Abstract
Air pollution is becoming an increasingly important global issue. Toxic gases such as ammonia, nitrogen dioxide, and volatile organic compounds (VOCs) like phenol are very common air pollutants. To date, various sensing methods have been proposed to detect these toxic gases. Researchers are trying their best to build sensors with the lowest detection limit, the highest sensitivity, and the best selectivity. As a 2D material, graphene is very sensitive to many gases and so can be used for gas sensors. Recent studies have shown that graphene with a 3D structure can increase the gas sensitivity of the sensors. The limit of detection (LOD) of the sensors can be upgraded from ppm level to several ppb level. In this review, the recent progress of the gas sensors based on 3D graphene frameworks in the detection of harmful gases is summarized and discussed.
1. Introduction
There is a huge demand for the development of simple and reliable gas sensors [1]. In many fields, such as agriculture, medical diagnosis, and industrial waste, especially in environmental monitoring, it is necessary to detect NOx (especially NO2), ammonia (NH3), and volatile organic compounds (VOCs), because of their possible toxicity and related risks to the ecosystem [2,3]. In many countries, air pollution is a major environmental problem caused by rapid industrialization. A large amount of NO2 is emitted into the environment every year due to the industrial combustions and automobile emissions [4]. Therefore, the detection of NO2 has aroused widespread concerns, because it is harmful to the plants and respiratory systems of people and animals [5]. Additionally, NO2 can cause acid rain and photochemical smog [6,7]. Therefore, the United States Environmental Protection Agency (EPA) defines NO2 as a typical air pollutant, and the exposure limit is only 53 ppb [8]. Ammonia (NH3) is also a common dangerous air pollutant, which is produced by the industrial process, agricultural production, and manufacturing process [9,10]. Specifically, any overexposure to the high concentrations of NH3 (>30 ppm, 10 min) can irritate the human eye, skin, and respiratory system [11,12,13]. VOCs are the hydrocarbons that exist as gases or vapor at room temperature, which can be emitted from numerous products and activities, e.g., detergents, paints, solvents, tools, clothes, toys, cleaning, and cooking [14]. Aldehyde, aromatic, aliphatic, halogenated, and terpenoid compounds are the VOCs commonly detected in commercial buildings [14,15]. Toxic VOCs that have been previously detected in air by any type of sensors include formaldehyde, acetaldehyde, benzene, toluene, xylenes, phenol, pyridine, acetone, acetic anhydride, carbon disulfide, dihydroxybenzene, and so on [14,15,16,17,18,19]. For example, phenol is a toxic VOC occurring both naturally but also from industrial processes, which can be rapidly absorbed through the skin and cause skin and eye burns upon contact [20]. It is considered as a serious pollutant because of the toxicity and persistence in the environment. The short-term exposure limit of phenol is 10 ppm, 60 min [21]. Because of the serious environmental pollution, phenol monitoring becomes an urgent problem. Therefore, with the monitoring development of air pollution, the demand for gas sensors will increase rapidly in the future.
As a 2D material, graphene has many advantages, such as large conjugated structure, high specific surface area, high conductivity, easy to be synthesized, sensitive to the gas molecules, and so on. It has been proven to be a promising high-performance gas detection material [22]. Graphene surface can easily absorb some molecules, such as NO2, NH3, CO2, and so on. Moreover, the conductivity of graphene will change after adsorption of target gas molecules. The concentration of target gas in the environment can be detected by monitoring the change of conductivity. There have been many reports on the application of graphene in gas sensors, including pure graphene [23,24,25,26] and graphene composite materials [27,28,29,30,31]. There are many factors affecting graphene-based sensors, including: synthetic method [32,33,34], chemical structure [35,36,37], interlaminar structure [34,38], testing environment [39,40,41,42], and surface properties [43,44,45,46,47]. Due to the π-π accumulation and Van Der Waals force binding between graphene, the 2D graphene nanocomposites tend to agglomerate, resulting in the reduction of specific surface area [48,49,50]. In order to make full use of the characteristics of graphene, 2D graphene is usually assembled into a three-dimensional (3D) framework state by a series of methods. In contrast, due to the combination of 3D porous structure and the inherent characteristics of graphene, 3D graphene provides more space and larger surface area to transport and store electrons. 3D graphene has good conductivity, large specific surface area, and versatile gas adsorption sites. Furthermore, the defects and edge positions on the 3D porous graphene play an important role in promoting gas adsorption [48]. In recent years, compared with 2D graphene structures, 3D porous graphene structures such as graphene hydrogels, graphene aerogels, and graphene foams have been used as high-performance gas sensors [49]. Although 3D graphene has broad prospects in the field of gas sensors with the super high sensitivity, the selectivity is not satisfactory. Different gas molecules may adsorb on the same 3D graphene sheets and lead to the total change of the resistance [50,51]. It is difficult to quantitatively distinguish one target gas from a gas mixture. To improve the selectivity, defect engineering is generally needed to modulate graphene [52].
Several reviews have presented the main development of graphene-based gas sensors. For example, in 2015, Meng et al. [49] reviewed the graphene-based hybrids for chemi-resistive gas sensors. They focused on the sensing principles and synthesis processes of the graphene-based hybrids with noble metals, metal oxides, and conducting polymers. In 2018, Xia et al. [50] summarized the 3D structure graphene/metal oxide hybrids for gas sensors. They concluded a variety of logical strategies to design the 3D nanohybrids of RGO and MOx. In 2020, Ilnicka et al. [51] summarized the graphene-based hydrogen gas sensors, a special case of gas sensitivity to H2. However, the above reviews did not reflect the whole progress of graphene gas sensors, especially for the air pollution monitoring applications. This paper aims to summarize the recent progress of the gas sensors based on 3D graphene frameworks in the detection of air pollutants.
2. Synthesis of 3D Graphene Frameworks
Graphene oxide (GO) and reduced graphene oxide (RGO) have a 2D conjugated structure with single-atom thickness and residual oxygen-containing groups, which can be regarded as 2D conjugated macromolecules, structurally. They have rich chemical activities, which are helpful for 3D self-assembly through a series of chemical modification methods to regulate the interaction between the layers [34,38].
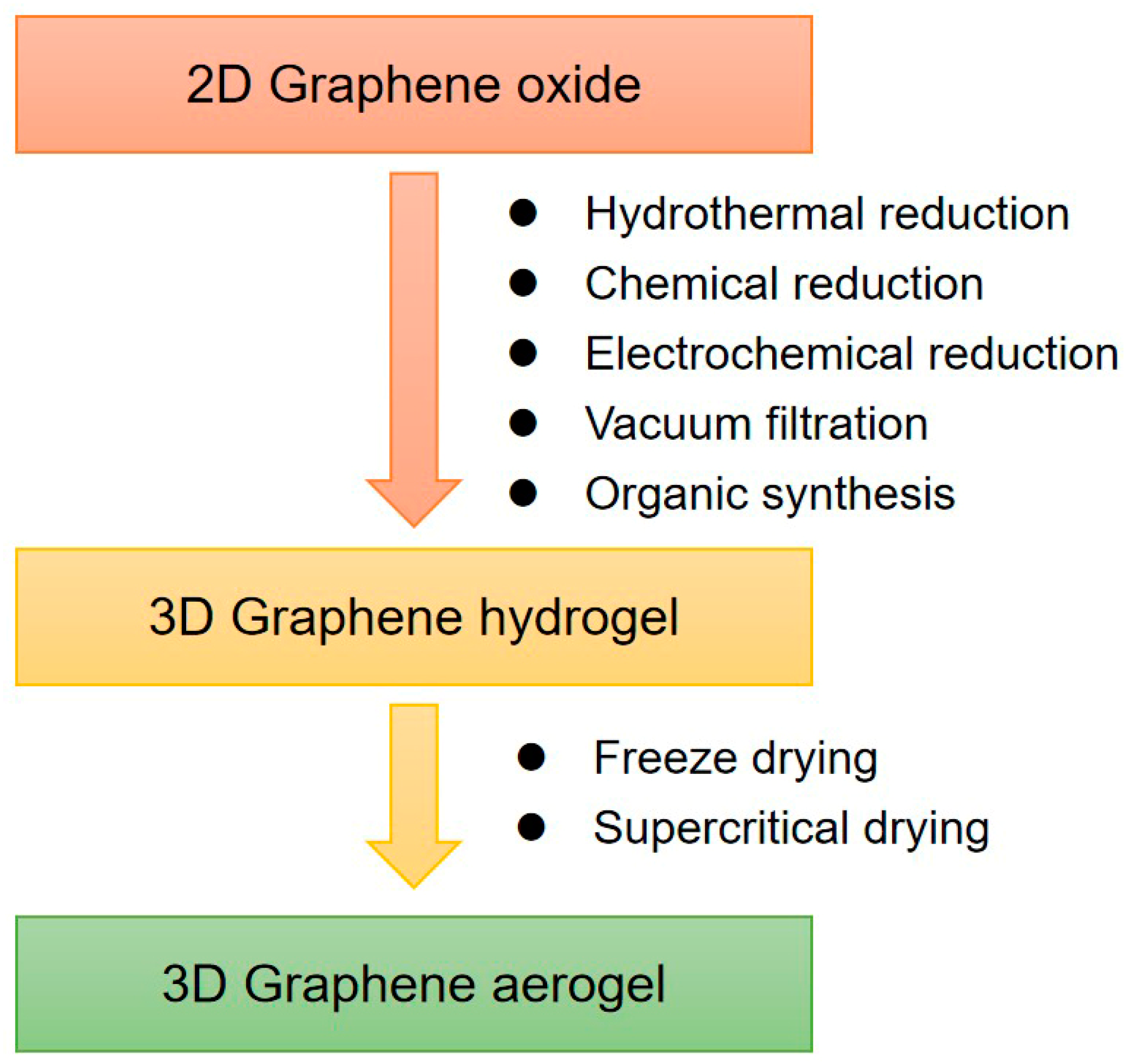
Graphene hydrogel is one of the major 3D assemblies. Chemically modified graphene (CMG) hydrogels prepared from GO or RGO can be used for large-scale production. As shown in Figure 1, RGO hydrogels (RGOHs) can be obtained by the following methods:
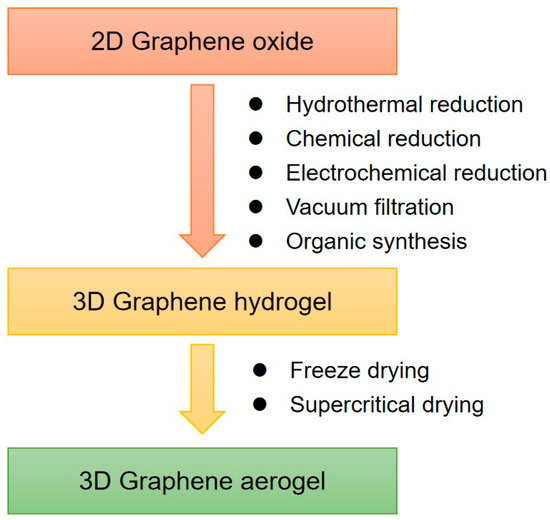
Figure 1.
