Wearable Devices for Biofeedback Rehabilitation: A Systematic Review and Meta-Analysis to Design Application Rules and Estimate the Effectiveness on Balance and Gait Outcomes in Neurological Diseases
Abstract
1. Introduction
2. Materials and Methods
2.1. Data Sources and Searches
2.2. Eligibility Criteria
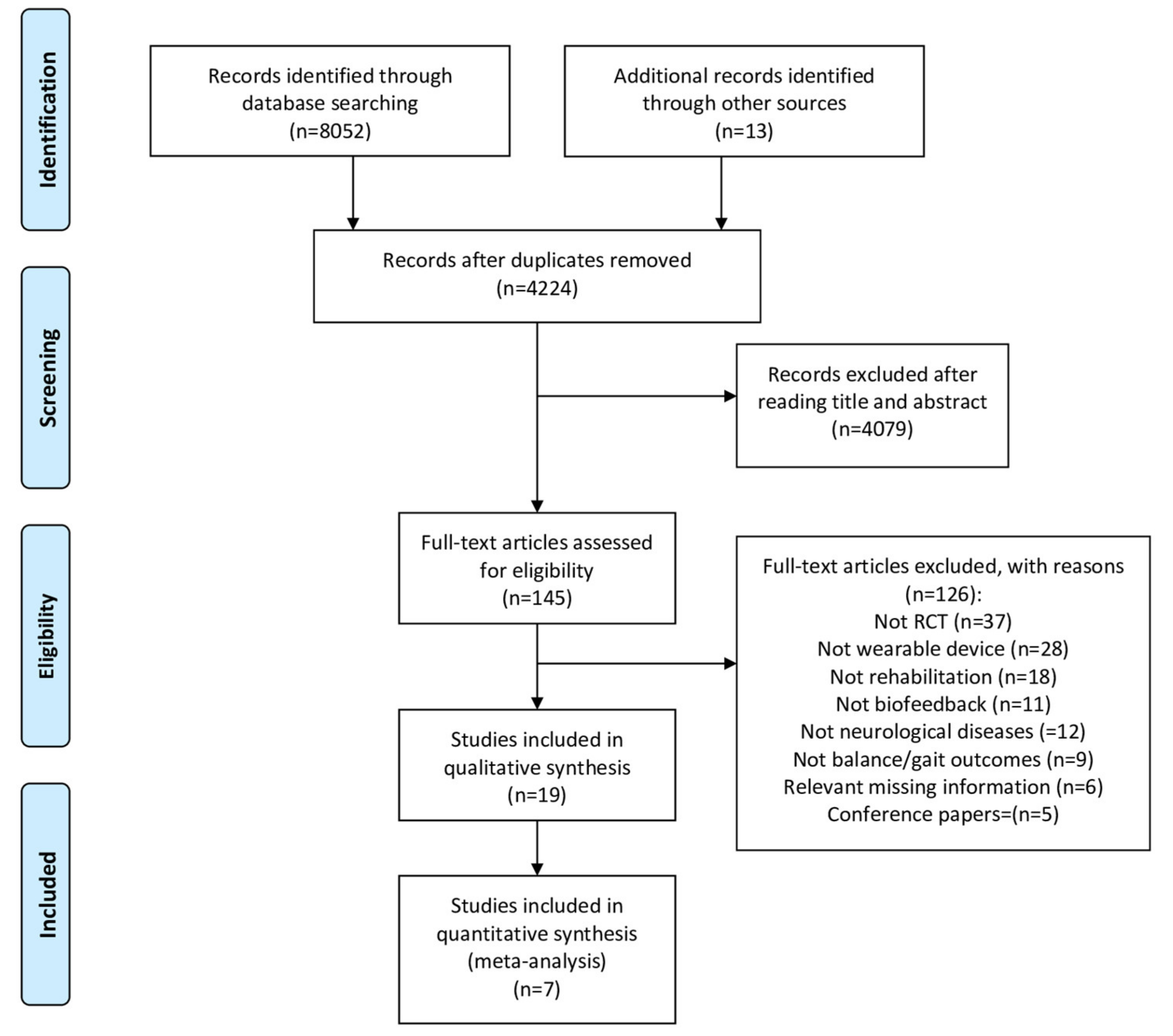
2.3. Study Selection and Data Extraction
2.4. Data Synthesis
2.5. Risk of Bias Assessment
3. Results
3.1. Study Characteristics
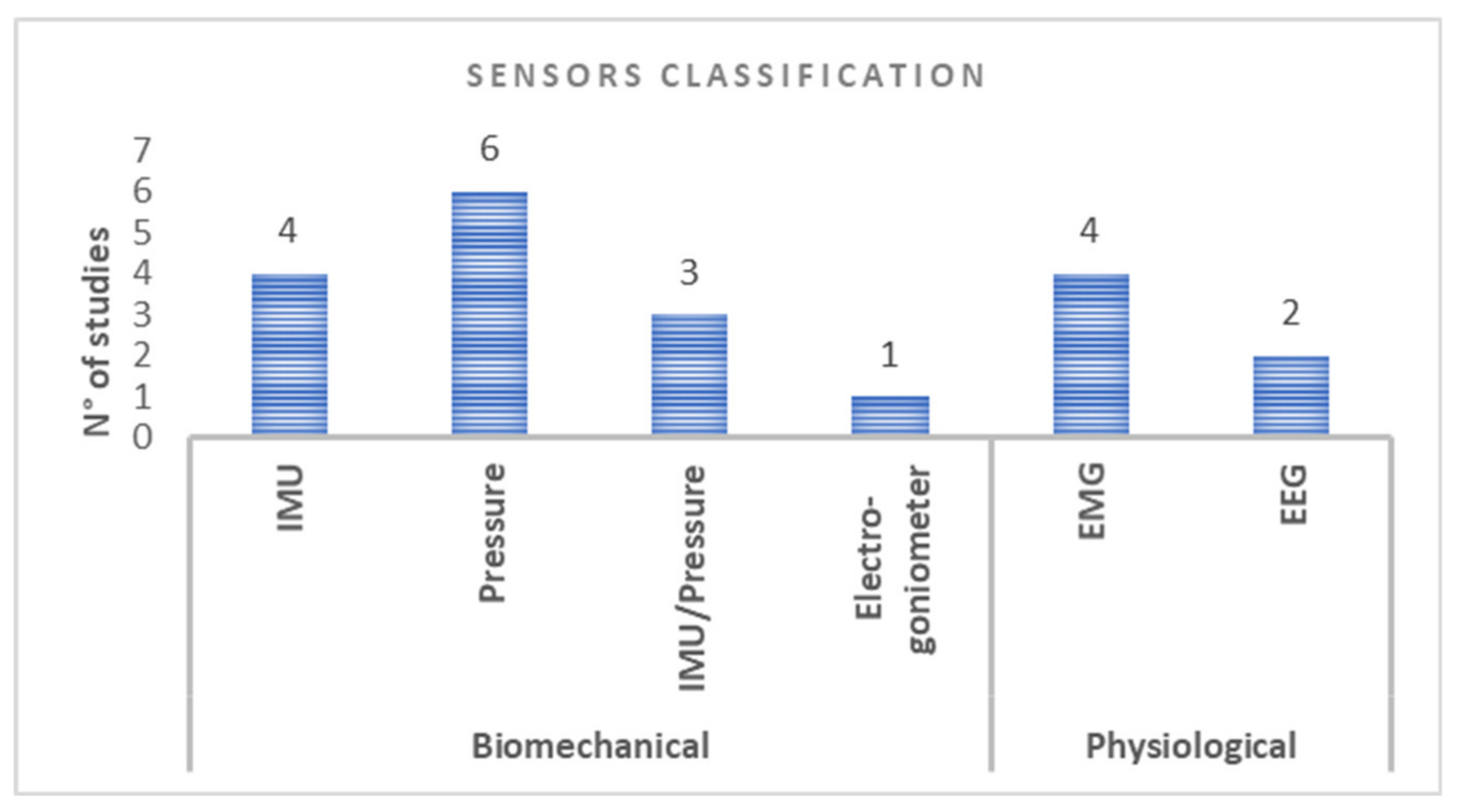
3.2. Wearable Device Biofeedback Rehabilitation: Sensors Classification and Configuration
3.3. Modalities of Exercise Interventions
3.4. Components of Balance and Gait Training
3.5. Biofeedback Components
3.6. WDBR Estimated Effectiveness on Balance and Gait Outcomes
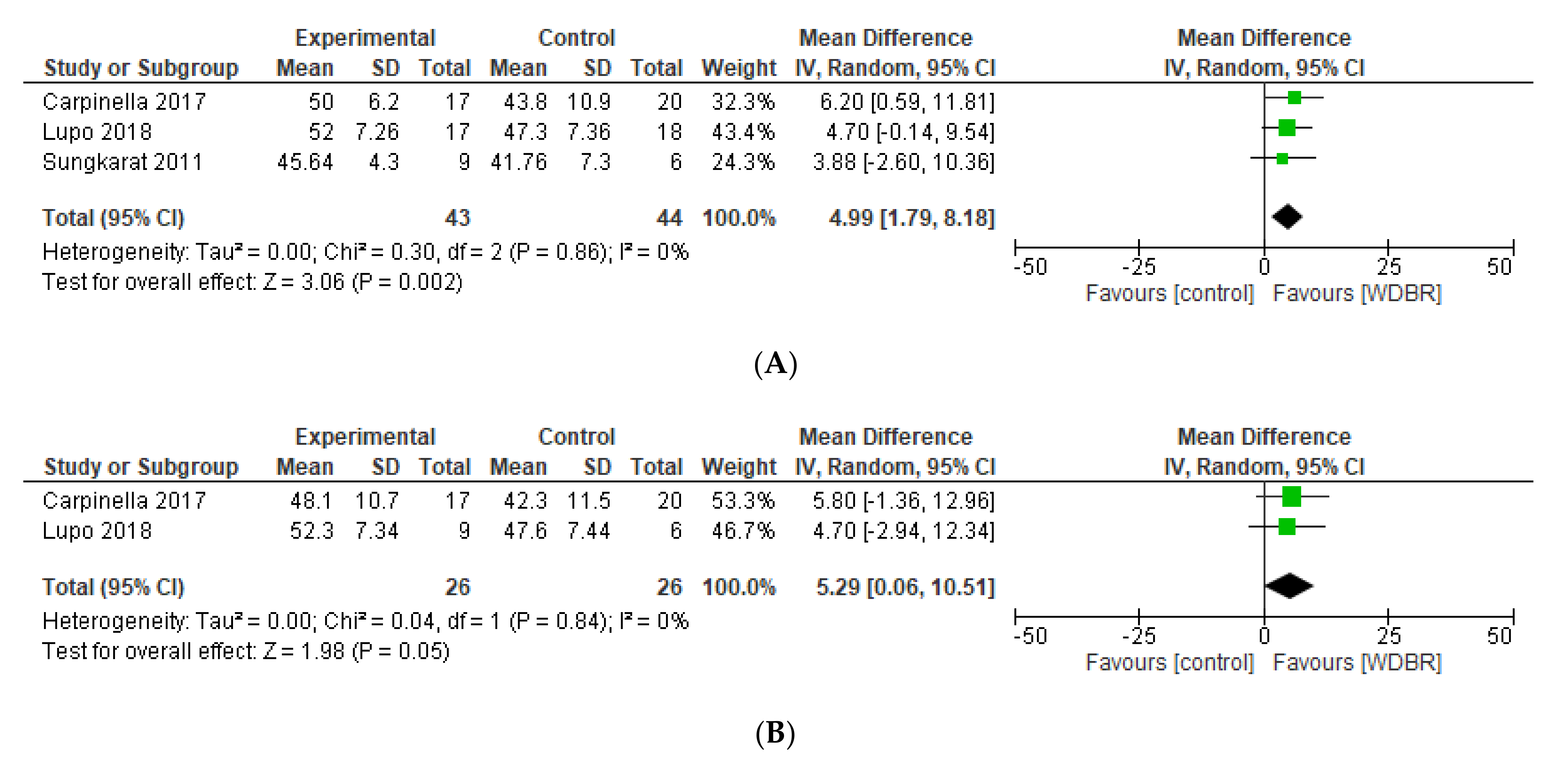
3.6.1. Berg Balance Scale (BBS)
3.6.2. Timed up and Go (TUG)
3.6.3. Gait Speed
3.6.4. Qualitative Synthesis
3.7. Feasibility and Usability of WS Training
3.8. Risk of Bias (RoB) Assessment
4. Discussion
4.1. Training Paradigm: Type and Configuration of Sensors
4.2. Training Paradigm: Biofeedback Components
4.3. Feasibility and Usability of Sensor-Based Training
4.4. Training Effectiveness
4.5. Suggestions for Design Rules and Implementation for Clinical Practice
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cattaneo, D.; Carpinella, I.; Aprile, I.; Prosperini, L.; Montesano, A.; Jonsdottir, J. Comparison of upright balance in stroke, Parkinson and multiple sclerosis. Acta Neurol. Scand. 2016, 133, 346–354. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.; Choi, H.; Pyun, S.B. Community Integration and Quality of Life in Aphasia after Stroke. Yonsei Med. J. 2015, 56, 1694–1702. [Google Scholar] [CrossRef] [PubMed]
- Minet, L.R.; Peterson, E.; von Koch, L.; Ytterberg, C. Occurrence and Predictors of Falls in People with Stroke: Six-Year Prospective Study. Stroke 2015, 46, 2688–2690. [Google Scholar] [CrossRef] [PubMed]
- Sudarsky, L. Gait disorders: Prevalence, morbidity, and etiology. Adv. Neurol. 2001, 87, 111–117. [Google Scholar] [PubMed]
- Stevens, V.; Goodman, K.; Rough, K.; Kraft, G.H. Gait impairment and optimizing mobility in multiple sclerosis. Phys. Med. Rehabil. Clin. N. Am. 2013, 24, 573–592. [Google Scholar] [CrossRef]
- Bowman, T.; Gervasoni, E.; Parelli, R.; Jonsdottir, J.; Ferrarin, M.; Cattaneo, D.; Carpinella, I. Predictors of mobility domain of health-related quality of life after rehabilitation in Parkinson’s disease: A pilot study. Arch. Physiother. 2018, 8, 10. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.H.; Wang, R.L.; Lioy, D.J.; Shaw, J.S. Gait disorders in Parkinson’s Disease: Assessment and Management. Int. J. Gerontol. 2013, 7, 189–193. [Google Scholar] [CrossRef]
- Balaban, B.; Tok, F. Gait disturbances in patients with stroke. PM&R 2014, 6, 635–642. [Google Scholar] [CrossRef]
- Pedersen, B.K.; Saltin, B. Exercise as medicine—Evidence for prescribing exercise as therapy in 26 different chronic diseases. Scand. J. Med. Sci. Sports 2015, 25 (Suppl. 3), 1–72. [Google Scholar] [CrossRef]
- Gunn, H.; Markevics, S.; Haas, B.; Marsden, J.; Freeman, J. Systematic Review: The Effectiveness of Interventions to Reduce Falls and Improve Balance in Adults With Multiple Sclerosis. Arch. Phys. Med. Rehabil. 2015, 96, 1898–1912. [Google Scholar] [CrossRef]
- Tomlinson, C.L.; Patel, S.; Meek, C.; Herd, C.P.; Clarke, C.E.; Stowe, R.; Shah, L.; Sackley, C.M.; Deane, K.H.; Wheatley, K.; et al. Physiotherapy versus placebo or no intervention in Parkinson’s disease. Cochrane Database Syst. Rev. 2013, 2013, CD002817. [Google Scholar] [CrossRef] [PubMed]
- Van Duijnhoven, H.J.; Heeren, A.; Peters, M.A.; Veerbeek, J.M.; Kwakkel, G.; Geurts, A.C.; Weerdesteyn, V. Effects of Exercise Therapy on Balance Capacity in Chronic Stroke: Systematic Review and Meta-Analysis. Stroke 2016, 47, 2603–2610. [Google Scholar] [CrossRef]
- Fernando, C.K.; Basmajian, J.V. Biofeedback in Physical Medicine and Rehabilitation. Biofeedback Self-Regul. 1978, 3, 435–455. [Google Scholar] [CrossRef] [PubMed]
- Tate, J.J.; Milner, C.E. Real-time kinematic, temporospatial, and kinetic biofeedback during gait retraining in patients: A systematic review. Phys. Ther. 2010, 90, 1123–1134. [Google Scholar] [CrossRef]
