1. Introduction
Recent scientific and technological advancements are enabling a plethora of new interaction and sensing possibilities that may eventually lead to a drastic change in how we connect with cells [
1,
2]. One important representative case is the convergence of disciplines like micro-, bio-information technologies and cognitive sciences. These technological advances have been crystallized in different brands and concepts known and used in multiple research fields at present, such as Lab-on-a-Chip (LoC) [
3] and System-on-a-Chip (SoC). For instance, SoC has sensing, processing and wireless interaction functionalities based on microfluidic and microwave subsystems [
4].
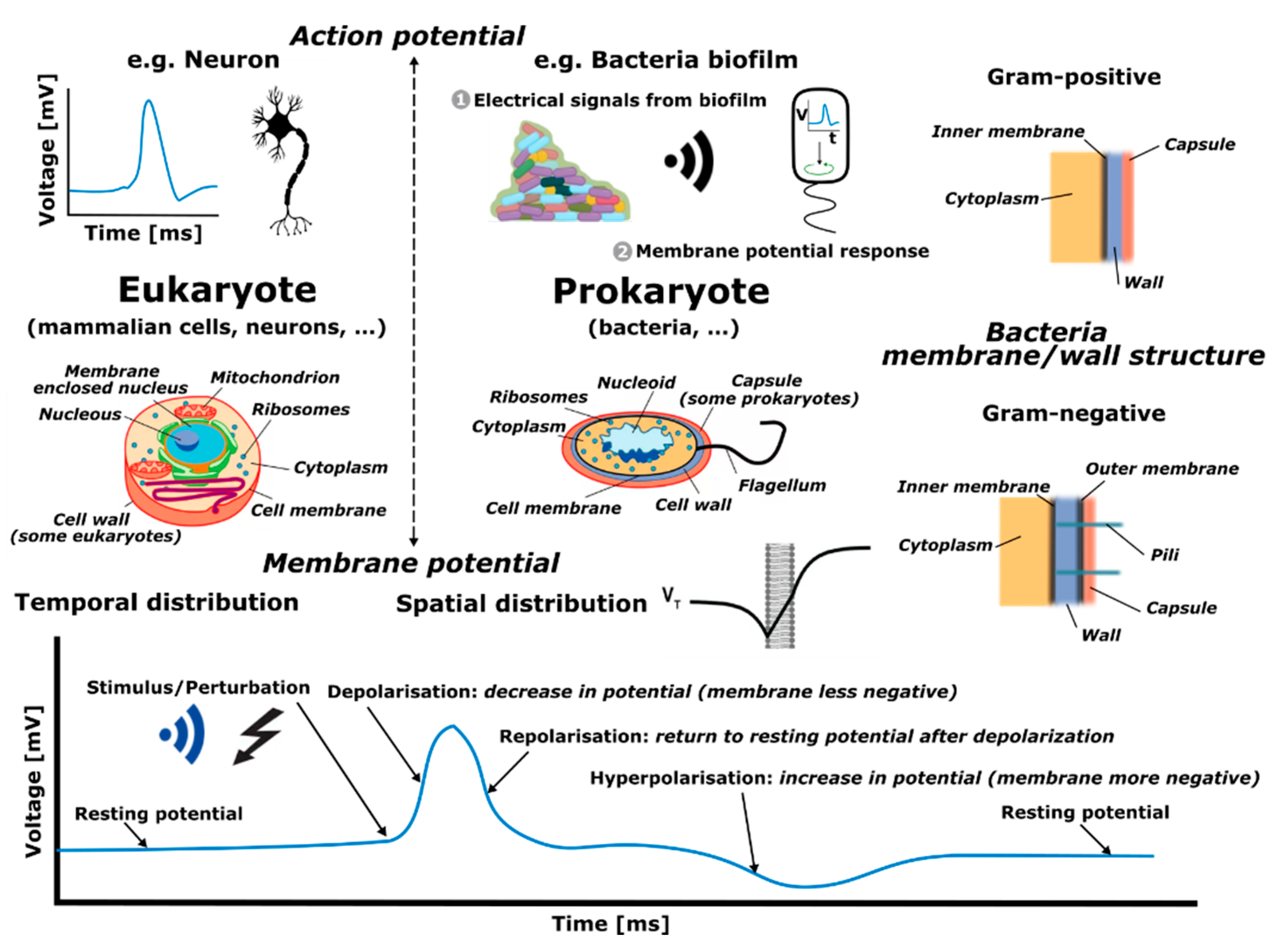
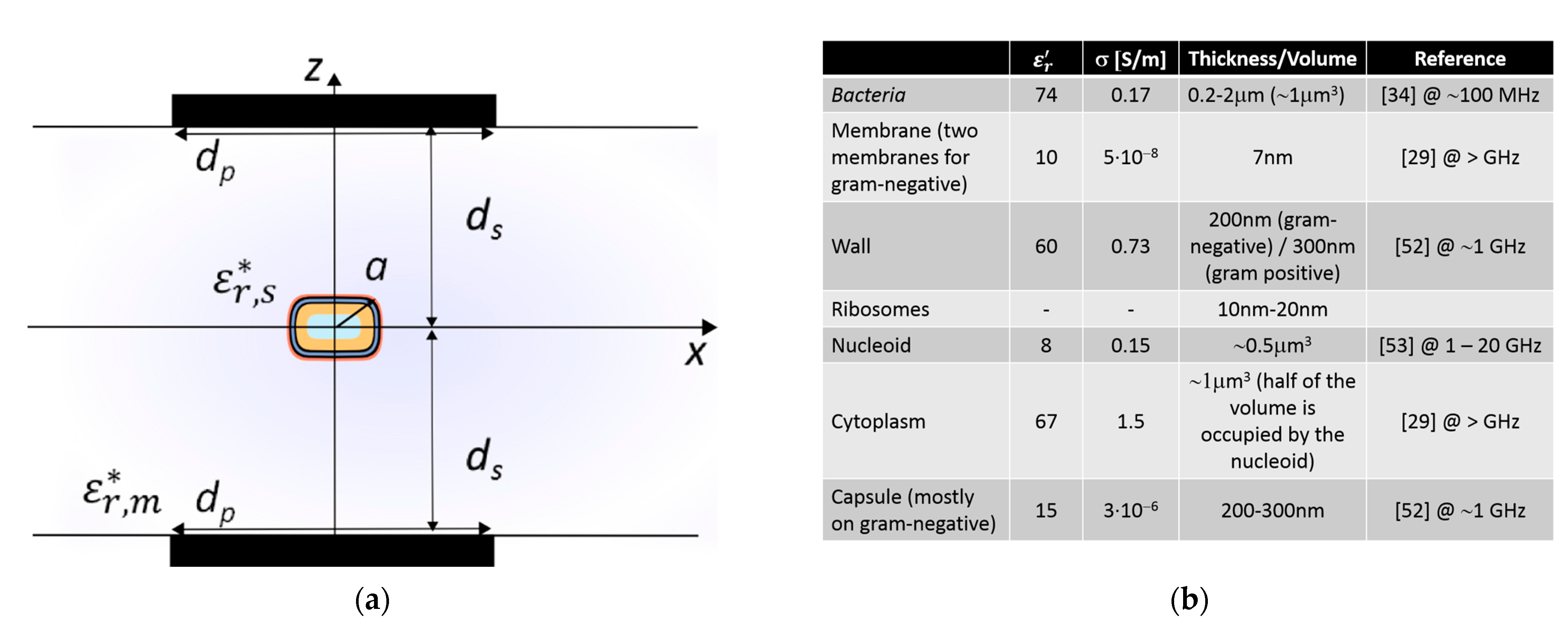
A main area of research in which such disciplines are converging is in biological solutions and technologies involving interaction with cells. A cell is often thought of as the smallest unit of a living organism, made up of even smaller parts, each with its own functionality, as shown in
Figure 1. Cells can be categorized into eukaryote (e.g., mammalian cells, neurons) and prokaryote cells (e.g., bacteria). There are several differences between the two, but a major distinction between them is that eukaryotic cells have a distinct nucleus containing genetic material, while prokaryotic cells do not have a distinct nucleus and have free-floating genetic material instead. Besides this distinction, there are several similarities in the different elements that form these two types of cells, such as the cell wall, cell membrane, cytoplasm and ribosomes, among others. Additionally, bacteria can be categorized according to Gram-negative and Gram-positive families (originally due to the permeability of specific dyes when observing them under the microscope), which, as depicted in
Figure 1, mostly relates to different membranes or wall structures. Generally, Gram-negative bacteria have an outer membrane, which expresses itself by different responses of the bacteria, for example virulence and antibiotic resistance.
Eukaryote and prokaryote cells, although inherently different as discussed above, present similarities in some functional activities, such as in action and membrane potentials. An action potential is a rapid rise and subsequent fall in voltage that propagates across a cell (such as in neurons), whereas membrane potential refers to the difference in charge between the inside and outside of a cell (such as in bacteria) created due to the unequal distribution of ions on its two sides. Specifically, the term action potential refers to the electrical signaling that occurs within neurons, or bacteria biofilms (aggregation of bacteria), resulting from rapid spatial changes in membrane potential when ion concentrations in the vicinity of membranes abruptly vary. Hence, action and membrane potentials are interconnected since they both depend on the electrophysical differences between the cell and the environment. However, action potentials are transient propagating events, while membrane potentials correspond to pseudo-static electrical characteristics. In this work, we consider sensing tasks as a way of monitoring and interacting with bacteria. In particular, the focus is placed on their membrane potential which, given a specific location, changes in time in response to a stimulus or perturbation, as depicted in the bottom graph of
Figure 1. The time response corresponds to an initial, or stable, resting potential (negative voltage value) which then, given a disruption, produces a depolarization (the membrane becomes less negative in voltage), succeeded by a repolarization (the membrane potential returns to the voltage resting potential), followed by a hyperpolarization (increase in negative voltage of the membrane potential), to finally end at the initial/stable resting voltage potential.
