Enhanced Monitoring of Sleep Position in Sleep Apnea Patients: Smartphone Triaxial Accelerometry Compared with Video-Validated Position from Polysomnography
Abstract
1. Introduction
2. Materials and Methods
2.1. Hospital Database and Acquisition Protocol
2.2. Signal Preprocessing
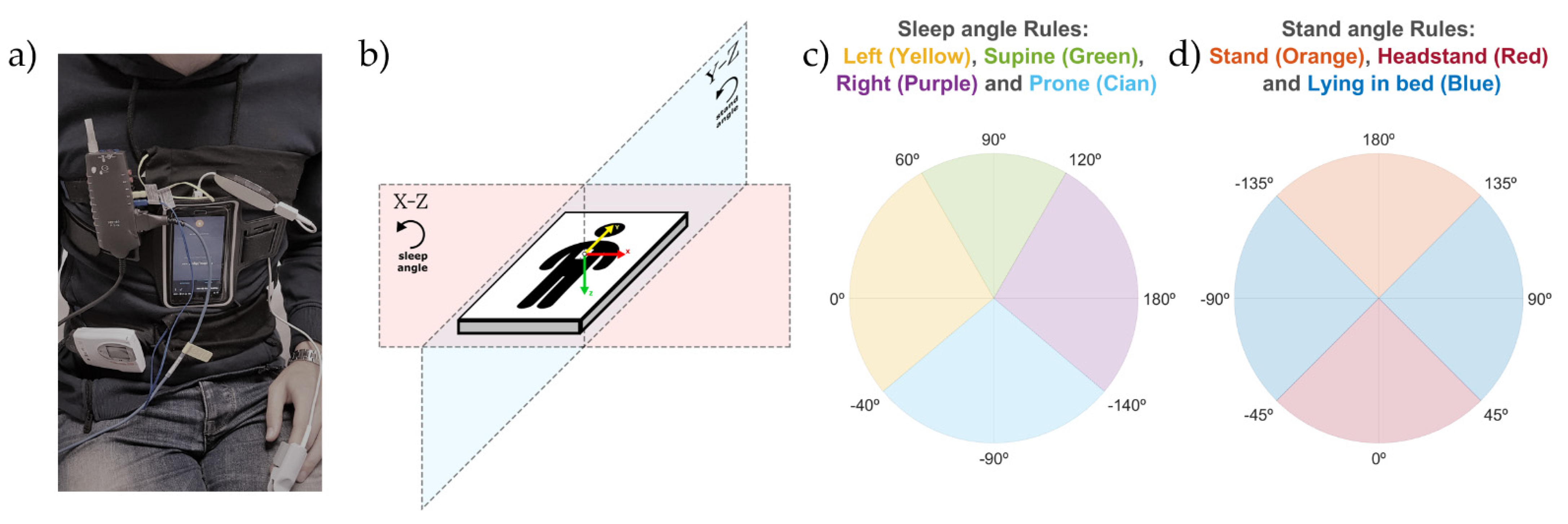
2.3. Sleep Position Monitoring: Sleep and Stand Angles
- Each of the triaxial accelerometer signals (X, Y, and Z) was filtered with a median filter with a window of 60 s to remove high-frequency noise and keep the signal baseline containing the gravity acceleration.
- For each sample of the triaxial accelerometry, two different angles were calculated using (1): the sleep position angle and the stand angle.
- 2.1.
- The sleep position angle was used to determine the four sleep positions (supine, prone, left and right). It was calculated by (1), where , with and being the values of the X and Z axis of the triaxial accelerometry at each specific timestamp; , which is a static reference aligned with the left sleep position; and , to be able to differentiate supine and prone positions. This angle explains the orientation of the accelerometry in the X-Z plane as shown in Figure 1b.
- 2.2.
- The stand angle was used to determine whether the patient is in a standing or lying position. It was calculated by (1), where , with and being the values of the Y and Z axis of the triaxial accelerometry at each specific timestamp; , which is a static reference aligned with the stand position; and , to be able to differentiate stand and headstand positions. This angle explains the orientation of the accelerometry in the Y-Z plane, as shown in Figure 1b.
- Two different corrections were made to ensure that the angles calculated provided the real patient position:
- 3.1.
- Module correction: to avoid unreal position angle calculations, due to small angle value variations in close-to-zero module vectors, both the sleep and stand angle values were replaced with their last module-correct angle value if the following criteria was found true:where is a threshold with a value of 0.5; belongs to the vectors declared for the sleep and stand angles in the steps 2.1 and 2.2 of this subsection; and , with , and being the values of the X, Y and Z axis of the triaxial accelerometry at each specific timestamp.
- 3.2.
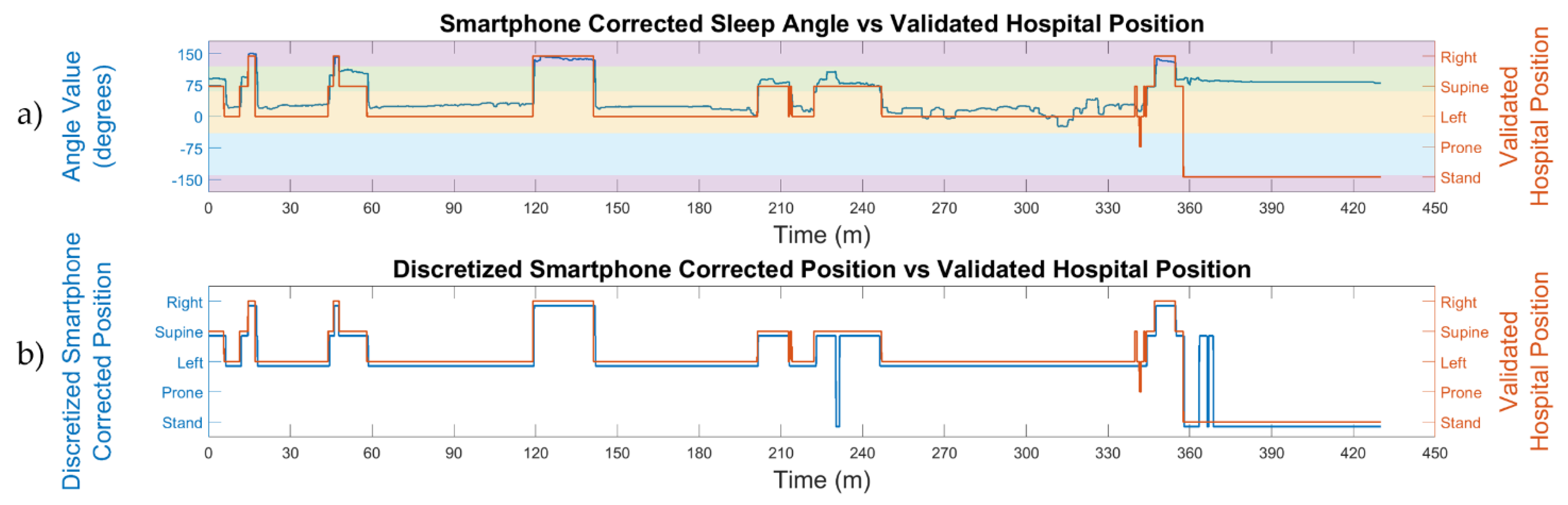
- Initial position correction: the supine positions in the first 10 min of the triaxial accelerometer signals were used to automatically self-correct the differences in smartphone placement due to anatomical variations in patients. The correction consisted of detecting the values of the angles associated with the supine positions within these initial 10 min and subtracting these values to correct the sleep and stand angles of the remaining data. Both the initial sleep and stand angles of the corrected position were 90° after the correction for the supine sleep position.