Synthesis methods of 3D graphene frameworks.
- (1)
- Hydrothermal reduction, which is simple, fast, and free of impurities. At present, the commonly used hydrothermal method is to prepare RGO dispersion by hydrothermal treatment at 180 °C [53,54,55].
- (2)
- Chemical reduction, which is beneficial for large-scale production, and various reducing agents can be selected [56,57,58,59,60,61,62,63,64,65].
- (3)
- Electrochemical reduction [66,67,68]. The hydrogel prepared by this method is applied to the electrode surface and can be directly applied to the electrode materials of electrochemical instruments.
- (4)
- Vacuum filtration. A simple vacuum filtration method was developed to prepare RGO hydrogels with high conductivity, anisotropy, and responsive stimuli [69,70].
In addition to the 3D self-assembly of graphene in a water system, the assembly of the graphene in an organic system can also be achieved by thermal solvent reduction [71,72,73].
Graphene aerogel composites are usually prepared by supercritical drying or freeze-drying of hydrogel precursors [74,75]. For example, highly compressible RGO aerogels can be obtained by freeze-drying and microwave treatment. Directional freezing is a well-known processing technology of porous materials. This technology can also be used for the preparation of graphene aerogels [76]. Moreover, the controllable heat treatment technology can also reduce GO to RGO and restore conductivity. The regulation of the chemical structure of GO can adjust the morphology and elasticity of aerogels, for example, the oxygen functional groups in GO have a significant effect on the morphology and elasticity of the gels [77].
3. NO2 Gas Sensors
The development of a highly selective NO2 gas sensor with ppb detection limit is an important requirement for continuous environmental monitoring [78] and early diagnosis of respiratory diseases [79]. However, the original graphene, RGO, and RGOH had limited reaction to NO2, and could not monitor NO2 gas below 100 ppb [80]. Therefore, researchers have explored a variety of methods to fabricate graphene composites. Compared with 2D RGO, 3D RGO is more sensitive to NO2 [81]. Table 1 lists the gas sensitivities of 3D graphene toward NO2. The response is generally given in the form of relative change of resistance (∆R/R0) or conductance (∆G/G0), which was tested at a set temperature and a set concentration of NO2. S3D and S2D are the sensitivities (responses per ppm) of 3D and 2D graphene, respectively. The graphene with a 3D structure can increase the gas sensitivity to one or two orders of magnitude higher. The LOD of the sensors can be upgraded from ppm level to several ppb level.

Table 1.
Gas sensitivities of 3D graphene toward NO2.
3.1. 3D MoS2/RGO
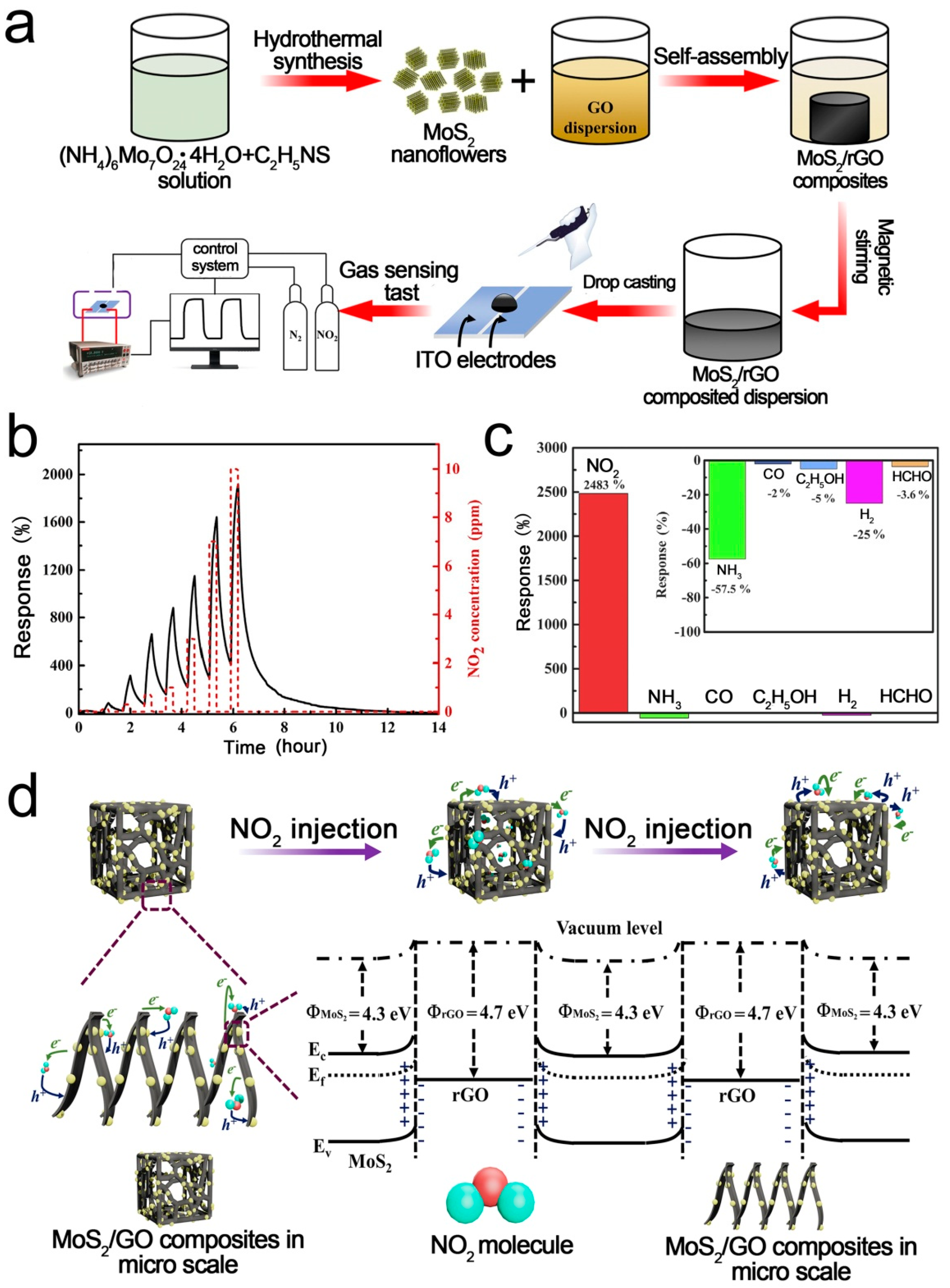
2D-layered MoS2 is an excellent gas sensing material due to its high surface/volume ratio and excellent electronic properties [90,91,92,93,94]. However, MoS2 nanoparticles tend to agglomerate, which limits their applications. Loading MoS2 on 3D RGO is a good choice [95,96,97]. Chen et al. [52] reported a highly sensitive NO2 sensor based on 3D MoS2/RGO composites. The composites were prepared using a novel self-assembly and hydrothermal method. The mild synthesis process enables MoS2 to uniformly disperse on the 3D RGO framework. The agglomeration of MoS2 was significantly alleviated, resulting in excellent low-temperature sensing performance. Figure 2 shows the fabrication process, gas responses, and sensing mechanism of the 3D MoS2/RGO sensor. The selectivity of the fabricated sensors was characterized using a variety of independent gases. The device shows higher sensing response towards N-based molecules (e.g., NO2, NH3) than the other gases (CO, C2H5OH, H2, HCHO). When the sensor is exposed to NO2 atmosphere, the minority charge carriers (electrons) in the MoS2/rGO composites will transfer to NO2 due to the strong electron negativity of NO2. Thus, the width and height of the heterojunction are reduced, leading to the increased conductivity. A small variation in barrier height and width caused by gas adsorption or desorption can have a significant influence on the resistance. A superior low-temperature NO2 sensing performance with a response of 2483% toward 10 ppm NO2 was achieved at 80 °C.