The location and time-varying signaling of cells can be described in terms of electromagnetic signals, and consequently research on electromagnetic biosensors is of main importance (an extended discussion is provided in
Section 2). Particularly, electromagnetic wave (EM-w) biosensors are attracting a lot of attention due to multiple benefits, such as being minimally invasive and cost effective [
5,
6,
7]. For instance, recent advances in microwave sensing [
8,
9,
10] enable the exploration of new frequency band windows for conducting novel research on membrane interactions. In particular, microwave frequencies allow to efficiently monitor biological elements and properties inside the human body, with considerable penetration distances (in the order of
) and resolutions (in the order of
) [
11]. When trying to approach this EM-w environment for basic microorganism interaction, the combination of EM biosensors with microfluidics has proven to be a very interesting pairing for testing, interacting and controlling functionality of microorganisms through the interplay with fluid streams [
12]; performing these functions without mechanical moving parts, and with little noise generation in the process. In particular, as it will be described in
Section 3, inertial and elasto-inertial microfluidics, using Newtonian and non-Newtonian fluids (respectively), can provide efficient single-particle flows in the center of a microfluidic channel and cell focusing that guarantees optimal sensitivity and accuracy [
13]. The benefits of such types of combinations will be leveraged in this work to enable the sensing and interaction with membrane potentials.
Therefore, the objectives of this work are to (i) develop a multidisciplinary theoretical framework for designing a microfluidic-based platform to wirelessly detect and interact with the membrane potential of microorganisms, (ii) provide an experimental demonstration of the capabilities that will be enabled with it, and (iii) discuss the results connected with a proposed microwave sensing strategy for the sensing of membrane potentials. In this regard, the paper is organized as follows. First, a description of state-of-the-art EM detection techniques utilized to detect properties of bacteria is provided in
Section 2. Next,
Section 3 presents the elasto-inertial particle-focusing strategy selected to focus microorganisms at the centerline of microchannels and describes the microfluidic platform designed, while
Section 4 focuses on characterizing the requirements for microwave detection of microorganisms. Experimental demonstration of the design and presentation of the results are provided in
Section 5. This is followed by
Section 6 which discusses the results connected with an original microwave sensing strategy for the detection of membrane potentials. Finally,
Section 7 summarizes the work, provides conclusions and proposes future research.
2. EM Detection Techniques
In many research studies, it is very convenient to detect bacteria individually to monitor and interact with their basic physiological parameters, and in particular with membrane potential on a one-by-one basis. Moreover, individually detecting and analyzing microorganisms is of capital importance in a variety of fields encompassing health, industry and environment technologies, and in applications ranging from diagnosis in clinics and pharma manufacturing to the production of beverages and fuel-treatment of solid deposits, among others [
14,
15,
16].
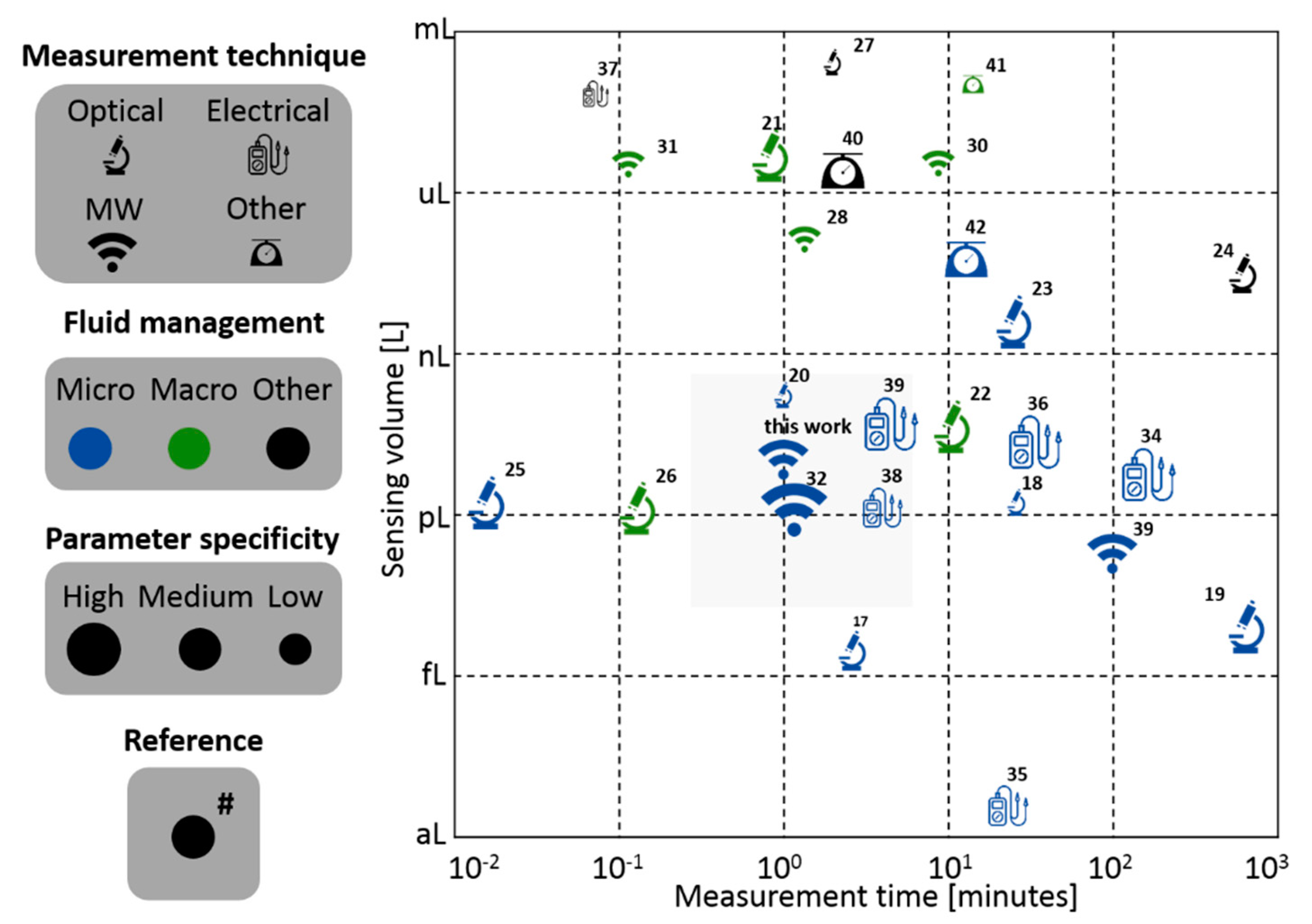
Figure 2 shows the comparison of several electromagnetic detection techniques reported in the literature based on the measurement time (in minutes) and the sensing volume (in liters). The values provided for the sensing volume correspond to either the geometrical dimension of the sensing volume or the actual liquid volume indicated by the analyzed systems. If multiple values were reported in the same study, the better value (i.e., best performance) is reflected in the chart. The experiments considered were performed with bacteria or particles of similar size to facilitate a better comparison between them on the chart.
The measurement techniques are grouped in four categories: optical, microwave, electric and others. Optical techniques refer to measurement systems where both the microorganism illumination and sensing is performed using light-based techniques [
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27]. Microwave systems make use of electromagnetic illumination and detection at higher frequencies in the order of
(
and
), where the equivalent wavelength is similar or larger than the fundamental characteristic dimension of the measurement system (this work) [
28,
29,
30,
31,
32,
33]. Electrical techniques consist mainly of approaches that make use of low frequency electrical signals (
) for measurement, where the equivalent wavelength is much larger than the fundamental characteristic dimension of the measured system and the electromagnetic dielectric properties substantially contribute to the detection process [
34,
35,
36,
37,
38,
39]. Other techniques consist of different physical-based (e.g., mass spectroscopy, magnetic detection) measurement systems that have been proposed or exist for detecting bacteria [
40,
41,
42].
In terms of fluid management, defined as the relevant fluid mechanics principles that govern the system, it is divided into three categories: micro, macro and other. Micro refers to managing fluids in flow channels with cross-sectional dimensions smaller than . Macro refers to flows handled in fluidic channels, or containers, with at least one cross-sectional dimension larger than . Other fluid-controlled techniques refer mainly to systems in which there is no intentional fluid flow management to make the particles flow through the sensing area.
Finally, the parameter specificity is divided in three levels: high, medium and low. The parameter specificity is defined for measurement techniques that aim to sense a particular physical property of the micro-organisms rather than solely detecting the element as a whole. The high level refers to experiments which have used a labeling technique or the physical measurement extracts high-level information from the bacteria. The medium level refers to studies where the physical measurement extracts qualitative information with little direct relation to the bacteria’s biological state or characteristics. The low level refers to studies that detect bacteria without any further insight.
Figure 2 shows a common tradeoff between measurement time and sensing volume. This is expected since (roughly) in order to have more sensibility (smaller volumes sensed) more time is needed to measure a large part of the sample. As seen in
Figure 2, there is an important gap in the literature with respect to sensing small volumes and using microwave techniques.
There is no specific technique that reins in all regions but it is a fact that, over the past 30 to 40 years, many advances have been performed in optical [
17,
18,
19,
20,
21,
22,
23,
24,
25,
26,
27] and low/medium-frequency electrical techniques [
34,
35,
36,
37,
43]. Differently, this work is focused on microwave techniques at the microfluidic scale, falling therefore in the center of the chart both in terms of sensibility and measurement time, which is of interest for bacteria detection applications, and especially relevant for sensing and interacting with bacterial functional parameters.