2.4. Discretization of Sleep and Stand Angles
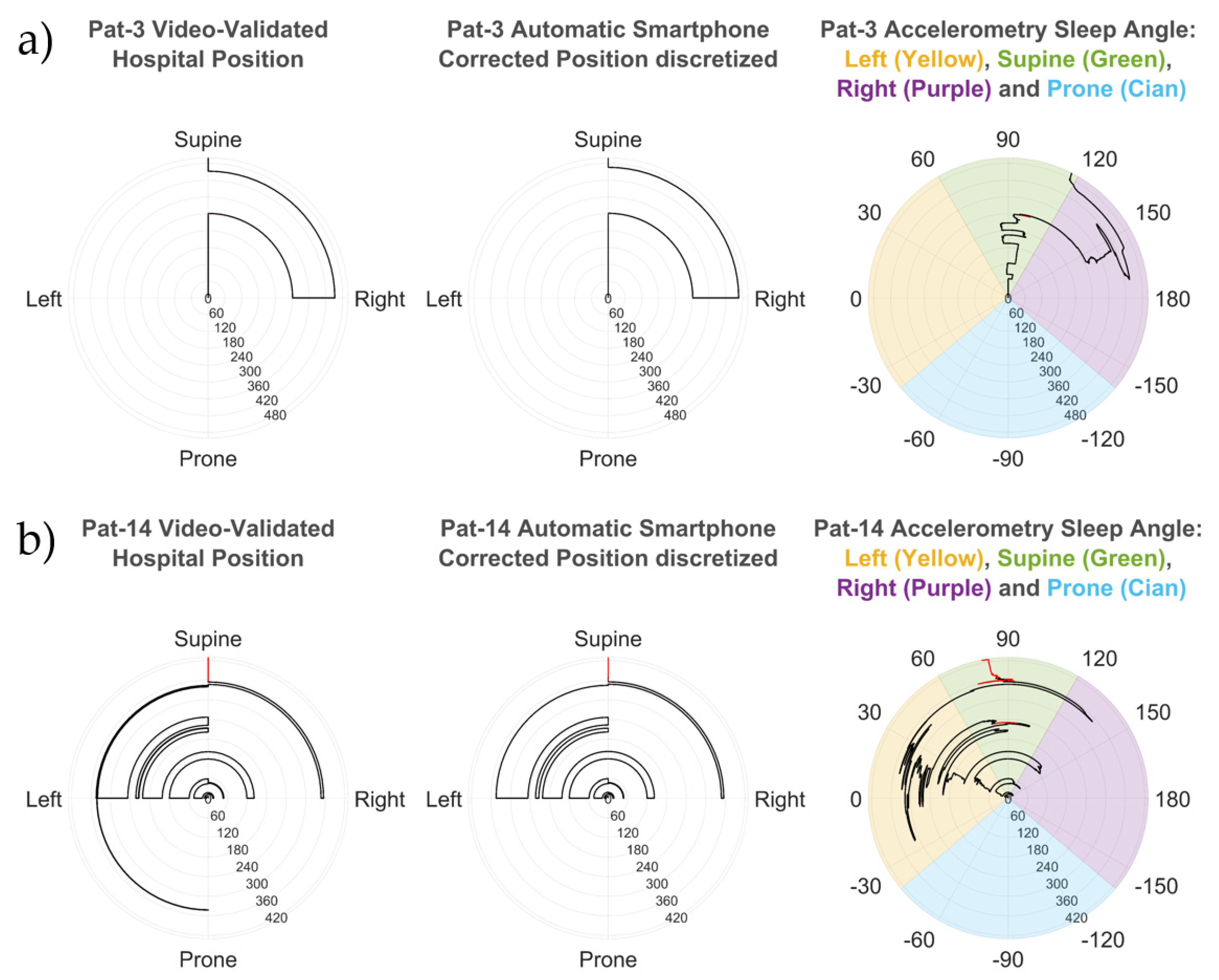
2.5. Sleep Position Validation: Agreement by Patient
- The automatic smartphone accelerometry position vs. the validated hospital position: this analysis was made to compare the position obtained from the discretized smartphone angles, without the initial position correction, to the hospital video-validated reference position.
- The automatic smartphone accelerometry corrected position vs. the validated hospital position: this analysis was made to compare the position obtained from the discretized smartphone angles with the initial position correction to the hospital video-validated reference position.
- The automatic hospital position vs. the validated hospital position: this comparison served to understand how the corrections introduced by sleep technicians can improve the automatic sleep position from the PSG system.