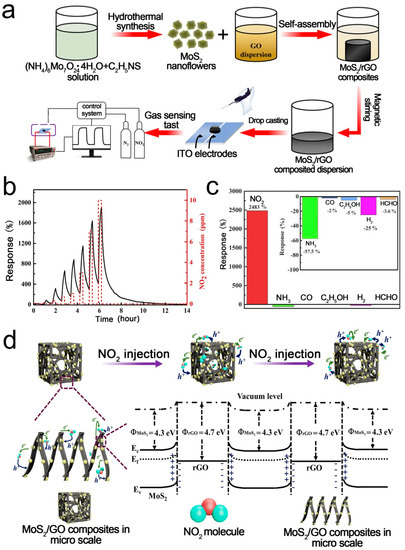
Figure 2.
(a) Fabrication process of 3D MoS2/RGO; (b) Dynamic gas responses of MoS2/RGO; (c) Responses of MoS2/RGO-5; (d) Schematic illustration of the sensing mechanism [52].
3.2. 3D SnS2/RGO
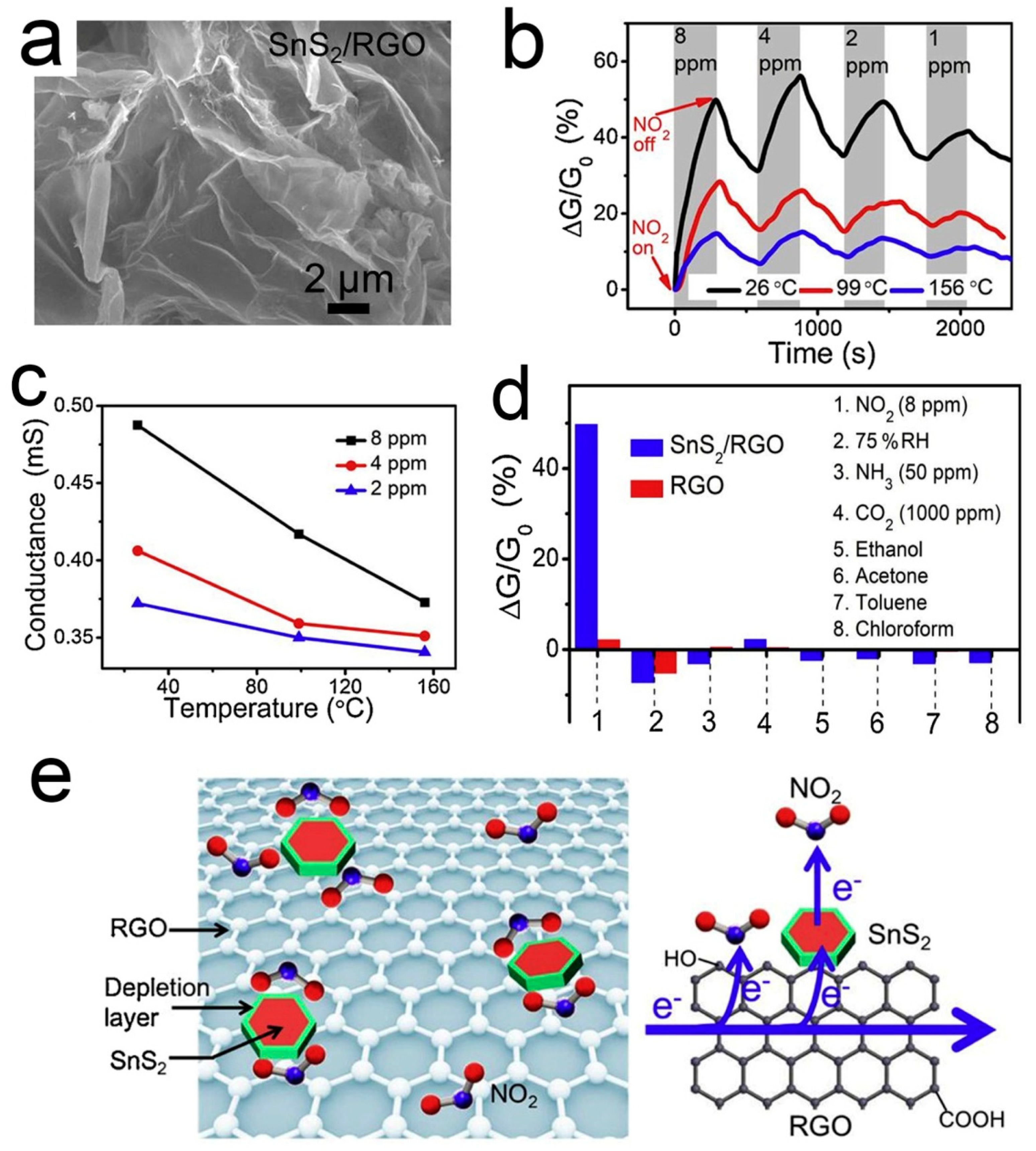
Different from p-type RGO, SnS2 is an n-type semiconductor with an indirect band gap of 2.2 ev [98]. SnS2 has a high affinity to NO2 because of its much weaker electronegativity than NO2 [99,100]. However, the resistance of SnS2 is too high to be measured at room temperature, so SnS2 is not suitable for monitoring NO2 at room temperature. When SnS2 is coupled with 3D graphene, their properties are complementary to each other. Wu et al. [86] demonstrated a kind of 3D structured SnS2/RGO heterojunction, which was synthesized through a facile hydrothermal route. Figure 3 shows the microstructure, gas responses, and sensing mechanism of the SnS2/RGO sensor. The 3D structure enhances the adsorption and diffusion of small NO2 molecules. SnS2 can facilitate the electron transfer from RGO to NO2 by forming a heterojunction with RGO. The depletion layer with an electrostatic field at the p-n heterojunction region could promote the dissociation of NO2 and thus enhance the NO2 adsorption at low temperatures. Upon NO2 adsorption on SnS2, the electron will transfer from SnS2 to NO2, leading to the increase both of the electron-depletion region and the hole concentration of the p-type RGO. Thus, the resistance of SnS2/RGO is reduced. The sensor displays impressive NO2 sensing performance, including high sensitivity (6.1 ppm−1), low LOD (8.7 ppb), good linearity, and reversibility.
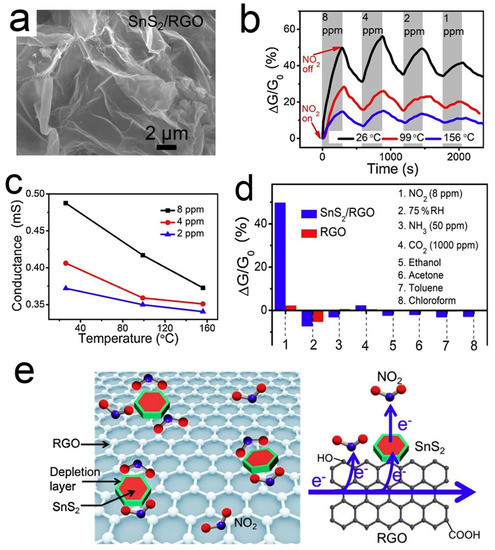
Figure 3.
(a) SEM image of 3D SnS2/RGO; (b) Dynamic responses of SnS2/RGO to NO2; (c) Conductance variation of SnS2/RGO versus operation temperature; (d) RT sensing responses of SnS2/RGO and RGO; (e) Schematic illustration of the NO2 sensing mechanism of the SnS2/RGO sensor [86].