3. Design of the Microfluidic Platform for Elasto-Inertial Focusing of Particles
Over the past decade, microfluidic technology has been proven essential in biological research to precisely control the motion and position of microorganisms in a fluid flow, and is consequently the methodology selected in this work to complement the microwave-based sensing technique (presented in
Section 4) to achieve hydrodynamic focusing of microorganisms. In this regard, inertial focusing is an effective technique for controlling particle positions (particles are utilized as a surrogate of biological species) in microfluidic devices at low-moderate Reynolds numbers (
), i.e.,
; in fluid mechanics [
44],
characterizes the ratio of inertial to viscous forces used to classify flow regimes as laminar (flow organized in layers) or turbulent (flow dominated by velocity fluctuations). As first studied in [
45] for Newtonian fluids in a cylindrical pipe, initially randomly distributed particles are known to focus to an annulus located between the center and wall of the pipe, while in square-section channels, following the symmetry of the system, particles instead focus to four equilibrium regions centered at the faces of the flow-bounding walls [
46]. However, when utilizing viscoelastic fluids, i.e., a type of non-Newtonian material that exhibits both viscous and elastic behavior when undergoing deformation, particles have been found to migrate toward the centerline of pipes/channels in the case of fluids with constant viscosity [
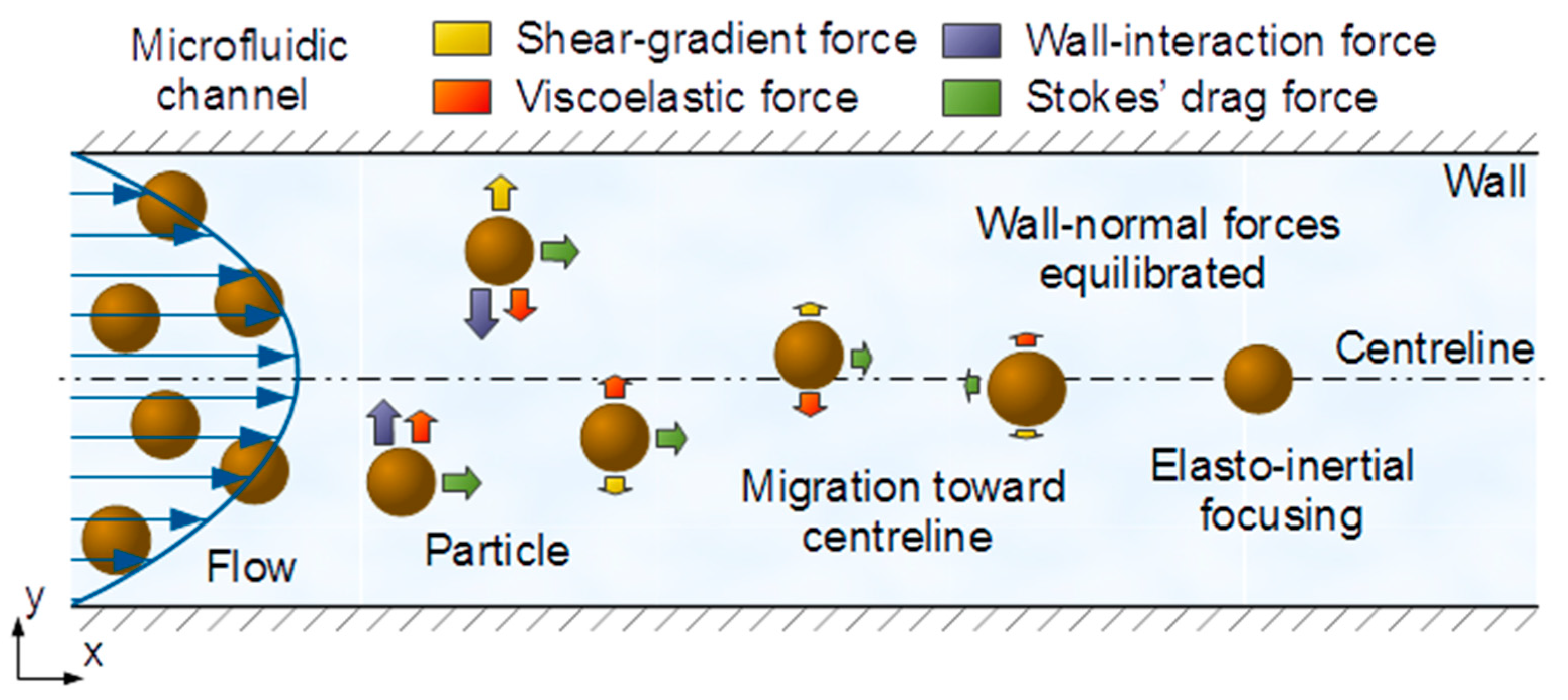
47]. The underlying principle of these phenomena is the balance of the hydrodynamic forces acting on the particles while advected by the flow. Particularly, as illustrated in
Figure 3, in the case of elasto-inertial microfluidic systems [
18], four main forces need to be considered: (i) wall-interaction force, (ii) shear-gradient lift force, (iii) viscoelastic force and (iv) Stokes’ drag force.
3.1. Dimensionless Numbers and Characteristic Regimes
The characterization of elasto-inertial fluid motion and particle focusing in wall-bounded microfluidic flows is efficiently assessed by considering the Reynolds number, defined as , where and are the density and dynamic zero-shear viscosity of the fluid, is the centerline fluid velocity, and is the hydraulic diameter with and the width and height of the microfluidic channel, respectively. This is in addition to the Weissenberg number, characterizing the ratio between elastic and viscous forces, given by , where is the fluid relaxation time, and is the characteristic shear rate with the volumetric flow rate and the mean fluid velocity in a laminar channel flow. The ratio between these two dimensionless numbers corresponds to the elasticity number , which only depends on the channel dimensions and fluid properties. Other parameters that are of second-order importance include the geometry of the channel, the strength of the shear-thinning effect, the initial position of particles and the blockage ratio defined as with as the particle diameter; when , blocking effects can be assumed to be negligible.
As studied in [
48], the equilibrium position for most particles in a viscoelastic fluid is either at
or at the channel axis, where
is the normalized vertical position away from the centerline. This is due to the occurrence of the inertial-force peak at
, which is explained below. For a second-order fluid, the viscoelastic force on a particle is
, with the negative sign indicating that the force drives the particle toward the center of the channel, in contrast to the shear-gradient lift force, which causes the particle to migrate away from the central axis given by [
30]
for
, where
is the shear-gradient lift coefficient, which is a positive function of
and has a maximum value of
at
, and is equal to zero at both
and
; the wall-interaction force
(
is the wall-interaction lift coefficient) is not considered in this analysis because it is assumed that the particle is close to the centerline and sufficiently away from the wall. The relative magnitude between
and
determines whether the particle can be focused at the centerline or not. In fluid flows with
, the viscoelastic force overcomes the maximum shear-gradient inertial force, and the particle migrates toward the centerline. In contrast, for fluid flows with
, the particle stops at a location before
reaches its maximum. Therefore, the balance between
and
at
leads to an estimate for the critical elasticity number of
.
The channel length
required for particles to achieve equilibrium positions at the centerline, i.e., particle focusing, is based upon the magnitudes of the forces described above and their variation along a particular channel. As an extremely important design parameter, this length must be estimated carefully. Following a scaling analysis for straight channels [
13], the focusing length
can be estimated based on the transversal particle migration velocity
[
49], which was calculated using the balance of
and Stokes’ drag force, defined as
, obtaining the expression
, where
takes values in the range 0.02–0.05;
is zero at the channel centerline and is consequently not considered to estimate
. In the case of significantly small particle Reynolds numbers, i.e.,
, the focusing mechanisms may be degraded due to the diffusion rate of particles becoming comparable to the inertial migration velocities [
50]. This limitation is evaluated by means of an equivalent particle Peclet number defined as
, where
is a characteristic length scale, and
is the Stokes-Einstein diffusion coefficient of a particle with
as the Boltzmann constant and
as the temperature of the system. For the diffusion effects to be negligible in inertial microfluidic systems, it is required that
.
In addition to the inertial and viscoelastic forces creating equilibrium positions for particles within the cross section of a channel, particles suspended in a fluid will interact in a flow with finite inertia to create particle trains with regular spacing in the streamwise direction of the flow. Therefore, particle concentration is also a critical factor affecting the focusing behavior and accuracy. Aside from the potential interparticle interactions, there are wake effects due to particles being concentrated in a few relatively narrow streamlines. In order to identify when these effects become important, the number of particle diameters per channel length, or length fraction, is defined, which is more appropriate than a volume fraction given that particles are focused to single streams. One can convert from a volume fraction to using the relation . For , focusing to a single stream is not to be expected due to wake interactions between particles, whereas the opposite is true for . Note that for a given , increases quadratically with decreasing , such that accurate focusing of smaller particles requires significantly more (quadratically) diluted solutions.