2.6. Sleep Position Validation: Agreement by Position
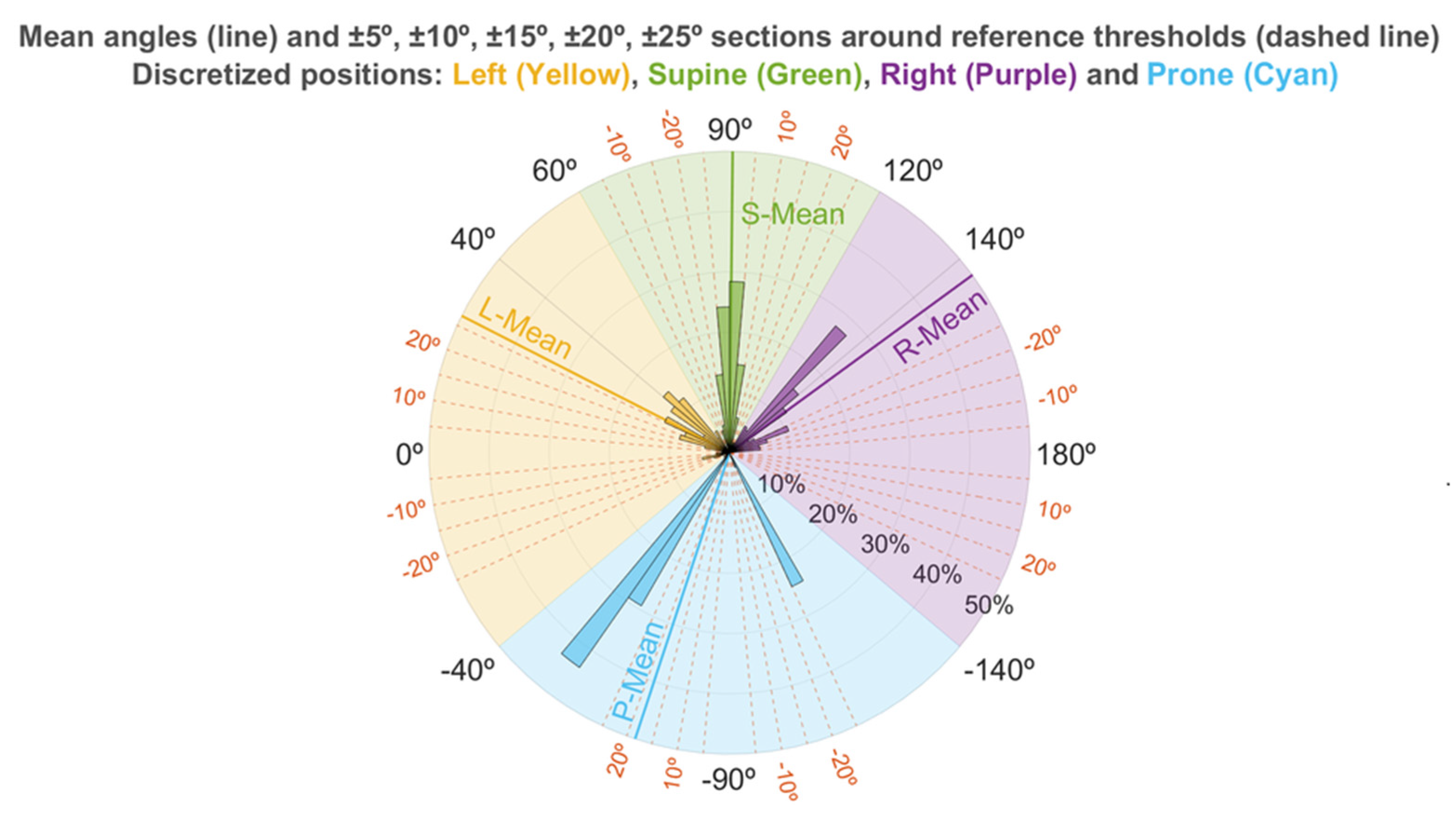
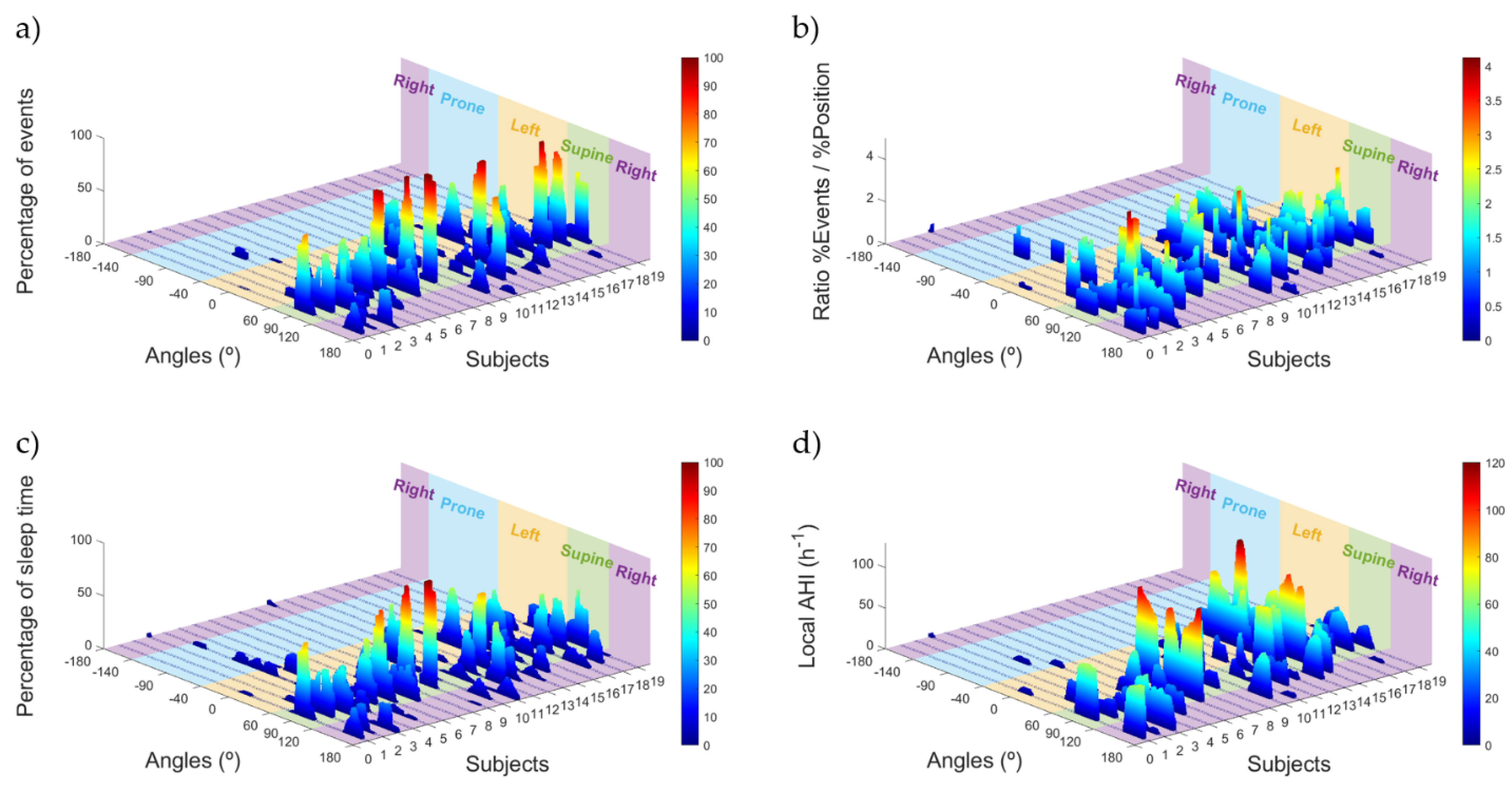
2.7. Sleep Position Characterization: Angle Distribution
2.8. OSA Events Related to Sleep Position Angle
3. Results
3.1. Sleep Position: Angles and Discretization
3.2. Sleep Position Performance: Patient Overlap
3.3. Sleep Position Performance: Position Overlap
3.4. Sleep Position Characterization: Angle Distribution
3.5. OSA Events Related to Sleep Position Angle
4. Discussion
4.1. Sleep Position: Discrete PSG vs. Discrete Sleep Angle
4.2. Enhanced Sleep Position Monitoring
4.3. Smartphone Sleep Monitors: Portable mHealth Tools
5. Conclusions
6. Patents
Author Contributions
Funding
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AASM | American academy of sleep medicine |
| Acc | Accuracy |
| AHI | Apnea hypopnea index |
| FN | False negative |
| FP | False positive |
| HRP | Home respiratory polygraphy |
| mHealth | Mobile health |
| Min | Minutes |
| NPV | Negative predictive value |
| OSA | Obstructive sleep apnea |
| Pos | Position |
| pOSA | Positional obstructive sleep apnea |
| PPV | Positive predictive value |
| PSG | Polysomnography |
| Se | Sensitivity |
| Sp | Specificity |
| Thld | Threshold |
| TN | True negative |
| TP | True positive |
| Win | Window |
References
- Bishop, T.M.; Walsh, P.G.; Ashrafioun, L.; Lavigne, J.E.; Pigeon, W.R. Sleep, suicide behaviors, and the protective role of sleep medicine. Sleep Med. 2019, 66, 264–270. [Google Scholar] [CrossRef]
- Stowkowy, J.; Brummitt, K.; Bonneville, D.; Goldstein, B.I.; Wang, J.; Kennedy, S.H.; Bray, S.; Lebel, C.; MacQueen, G.; Addington, J. Sleep disturbances in youth at-risk for serious mental illness. Early Interv. Psychiatry 2019, 14, 373–383. [Google Scholar] [CrossRef]
- Augner, C. Associations of Subjective Sleep Quality with Depression Score, Anxiety, Physical Symptoms and Sleep Onset Latency in Young Students. Cent. Eur. J. Public Health 2011, 19, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Bartlett, D.J.; Marshall, N.S.; Williams, A.; Grunstein, R.R. Sleep health New South Wales: Chronic sleep restriction and daytime sleepiness. Intern. Med. J. 2007, 38, 24–31. [Google Scholar] [CrossRef] [PubMed]
- Yoshihisa, A.; Takeishi, Y. Sleep Disordered Breathing and Cardiovascular Diseases. J. Atheroscler. Thromb. 2019, 26, 315–327. [Google Scholar] [CrossRef] [PubMed]
- Durgan, D.J.; Bryan, R.M. Cerebrovascular Consequences of Obstructive Sleep Apnea. J. Am. Heart Assoc. 2012, 1, e000091. [Google Scholar] [CrossRef]
- Eckert, D.J.; Malhotra, A. Pathophysiology of Adult Obstructive Sleep Apnea. Proc. Am. Thorac. Soc. 2008, 5, 144–153. [Google Scholar] [CrossRef]
- Patil, S.P.; Schneider, H.; Schwartz, A.R.; Smith, P.L. Adult Obstructive Sleep Apnea. Chest 2007, 132, 325–337. [Google Scholar] [CrossRef]
- Osman, A.M.; Carter, S.G.; Carberry, J.C.; Eckert, D.J. Obstructive sleep apnea: Current perspectives. Nat. Sci. Sleep 2018, 10, 21–34. [Google Scholar] [CrossRef]
- Rundo, J.V. Obstructive sleep apnea basics. Cleve. Clin. J. Med. 2019, 86, 2–9. [Google Scholar] [CrossRef] [PubMed]
- Berry, R.B.; Budhiraja, R.; Gottlieb, D.J.; Gozal, D.; Iber, C.; Kapur, V.K.; Marcus, C.L.; Mehra, R.; Parthasarathy, S.; Quan, S.F.; et al. Rules for scoring respiratory events in sleep: Update of the 2007 AASM Manual for the Scoring of Sleep and Associated Events. Deliberations of the Sleep Apnea Definitions Task Force of the American Academy of Sleep Medicine. J. Clin. Sleep Med. 2012, 8, 597–619. [Google Scholar] [CrossRef] [PubMed]