3.3. 3D Eu(TPyP)(Pc)/RGO
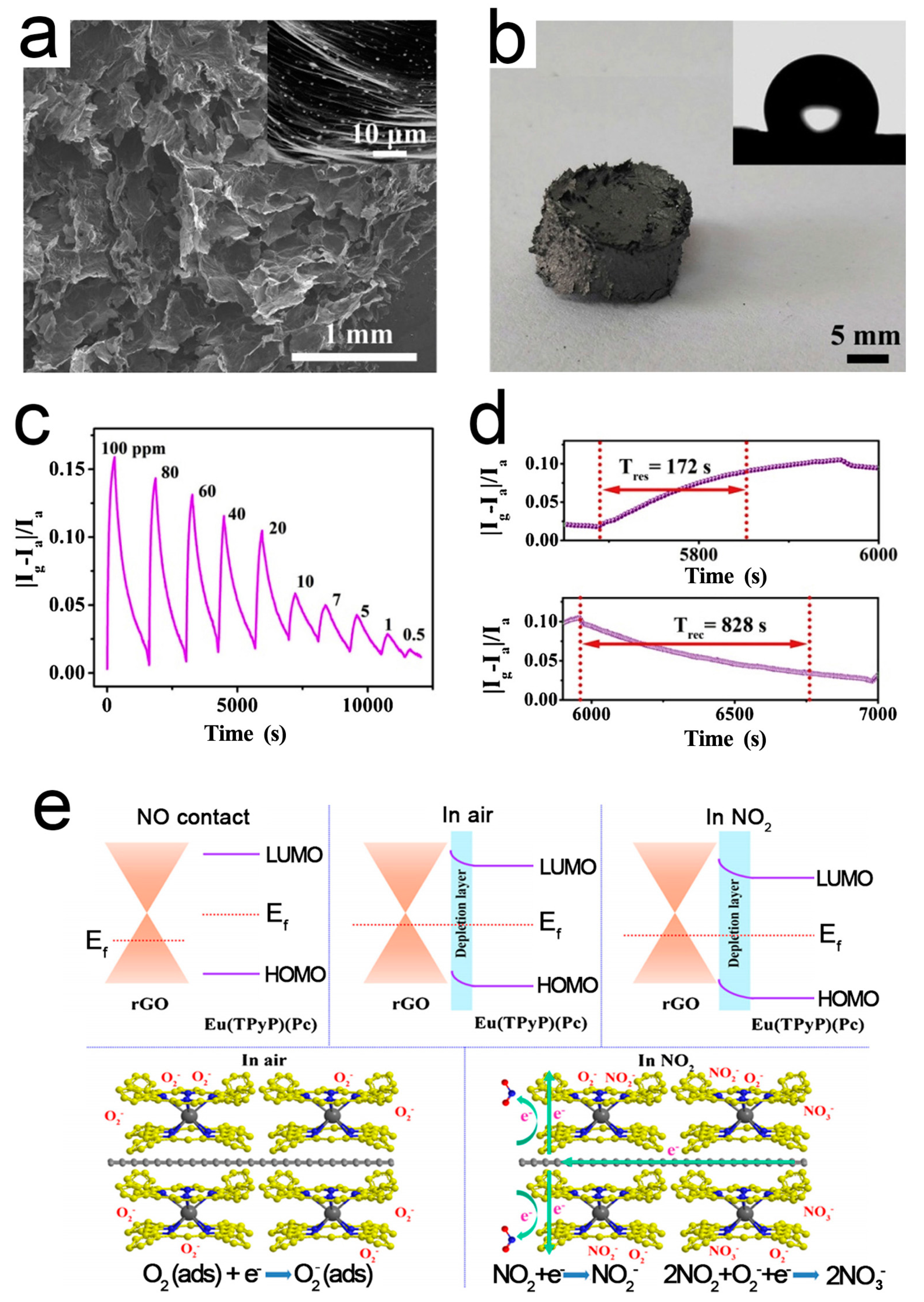
Pure RGO sensors have poor selectivity, slow response, and long recovery time, which limits their wide applications [89]. Chemical/physical modification with the external groups or atoms is an effective method [101,102]. Zhu et al. [88] reported a sandwich-type double-decker complex Eu(TPyP)(Pc) (TPyP = meso-tetra(4-pyridyl)porphyrin; Pc = phthalocyanine), which was in situ self-assembled on the surface of RGO driven by the π–π interaction, forming a 3D RGO/Eu(TPyP)(Pc) hybrid aerogel. Eu(TPyP)(Pc) not only acts as a sensor recognition unit, but also helps to enhance the amplification effect of the p-n heterojunction. At the same time, it provides enough space for the efficient transmission of RGO. The resulting aerogel not only effectively integrates the gas sensing of Eu(TPyP)(Pc) and good conductivity of RGO, but also exhibits a prominent synergy effect. Figure 4 shows the microstructure, responses, and working mechanism of the RGO/Eu(TPyP)(Pc) sensor. A good linear ratio between signal response and target gas concentration is achieved in the range of 0.5–20 and 20–100 ppm.
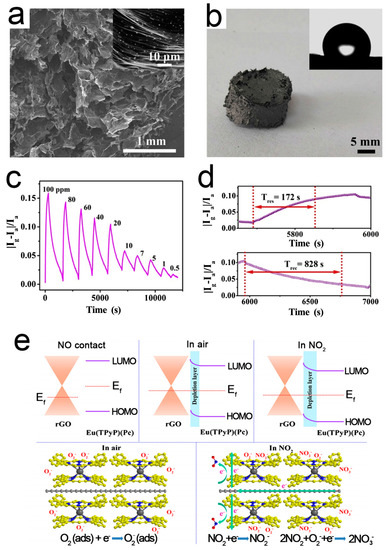
Figure 4.
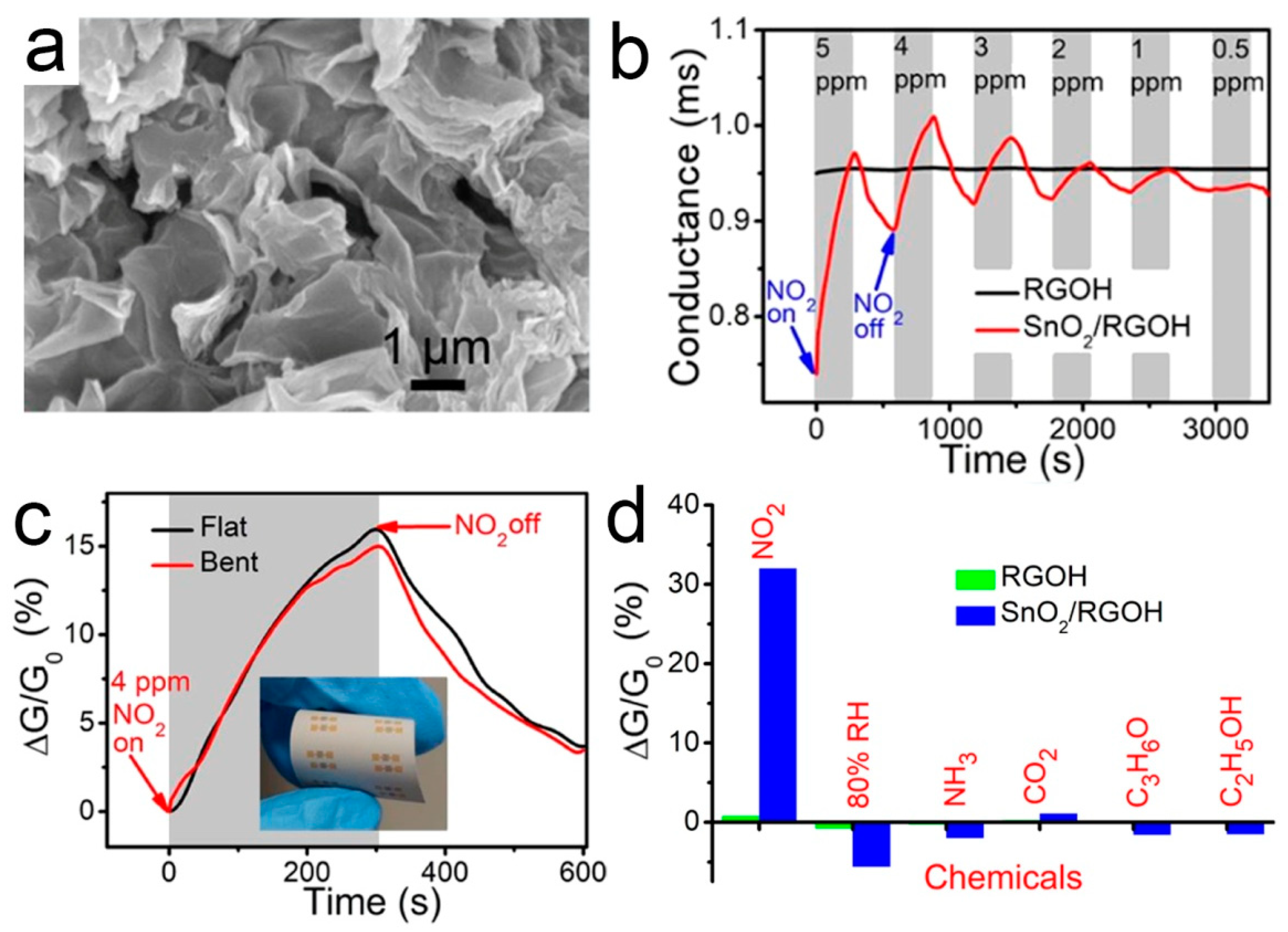
(a) SEM image of 3D SnO2/RGOH; (b) Dynamic responses of RGOH and SnO2/RGOH to NO2; (c) RT responses of SnO2/RGOH and RGOH; (d) Real-time responses of the flexible SnO2/RGOH sensor. Inset: Photograph of the sensor with the bent angle of 150° [85].
3.4. 3D SnO2/RGOH
Many studies have been devoted to improving the gas sensitivity by immobilizing SnO2 crystals on RGO [103,104]. Li and co-workers used SnO2 nanocrystals supported by the 3D mesoporous graphene aerogels to detect NO2 gas at low temperature [84]. Wu et al. [85] reported a facile preparation of SnO2-modified graphene hydrogel (SnO2/RGOH) via the one-step hydrothermal method. 3D SnO2/RGOH was synthesized directly from Sn2+ and GO precursors without any surfactant. The results show that it is feasible to optimize the gas sensing performance by combining reasonable material hybridization, 3D structure, and temperature modulation. The SnO2/RGO hybrid showed a high sensitivity to NO2 [105]. The improved sensitivity is due to the agglomeration of SnO2 nanoparticles and the formation of p-n heterojunction at the interface between SnO2 and RGO [106]. The p-n heterojunction formed at the interface of RGOH and SnO2 promotes the charge transfer. A micro-heater is integrated on the other side of the substrate to increase the substrate temperature locally, so as to suppress the interference of humidity in NO2 sensing. Figure 5 shows the microstructure, gas responses, and selectivity of the flexible SnO2/RGOH sensor. When exposed to 0.5–5 ppm NO2, SnO2/RGOH showed an immediately increased conductivity.