3.2. Microfluidic Design for Microwave-Based Sensing of Bacteria
Elasto-inertial microfluidics can be prepared by additives comprising biological, or synthetic, polymeric powders. For example, 500 ppm of polyethylene oxide (PEO) polymer in a phosphate-buffered saline (PBS) solution can be used to force particles to focus into a single stream at the center of a microfluidic channel [
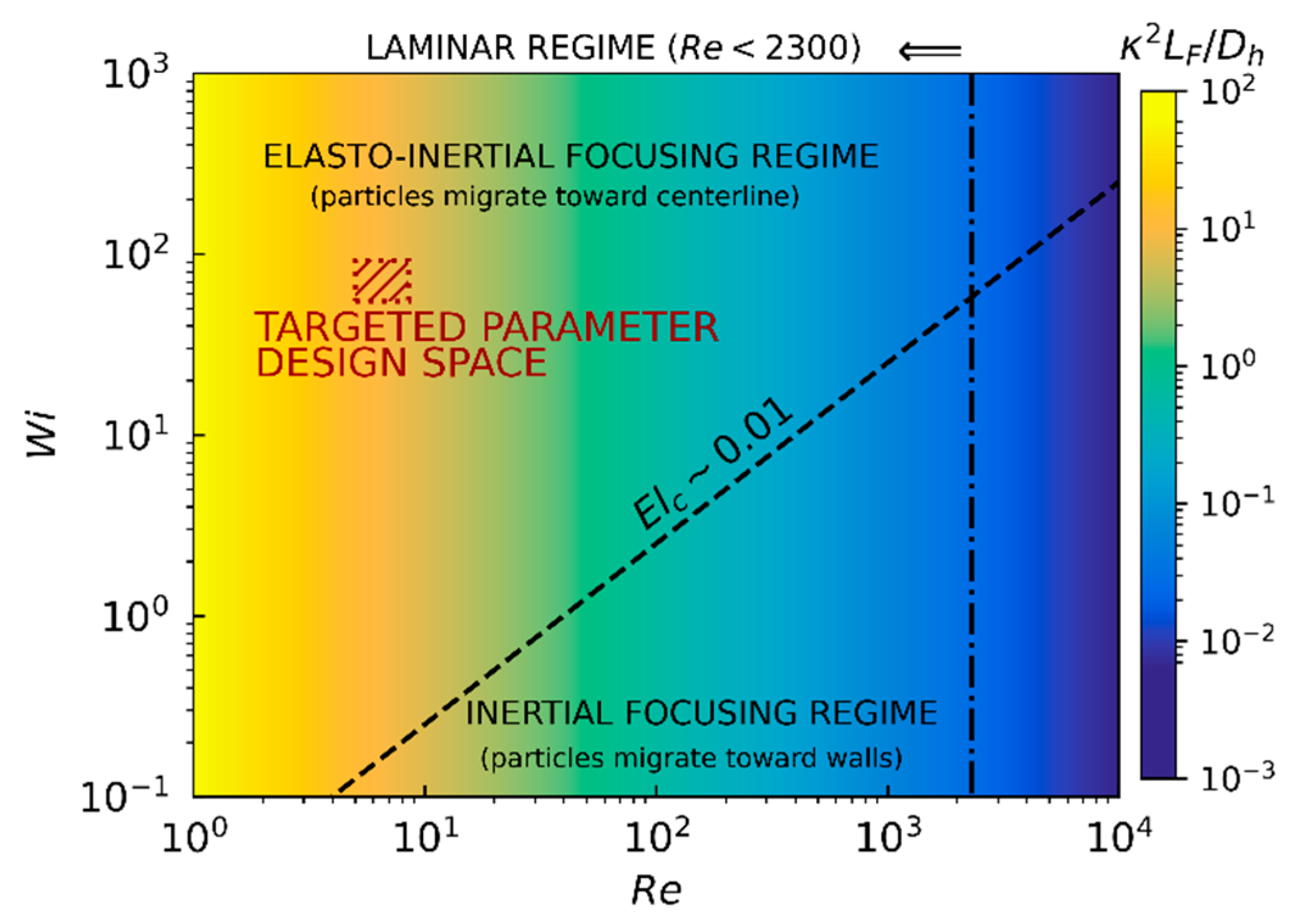
17]. As explained above, elasto-inertial focusing is achieved under specific conditions which depend mainly on the Reynolds and Weissenberg numbers and, to a second-order approximation, on the blockage ratio and length fraction. The interplay between these different parameters is visually summarized on the regime diagram depicted in
Figure 4. The vertical critical-Reynolds-number (
) line separates the laminar flow region, where the balance between forces discussed in this section applies, from the transitional/turbulent region not typically achieved in microfluidic applications due to their small characteristic sizes and velocities. In parallel, the diagonal critical-elasticity-number (
) line delineates the separation between elasto-inertial and inertial-focusing regimes. The red dashed rectangle indicates the parameter design space, which is detailed below, targeted for sensing bacteria using microwave-based detection techniques.
The constraints of the designed microfluidic channel are imposed by manufacturing, sensing, fluid properties and biological limitations, and correspond to: (i) the width, height and length of the channel are
and
as standardized by manufactured microchips; (ii) the average fluid velocity provided by the pressure pump is tuned to
(the range
is considered to calculate the targeted parameter design space) to provide sufficient exposure time (approximately
) for sensing the biological species on a field of view of
as typically required by a microscope; (iii) the viscoelastic fluid utilized is based on a solution of water with
of
PEO solution and resulting in
,
and
[
18]; and (iv) the diameter of the bacteria considered is
with a reasonable particle number density for preparing solutions in the order of
. This set of values results in the elasto-inertial microfluidic design located within the red dashed rectangle depicted in
Figure 4 and corresponding to
, which is smaller than
(laminar regime),
which is larger than
(viscoelastic fluid),
which is larger than
(elasto-inertial focusing regime),
which is sufficiently larger than
(inertial regime),
which is smaller than
(blocking effects are negligible),
,
which is much smaller than
(dilute solution), and
(a value of
has been utilized) which is more than an order of magnitude larger than
.
The inadequacy of the design in terms of focusing length, i.e.,
, is expected due to the small diameter of the particles considered; elasto-inertial manipulation of particles is typically limited to
as
scales inversely with
. A solution recently proposed to overcome this challenge is based on oscillatory microfluidics [
50]. Unlike traditional steady-flow microfluidics, oscillatory microfluidics switches the streamwise direction of the flow at a specific frequency. Due to the symmetry of the velocity field along the flow axis, the elastic and inertial forces acting on the particles preserve their wall-normal directionality when the flow is switched, and consequently, by exploiting this symmetry, the focusing length can be extended indefinitely, even though the channel itself has a much shorter fixed length. Based on the dimensionless numbers described above, the frequency of the oscillations
and the corresponding distances traveled by the particles can be defined for a given system. In particular,
is limited in the lower end by the distance
that the particles can travel within the microchip. On the higher end,
is limited by the entrance length, viz. streamwise distance required for the flow to develop. In the case of laminar flow, this upper limit is quantified by the dimensionless Womersley number, which expresses the pulsatile flow frequency in relation to viscous effects, defined as
. Small Womersley numbers, i.e.,
, indicate that the flow is fully developed for most of the oscillation period, and consequently entrance-length effects can be ignored. In general, the wall-normal particle focusing positions achieved by this oscillatory flow method are the same as for traditional steady-flow systems [
51]. Particularly for the microfluidic design considered in this work, selecting an oscillation frequency based on the mean velocity of the fluid and a length equal to the channel length, i.e.,
, the Womersley number obtained is
, which is sufficiently small for entrance-length effects to not impact the elasto-inertial focusing of particles.
5. Experimental Results
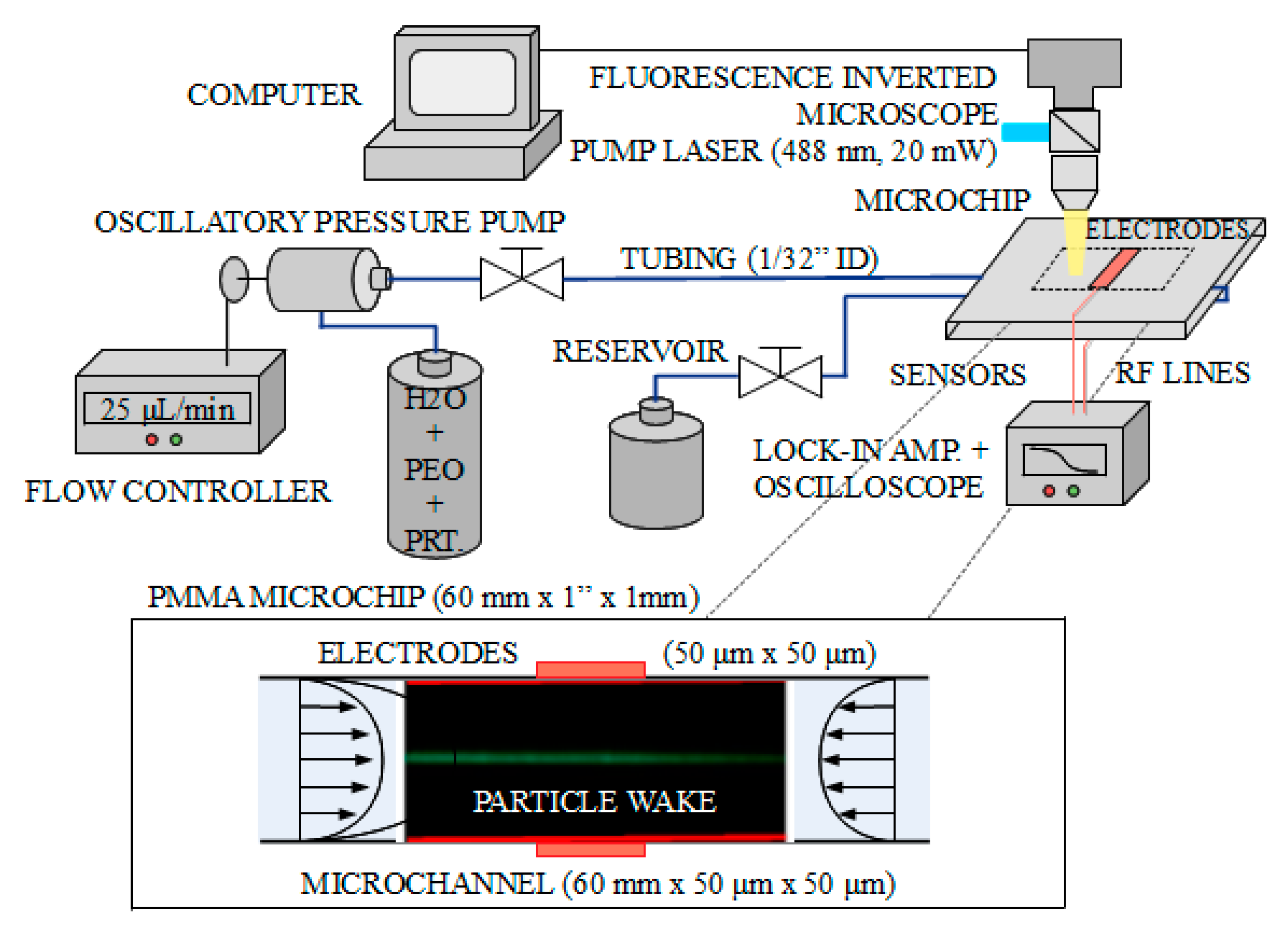
As described in
Section 3, to achieve single-cell detection (also for membrane potential sensing, as proposed in
Section 6) elasto-inertial hydrodynamic focusing combined with oscillatory-fluid operation is utilized in this work to (i) position a single bio-particle at the centerline of the microchannel (equidistantly from the measuring electrodes located at the microchannel walls), and (ii) to allow sufficient time to sense/measure the biological parameters of interest. In this regard,
Figure 7 provides a schematic illustration of the experimental setup combining the microfluidic platform, electromagnetic measurement platform and microscope for visualization and measurement purposes. The system is computer controlled and consists of an inverted fluorescence microscope (different microscope objectives available ranging from
to
) with an integrated color image sensor (CMOS camera with
frame rate with external triggering,
sensitivity and
minimum integration time) and equipped with a manually controlled micro-positioning stage with a custom
pump laser. The microscope fluorescence cube consists of a band-pass
excitation filter, a
dichroic mirror and a long-pass
emission filter. On the microfluidics side, the fluid is driven by means of a pressure-driven flow-controlled pump, capable of providing
of maximum pressure. The different microfluidic components are connected using tubing of 1/32” of internal diameter, combining polyetheretherketone (PEEK) and polytetrafluoroethylene (PTFE) tubing across the microfluidic system. The internal diameter (ID) is constant throughout the system to reduce the quantity of bubbles generated and enhance the stability of the flow. The oscillatory fluid operation is achieved using standard slow-switching three-way valves. The electromagnetic measurement station is connected using
-matched coaxial cables from the microfluidic chip to a lock-in amplifier, and visualized in a low bandwidth time oscilloscope. All the electromagnetic parts are properly shielded and grounded to avoid and reduce possible electromagnetic interferences present in the laboratory.