- Schmidt, R.; Lee, T. Motor Learning and Performance: From Principles to Application, 5th ed.; Human Kinetics: Windsor, ON, Canada, 2013; p. 336. [Google Scholar]
- Giggins, O.M.; Persson, U.M.; Caulfield, B. Biofeedback in rehabilitation. J. Neuroeng. Rehabil. 2013, 10, 60. [Google Scholar] [CrossRef]
- Sigrist, R.; Rauter, G.; Riener, R.; Wolf, P. Augmented Visual, Auditory, Haptic, and Multimodal Feedback in Motor Learning: A Review. Psychon. Bull. Rev. 2013, 20, 21–53. [Google Scholar] [CrossRef]
- Brennan, L.; Dorronzoro Zubiete, E.; Caulfield, B. Feedback Design in Targeted Exercise Digital Biofeedback Systems for Home Rehabilitation: A Scoping Review. Sensors 2019, 20, 181. [Google Scholar] [CrossRef] [PubMed]
- Sigrist, R.; Rauter, G.; Reiner, R.; Wolf, P. Terminal feedback outperforms concurrent visual, auditory, and haptic feedback in learning a complex rowing-type task. J. Motor Behav. 2013, 20, 21–53. [Google Scholar] [CrossRef]
- Gorman, A.J.; Willmott, A.P.; Mullineaux, D.R. The effects of concurrent biomechanical biofeedback on novel skill acquisition. Sports Biomech. 2019, 26, 1–15. [Google Scholar] [CrossRef]
- Park, J.H.; Shea, C.H.; Wright, D.L. Reduced–Frequency concurrent and terminal feedback: A test of the guidance hypothesis. J. Motor Behav. 2000, 32, 287–296. [Google Scholar] [CrossRef]
- Trotter, A.B.; Inman, D.A. The use of positive reinforcement in physical therapy. Phys. Ther. 1968, 48, 347–352. [Google Scholar] [CrossRef] [PubMed]
- Galea, J.M.; Mallia, E.; Rothwell, J.; Diedrichsen, J. The dissociable effects of punishment and reward on motor learning. Nat. Neurosci. 2015, 18, 597–602. [Google Scholar] [CrossRef]
- Huang, H.; Wolf, S.L.; He, J. Recent Developments in Biofeedback for Neuromotor Rehabilitation. J. Neuroeng. Rehabil. 2006, 3, 11. [Google Scholar] [CrossRef] [PubMed]
- Kitago, T.; Krakauer, J.W. Motor learning principles for neurorehabilitation. Handb. Clin. Neurol. 2013, 110, 93–103. [Google Scholar] [CrossRef] [PubMed]
- Dunn, J.; Runge, R.; Snyder, M. Wearables and the medical revolution. Pers. Med. 2018, 15, 429–448. [Google Scholar] [CrossRef] [PubMed]
- Porciuncula, F.; Roto, A.V.; Kumar, D.; Davis, I.; Roy, S.; Walsh, C.J.; Awad, L.N. Wearable Movement Sensors for Rehabilitation: A Focused Review of Technological and Clinical Advances. PM&R 2018, 10, S220–S232. [Google Scholar] [CrossRef]
- Tao, W.; Liu, T.; Zheng, R.; Feng, H. Gait analysis using wearable sensors. Sensors 2012, 12, 2255–2283. [Google Scholar] [CrossRef]
- Schepers, M. Ambulatory Assessment of Human Body Kinematics and Kinetics. Ph.D. Thesis, University of Twente, Enschede, The Netherlands, 2009. [Google Scholar]
- Crea, S.; Edin, B.B.; Knaepen, K.; Meeusen, R.; Vitiello, N. Time-Discrete Vibrotactile Feedback Contributes to Improved Gait Symmetry in Patients With Lower Limb Amputations: Case Series. Phys. Ther. 2017, 97, 198–207. [Google Scholar] [CrossRef]
- Omejc, N.; Rojc, B.; Battaglini, P.P.; Marusic, U. Review of the therapeutic neurofeedback method using electroencephalography: EEG Neurofeedback. BOSN J. Basic Med. Sci. 2019, 19, 213–220. [Google Scholar] [CrossRef]
- Merletti, R.; Muceli, S. Tutorial. Surface EMG detection in space and time: Best practices. J. Electromyogr. Kinesiol. 2019, 49, 102363. [Google Scholar] [CrossRef] [PubMed]
- Peake, J.M.; Kerr, G.; Sullivan, J.P. A Critical Review of Consumer Wearables, Mobile Applications, and Equipment for Providing Biofeedback, Monitoring Stress, and Sleep in Physically Active Populations. Front. Physiol. 2018, 9, 743. [Google Scholar] [CrossRef]
- Gordt, K.; Gerhardy, T.; Najafi, B.; Schwenk, M. Effects of Wearable Sensor-Based Balance and Gait Training on Balance, Gait, and Functional Performance in Healthy and Patient Populations: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. Gerontology 2018, 64, 74–89. [Google Scholar] [CrossRef]
- Moher, D.; Liberati, A.; Tetzlaff, J.; Altman, D.G.; PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA statement. PLoS Med. 2009, 6, e1000097. [Google Scholar] [CrossRef]
- Horak, F.B. Postural orientation and equilibrium: What do we need to know about neural control of balance to prevent falls? Age Ageing 2006, 35, ii7–ii11. [Google Scholar] [CrossRef] [PubMed]
- Grissom, R.J.; Kim, J.J. Effect Sizes for Research: A Broad Practical Approach; Lawrence Erlbaum: Mahwah, NJ, USA, 2005. [Google Scholar]