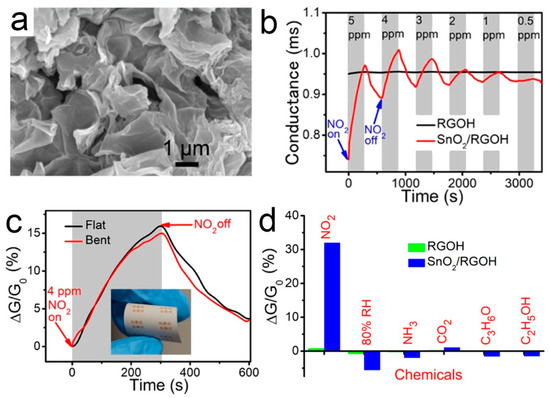
Figure 5.
(a) SEM image of RGO/Eu(TPyP)(Pc); (b) Photograph of RGO/Eu(TPyP)(Pc); inset: water contact angle. (c) Dynamic responses of RGO/Eu(TPyP)(Pc) to NO2; (d) Response-recovery time of RGO/Eu(TPyP)(Pc) to 20 ppm NO2; (e) Working mechanism of the RGO/Eu(TPyP)(Pc) sensor [88].
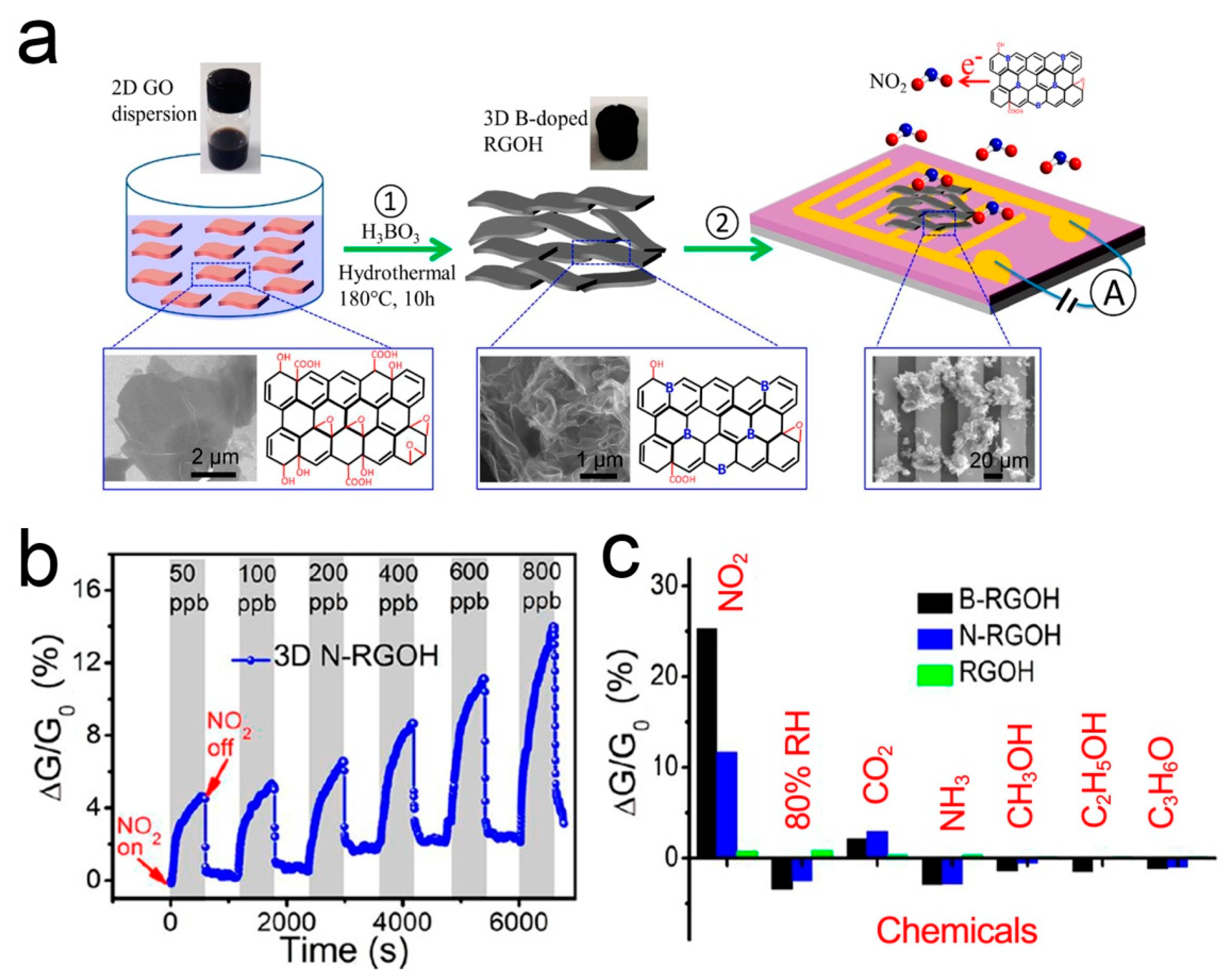
3.5. 3D Porous B- and N-Doped RGOH
Recent studies have shown that doping is a feasible strategy to adjust the physical, electronic, and chemical properties of graphene by creating a band gap [107]. Under the stable adsorption configuration, different elements in graphene can exhibit different gas sensing behaviors due to the different adsorption energy and the distance between doped atoms and gas molecules [108,109,110]. B- and N-doping can improve the selectivity of the graphene-based NO2 sensor [111,112,113,114]. Wu et al. [87] reported a 3D porous B- and N-doped RGOH chemical resistor. B- and N-RGOH were synthesized using a hydrothermal self-assembly method with the aid of boric acid (H3BO3) and dicyandiamide (C2H4N4). Figure 6 shows the fabrication process, microstructures, and gas responses of the sensors. It shows that the combination of 3D hierarchical structure and the doping of B- and N-heteroatoms can significantly improve the sensing performance. The response of B- and N-RGOH to NO2 is more than one order of magnitude higher than that of RGOH. It is worth noting that the response of B- and N-RGOH sensors varies almost linearly with the concentration of NO2.
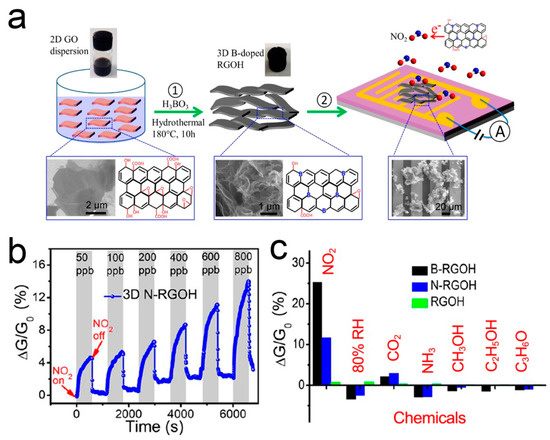
Figure 6.
(a) Fabrication process of B-RGOH sensors. (b) Responses of B- and N-RGOH sensors to NO2. (c) Responses of B- and N-RGOH sensors to different vapors [87].
4. NH3 Gas Sensors
The ammonia (NH3) sensor is indispensable in many industries and daily life. However, due to its complex preparation process, strict environmental requirements, and desorption of residual ammonia molecules, the production cost is high, which hinders the market acceptance [115,116,117]. The ammonia sensor might exhibit a reduced response during the recovery process in the co-presence of ammonia and ethanol, due to the result of multistep gas adsorption and desorption processes on material surface [115]. In general, the ammonia sensing behavior of the semi-conductive graphene-based sensors is negative to that of NO2 sensing, because NH3 is the reductive gas and NO2 is the oxidative gas [116,117]. For the same graphene-based sensors, however, the sensing performance toward NH3 is mostly reported as inferior to that toward NO2, for example, in 2020, Wu et al. [89] reported a green synthesis method of 3D chemically functionalized graphene for the high-performance detection of NH3 and NO2 at room temperature. They found that the LOD of NH3 and NO2 were 500 and 100 ppb, respectively.