The microfluidic channels available for the measurements correspond to commercially available components with optical-grade-quality polymethyl methacrylate (PMMA) and two different cross-sections (
and
, are
long, and contain integrated
electrodes.
Figure 7 (bottom) shows an example of the microfluidic measurement provided by the microscope system. The focusing of particles (polystyrene fluorescent beads) was demonstrated for
(mean size of
with range 10–14 μm) and
(mean size of
with range 0.8–2 μm). The different samples were prepared in
of volume using
-filtered water, more than four-weeks-cured
PEO from 10,000 ppm of 0.4 MDa prepared stock, and with
. The procedure to operate the experiment is the same for the two channel cross-sections. First, the pressure controller (
) transmits pressurized air (positive pressure pump, with maximum of
and adjusted to
) to the first reservoir containing the sample preparation and this initiates the circulation of the fluid. The fluid then passes through a debubbler (porous PTFE with
pores,
internal volume), whose function is to remove any air bubbles that are contained within the fluid. It then passes through the flow sensor (
accuracy and
maximum flow rate), and enters the microchip oscillating at
, to complete the cycle by finally ending in the waste reservoir.
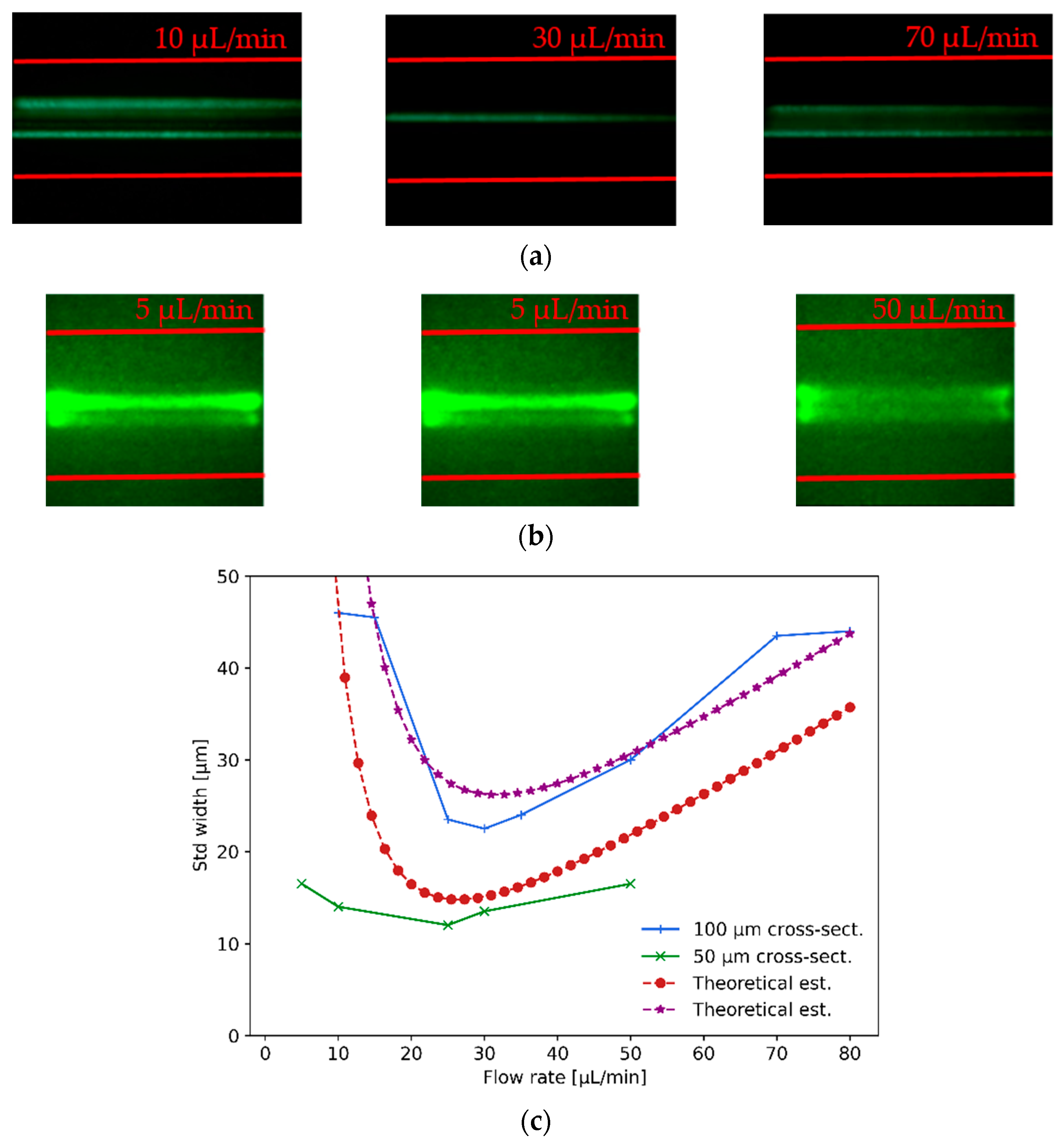
As shown in
Figure 8a, the first test consisted of hydrodynamically focusing
-size polystyrene fluorescent beads in the
cross-section microfluidic channel by varying the flow rate from
to
. In
Figure 8a, the utmost left image shows different beads passing through the channel (particle wake) at relatively off-centered distances when the flow rate is below the optimal focusing value (the red lines indicate the microchannel walls). Idem for the utmost right image where the flow rate is above the optimal one. On the contrary, the center image in
Figure 8a depicts the beads passing mostly through the centerline of the microfluidic channel with a high degree of hydrodynamic focusing (achieved for
flow rate). From left to right, the images for the
beads in the
cross-section channel correspond to flow rates of
,
and
, respectively. Similarly, the hydrodynamic focusing of
-size beads was assessed, shown in
Figure 8b, in the
cross-section microchannel. Once again, when the flow rate was too small or too large, optimal focusing was not achieved, while for
the optimal flow rate provided an efficient hydrodynamic focusing of particles. The images from left to right for the
beads in the
cross-section channel correspond to flow rates of
,
and
. The images were captured with
and
microscope objectives in fluorescence configuration, respectively, and were processed with standard image processing techniques to compute the standard deviation width of their recorded wakes.
In
Figure 8c, it is shown that the measured values (green crosses and blue plus signs) of hydrodynamic particle focusing agree with the results obtained from the theoretical estimations (red dotted and purple star curves) described below. An estimation of the lateral migration velocity
can be derived (first-order approximation) in the vicinity of the channel centerline by balancing the shear-gradient inertial lift force with the Stokes drag force as
. Noticing that the Stokes-Einstein diffusion coefficient of the particle is
, the transversal standard deviation width is then limited to
; in the plot, the estimation has been corrected to account for high-order non-linear effects at relatively large flow rates (
). As shown in
Figure 8c, the expected standard deviation width obtained from the theoretical estimation is
at a flow rate of
, whilst the experimental measurements fall just above and below these expected values with standard deviation values of
and
for the
and
cross-section channels, respectively.