- Oksenberg, A.; Silverberg, D.S.; Arons, E.; Radwan, H. Positional vs nonpositional obstructive sleep apnea patients: Anthropomorphic, nocturnal polysomnographic, and multiple sleep latency test data. Chest 1997, 112, 629–639. [Google Scholar] [CrossRef] [PubMed]
- Yalciner, G.; Babademez, M.A.; Gul, F. Association of sleep time in supine position with apnea-hypopnea index as evidenced by successive polysomnography. Sleep Breath 2017, 21, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Alevi, D.; Perry, H.D.; Wedel, A.; Rosenberg, E.; Alevi, L.; Donnenfeld, E.D. Effect of sleep position on the ocular surface. Cornea 2017, 36, 567–571. [Google Scholar] [CrossRef]
- Kaplowitz, K.; Dredge, J.; Honkanen, R. Relationship between sleep position and glaucoma progression. Curr. Opin. Ophthalmol. 2019. [Google Scholar] [CrossRef]
- Kaplowitz, K.; Blizzard, S.; Blizzard, D.J.; Nwogu, E.; Hamill, C.E.; Weinreb, R.N.; Mohsenin, V.; Loewen, N.A. Time spent in lateral sleep position and asymmetry in glaucoma. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3869–3874. [Google Scholar] [CrossRef][Green Version]
- Buysse, D.J.; Reynolds, C.F.; Monk, T.H.; Berman, S.R.; Kupfer, D.J. The Pittsburgh sleep quality index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989, 28, 193–213. [Google Scholar] [CrossRef]
- Johns, M.W. A New Method for Measuring Daytime Sleepiness: The Epworth Sleepiness Scale. Sleep 1991, 14, 540–545. [Google Scholar] [CrossRef] [PubMed]
- Chung, F.; Yegneswaran, B.; Liao, P.; Chung, S.A.; Vairavanathan, S.; Islam, S.; Khajehdehi, A.; Shapiro, C.M. STOP Questionnaire. Anesthesiology 2008, 108, 812–821. [Google Scholar] [CrossRef]
- Netzer, N.C.; Stoohs, R.A.; Netzer, C.M.; Clark, K.; Strohl, K.P. Using the Berlin Questionnaire To Identify Patients at Risk for the Sleep Apnea Syndrome. Ann. Intern. Med. 1999, 131, 485. [Google Scholar] [CrossRef]
- Lomeli, H.A.; Pérez-Olmos, I.; Talero-Gutiérrez, C.; Moreno, C.B.; González-Reyes, R.; Palacios, L.; de la Peña, F.; Muñoz-Delgado, J. Sleep evaluation scales and questionaries: A review. Actas Esp. Psiquiatr. 2008, 36, 50–59. [Google Scholar] [PubMed]
- Young, T.; Palta, M.; Dempsey, J.; Skatrud, J.; Weber, S.; Badr, S. The Occurrence of Sleep-Disordered Breathing among Middle-Aged Adults. N. Engl. J. Med. 1993, 328, 1230–1235. [Google Scholar] [CrossRef]
- Dalewski, B.; Kamińska, A.; Syrico, A.; Kałdunska, A.; Pałka, Ł.; Sobolewska, E. The Usefulness of Modified Mallampati Score and CT Upper Airway Volume Measurements in Diagnosing OSA among Patients with Breathing-Related Sleep Disorders. Appl. Sci. 2021, 11, 3764. [Google Scholar] [CrossRef]
- Philip, P.; Bailly, S.; Benmerad, M.; Micoulaud-Franchi, J.A.; Grillet, Y.; Sapène, M.; Jullian-Desayes, I.; Joyeux-Faure, M.; Tamisier, R.; Pépin, J.L. Self-reported sleepiness and not the apnoea hypopnoea index is the best predictor of sleepiness-related accidents in obstructive sleep apnoea. Sci. Rep. 2020, 10, 16267. [Google Scholar] [CrossRef]
- Russo, K.; Bianchi, M.T. How reliable is self-reported body position during sleep? J. Clin. Sleep Med. 2016, 12, 127–128. [Google Scholar] [CrossRef][Green Version]