- Higgins, J.P.; Green, S. (Eds.) Cochrane Handbook for Systematic Reviews of Interventions; Cochrane Book Series; John Wiley & Sons, Ltd.: Chichester, UK, 2008; pp. 51–79. [Google Scholar]
- Higgins, J.P.T.; Altman, D.G.; Gøtzsche, P.C.; Jüni, P.; Moher, D.; Oxman, A.D.; Savović, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A.C.; et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ 2011, 343, d5928. [Google Scholar] [CrossRef]
- Azarpaikan, A.; Torbati, H.T.; Sohrabi, M. Neurofeedback and physical balance in Parkinson’s patients. Gait Posture 2014, 40, 177–181. [Google Scholar] [CrossRef]
- Byl, N.; Zhang, W.; Coo, S.; Tomizuka, M. Clinical impact of gait training enhanced with visual kinematic biofeedback: Patients with Parkinson’s disease and patients stable post stroke. Neuropsychologia 2015, 79, 332–343. [Google Scholar] [CrossRef]
- Carpinella, I.; Cattaneo, D.; Bonora, G.; Bowman, T.; Martina, L.; Montesano, A.; Ferrarin, M. Wearable Sensor-Based Biofeedback Training for Balance and Gait in Parkinson Disease: A Pilot Randomized Controlled Trial. Arch. Phys. Med. Rehabil. 2017, 98, 622–630.e3. [Google Scholar] [CrossRef] [PubMed]
- El-Tamawy, M.S.; Darwish, M.H.; Khallaf, M.E. Effects of augmented proprioceptive cues on the parameters of gait of individuals with Parkinson’s disease. Ann. Indian Acad. Neurol. 2012, 15, 267–272. [Google Scholar] [CrossRef] [PubMed]
- Ginis, P.; Nieuwboer, A.; Dorfman, M.; Ferrari, A.; Gazit, E.; Canning, C.G.; Rocchi, L.; Chiari, L.; Hausdorff, J.M.; Mirelman, A. Feasibility and effects of home-based smartphone-delivered automated feedback training for gait in people with Parkinson’s disease: A pilot randomized controlled trial. Parkinsonism Relat. Disord. 2016, 22, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Van den Heuvel, M.R.; Kwakkel, G.; Beek, P.J.; Berendse, H.W.; Daffertshofer, A.; van Wegen, E.E. Effects of augmented visual feedback during balance training in Parkinson’s disease: A pilot randomized clinical trial. Parkinsonism Relat. Disord. 2014, 20, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Cha, Y.J.; Kim, J.D.; Choi, Y.R.; Kim, N.H.; Son, S.M. Effects of gait training with auditory feedback on walking and balancing ability in adults after hemiplegic stroke: A preliminary, randomized, controlled study. Int. J. Rehabil. Res. 2018, 41, 239–243. [Google Scholar] [CrossRef] [PubMed]
- Cho, S.H.; Shin, H.K.; Kwon, Y.H.; Lee, M.Y.; Lee, Y.H.; Lee, C.H.; Yang, D.S.; Jang, S.H. Cortical activation changes induced by visual biofeedback tracking training in chronic stroke patients. NeuroRehabilitation 2007, 22, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Choi, Y.H.; Kim, J.D.; Lee, J.H.; Cha, Y.J. Walking and balance ability gain from two types of gait intervention in adult patients with chronic hemiplegic stroke: A pilot study. Assist. Technol. 2019, 31, 112–115. [Google Scholar] [CrossRef]
- Cozean, C.D.; Pease, W.S.; Hubbell, S.L. Biofeedback and functional electric stimulation in stroke rehabilitation. Arch. Phys. Med. Rehabil. 1988, 69, 401–405. [Google Scholar]
- Intiso, D.; Santilli, V.; Grasso, M.G.; Rossi, R.; Caruso, I. Rehabilitation of walking with electromyographic biofeedback in foot-drop after stroke. Stroke 1994, 25, 1189–1192. [Google Scholar] [CrossRef]
- Jonsdottir, J.; Cattaneo, D.; Recalcati, M.; Regola, A.; Rabuffetti, M.; Ferrarin, M.; Casiraghi, A. Task-oriented biofeedback to improve gait in individuals with chronic stroke: Motor learning approach. Neurorehabil. Neural Repair 2010, 24, 478–485. [Google Scholar] [CrossRef]
- Jung, K.; Kim, Y.; Cha, Y.; In, T.S.; Hur, Y.G.; Chung, Y. Effects of gait training with a cane and an augmented pressure sensor for enhancement of weight bearing over the affected lower limb in patients with stroke: A randomized controlled pilot study. Clin. Rehabil. 2015, 29, 135–142. [Google Scholar] [CrossRef]
- Ki, K.I.; Kim, M.S.; Moon, Y.; Choi, J.D. Effects of auditory feedback during gait training on hemiplegic patients’ weight bearing and dynamic balance ability. J. Phys. Ther. Sci. 2015, 27, 1267–1269. [Google Scholar] [CrossRef]
- Lee, Y.S.; Bae, S.H.; Lee, S.H.; Kim, K.Y. Neurofeedback training improves the dual-task performance ability in stroke patients. Tohoku J. Exp. Med. 2015, 236, 81–88. [Google Scholar] [CrossRef]