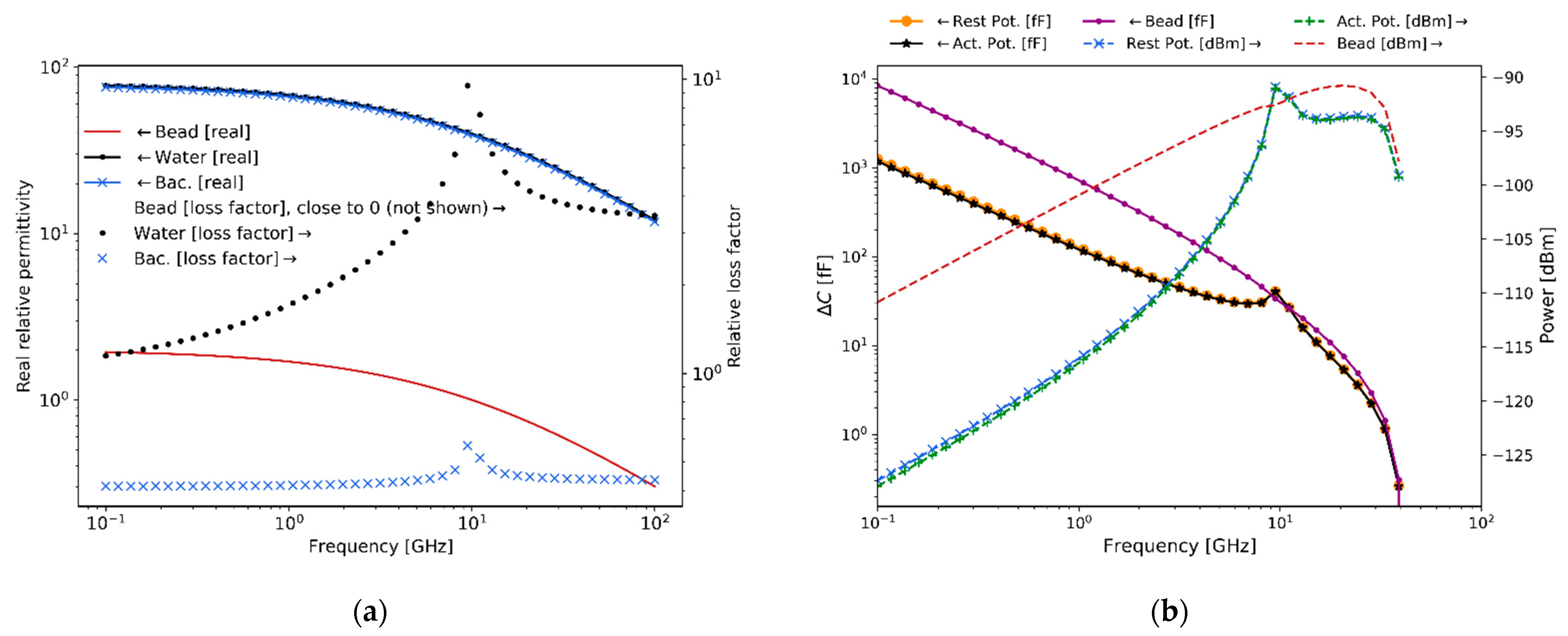
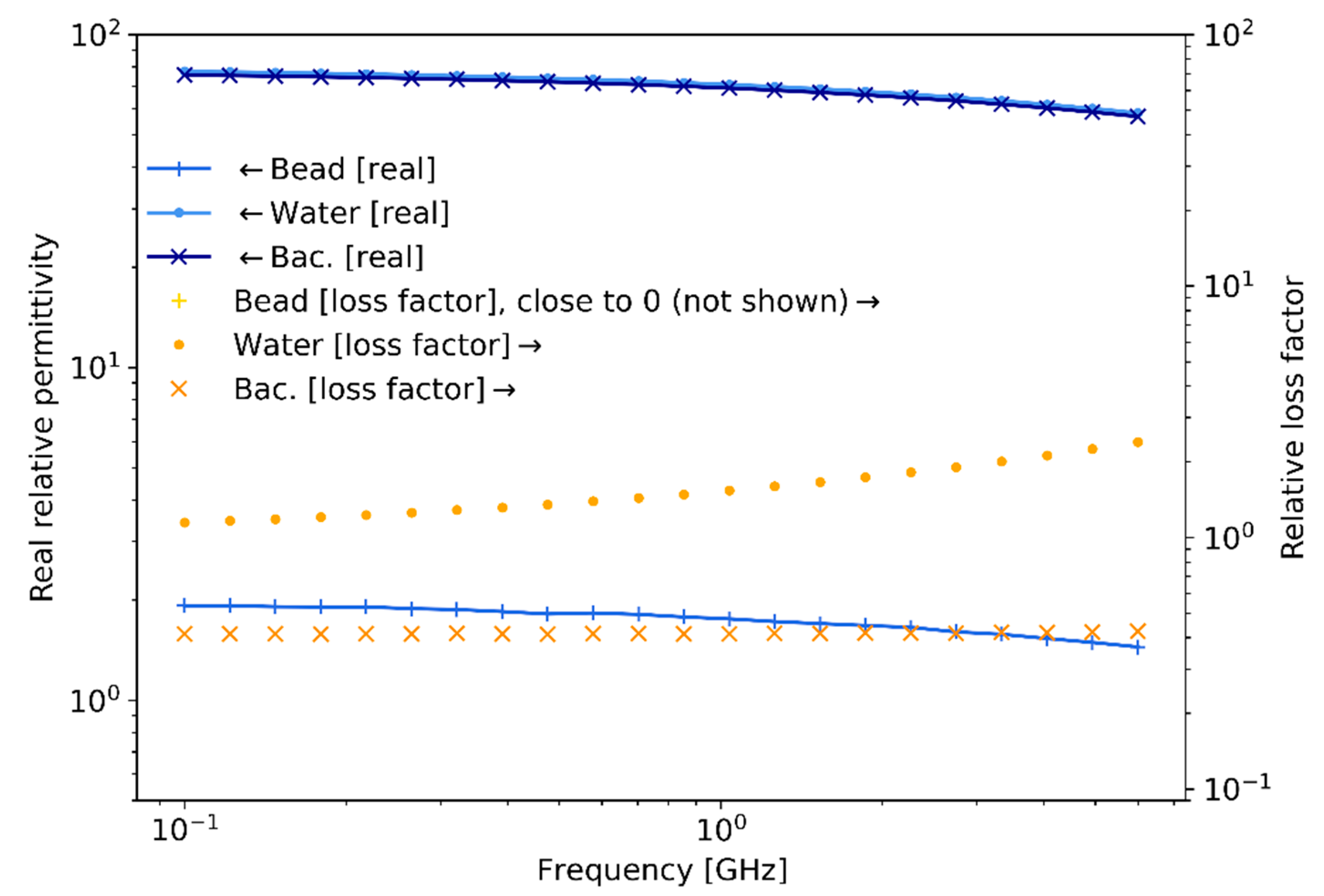
Figure 9 summarizes the measured experimental values of bacteria complex relative permittivity in the frequency range 0.1–6 GHz using the technique described in [
63,
64]. A vector network analyzer (VNA) with two built-in ports, with
of dynamic range, 2 MHz–6 GHz of frequency range, and
of output power, is employed to measure the S-parameters, which are post-processed and transformed to complex relative permittivity. These results, although measured for bacteria in a culture sample, are still indicative of the trends that would be observed for single-bacteria measurements and are consequently useful for estimating the expected measurable capacitance change and scattering levels for the latter case.
As expected, the real relative permittivity for water and bacteria are of a similar value of around 80, while they are approximately 2 for the polystyrene beads. These values remain relatively constant across the frequency range. On the contrary, the relative loss factor for water and bacteria presents a uniform difference of a half-decade for almost all frequencies. In addition, the value of the relative loss factor for polystyrene beads (not shown) remains close to 0 throughout the frequency range.
The electromagnetic measurement is centered in a lock-in amplifier, which generates the reference 100 MHz signal and is directly applied to the microfluidic chip through the available pads of the DC-high-impedance electrodes, as indicated in
Figure 7. This signal is also utilized internally for the phase-lock receiver to detect small voltage signals embedded in the noise. The lock-in amplifier is
-matched, with a single-ended input channel, working with voltage signals, a
noise level and maximum working frequency of
. The applied
signal on the electrodes is adjusted to
on a
-matched circuit prior to being transferred to the electrodes; this approach is equivalent to applying an irradiance of around
through the micro-channel. While the impedance of the micro-channel at DC frequencies is equivalent to an open circuit (the resistance between the electrodes is in the order of giga-ohms), it reduces to hundreds of ohms at the MHz–GHz range due to the equivalent parallel capacitance produced by the microchannel. The time integration filter of the lock-in amplifier is set to
, which corresponds to the time-of-flight duration of the particles within the effective field of view of the electrodes for each pass of the oscillatory flow regime. The interference noise above
is fairly well reduced by the lock-in amplifier, while the
electric grid noise (Europe) is properly shielded and filtered out by enclosing the open electromagnetic circuits in a metallic-box structure (with anti-reflection foams in the inner face to avoid electromagnetic self-resonances within the enclosure).