- Vonk, P.E.; de Vries, N.; Ravesloot, M.J.L. Polysomnography and sleep position, a Heisenberg phenomenon?: A large-scale series. HNO 2019, 67, 679–684. [Google Scholar] [CrossRef]
- Metersky, M.L.; Castriotta, R.J. The effect of polysomnography on sleep position: Possible implications on the diagnosis of positional obstructive sleep apnea. Respiration 1996, 63, 283–287. [Google Scholar] [CrossRef]
- Simpson, L.; Hillman, D.R.; Cooper, M.N.; Ward, K.L.; Hunter, M.; Cullen, S.; James, A.; Palmer, L.J.; Mukherjee, S.; Eastwood, P. High prevalence of undiagnosed obstructive sleep apnoea in the general population and methods for screening for representative controls. Sleep Breath. 2013, 17, 967–973. [Google Scholar] [CrossRef]
- Aurora, R.N.; Collop, N.A.; Jacobowitz, O.; Thomas, S.M.; Quan, S.F.; Aronsky, A.J. Quality measures for the care of adult patients with obstructive sleep apnea. J. Clin. Sleep Med. 2015, 11, 357–383. [Google Scholar] [CrossRef] [PubMed]
- Xia, F.; Sawan, M. Clinical and Research Solutions to Manage Obstructive Sleep Apnea: A Review. Sensors 2021, 21, 1784. [Google Scholar] [CrossRef]
- Jane, R. Engineering Sleep Disorders: From classical CPAP devices toward new intelligent adaptive ventilatory therapy. IEEE Pulse 2014, 5, 29–32. [Google Scholar] [CrossRef]
- Epstein, L.J.; Kristo, D.; Strollo, P.J.; Friedman, N.; Malhotra, A.; Patil, S.P.; Ramar, K.; Rogers, R.; Schwab, R.J.; Weaver, E.M.; et al. Clinical guideline for the evaluation, management and long-term care of obstructive sleep apnea in adults. J. Clin. Sleep Med. 2009, 5, 263–276. [Google Scholar] [PubMed]
- Al-Mardini, M.; Aloul, F.; Sagahyroon, A.; Al-Husseini, L. On the use of smartphones for detecting obstructive sleep apnea. In Proceedings of the 13th IEEE International Conference on BioInformatics and BioEngineering, Chania, Greece, 10–13 November 2013. [Google Scholar]
- Imtiaz, S.A. A Systematic Review of Sensing Technologies for Wearable Sleep Staging. Sensors 2021, 21, 1562. [Google Scholar] [CrossRef] [PubMed]
- Nakano, H.; Hirayama, K.; Sadamitsu, Y.; Toshimitsu, A.; Fujita, H.; Shin, S.; Tanigawa, T. Monitoring sound to quantify snoring and sleep apnea severity using a smartphone: Proof of concept. J. Clin. Sleep Med. 2014, 10, 73–78. [Google Scholar] [CrossRef] [PubMed]
- Camara, M.A.; Castillo, Y.; Blanco-Almazan, D.; Estrada, L.; Jane, R. MHealth tools for monitoring Obstructive Sleep Apnea patients at home: Proof-of-concept. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Jeju Island, Korea, 11–15 July 2017; pp. 1555–1558. [Google Scholar]
- Castillo-Escario, Y.; Ferrer-Lluis, I.; Montserrat, J.M.; Jane, R. Automatic Silence Events Detector from Smartphone Audio Signals: A Pilot mHealth System for Sleep Apnea Monitoring at Home. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 4982–4985. [Google Scholar] [CrossRef]
- Castillo-Escario, Y.; Ferrer-Lluis, I.; Montserrat, J.M.; Jane, R. Entropy Analysis of Acoustic Signals Recorded With a Smartphone for Detecting Apneas and Hypopneas: A Comparison With a Commercial System for Home Sleep Apnea Diagnosis. IEEE Access 2019, 7, 128224–128241. [Google Scholar] [CrossRef]
- Behar, J.; Roebuck, A.; Shahid, M.; Daly, J.; Hallack, A.; Palmius, N.; Stradling, J.; Clifford, G.D. SleepAp: An Automated Obstructive Sleep Apnoea Screening Application for Smartphones. IEEE J. Biomed. Health Inform. 2015, 19, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Ferrer-Lluis, I.; Castillo-Escario, Y.; Montserrat, J.M.; Jane, R. Automatic Event Detector from Smartphone Accelerometry: Pilot mHealth Study for Obstructive Sleep Apnea Monitoring at Home. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, EMBS, Berlin, Germany, 23–27 July 2019. [Google Scholar]