- Lupo, A.; Cinnera, A.M.; Pucello, A.; Iosa, M.; Coiro, P.; Personeni, S.; Gimigliano, F.; Iolascon, G.; Paolucci, S.; Morone, G. Effects on balance skills and patient compliance of biofeedback training with inertial measurement units and exergaming in subacute stroke: A pilot randomized controlled trial. Funct. Neurol. 2018, 33, 131–136. [Google Scholar]
- Mandel, A.R.; Nymark, J.R.; Balmer, S.J.; Grinnell, D.M.; O’Riain, M.D. Electromyographic versus rhythmic positional biofeedback in computerized gait retraining with stroke patients. Arch. Phys. Med. Rehabil. 1990, 71, 649–654. [Google Scholar] [PubMed]
- Sungkarat, S.; Fisher, B.E.; Kovindha, A. Efficacy of an insole shoe wedge and augmented pressure sensor for gait training in individuals with stroke: A randomized controlled trial. Clin. Rehabil. 2011, 25, 360–369. [Google Scholar] [CrossRef] [PubMed]
- Schwenk, M.; Sabbagh, M.; Lin, I.; Morgan, P.; Grewal, G.S.; Mohler, J.; Coon, D.W.; Najafi, B. Sensor-based balance training with motion feedback in people with mild cognitive impairment. J. Rehabil. Res. Dev. 2016, 53, 945–958. [Google Scholar] [CrossRef] [PubMed]
- Cirstea, C.M.; Ptito, A.; Levin, M.F. Feedback and cognition in arm motor skill reacquisition after stroke. Stroke 2006, 37, 1237–1242. [Google Scholar] [CrossRef] [PubMed]
- Kimmeskamp, S.; Hennig, E.M. Heel to toe motion characteristics in Parkinson patients during free walking. Clin. Biomech. 2001, 16, 806–812. [Google Scholar] [CrossRef]
- Winfree, K.N.; Pretzer-Aboff, I.; Hilgart, D.; Aggarwal, R.; Behari, M.; Agrawal, S. An untethered shoe with vibratory feedback for improving gait of Parkinson’s patients: The PDShoe. In Proceedings of the 2012 Annual International Conference of the IEEE Engineering in Medicine and Biology Society, San Diego, CA, USA, 28 August–1 September 2012; pp. 1202–1205. [Google Scholar] [CrossRef]
- Widmer, M.; Ziegler, N.; Held, J.; Luft, A.; Lutz, K. Rewarding feedback promotes motor skill consolidation via striatal activity. Prog. Brain Res. 2016, 229, 303–323. [Google Scholar] [CrossRef]
- Blum, L.; Korner-Bitensky, N. Usefulness of the Berg Balance Scale in Stroke Rehabilitation: A Systematic Review. Phys. Ther. 2008, 88, 559–566. [Google Scholar] [CrossRef]
- Oermann, M.H.; Muckler, V.C.; Morgan, B. Framework for Teaching Psychomotor and Procedural Skills in Nursing. J. Contin. Educ. Nurs. 2016, 47, 278–282. [Google Scholar] [CrossRef]
- Vienne, A.; Barrois, R.P.; Buffat, S.; Ricard, D.; Vidal, P.P. Inertial Sensors to Assess Gait Quality in Patients with Neurological Disorders: A Systematic Review of Technical and Analytical Challenges. Front. Psychol. 2017, 8, 817. [Google Scholar] [CrossRef]





| AND | (Humans[mh] OR Adult[mh] OR Nervous System Diseases[mh] OR Gait Disorders, Neurologic[mh] AND (Neurofeedback[mh] OR Feedback, Sensory[mh] OR feedback[tiab] OR Biofeedback[tiab] OR Cues[mh] OR Physical Therapy modalities[mh] OR Rehabilitation[mh] OR Rehab*[tiab] OR Conservative treatment[mh] OR Training[tiab] OR Exercise*[tiab]) |
| (Wearable Electronic Devices[mh] OR wearable[tiab] OR Device[tiab] OR Accelerometry[mh] OR Acceleromet*[tiab] OR gyroscope*[tiab] OR sensor*[tiab] OR shoe*[tiab] OR Insole*[tiab]) | |
| (Walking[mh] OR Walk*[tiab] OR Ambulation[tiab] OR Gait[mh] OR Gait[tiab] OR Postural Balance[mh] OR Balance[tiab] OR Equilibrium[tiab] OR Recovery of function[mh] OR Motor Activity[mh]) | |
| (Randomized controlled trial[pt] OR randomized controlled trials as topic[mh] OR random*[tiab]) |
| First Author [Ref] | Aim | Participant Characteristics | Wearable Device | Sensors Type (Short) | Experimental Intervention | Control Intervention | Timing | Balance and Gait Outcome Measure | Evaluation Time Points |
|---|---|---|---|---|---|---|---|---|---|
| Azerpaikan et al. [40] | To study the effect of a neurofeedback training on balance problems associated with Parkinson’s disease. | TOT n = 16 patients with PD EG: n = 8 Mean age: 74.23 ± 3.51 years Female: n = 4 CG: n = 8 Mean age: 75.16 ± 3.64 years Female: n = 4 | Biograph Infiniti Software system (version 5.0), the ProComp differential amplifier (Thought Technology Ltd, Montreal, Quebec) for NeuroFeedback training sessions (FlexComp Infiniti encoder, TT-USB interface unit, fiber optic cable, USB cable) | EEG SENSORS | EG: Neurofeedback training with EEG generator | EG: Sham Neuro Feedback Training using sham EEG generator | D:8 sessions T: 30 min F: 3 days/week | BBS*, Limit of stability * | PRE, POST |