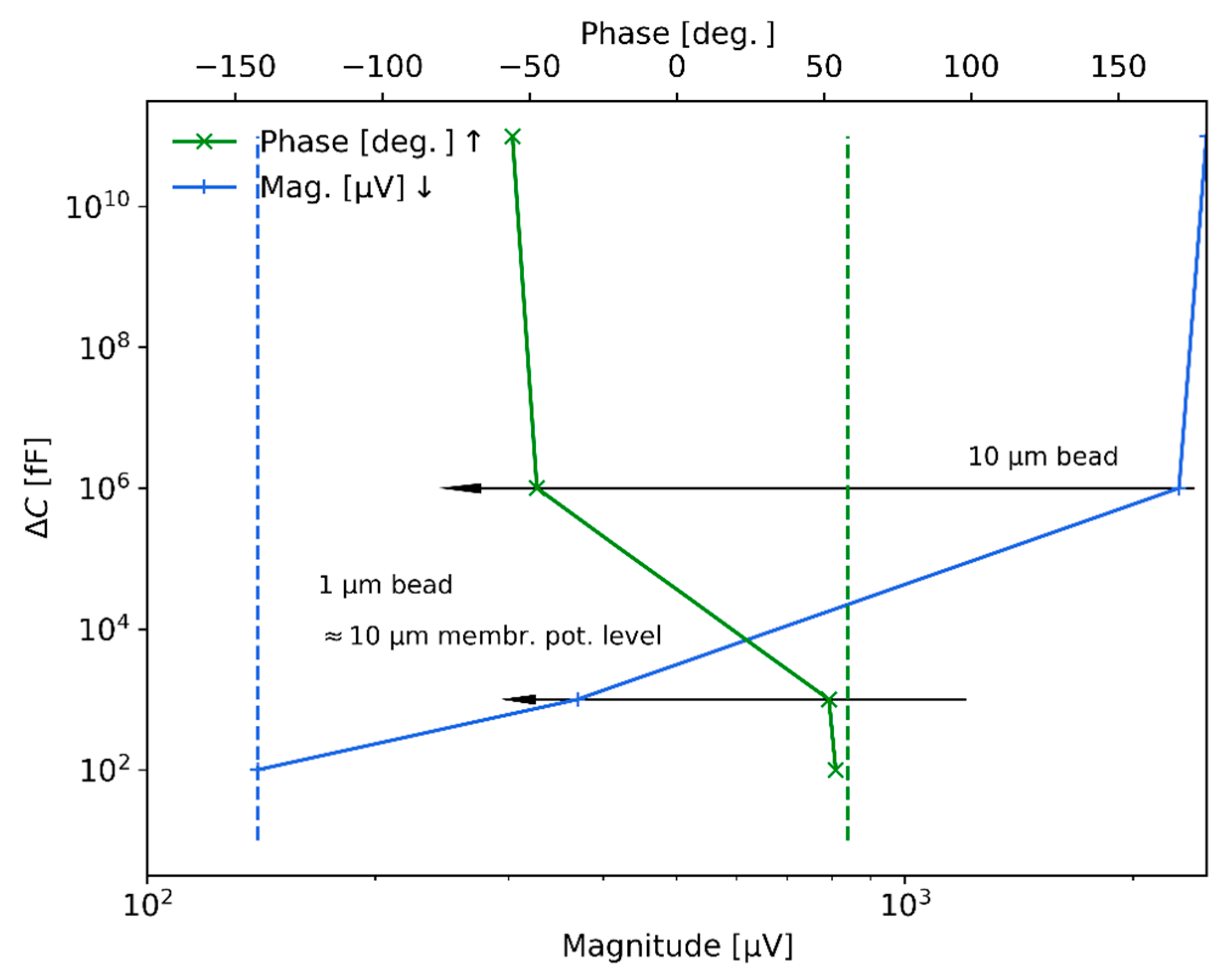
Figure 10 shows the measured results with the lock-in amplifier when measuring
and
polystyrene beads in the
cross-section chip with integrated electrodes separated by
(approximately
of separation from the top/bottom electrode to the particles) at a flow rate of
. The bottom horizontal axis represents the magnitude signal in
of the detected peaks when the beads pass through the sensing zone of the microchannel, while the top horizontal axis indicates the phase signal change detected. In particular, the measured values were in the order of 2
(around
phase value) for the
bead, while they significantly reduced to
(around
) for the
beads; as a reference, for the case of the microchannel full of air and without particles, the detected magnitude was
(around
. The signal sensitivity level achieved with the electromagnetic measurement system is around
in magnitude and
in phase (shown in
Figure 10 by dashed lines for the voltage magnitude and the phase). Regarding the vertical axis of the figure and considering the theoretical descriptions provided in
Section 4, the levels of signal detected are transformed to equivalent capacitance change units. In particular, the transformed capacitance changes for the
beads are around nano-farads, while the equivalent capacitance change level for
beads is found in the range of pico-farads. Hence, the sensitivity achieved with the current measurement system is around hundreds of femto-farads.
The levels of hydrodynamic focusing achieved and electromagnetic capacitance changes (or sensitivity) measured from the experimental rig and discussed in this section are of main importance for demonstrating the capability of the designed system to sense the biological signals of bacteria and more generally of other potential microorganisms, for example neurons and their action potential. Moreover, as proposed in this work and presented in the following section, it is also important to notice that being able to measure the signal produced by a bead shows, as will be discussed in the next Section, that the system could be used to measure the second-order non-linear membrane potential response of bio-particles since both signals are expected to be of the same order of magnitude.
6. Discussion on Membrane Potential Detection
Cellular, as well as intracellular, membranes exhibit a distinct nonlinear electrical behavior due to the potential barrier resulting from the difference between the inner and outer electrolytes and the action of ion-pumps [
65]. In the absence of an applied electromagnetic field, the transmembrane potential difference
is equal to the cell resting potential
(
for a typical cell). When a cell enters into an active membrane potential state it generates itself a low frequency voltage transmembrane voltage excess potential
. On top of the self-generated low frequency voltage, the illuminating signal at microwave frequencies (the cell is relatively sensitive to this electromagnetic frequencies) adds a high frequency small-signal transmembrane voltage excess potential on the cell, which is proportional to the electric field between the electrodes. As a result, a transmembrane current density
is generated. The current-voltage response is known to be fairly well approximated by a nonlinear diode-like relationship of the form
with typical values of order
and
[
66,
67].
In terms of electromagnetic flux, the current density associated with the membrane potential generated by bacteria is typically in the order of
. This relatively high, but extraordinarily localized in space (membrane of bacteria) and time (10–100 ms), density current could generate two-way coupling phenomena between the electromagnetic signal and fluid flow as analyzed in [
68]; in their work, the fluid studied is dilute in electrical charges, and consequently the electromagnetic forces are finally neglected. Similarly in this problem, the impact of the electromagnetic signal on the fluid phase, which is also electrically dilute, in addition to be activated sporadically and for a small period of time, exponentially decays as
(diffusion term in Poisson-Nernst-Planck-Stokes equation in spherical coordinates [
69]). As a result, the net effect will not be powerful enough to substantially modify the balance of hydrodynamic forces governing the advection and focusing of bacteria in the centerline of the microchannel.
In general, at optical frequencies the use of a generated second-order signal to sense the potential variation of cells has been demonstrated by making use of the nonlinear susceptibility tensor [
70,
71]. In this work, we proposed to sense the membrane’s action potential at microwave frequencies by leveraging the non-linear behavior of the voltage-current relationship of the membrane potential. Ideally, the charges respond to the rapid microwave wavelength, as indicated in [
72]. As shown in
Figure 11, the cell’s membrane is illuminated with a microwave field (e.g.,
), while the membrane’s voltage working point in the non-linear voltage-current model will be determined by the cell’s self-potential state, as shown in
Figure 11a. Depending on the state of the membrane potential, the scattered signals will have multiple generated frequency harmonics (due to the non-linear voltage-current model of the membrane) in comparison to the incident illuminating signal. In particular, using a Taylor series expansion for the first two terms of the transmembrane current density
, the expected efficiency of the second-order process at
compared to
is
times larger, while the second-order process at
compared to the first-order one is
, which is still significantly efficient [
73].
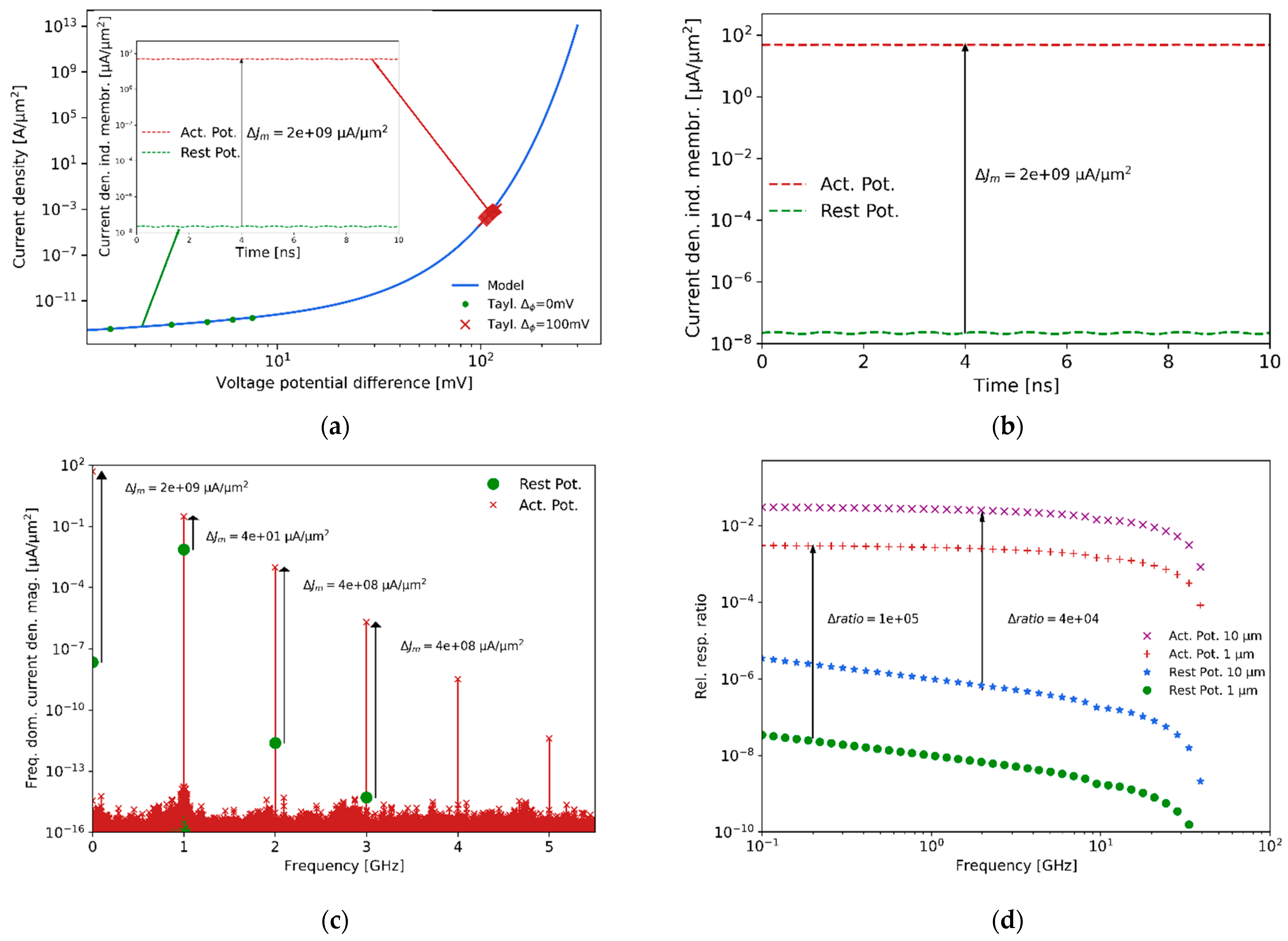
In addition,
Figure 11b, which is the insight plot of
Figure 11a, shows the induced current density due to the self-generated transmembrane potential and the impinging field at two different membrane potential states, while (i) at rest and (ii) generating an active potential.