4.1. 3D Graphene
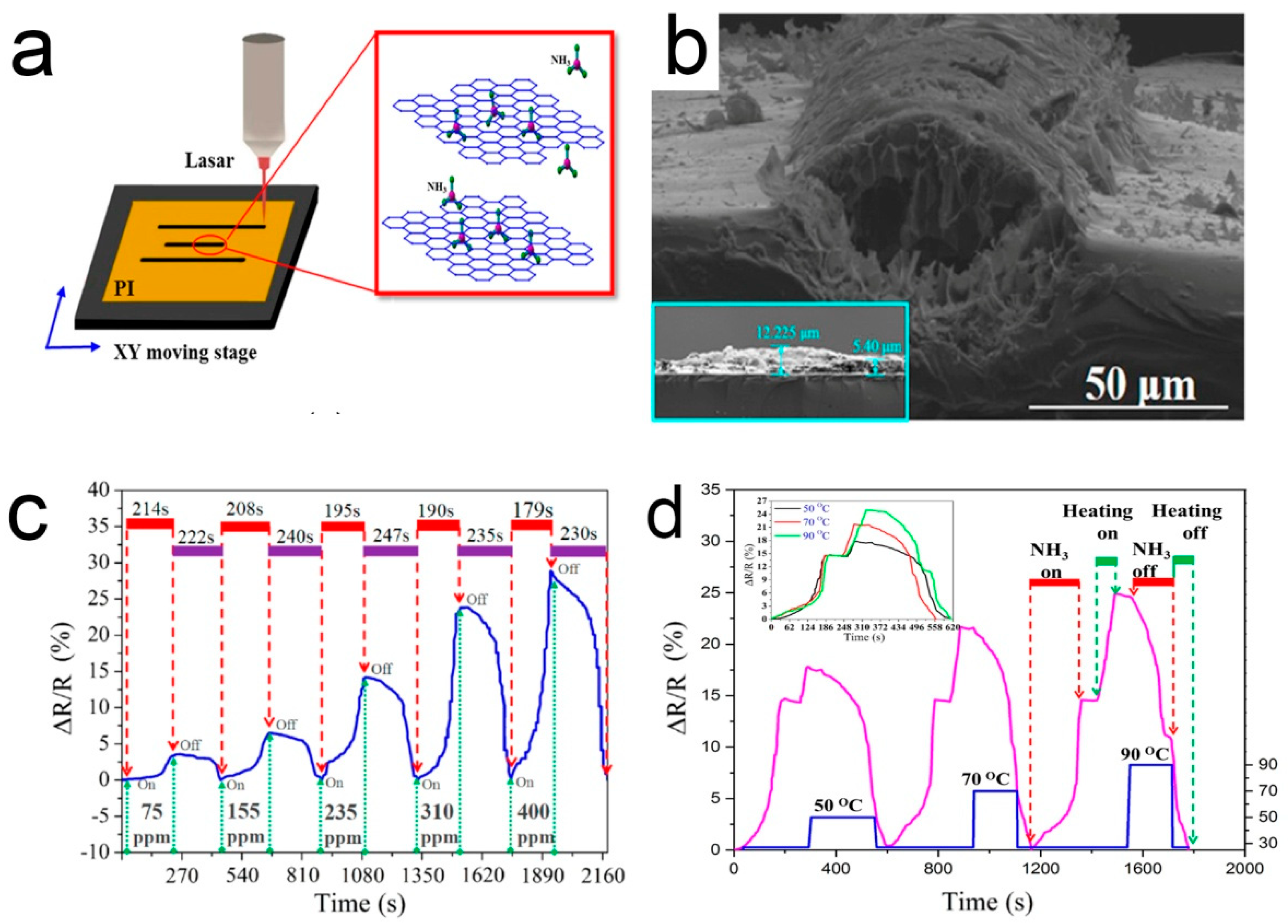
Generally, when a sensor is recovered at room temperature, the gas molecules cannot be completely desorbed, resulting in poor stability and long recovery time [118]. The desorption of ammonia can be promoted using the infrared light source. This is attributed to the generation of charge carriers by absorbing infrared light [119]. Most of the others used heating to accelerate the desorption process, based on the principle of thermally excited gas molecules [120]. Wu et al. [121] used laser direct writing to fabricate three parallel porous 3D graphene lines on a polyimide (PI) tape to simply construct an ammonia gas sensor. In this study, the ammonia sensor is located in the middle and the two sides are used as heaters. Voltage can be applied to the heater to promote desorption, as shown in Figure 7. The recovery time is relatively stable with the increase of the number of cycles, indicating that the residual ammonia molecules in the element are almost fully desorbed.
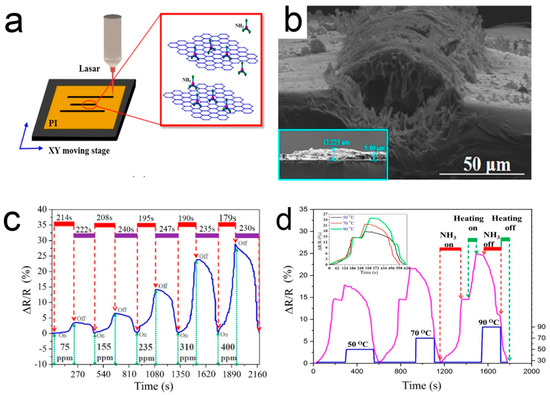
Figure 7.
(a) A diagram of laser direct writing; (b) SEM side view image of the laser-irradiated PI; (c) The real-time response/recovery behaviors of the sensor; (d) The normalized real-time response/recovery behaviors of the sensor [121].
4.2. 3D RGO/PPy
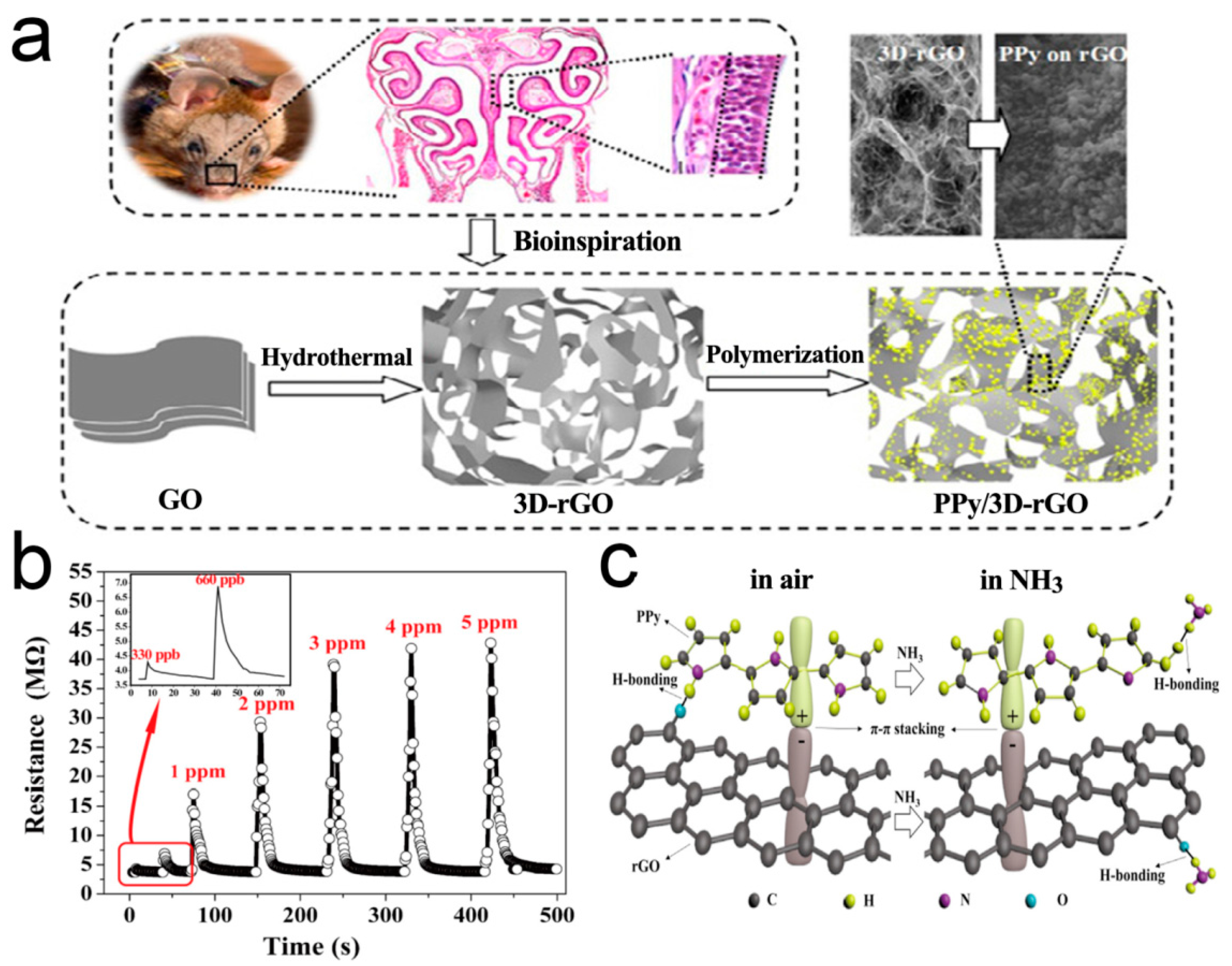
Recent studies have witnessed the significant progress of gas sensors based on polypyrrole (PPy) conducting polymers [122,123,124,125]. However, the serious agglomeration of the nanoparticles leads to a significant decrease in the visible active surface of gas molecules, which leads to the decrease of response sensitivity [126,127,128]. Qin et al. [129] used 3D RGO as a 3D skeleton support for the sensitive PPy nanoparticles. It has made an important contribution to improve the conductivity, dynamic performance, and sensing response, as shown in Figure 8. Highly dispersed and low agglomerated PPy nanoparticles are uniformly distributed on the pore walls of 3D RGO. The response to NH3 can rapidly reach sub-ppm level, 4–5 times more sensitive than that of pure PPy. The sensor has perfect stability.