| Byl et al. [41] | To evaluate the effectiveness of supervised gait training with and without visual kinematic feedback | TOT: n = 24 stroke and PD EG: n = 12 Stroke: n = 5 Mean age: 66.2 ± 5.0 years Female: n = 3 PD: n = 7 Mean age: 68.5 ± 3.6 years Female: n = 4 CG: n = 12 Stroke: n = 7 Mean age: 60.8 ± 5.4 years Female: n = 5PD: n = 5 Mean age: 70.0 ± 2.9 years Female: n = 2 | Wireless joint angle sensors and Smart Shoes | IMU, PRESSURE SENSORS | EG: visual kinematic feedback on the computer screen during progressive and task-oriented balance and gait training activities | CG: balance and gait training activities | D: 12 sessions (6–8 weeks) T: 90 min (30 min visual kinematic feedback in the EG) F: NA | Gait Speed; Step Length; TBS, 6MWT, DGI, 5TSS, TUG, BBS, FOG-Q | PRE, POST |
| Carpinella et al. [42] | To test the feasibility of a wearable biofeedback system in a typical rehabilitation gym and analyze the effect on balance and gait outcome measures compared to physiotherapy without feedback. | TOT: n = 42 subjects with PD EG: n = 17 Mean Age: 73 ± 7.1 years Female: n = 3 CG: n = 20 Mean Age: 75.6 ± 8.2 years Female: n = 11 | Gamepad System (IMU, PC and Customized software) | IMU SENSORS | EG: balance and gait functional tailored exercises using Gamepad System | CG: personalized balance and gait exercises defined by the clinical staff | D: 20sessions T:45 min F:3 times/week | BBS *, Gait Speed, UPDRSIII, TUG, ABC, FOGQ, COP ML sway *, COP AP sway, Tele-healthcare Satisfaction Questionnaire-Wearable Technologies | PRE, POST, FU |
| Cha et al. [46] | To compare the effectiveness of auditory feedback stimulation from the heel and forefoot areas in terms of ambulatory functional improvements in stroke patients | TOT n = 31 stroke subjects EG1: n = 11 Mean age: 64.6 ± 10.6 Female: n = 3 EG2: n = 10 Mean age: 63.0 ± 4.7 Female: n = 3 CG: n = 10 Mean age: 61.8 ± 9.8 Female: n = 4 | A PedAlert Monitor 120 (PattersonMedical Holdings Inc., Warrenville, IL, USA) | PRESSURE SENSORS | EG1: gait training with active weight bearing on the paretic heel with auditory feedback EG2: gait training with auditory feedback from paretic metatarsals | CG= gait intervention | D: 6 weeks T: 50 min (30 min of conventional therapy + 20 min of gait intervention with or without auditory feedback) F: 3 × week | Gait speed, FGA *, TUG, COP length EO *, COP length EC, COP velocity EO *, COP velocity EC | PRE, POST |
| Cho et al. [47] | To examine whether visual biofeedback tracking training can improve gait performance in chronic stroke patients. | TOT n = 10 Stroke subjects EG: n = 5 Mean age: 46.2 ± 7.3 years Female: n = 2 CG: n = 5 Mean age: 48.8 ± 6.3 years Female: n = 1 | A double-axis electrogoniometer (Biometrics Ltd. Ladysmith, VA) was used to record the instant degrees of knee joint flexion–extension. Series of PC generated sine waves at 0.2 Hz were displayed on a PC monitor at 80 cm distance from the eyes of the subject | ELECTRO-GONIOMETER | EG: visual biofeedback tracking training | CG: not Reported | D:20 sessions (4 week) T: 39 min F: 5 days/week | Motoricity Index, Modified Motor Assessment Scale, Gait Speed. | PRE, POST |
| Choi et al. [48] | To compare gait intervention with auditory feedback induced by active weight bearing on the paralyzed side with the effects of the general gait training method | TOT n = 24 stroke subjects EG: n = 12 Mean age: 62.8 ± 4.8 Female: n = 4 CG: n = 12 Mean age: 59.7 ± 10.2 Female: n = 4 | PedAlert Monitor 120, (Patterson Medical Holdings, Inc.). | PRESSURE SENSORS | EG: gait intervention with auditory feedback | CG: general gait training over the ground. | D: 6 weeks T: 50 min (30 min of conventional therapy + 20 mins of gait training with or without bfb) F: 3 × week | Gait Speed (10MWT) *, FGA *, TUG *, COP length EO *, COP length EC* | PRE, POST |
| Cozean et al. [49] | To study the effect of EMG Biofeedback and FES as therapies for gait dysfunction in patients with hemiplegia after stroke. | TOT n = 36 Patients with stroke EG1: n = 9 Mean age:51 ± (not specified) Female: n = 4 EG2: n = 10 Mean age: 52 ± (not specified) Female: n = 2 G3: n = 8 Mean age: 56 ± (not specified) Female: n = 6 CG: n = 9 Mean age: 62 ± (not specified) Female: n = 2 | Not reported | EMG SENSORS | EG1: EMG biofeedback during static and dynamic activities EG2: FES during static and dynamic activities EG3: EMG biofeedback+FES during static and dynamic activities | CG: conventional Physical Therapy | D: 6 week T: 30 min F: 3 × Week | ankle angle (swing phase) *, knee angle (swing phase) *, stride length, gait speed* | PRE, POST |