- Ferrer-Lluis, I.; Castillo-Escario, Y.; Montserrat, J.M.; Jane, R. Analysis of Smartphone Triaxial Accelerometry for Monitoring Sleep-Disordered Breathing and Sleep Position at Home. IEEE Access 2020, 8, 71231–71244. [Google Scholar] [CrossRef]
- Haas, D.; Birk, R.; Maurer, J.T.; Hörmann, K.; Stuck, B.A.; Sommer, J.U. Treatment of supine position-related obstructive sleep apnea with smartphone applications. HNO 2017, 65, 148–153. [Google Scholar] [CrossRef]
- Nam, Y.; Kim, Y.; Lee, J. Sleep Monitoring Based on a Tri-Axial Accelerometer and a Pressure Sensor. Sensors 2016, 16, 750. [Google Scholar] [CrossRef] [PubMed]
- Marino, M.; Li, Y.; Rueschman, M.N.; Winkelman, J.W.; Ellenbogen, J.M.; Solet, J.M.; Dulin, H.; Berkman, L.F.; Buxton, O.M. Measuring Sleep: Accuracy, Sensitivity, and Specificity of Wrist Actigraphy Compared to Polysomnography. Sleep 2013, 36, 1747–1755. [Google Scholar] [CrossRef]
- Ancoli-Israel, S.; Cole, R.; Alessi, C.; Chambers, M.; Moorcroft, W.; Pollak, C.P. The Role of Actigraphy in the Study of Sleep and Circadian Rhythms. Sleep 2003, 26, 342–392. [Google Scholar] [CrossRef] [PubMed]
- Dafna, E.; Tarasiuk, A.; Zigel, Y. OSA severity assessment based on sleep breathing analysis using ambient microphone. In Proceedings of the 2013 35th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Osaka, Japan, 3–7 July 2013; pp. 2044–2047. [Google Scholar]
- Brouillette, R.T.; Morielli, A.; Leimanis, A.; Waters, K.A.; Luciano, R.; Ducharme, F.M. Nocturnal Pulse Oximetry as an Abbreviated Testing Modality for Pediatric Obstructive Sleep Apnea. Pediatrics 2000, 105, 405–412. [Google Scholar] [CrossRef] [PubMed]
- Garde, A.; Dehkordi, P.; Wensley, D.; Ansermino, J.M.; Dumont, G.A. Pulse oximetry recorded from the Phone Oximeter for detection of obstructive sleep apnea events with and without oxygen desaturation in children. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 7692–7695. [Google Scholar]
- Kang, S.; Lee, Y.; Lim, Y.-H.; Park, H.-K.; Cho, S.H.; Cho, S.H. Validation of noncontact cardiorespiratory monitoring using impulse-radio ultra-wideband radar against nocturnal polysomnography. Sleep Breath. 2020, 24, 841–848. [Google Scholar] [CrossRef] [PubMed]
- Tran, V.P.; Al-Jumaily, A.A.; Islam, S.M.S. Doppler Radar-Based Non-Contact Health Monitoring for Obstructive Sleep Apnea Diagnosis: A Comprehensive Review. Big Data Cogn. Comput. 2019, 3, 3. [Google Scholar] [CrossRef]
- Qi, F.; Li, C.; Wang, S.; Zhang, H.; Wang, J.; Lu, G. Contact-Free Detection of Obstructive Sleep Apnea Based on Wavelet Information Entropy Spectrum Using Bio-Radar. Entropy 2016, 18, 306. [Google Scholar] [CrossRef]
- Tate, A.; Walsh, J.; Kurup, V.; Shenoy, B.; Mann, D.; Freakley, C.; Eastwood, P.; Terrill, P. An emerging technology for the identification and characterization of postural-dependent obstructive sleep apnea. J. Clin. Sleep Med. 2020, 16, 309–318. [Google Scholar] [CrossRef]
- Akhadov, S. Sensors Logger Android 6.0.1 Application for Acquiring Smartphone’s Sensor Data. Available online: https://apkcombo.com/sensors-logger-demo/com.sensorsloggerdemo/ (accessed on 8 May 2021).