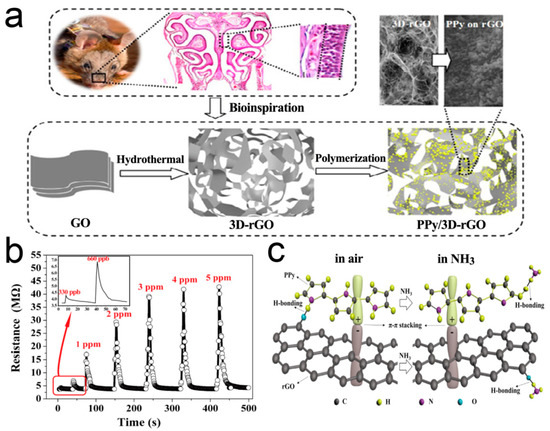
Figure 8.
(a) Fabrication process for the 3D crumpled PPy/3D-rGO nanocomposite; (b) Dynamic response of the PPy/3D-RGO sensor to NH3; (c) Schematic illustration depicting the interaction of NH3 with PPy/3D-RGO [129].
4.3. PANI/CuO@3D-NGF
CuO nanoparticles are suitable for the gas detection due to their narrow band gap and adjustable morphology [130,131,132]. However, the problem is that they can only operate effectively at high temperatures. To improve this problem, nanoparticles can be incorporated into porous materials to construct the functional composites [102,133]. Tabar et al. [134] prepared a chemical-resistant ammonia sensor based on polyaniline (PANI)/CuO nanoparticles, supported on a 3D N-doped graphene framework (NGF). The sensor has an excellent response to 100 ppm NH3, with an outstanding LOD down to 50 ppb. The average response time is 30 s at room temperature. It is not sensitive to other gases and has good selectivity to NH3. The excellent sensing performance was attributed to 3D interconnected porous structure, remarkable enhancement of charge carriers, and modified π-interactions between molecules [134].
5. Phenol Gas Sensors
Phenol is the natural component of many substances and can be emitted from the combustion of fossil fuels and tobacco [20]. It is also present in animal wastes and decomposing organic material. More importantly, it is a chemical product produced at a rate of about 6 million ton/year worldwide [20]. The manufacture and transportation of phenol as well as its many uses may lead to worker exposures to this substance with health risks [135]. In 2013, Liu et al. [136] prepared an electrochemical sensor using assembled 3D graphene as the electrode. The LOD of the phenol sensor mediated by tyrosine was successfully achieved down to 50 ppm. In 2016, Guo et al. [137] reported RGO/metal-oxide p-n heterojunction aerogels as efficient 3D sensing frameworks for phenol detection. Upon the detection of phenol at room temperature, the sensor has good sensitivity, repeatability, and stability. The linear relationship is in the range of 10–80 ppb. Compared with the detection results of ethanol, toluene, and methanol, the gas sensor based on RGO/SnO2 composite aerogel has much higher sensitivity to phenol with the LOD down to 5 ppb. In the same year, Gao et al. [138] reported highly sensitive electrocatalytic determination of phenols based on coupled cMWCNT/cyclodextrin edge-functionalized graphene composite. The sensor has excellent performance towards trace detection of three typical phenols (4-aminophenol, 4-AP; 4-chlorophenol, 4-CP; 4-nitrophenol, 4-NP). Under optimal conditions, the current responses of 4-AP, 4-CP, and 4-NP are linear to concentrations over two different ranges, with the LODs of 0.019, 0.017, and 0.027 ppm (S/N = 3), respectively. In 2019, Qi et al. [139] reported a facile synthesis of 3D S/N co-doped graphene derived from GO hydrogel. This newly fabricated sensor was used in the simultaneous detection of catechol and hydroquinone, with the LODs of 0.28 and 0.15 ppm, respectively.
6. Conclusions
Compared with the 2D graphene nanosheets, the signal transduction of 3D graphene frameworks are more than ten times higher in most gas detection, due to the increase of the interaction surface area and the number of active adsorption sites [89]. In addition to the original 2D structural properties of graphene, its 3D porous frameworks are more favorable. It is good for adsorption and diffusion of gas molecules. In general, the LOD can be improved from ppm level to several ppb level. This has a great impact on the prevention of toxic and harmful air pollution to the atmosphere.
In addition, 3D graphene has a strong mechanical strength and a high temperature resistance, capable of using in harsh environments. These properties are very promising for practical application. It is a potential research direction to combine 3D graphene with flexible substrate to make wearable flexible sensors.
However, 3D graphene is still limited in wide applications due to its limited gas sensing types. The main toxic pollutants in the air are NOx (main NO2), NH3, CO, CH2O, and phenol. Compared with traditional metal oxide sensing materials, graphene is superior due to the much lower operating temperature and much lower resistance. It requires a low energy consumption in operation [83,87,115]. As shown in Table 2, traditional semiconductor-based gas sensors generally work at 200–400 °C [98,140,141,142]. Some commercial gas sensors could work well at room temperature, but with some performance loss of the LOD [143,144]. Compared to individual MoS2, SnS2, organic compounds (e.g., PPy), and semiconductor metal oxides (e.g., SnO2), the sensors based on 3D graphene composites could see an increase of the sensitivity.

Table 2.
Sensing performance comparison of 3D graphene and other semiconductor materials including commercial sensors.
At present, 3D graphene is mainly sensitive to NO2 and NH3, and more gas sensors need to be discovered. The adsorption and desorption of 3D graphene need to be accelerated so as to reduce the response and recovery times of the sensor. Finally, 3D graphene sensors are still in the stage of laboratory investigation, and more work is needed to put forward these developments to the commercialization stage.
Author Contributions
Conceptualization, Q.D., M.X. and Z.C.; methodology, M.X.; software, Q.D.; validation, G.L., Y.Z. and Z.C.; formal analysis, Q.D.; investigation, Z.C.; resources, M.X.; data curation, Q.D.; writing—original draft preparation, Q.D. and M.X.; writing—review and editing, Z.C.; visualization, Q.D.; supervision, Y.Z. and Z.C.; project administration, Z.C.; funding acquisition, Z.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Koolen, C.D.; Rothenberg, G. Air pollution in Europe. ChemSusChem 2018, 12, 164–172. [Google Scholar] [CrossRef]
- Tong, S. Air pollution and disease burden. Lancet Planet. Health 2019, 3, 49–50. [Google Scholar] [CrossRef]
- Ranscombe, P. Wearable technology for air pollution. Lancet Respir. Med. 2019, 7, 567–568. [Google Scholar] [CrossRef]
- Liu, J. Mapping high resolution national daily NO2 exposure across mainland China using an ensemble algorithm. Environ. Pollut. 2021, 279, 116932. [Google Scholar] [CrossRef] [PubMed]
- Sinharoy, S.S.; Clasen, T.; Martorell, R. Air pollution and stunting: A missing link? Lancet Glob. Health 2020, 8, 472–475. [Google Scholar] [CrossRef]
- Chen, C.; Li, W.; Dong, L.; Li, X. The effect of meteorological factors, seasonal factors and air pollutions on the formation of particulate matter. IOP Conf. Ser. Earth Environ. Sci. 2020, 450, 0120121. [Google Scholar] [CrossRef]
- Siregar, A.M.; Siregar, C.A.; Yani, M. Engineering of motorcycle exhaust gases to reduce air pollution. IOP Conf. Ser. Mater. Sci. Eng. 2020, 821, 012048. [Google Scholar] [CrossRef]
- U. S. Environmental Protection Agency. Review of the Primary National Ambient Air Quality Standards for Nitrogen Dioxide. 2015. Available online: https://www.epa.gov/sites/production/files/2020-07/documents/20150504reaplanning.pdf (accessed on 4 May 2021).
- Zhang, Y.; Xu, W.; Wen, Z.; Wang, D.; Hao, T.; Tang, A.; Liu, X. Atmospheric deposition of inorganic nitrogen in a semi-arid grassland of Inner Mongolia, China. J. Arid Land 2017, 9, 810–822. [Google Scholar] [CrossRef]
- Widiana, D.R.; Wang, Y.F.; You, S.J.; Yang, H.H.; Wang, L.C.; Tsai, J.H.; Chen, H.M. Air pollution profiles and health risk assessment of ambient volatile organic compounds above a municipal wastewater treatment plant, Taiwan. Aerosol. Air Qual. Res. 2019, 19, 375–382. [Google Scholar] [CrossRef]
- Naseem, S.; King, A.J. Ammonia production in poultry houses can affect health of humans, birds, and the environment—techniques for its reduction during poultry production. Environ. Sci. Pollut. Res. 2018, 25, 15269–15293. [Google Scholar] [CrossRef]
- Börü, Ü.T.; Bölük, C.; Taşemir, M.; Gezer, T.; Serim, V.A. Air pollution, a possible risk factor for multiple sclerosis. Acta Neurol. Scand. 2020, 141, 431–437. [Google Scholar] [CrossRef] [PubMed]
- National Research Council. Acute Exposure Guideline Levels for Selected Airborne Chemicals: Volume 6; The National Academies Press: Washington, DC, USA, 2003. Available online: https://www.ncbi.nlm.nih.gov/books/NBK207883/ (accessed on 4 May 2021).