Figure 11c shows the resulting frequency harmonics generated due to the nonlinear voltage-current response of the membrane potential. The fundamental frequency scattering intensity due to the membrane thickness (around
) is obscured compared to the scattering generated by the cell’s bulk body (difference ratio of intensities around 4), making it very difficult to sense the difference between the bacteria at rest and in an active potential state. Instead, the second-order, for instance, is characterized by a difference of approximately
orders of magnitude between the two states. The reason for this result is that the frequency mixing involved in the transmembrane voltage excess potential is directly sensing the effect of the membrane potential and not the entire body of the bacteria. The expected relative second-order response at different illumination frequencies, with respect to the fundamental frequency at rest and at an action potential state, are shown in
Figure 11d. As a note, the size of the bacteria scales the response with the cube of the radius, and consequently it is more challenging to detect
than
cells.
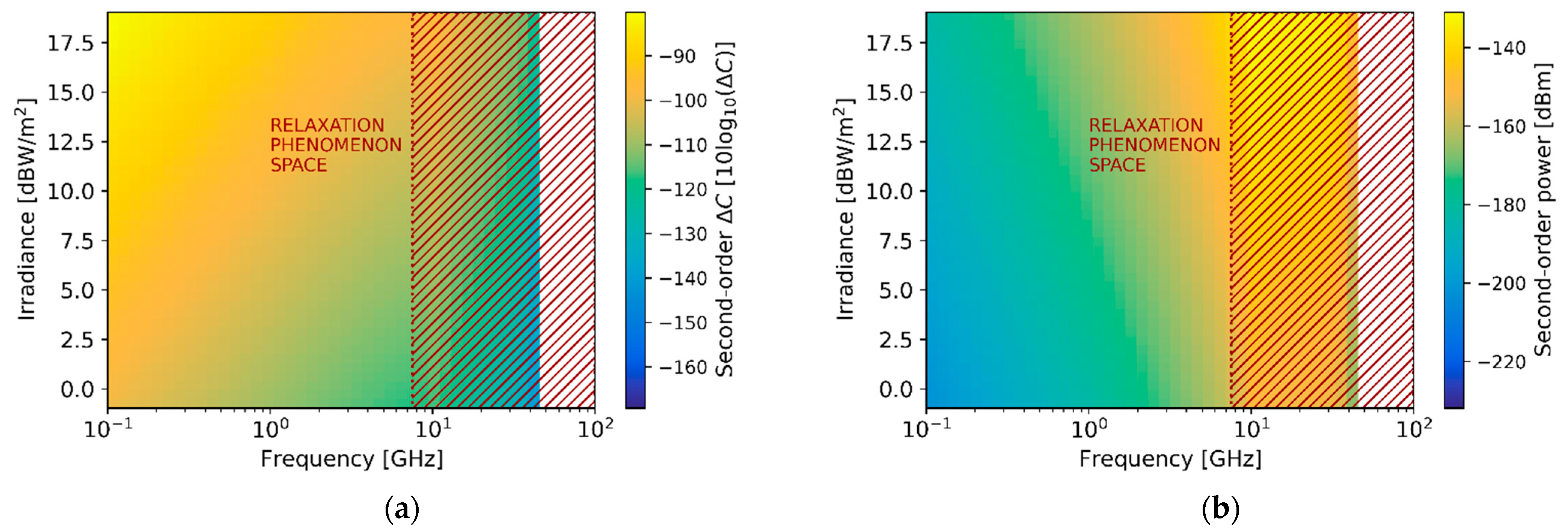
Figure 12 shows the expected magnitude of the second-order signal with respect to the illumination frequency in the 0.1–100 GHz range and the irradiance power applied in the tens of
range. In particular,
Figure 12a depicts the response in units of capacitance change, while
Figure 12b presents it in units of scattering signal level. The figure, as it is common for frequency conversion, shows the non-linear relation between irradiance intensity and power of the generated harmonic. Moreover, the second-order non-linear response vanishes strongly above
due to the low efficiency of the processes caused by the relaxation phenomena (red-dashed rectangles).
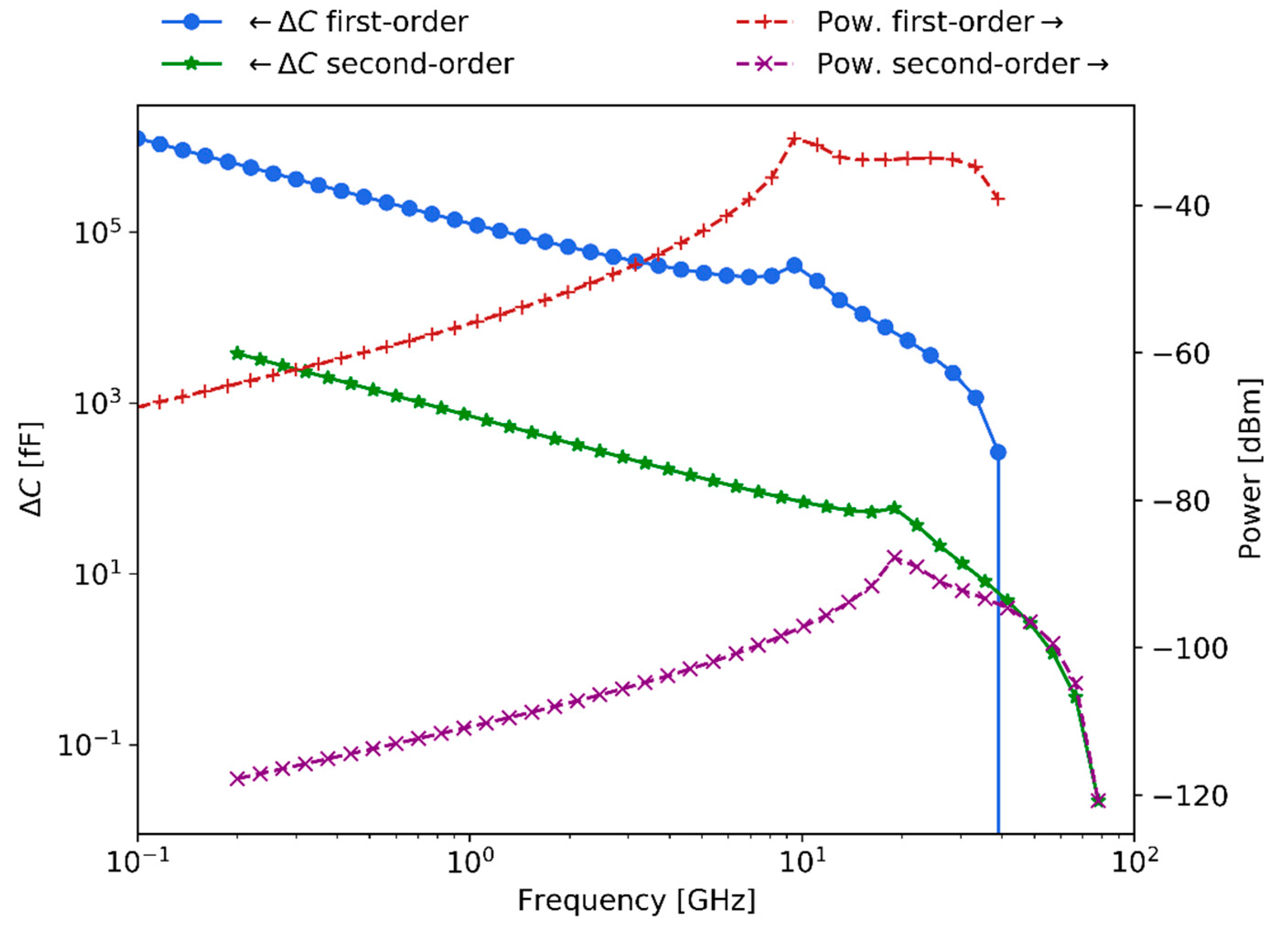
Finally,
Figure 13 presents the first-order expected capacitance change and scattering levels of detection when considering an irradiance power of
and the illumination geometry chosen in this work. In addition, the figure also depicts the second-order response, which in this work is proposed to be related to the sensing of the membrane potential.
The capacitance change of the first-order curve is in the
range, while the second-order response is in the
range. Equivalently, the scattering signal level of the first-order is in the
power range, whereas the second-order response is in the
power range. As expected, the capacitance change diminishes with increasing frequencies while scattering signals increase. Hence, the calculations indicate that at large frequencies scattering detection techniques are convenient since larger signal responses are expected to be generated. Relevantly, the expected membrane potential sensitivity levels for
bio-particles fall within the experimentally demonstrated sensitivity results for the
polystyrene beads, presented in
Section 5.