- Skarpsno, E.S.; Mork, P.J.; Nilsen, T.I.L.; Holtermann, A. Sleep positions and nocturnal body movements based on free-living accelerometer recordings: Association with demographics, lifestyle, and insomnia symptoms. Nat. Sci. Sleep 2017, 9, 267. [Google Scholar] [CrossRef]
- Soll, B.A.G.; Yeo, K.K.; Davis, J.W.; Seto, T.B.; Schatz, I.J.; Shen, E.N. The effect of posture on Cheyne-Stokes respirations and hemodynamics in patients with heart failure. Sleep 2009, 32, 1499–1507. [Google Scholar] [CrossRef] [PubMed]
- Bates, A.; Ling, M.J.; Mann, J.; Arvind, D.K. Respiratory rate and flow waveform estimation from tri-axial accelerometer data. In Proceedings of the 2010 International Conference on Body Sensor Networks (BSN 2010), Biopolis, Singapore, 7–9 June 2010; pp. 144–150. [Google Scholar]
- Hung, P.D.; Bonnet, S.; Guillemaud, R.; Castelli, E.; Yen, P.T.N. Estimation of respiratory waveform using an accelerometer. In Proceedings of the 2008 5th IEEE International Symposium on Biomedical Imaging: From Nano to Macro, Paris, France, 14–17 May 2008; pp. 1493–1496. [Google Scholar]
- Bricout, A.; Fontecave-Jallon, J.; Colas, D.; Gerard, G.; Pepin, J.-L.; Gumery, P.-Y. Adaptive Accelerometry Derived Respiration: Comparison with Respiratory Inductance Plethysmography during Sleep. In Proceedings of the 2019 41st Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Berlin, Germany, 23–27 July 2019; pp. 6714–6717. [Google Scholar]
- Estrada, L.; Torres, A.; Sarlabous, L.; Jane, R. Respiratory signal derived from the smartphone built-in accelerometer during a Respiratory Load Protocol. In Proceedings of the 2015 37th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Milan, Italy, 25–29 August 2015; pp. 6768–6771. [Google Scholar]
- Laub, R.R.; Tønnesen, P.; Jennum, P.J. A Sleep Position Trainer for positional sleep apnea: A randomized, controlled trial. J. Sleep Res. 2017, 26, 641–650. [Google Scholar] [CrossRef]
- Armas, L.H.; Turino, C.; Cordero-Guevara, J.; Manjón, J.L.; Durán-Carro, J.; Barbé, F.; Vaca, R.; Durán-Cantolla, J. A new postural device for the treatment of positional obstructive sleep apnea. A pilot study. Respir. Med. 2019, 151, 111–117. [Google Scholar] [CrossRef] [PubMed]

| Validated Hospital Position | Automatic Smartphone Accelerometry Corrected Position | |||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Right | Supine | Left | Prone | Stand | Right | Supine | Left | Prone | Stand | |||||||||||
| Patient | Min. | % | Min. | % | Min. | % | Min. | % | Min. | % | Min. | % | Min. | % | Min. | % | Min. | % | Min. | % |
| 1 | 122.8 | 26.8 | 327.1 | 71.4 | 8.2 | 1.8 | - | - | - | - | 121.3 | 26.5 | 328.1 | 71.6 | 8.5 | 1.9 | - | - | 0.1 | 0.0 |
| 2 | 96.4 | 24.6 | 276.7 | 70.5 | 19.3 | 4.9 | - | - | - | - | 95.3 | 24.3 | 277.7 | 70.8 | 19.3 | 4.9 | - | - | - | - |
| 3 | 150.7 | 30.2 | 346.4 | 69.3 | - | - | - | - | 2.8 | 0.6 | 163.9 | 32.8 | 333.7 | 66.7 | - | - | - | - | 2.3 | 0.5 |
| 4 | 86.4 | 17.0 | 188.0 | 37.0 | 72.7 | 14.3 | 156.2 | 30.8 | 4.2 | 0.8 | 22.9 | 4.5 | 250.9 | 49.4 | 167.1 | 32.9 | 61.4 | 12.1 | 5.3 | 1.1 |
| 5 | 51.0 | 12.7 | 277.5 | 69.1 | 72.9 | 18.2 | - | - | - | - | 35.6 | 8.9 | 290.1 | 72.3 | 75.6 | 18.8 | - | - | - | - |
| 6 | - | - | 355.3 | 100.0 | - | - | - | - | - | - | - | - | 355.3 | 100.0 | - | - | - | - | - | - |
| 7 | 44.8 | 10.4 | 232.7 | 53.9 | 154.3 | 35.7 | - | - | - | - | - | - | 277.5 | 64.3 | 154.4 | 35.7 | - | - | - | - |
| 8 | - | - | 504.5 | 98.7 | - | - | - | - | 6.8 | 1.3 | - | - | 505.8 | 98.9 | - | - | - | - | 5.5 | 1.1 |
| 9 | 2.6 | 0.7 | 367.1 | 99.3 | - | - | - | - | - | - | 2.6 | 0.7 | 367.1 | 99.3 | - | - | - | - | - | - |
| 10 | 68.9 | 16.4 | 3.5 | 0.8 | 345.8 | 82.1 | - | - | 2.8 | 0.7 | 69.7 | 16.6 | 1.9 | 0.5 | 346.7 | 82.3 | - | - | 2.8 | 0.7 |
| 11 | 105.7 | 24.0 | 68.4 | 15.5 | 256.2 | 58.2 | - | - | 10.0 | 2.3 | 102.7 | 23.3 | 74.2 | 16.8 | 256.6 | 58.3 | - | - | 6.9 | 1.6 |
| 12 | 239.5 | 56.3 | 161.8 | 38.0 | 19.8 | 4.7 | - | - | 4.7 | 1.1 | 237.8 | 55.9 | 165.3 | 38.8 | 19.2 | 4.5 | - | - | 3.4 | 0.8 |
| 13 | 105.0 | 29.5 | 242.1 | 68.0 | - | - | 0.8 | 0.2 | 8.1 | 2.3 | 104.9 | 29.5 | 244.5 | 68.7 | - | - | - | - | 6.6 | 1.8 |
| 14 | 34.7 | 8.1 | 65.1 | 15.1 | 257.2 | 59.8 | 0.7 | 0.2 | 72.5 | 16.8 | 34.4 | 8.0 | 66.4 | 15.4 | 260.3 | 60.5 | - | - | 68.9 | 16.0 |
| 15 | 112.3 | 22.3 | 152.6 | 30.3 | 233.3 | 46.4 | - | - | 4.8 | 1.0 | 112.2 | 22.3 | 157.4 | 31.3 | 230.2 | 45.8 | - | - | 3.3 | 0.7 |
