar] [CrossRef]
- Cui, X.; Martin, D.C. Fuzzy Gold Electrodes for Lowering Impedance and Improving Adhesion with Electrodeposited Conducting Polymer Films. Sens. Actuators A Phys. 2003, 103, 384–394. [Google Scholar] [CrossRef]
- Raub, C.J.; Knödler, A. The Electrodeposition of Gold by Pulse Plating. Gold Bull 1977, 10, 38–44. [Google Scholar] [CrossRef]
- Schlesinger, M.; Paunovic, M. Modern Electroplating, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 2011; ISBN 978-1-118-06314-9. [Google Scholar]
- Ha, H.; Payer, J. The Effect of Silver Chloride Formation on the Kinetics of Silver Dissolution in Chloride Solution. Electrochim. Acta 2011, 56, 2781–2791. [Google Scholar] [CrossRef]
- Choi, Y.-J.; Luo, T.-J.M. Electrochemical Properties of Silver Nanoparticle Doped Aminosilica Nanocomposite. Int. J. Electrochem. 2011. [Google Scholar] [CrossRef]
- Yakushenko, A.; Mayer, D.; Buitenhuis, J.; Offenhäusser, A.; Wolfrum, B. Electrochemical Artifacts Originating from Nanoparticle Contamination by Ag/AgCl Quasi-Reference Electrodes. Lab Chip 2014, 14, 602–607. [Google Scholar] [CrossRef] [PubMed]
- Hwang, J.C.M.; Pan, J.D.; Balluffi, R.W. Measurement of Grain-boundary Diffusion at Low Temperature by the Surface-accumulation Method. II. Results for Gold-silver System. J. Appl. Phys. 1979, 50, 1349–1359. [Google Scholar] [CrossRef]
- Wu, Q.; Soppa, K.; Scherrer, N.; Watts, B.; Yokosawa, T.; Bernard, L.; Araki, T.; Döbeli, M.; Meyer, M.; Spiecker, E.; et al. Investigation of the Foil Structure and Corrosion Mechanisms of Modern Zwischgold Using Advanced Analysis Techniques. J. Cult. Herit. 2018, 31, 122–132. [Google Scholar] [CrossRef]
- Jamil, Z.; Ruiz-Trejo, E.; Brandon, N.P. Nickel Electrodeposition on Silver for the Development of Solid Oxide Fuel Cell Anodes and Catalytic Membranes. J. Electrochem. Soc. 2017, 164, D210–D217. [Google Scholar] [CrossRef]
- Yasin, H.M.; Denuault, G.; Pletcher, D. Studies of the Electrodeposition of Platinum Metal from a Hexachloroplatinic Acid Bath. J. Electroanal. Chem. 2009, 633, 327–332. [Google Scholar] [CrossRef]
- Djokic, S.S. Electrodeposition of Platinum from an Alkaline Electrolyte. Plat. Surf. Finish. 2000, 87, 145–147. [Google Scholar]
- Hussain, G.; Aldous, L.; Silvester, D.S. Preparation of Platinum-Based ‘cauliflower Microarrays’ for Enhanced Ammonia Gas Sensing. Anal. Chim. Acta 2019, 1048, 12–21. [Google Scholar] [CrossRef]