| El-Tamawy et al. [43] | To examine the influence of paired proprioceptive cues on gait parameters of individuals with PD. | TOT n = 30 subjects with PD EG: n = 15 Mean age: 61.4 ± 7.28 Female: not specified CG: n = 15 Mean age: 63.2 ± 5.6 Female: not specified | The vibratory device, OPTEC Co. LLtd. | PRESSURE SENSORS | EG: individually designed physiotherapy and traditional gait training plus treadmill training with vibratory stimuli | CG: individually designed physiotherapy and traditional gait training including instructions to walk with long steps. | EG: D: 8 weeks T: 51–70 min F: 3 sess/week, CG: D: 8 weeks T: 45 min F: 3 sess/week | Cadence *, Stride length *, Gait speed *, Walking distance * | PRE, POST |
| Ginis et al. [44] | To test the feasibility of CuPID system in the home environment and verify differential effects of CuPID training versus conventional home-based gait intervention | TOT n = 40 subjects with PD EG: n = 22 Mean age: not specified Female: not specified CG: n = 18 Mean age: not specified Female: not specified | The CuPiD system consisted of a smartphone (Galaxy S3-mini, Samsung, South Korea), a docking station and two IMUs (EXLs3, EXEL srl., Italy) | IMU SENSORS | EG: received weekly home visit and patients were instructed to walk with the CUPID system | CG: received weekly home visit by the researcher who gave advice on gait and freezing, and patients were instructed to walk without using the CUPID system | D: 6 weeks T: 30 min F:3 times/week | MiniBEST *, FSST, FES-I, 2MWT, UPDRS III, NFOG-Q, Comfortable gait and Dual task activities (gait speed, stride length, DS time); | PRE, POST, FU |
| Intiso et al. [50] | to evaluate the efficacy of electromyographic biofeedback compared with physical therapy. | TOT n = 16 Patients with stroke EG: n = 8 Mean age: 61.3 ± 12.3 years Female: n = 4CG: n = 8 Mean age: 53.5 ± 18.5 years Female: n = 3 | table Satem PT 1015 and a walking Satem EMG Combitrainer PT 9115. | EMG SENSORS | EG: EMG Biofeedback and Physical Therapy (standard exercise bobath, facilitation, and inhibition techniques, neurofacilitatory techniques) | CG: Physical therapy (standard exercise bobath, facilitation and inhibition techniques, neurofacilitatory techniques) | D: 2 months T: 60 min F: daily physical therapy (Only EG 30 session of EMG BFB) | Basmajian scale *, Gait speed, step length, ankle angle (swing phase) *, ankle angle (heel contact) | PRE, POST |
| Jonsdottir et al. [51] | to assess the efficacy of EMG-BFB applied in a task-oriented approach based on principles of motor learning to increase peak ankle power of the affected leg and gait velocity in patients with chronic mild to moderate hemiparesis | TOT n = 20 Patients with stroke EG: n = 10 Mean age: 61.6 ± 13.1 years Female: not specified CG: n = 10 Mean age: 62.6 ± 9.5 years Female: not specified | BFB device: (SATEM Mygotron, SATEM srl, Rome, Italy); EMG, system (band-pass filtered at 20 to 950 Hz and then amplified with a gain of 40 000 (50 mVrms range), | EMG SENSORS | EG=Task-oriented gait training with EMG BFB device | CG= conventional physical therapy (at least 15 mins of gait training in each session) | D: 20 session T: 45 min F: 3 × Week | Gait speed *, ankle power peak at push-off *, stride length *, knee flexion peak | PRE, POST, FU |
| Jung et al. [52] | to examine the effect of gait training using a cane with an augmented pressure sensor to improve weight bearing on the nonparetic leg in patients with stroke | TOT 22 stroke subjects EG: n = 12 Mean age: 56.4 ± 11.1 Female: n = 4 CG: n = 10 Mean age: 56.3 ± 17.1 Female: n = 3 | An instrumented cane, outfitted with a pressure sensor (CD 210-K200, Dacell Co. Ltd., Korea) connected to an indicator (DN30W, Dacell Co. Ltd., Korea) | PRESSURE SENSORS | EG: gait training with auditory feedback | CG: gait training without auditory feedback | D: 4 weeks T: 60 min (30 min +30 min gait training with or without bfb) F: 5 × week | Peak force cane *, Gait speed *, single support time * | PRE, POST |