| 16 | - | - | 126.6 | 27.0 | 338.0 | 72.1 | - | - | 4.2 | 0.9 | - | - | 123.7 | 26.4 | 341.3 | 72.8 | - | - | 3.6 | 0.8 |
| 17 | 76.7 | 21.0 | 221.6 | 60.6 | 67.3 | 18.4 | - | - | - | - | 77.0 | 21.1 | 201.7 | 55.2 | 86.8 | 23.8 | - | - | - | - |
| 18 | 106.3 | 28.5 | 207.9 | 55.8 | 58.4 | 15.7 | - | - | - | - | 105.8 | 28.4 | 208.4 | 55.9 | 58.3 | 15.7 | - | - | - | - |
| 19 | 6.8 | 1.5 | 262.7 | 59.0 | 172.1 | 38.7 | - | - | 3.5 | 0.8 | 4.5 | 1.0 | 259.3 | 58.3 | 177.4 | 39.9 | - | - | 3.9 | 0.9 |
| Total | 1410.6 | 17.3 | 4387.4 | 53.8 | 2075.4 | 25.4 | 157.7 | 1.9 | 124.2 | 1.5 | 1290.5 | 15.8 | 4489.0 | 55.0 | 2201.8 | 27.0 | 61.4 | 0.8 | 112.6 | 1.4 |
| Patient | Automatic Smartphone Accelerometry Position vs. Validated Hospital Position | Automatic Smartphone Accelerometry Corrected Position vs. Validated Hospital Position | Automatic Hospital Position vs. Validated Hospital Position |
|---|---|---|---|
| 1 | 99.4 | 99.5 | 94.5 |
| 2 | 99.7 | 99.7 | 91.6 |
| 3 | 90.0 | 96.8 | 89.6 |
| 4 | 61.1 | 67.4 | 58.0 |
| 5 | 95.0 | 95.3 | 75.6 |
| 6 | 100.0 | 100.0 | 100.0 |
| 7 | 89.4 | 89.4 | 58.8 |
| 8 | 99.4 | 99.8 | 98.7 |
| 9 | 100.0 | 100.0 | 100.0 |
| 10 | 99.2 | 99.2 | 75.1 |
| 11 | 96.5 | 96.5 | 86.1 |
| 12 | 98.4 | 98.6 | 95.1 |
| 13 | 98.2 | 99.2 | 97.5 |
| 14 | 81.6 | 96.0 | 68.8 |
| 15 | 99.1 | 98.4 | 52.5 |
| 16 | 94.7 | 95.8 | 78.4 |
| 17 | 93.4 | 93.3 | 97.6 |
| 18 | 99.4 | 99.4 | 99.4 |
| 19 | 99.0 | 98.0 | 61.2 |
| Average | 94.4 | 95.9 | 83.1 |
| Automatic Smartphone Accelerometry Corrected Position | Row Total | |||||||
|---|---|---|---|---|---|---|---|---|
| Position | Right | Supine | Left | Prone | Stand | Min. | % | |
| Validated Hospital Position | Right | 1268.8 | 139.6 | 1.6 | 0.0 | 0.6 | 1410.6 | 17.3 |
| Supine | 19.9 | 4308.1 | 55.6 | 0.0 | 3.9 | 4387.4 | 53.8 | |
| Left | 1.9 | 23.4 | 2049.9 | 0.0 | 0.3 | 2075.4 | 25.4 | |
| Prone | 0.0 | 0.4 | 94.6 | 61.4 | 1.3 | 157.7 | 1.9 | |
| Stand | 0.0 | 17.6 | 0.1 | 0.0 | 106.5 | 124.2 | 1.5 | |
| Column Total | Min. | 1290.5 | 4489.0 | 2201.8 | 61.4 | 112.6 | 8155.3 | |
| % | 15.8 | 55.0 | 27.0 | 0.8 | 1.4 | |||
| Automatic Smartphone Accelerometry Corrected Position | ||||||
|---|---|---|---|---|---|---|
| Position | Se (%) | Sp (%) | PPV (%) | NPV (%) | Acc (%) | |
| Validated Hospital Position | Right | 89.9 | 99.7 | 98.3 | 97.9 | 98.0 |
| Supine | 98.2 | 95.2 | 96.0 | 97.8 | 96.8 | |
| Left | 98.8 | 97.5 | 93.1 | 99.6 | 97.8 | |
| Prone | 38.9 | 100.0 | 100.0 | 98.8 | 98.8 | |
| Stand | 85.7 | 99.9 | 94.6 | 99.8 | 99.7 | |
| Average | 82.3 | 98.5 | 96.4 | 98.8 | 98.2 | |
| Automatic Smartphone Accelerometry Classified by the Discretized Positions | |||||
|---|---|---|---|---|---|
| Right | Supine | Left | Prone | Stand | |
| Angle: Mean | 143.9 | 90.6 | 27.0 | −71.7 | 166.9 |
| Angle: Standard deviation | 14.0 | 8.7 | 21.2 | 26.6 | 12.1 |
| % Angles: Reference angle ± 5° window | 0.7 | 52.6 | 3.6 | 0 | 9.7 |
| % Angles: Reference angle ± 10° window | 5.9 | 80.2 | 7.0 | 0 | 24.8 |
| % Angles: Reference angle ± 15° window | 10.9 | 90.8 | 15.8 | 0 | 31.3 |
| % Angles: Reference angle ± 20° window | 17.4 | 95.9 | 27.1 | 0 | 76.8 |
| % Angles: Reference angle ± 25° window | 27.9 | 97.8 | 35.4 | 2.6 | 94.4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrer-Lluis, I.; Castillo-Escario, Y.; Montserrat, J.M.; Jané, R. Enhanced Monitoring of Sleep Position in Sleep Apnea Patients: Smartphone Triaxial Accelerometry Compared with Video-Validated Position from Polysomnography. Sensors 2021, 21, 3689. https://doi.org/10.3390/s21113689
Ferrer-Lluis I, Castillo-Escario Y, Montserrat JM, Jané R. Enhanced Monitoring of Sleep Position in Sleep Apnea Patients: Smartphone Triaxial Accelerometry Compared with Video-Validated Position from Polysomnography. Sensors. 2021; 21(11):3689. https://doi.org/10.3390/s21113689
Chicago/Turabian StyleFerrer-Lluis, Ignasi, Yolanda Castillo-Escario, Josep Maria Montserrat, and Raimon Jané. 2021. "Enhanced Monitoring of Sleep Position in Sleep Apnea Patients: Smartphone Triaxial Accelerometry Compared with Video-Validated Position from Polysomnography" Sensors 21, no. 11: 3689. https://doi.org/10.3390/s21113689
APA StyleFerrer-Lluis, I., Castillo-Escario, Y., Montserrat, J. M., & Jané, R. (2021). Enhanced Monitoring of Sleep Position in Sleep Apnea Patients: Smartphone Triaxial Accelerometry Compared with Video-Validated Position from Polysomnography. Sensors, 21(11), 3689. https://doi.org/10.3390/s21113689