- Yakushenko, A.; Schöps, V.; Mayer, D.; Offenhäusser, A.; Wolfrum, B. On-Chip Fast Scan Cyclic Voltammetry for Selective Detection of Redox Active Neurotransmitters. Phys. Status Solidi 2014, 211, 1364–1371. [Google Scholar] [CrossRef]
- Yamamoto, H.; Grob, L.; Sumi, T.; Oiwa, K.; Hirano-Iwata, A.; Wolfrum, B. Ultrasoft Silicone Gel as a Biomimetic Passivation Layer in Inkjet-Printed 3D MEA Devices. Adv. Biosyst. 2019, 3, 1900130. [Google Scholar] [CrossRef] [PubMed]
- Roshanghias, A.; Krivec, M.; Baumgart, M. Sintering Strategies for Inkjet Printed Metallic Traces in 3D Printed Electronics. Flex. Print. Electron. 2017, 2, 045002. [Google Scholar] [CrossRef]


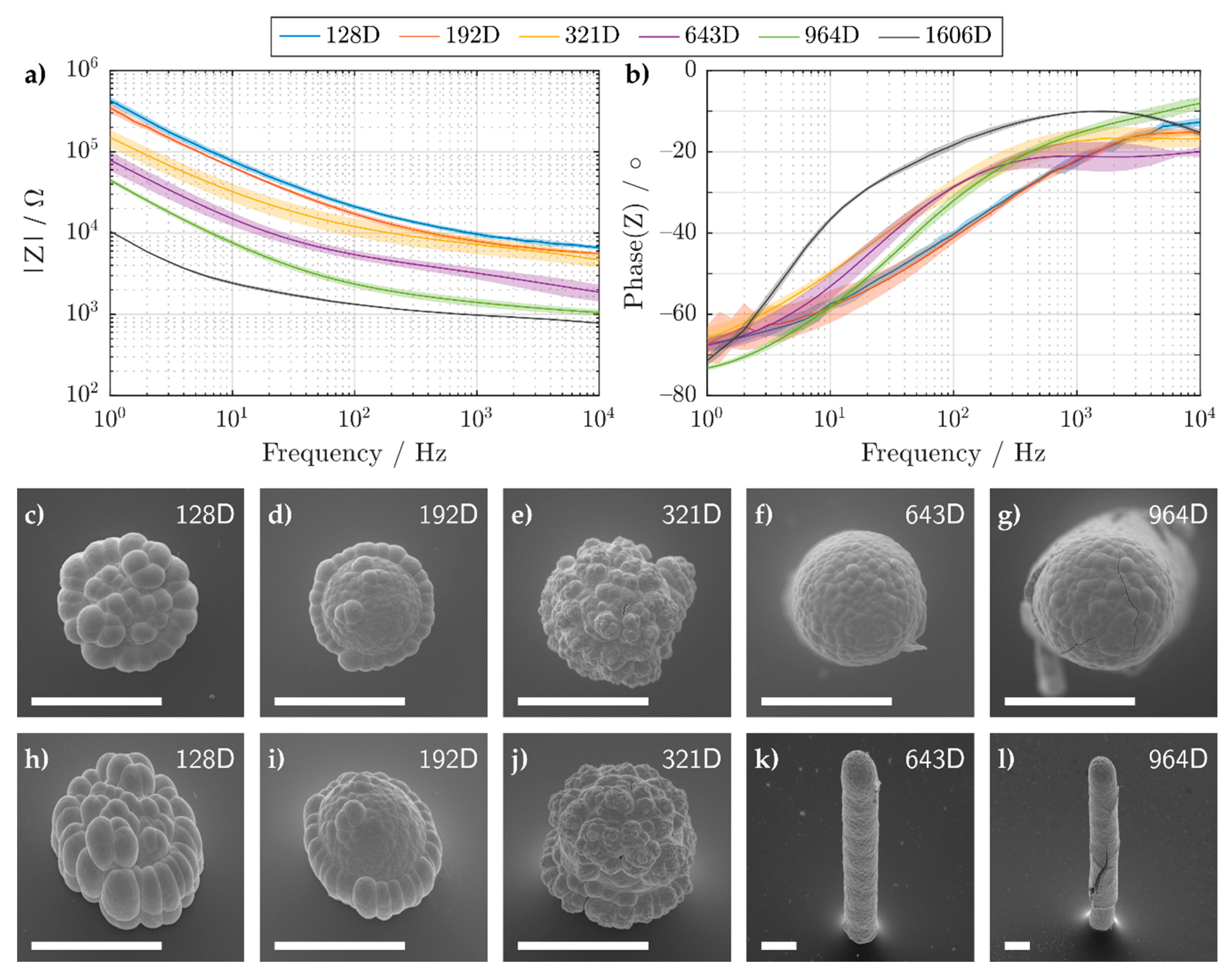


| Droplet Num. | 128 | 192 | 321 | 643 | 964 | 1606 |
|---|---|---|---|---|---|---|
| Q/µFs(α−1) | 0.7 ± 0.1 | 0.9 ± 0.2 | 2.3 ± 0.7 | 4 ± 1 | 5.7 ± 0.2 | 33 ± 4 |
| α | 0.71 ± 0.03 | 0.69 ± 0.05 | 0.65 ± 0.01 | 0.68 ± 0.02 | 0.77 ± 0.01 | 0.64 ± 0.05 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grob, L.; Rinklin, P.; Zips, S.; Mayer, D.; Weidlich, S.; Terkan, K.; Weiß, L.J.K.; Adly, N.; Offenhäusser, A.; Wolfrum, B. Inkjet-Printed and Electroplated 3D Electrodes for Recording Extracellular Signals in Cell Culture. Sensors 2021, 21, 3981. https://doi.org/10.3390/s21123981
Grob L, Rinklin P, Zips S, Mayer D, Weidlich S, Terkan K, Weiß LJK, Adly N, Offenhäusser A, Wolfrum B. Inkjet-Printed and Electroplated 3D Electrodes for Recording Extracellular Signals in Cell Culture. Sensors. 2021; 21(12):3981. https://doi.org/10.3390/s21123981
Chicago/Turabian StyleGrob, Leroy, Philipp Rinklin, Sabine Zips, Dirk Mayer, Sabrina Weidlich, Korkut Terkan, Lennart J. K. Weiß, Nouran Adly, Andreas Offenhäusser, and Bernhard Wolfrum. 2021. "Inkjet-Printed and Electroplated 3D Electrodes for Recording Extracellular Signals in Cell Culture" Sensors 21, no. 12: 3981. https://doi.org/10.3390/s21123981
APA StyleGrob, L., Rinklin, P., Zips, S., Mayer, D., Weidlich, S., Terkan, K., Weiß, L. J. K., Adly, N., Offenhäusser, A., & Wolfrum, B. (2021). Inkjet-Printed and Electroplated 3D Electrodes for Recording Extracellular Signals in Cell Culture. Sensors, 21(12), 3981. https://doi.org/10.3390/s21123981