| Ki et al. [53] | to examine the effects of auditory feedback during gait on the weight bearing of patients with hemiplegia resulting from a stroke. | TOT n = 30 stroke subjects EG: n = 12 Mean age: 55.3 ± 9.2 Female: n = 4 CG: n = 13 Mean age: 60.1 ± 12.3 Female: n = 2 | A pressure gauge Ped-AlertTM120 (ORBITEC, USA) | PRESSURE SENSORS | EG: neurodevelopmental treatment with auditory feedback | CG: neurodevelopmental treatment | D: 4 weeks T: NA F: NA. | TUG *, Stance phase duration, Single support time. | PRE, POST |
| Lee et al [54] | to examine the effect of neurofeedack training on brain waves control and gait performed under dual-task conditions. | TOT n = 20 stroke subjects EG: n = 10 Mean age: 53.2 ± 6.46 Female: n = 4 CG: n = 10 Mean age: 54.7 ± 3.77 Female: n = 3 | The Procomp Infiniti system (SA7951 version 5.1, Thought Technology, Canada) was used for neurofeedback training. The QEEG-8 (LXE3208, LAXHA Inc., Korea) system was used to measure brain waves | EEG SENSORS | EG: neurofeedback training | CG: pseudo-neurofeedback training (sham neurofeedback) | D: 8 weeks T: 30 min F: 3 × week | gait speed *, cadence *, stance phase percentage, and plantar foot pressure (dual task) * | PRE, POST |
| Lupo et al. [55] | To evaluate the efficacy of training involving the use of a combined biofeedback system versus conventional balance training | TOT n = 15 stroke subjects EG: n = 9 Mean Age: 52.56 ± 13.92 Female: n = 3 CG: n = 6 Mean Age: 65.66 ± 9.64 Female: n = 1 | The RIABLO™ (CoRehab, Trento, Italy) system comprised of several inertial measurement unitsand a force platform connected wirelessly to a computer | IMU SENSORS AND FORCE PLATFORM | EG: balance training with RIABLO biofeedback system using a video interface. | CG: conventional balance training without the use of the RIABLO biofeedback system | D: 10 sessions T: 20 min F:3 times/week | BBS *, RMI, COP length EO *, COP length EC * | PRE, POST, FU |
| Mandel et al. [56] | to investigate the efficacy of electromyographic (EMG) versus a novel biofeedback (BFB) approach to improve ankle control and functional gait in stroke patients | TOT n = 37 stroke subjects EG1: n = 13 Mean age: 54.7 ± 13.9 years Female: n = 5 EG2: n = 13 Mean age: 57.5 ± 14.2 years Female: n = 5 CG: n = 11 Mean age: 56.8 ± 12.8 years Female: n = 1 | Two channels of EM-BFB, a Lamoureux-type parallelogram electrogoniometer was and a computerized system to provide audiovisual feedback of ankle position during dorsiflexion and plantar flexion. | EMG SENSORS and ELECTRO-GONIOMETER | EG1: EMG biofeedback training (computer-generated auditory and visual feedback of calf and pretibial muscle activity during active ankle movements) EG2: EMG biofeedback followed by rhythmic positional biofeedback (computer-generated single-channel feedback of dorsiflexion and plantar flexion) | CG: No training | D: 24 session (EG2 performed 12 session of EMG biofeedback and 12 session of Rythmical positional biofeedback) T: not specified F: 3 × Week | Gait speed *, ROM * | PRE, POST, FU |
| Schwenk et al. [58] | To evaluate the feasibility and experience in using the new sensor-based training in a sample of patients with clinically confirmed amnestic MCI | TOT n = 32 subjects with MCI EG: mean age 77.8 ± 6.9 Female: n = 7 CG: mean age 79 ± 10.4 Female: n = 5 | The technology consisted of a 24 inch computer screen, an interactive virtual user interface, and 5 inertial sensors (LegSysTM, BioSensics LLC, MA, USA) | IMU SENSORS AND FORCE PLATFORM | EG: postural balance exercises during standing (ankle point-to-point reaching tasks and virtual obstacles crossing tasks) using biofeedback training | CG: No training | EG: D: 4 weeks T: 45 mins F: 2 sessions/week | CoM area EO *, CoM area EC, CoM sway ML EO *, CoM swayML EC, CoM sway AP EO *, CoM sway AP EC, Gait Speed, Gait stride time variability, FES-I * | PRE, POST |
| Sungkarat et al. [57] | To determine whether improved symmetrical weight bearing somatosensory feedback would result in improved gait and balance in people with stroke | TOT n = 35 people with stroke EG: n = 17 Mean Age: 52.12 ± 7.17 years Female: n = 5 CG: n = 18 Mean Age: 53.83 ± 11.18 years Female: n = 6 | Insole Shoe Wedge and Sensors (I-ShoWS) | PRESSURE SENSORS | EG: conventional rehabilitation and gait training with I-ShoWS set-up | CG: conventional rehabilitation and gait training without I-ShoWS set-up | D:15 sessions T: 60 min/session, (30 min gait training and 30 min conventional) F: 5 days/week | Gait speed *, Step Length asymmetry ratio*, Single Support time asymmetry ratio *, Load on paretic leg during stance (% Body Weight) *, BBS *, TUG * | PRE, POST |
| van den Heuvel et al. [45] | to investigate the feasibility of visual feedback-based balance training (VFT) and to compare the effects of the training program with conventional training | TOT n = 33 subjects with PD EG: mean age 63.9 ± 6.39 Female: n = 5 CG:mean age 68.8 ± 9.68 Female: n = 8 | Flat-panel LCD monitor connected to a PC (Motek Medical, Amsterdam, The Netherlands), Force plate (Forcelink, Culemborg, and The Netherlands) and Inertial sensors (X sens, Enschede, The Netherlands) | IMU SENSORS | EG: interactive balance games with explicit augmented visual feedback | CG: conventional balance training recommended by the guidelines for physical therapy. | D: 5 weeks T: 60 min (45 min balance workstation) F: 2 sess/week | BBS; Single leg stance test; Gait speed, FES-I, UPDRSIII* | PRE, POST, FU |
| Author, Year | Biofeedback Mode | Biofeedback Content | Biofeedback Frequency | Biofeedback Timing | Reinforce Type |
|---|---|---|---|---|---|
| Azerpaikan, 2014 | Visual | Performance | Constant | Concurrent | Positive |
| Byl, 2015 | Visual | Performance, Result | Constant | Terminal | Positive, Negative |
| Carpinella, 2017 | Auditory, Visual | Performance, Result | Fading | Concurrent, Terminal | Positive, Negative |
| Cha, 2018 | Auditory | Performance | Constant | Concurrent | Positive |
| Cho, 2007 | Visual | Performance | Constant | Concurrent | Not specified |
| Choi, 2019 | Auditory | Performance | Constant | Concurrent | Positive |
| Cozean, 1988 | Auditory, Visual | Performance | Constant | Concurrent | Positive |
| El-Tamawy, 2012 | Vibrotactile | Performance | Constant | Concurrent | Positive |
| Ginis, 2016 | Auditory | Performance | Fading | Concurrent | Positive, Negative |
| Intiso, 1994 | Auditory | Performance | Constant | Concurrent | Positive |
| Jonsdottir, 2010 | Auditory | Performance | Fading | Concurrent | Positive |
| Jung, 2015 | Auditory | Performance | Constant | Concurrent | Negative |
| Ki, 2015 | Auditory | Performance | Constant | Concurrent | Positive |
| Lee, 2015 | Visual | Performance | Constant | Concurrent | Positive |
| Lupo, 2018 | Auditory, Visual | Performance, Result | Constant | Concurrent | Not specified |
| Mandel, 1990 | Auditory, Visual | Performance | Constant | Concurrent | Positive |
| Schwenk, 2016 | Auditory, Visual | Performance | Constant | Concurrent | Positive, Negative |
| Sungkarat, 2011 | Auditory | Performance | Constant | Concurrent | Positive |
| van den Heuvel, 2014 | Visual | Performance, Result | Constant | Concurrent | Positive |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bowman, T.; Gervasoni, E.; Arienti, C.; Lazzarini, S.G.; Negrini, S.; Crea, S.; Cattaneo, D.; Carrozza, M.C. Wearable Devices for Biofeedback Rehabilitation: A Systematic Review and Meta-Analysis to Design Application Rules and Estimate the Effectiveness on Balance and Gait Outcomes in Neurological Diseases. Sensors 2021, 21, 3444. https://doi.org/10.3390/s21103444
Bowman T, Gervasoni E, Arienti C, Lazzarini SG, Negrini S, Crea S, Cattaneo D, Carrozza MC. Wearable Devices for Biofeedback Rehabilitation: A Systematic Review and Meta-Analysis to Design Application Rules and Estimate the Effectiveness on Balance and Gait Outcomes in Neurological Diseases. Sensors. 2021; 21(10):3444. https://doi.org/10.3390/s21103444
Chicago/Turabian StyleBowman, Thomas, Elisa Gervasoni, Chiara Arienti, Stefano Giuseppe Lazzarini, Stefano Negrini, Simona Crea, Davide Cattaneo, and Maria Chiara Carrozza. 2021. "Wearable Devices for Biofeedback Rehabilitation: A Systematic Review and Meta-Analysis to Design Application Rules and Estimate the Effectiveness on Balance and Gait Outcomes in Neurological Diseases" Sensors 21, no. 10: 3444. https://doi.org/10.3390/s21103444
APA StyleBowman, T., Gervasoni, E., Arienti, C., Lazzarini, S. G., Negrini, S., Crea, S., Cattaneo, D., & Carrozza, M. C. (2021). Wearable Devices for Biofeedback Rehabilitation: A Systematic Review and Meta-Analysis to Design Application Rules and Estimate the Effectiveness on Balance and Gait Outcomes in Neurological Diseases. Sensors, 21(10), 3444. https://doi.org/10.3390/s21103444