- Jia, C.; Cao, K.; Valaulikar, R.; Fu, X.; Sorin, A.B. Variability of total volatile organic compounds (TVOC) in the indoor air of retail stores. Int. J. Environ. Res. Public Health 2019, 16, 4622. [Google Scholar] [CrossRef] [PubMed]
- Spinelle, L.; Gerboles, M.; Kok, G.; Persijn, S.; Sauerwald, T. Review of portable and low-cost sensors for the ambient air monitoring of benzene and other volatile organic compounds. Sensors 2017, 17, 1520. [Google Scholar] [CrossRef]
- Bocos-Bintintan, V.; Ratiu, I.A. Hunting for toxic industrial chemicals: Real-time detection of carbon disulfide traces by means of ion mobility spectrometry. Toxics 2020, 8, 121. [Google Scholar] [CrossRef] [PubMed]
- Bocos-Bintintan, V.; Ghira, G.B.; Anton, M.; Martiniuc, A.-V.; Ratiu, I.A. Sensing precursors of illegal drugs—Rapid detection of acetic anhydride vapors at trace levels using photoionization detection and ion mobility spectrometry. Molecules 2020, 61, 1852. [Google Scholar] [CrossRef]
- Ghira, G.B.; Ratiu, I.A.; Bocos-Bintintan, V. Fast characterization of pyridine using ion mobility spectrometry and photoionization detection. Environ. Eng. Manag. J. 2013, 12, 251–256. [Google Scholar] [CrossRef]
- Patel, B.R.; Noroozifar, M.; Kerman, K. Review-Nanocomposite-based sensors for voltammetric detection of hazardous phenolic pollutants in water. J. Electrochem. Soc. 2020, 167, 037568. [Google Scholar] [CrossRef]
- Busca, G.; Berardinelli, S.; Resini, C.; Arrighi, L. Technologies for the removal of phenol from fluid streams: A short review of recent developments. J. Hazard. Mater. 2008, 160, 265–288. [Google Scholar] [CrossRef]
- Wolkoff, P.; Wilkins, C.K.; Clausen, P.A.; Nielsen, G.D. Organic compounds in office environments—Sensory irritation, odor, measurements and the role of reactive chemistry. Indoor Air 2006, 16, 7–19. [Google Scholar] [CrossRef]
- Zong, P.A.; Liang, J.; Zhang, P.; Wan, C.; Wang, Y.; Koumoto, K. Graphene-based thermoelectrics. ACS Appl. Energy Mater. 2020, 3, 2224–2239. [Google Scholar] [CrossRef]
- Varghese, S.S.; Lonkar, S.; Singh, K.K.; Swaminathan, S.; Abdala, A. Recent advances in graphene based gas sensors. Sens. Actuator B Chem. 2015, 218, 160–183. [Google Scholar] [CrossRef]
- Cui, Y.; Liu, Q.; He, Z.; Gao, X.; Wu, E.; Guo, G.; Zhou, C.; Feng, Z. Epitaxial graphene gas sensors on SiC substrate with high sensitivity. J. Semicond. 2020, 41, 032101. [Google Scholar] [CrossRef]
- Yoon, H.J.; Jun, D.H.; Yang, J.H.; Zhou, Z.X.; Yang, S.S.; Cheng, M.M.C. Carbon dioxide gas sensor using a graphene sheet. Sens. Actuator B Chem. 2011, 157, 310–313. [Google Scholar] [CrossRef]
- Chung, M.G.; Kim, D.H.; Lee, H.M.; Kim, T.; Choi, J.H.; Seo, D.K.; Yoo, J.B.; Hong, S.H.; Kang, T.J.; Kim, Y.H. Highly sensitive NO2 gas sensor based on ozone treated graphene. Sens. Actuator B Chem. 2012, 166–167, 172–176. [Google Scholar] [CrossRef]
- Zhou, L.; Shen, F.; Tian, X.; Wang, D.; Zhang, T.; Chen, W. Stable Cu2O nanocrystals grown on functionalized graphene sheets and room temperature H2S gas sensing with ultrahigh sensitivity. Nanoscale 2013, 5, 1564–1569. [Google Scholar] [CrossRef]
- Bian, S.; Shen, C.; Qian, Y.; Liu, J.; Xi, F.; Dong, X. Facile synthesis of sulfur-doped graphene quantum dots as fluorescent sensing probes for Ag+ ions detection. Sens. Actuator B Chem. 2017, 242, 231–237. [Google Scholar] [CrossRef]
- Li, X.; Li, X.; Li, Z.; Wang, J.; Zhang, J. WS2 nanoflakes based selective ammonia sensors at room temperature. Sens. Actuator B Chem. 2017, 240, 273–277. [Google Scholar] [CrossRef]
- Karmakar, N.S.; Fernandes, R.P.; Jain, S.; Patil, U.V.; Shimpi, N.G.; Bhat, N.V.; Kothari, D.C. Room temperature NO2 gas sensing properties of p-toluenesulfonic acid doped silver-polypyrrole nanocomposite. Sens. Actuator B Chem. 2017, 242, 118–126. [Google Scholar] [CrossRef]
- Zou, J.; Liu, Z.; Guo, Y.; Dong, C. Electrochemical sensor for the facile detection of trace amounts of bisphenol A based on cyclodextrin-functionalized graphene/platinum nanoparticles. Anal. Methods 2016, 9, 134–140. [Google Scholar] [CrossRef]
- Wu, W.; Liu, Z.; Jauregui, L.A.; Yu, Q.; Pillai, R.; Cao, H.; Bao, J.; Chen, Y.P.; Pei, S.S. Wafer-scale synthesis of graphene by chemical vapor deposition and its application in hydrogen sensing. Sens. Actuator B Chem. 2010, 150, 296–300. [Google Scholar] [CrossRef]
- Pak, Y.; Kim, S.M.; Jeong, H.; Kang, C.G.; Park, J.S.; Song, H.; Lee, R.; Myoung, N.S.; Lee, B.H.; Seo, S.; et al. Palladium-decorated hydrogen-gas sensors using periodically aligned graphene nanoribbons. ACS Appl. Mater. Interfaces 2014, 6, 13293–13298. [Google Scholar] [CrossRef]
- Ritikos, R.; Whitcher, T.J.; Razib, N.M.; Bien, D.C.S.; Chanlek, N.; Nakajima, H.; Saisopa, T.; Songsiriritthigul, P.; Huang, N.M.; Rahman, S.A. A practical carbon dioxide gas sensor using room-temperature hydrogen plasma reduced graphene oxide. Sens. Actuator B Chem. 2014, 193, 692–700. [Google Scholar] [CrossRef]
- Yasaei, P.; Kumar, B.; Hantehzadeh, R.; Kayyalha, M.; Baskin, A.; Repnin, N.; Wang, C.H.; Klie, F.R.; Chen, Y.P.; Král, P.; et al. Chemical sensing with switchable transport channels in graphene grain boundaries. Nat. Commun. 2014, 5, 4911. [Google Scholar] [CrossRef]
- Wu, Z.; Chen, X.; Zhu, S.; Zhou, Z.; Yao, Y.; Quan, W.; Liu, B. Enhanced sensitivity of ammonia sensor using graphene/polyaniline nanocomposite. Sens. Actuator B Chem. 2013, 178, 485–493. [Google Scholar] [CrossRef]
- Johnson, J.L.; Behnam, A.; Pearton, S.J.; Ural, A. Hydrogen sensing using Pd-functionalized multi-layer graphene nanoribbon networks. Adv. Mater. 2010, 22, 4877–4880. [Google Scholar] [CrossRef]
- Kumar, R.; Avasthi, D.K.; Kaur, A. Fabrication of chemiresistive gas sensors based on multistep reduced graphene oxide for low parts per million monitoring of sulfur dioxide at room temperature. Sens. Actuator B Chem. 2017, 242, 461–468. [Google Scholar] [CrossRef]
- Akhter, F.; Alahi, M.E.E.; Siddiquei, H.R.; Gooneratne, C.P.; Mukhopadhyay, S.C. Graphene oxide (GO) coated impedimetric gas sensor for selective detection of carbon dioxide (CO2) with temperature and humidity compensation. IEEE Sens. J. 2021, 21, 4241–4249. [Google Scholar] [CrossRef]
- Ratinac, K.R.; Yang, W.; Ringer, S.P.; Braet, F. Toward ubiquitous environmental gas sensors—Capitalizing on the promise of graphene. Environ. Sci. Technol. 2010, 44, 1167–1176. [Google Scholar] [CrossRef] [PubMed]
- Ali, S.; Hassan, A.; Hassan, G.; Bae, J.; Lee, C.H. All-printed humidity sensor based on gmethyl-red/methyl-red composite with high sensitivity. Carbon 2016, 105, 23–32. [Google Scholar] [CrossRef]
- Jang, J.; Han, J.I. High performance cylindrical capacitor as a relative humidity sensor for wearable computing device. J. Electrochem. Soc. 2017, 164, B136–B141. [Google Scholar] [CrossRef]
- Chung, M.G.; Kim, D.H.; Seo, D.K.; Kim, T.; Im, H.U.; Lee, H.M.; Yoo, J.B.; Hong, S.H.; Kang, T.J.; Kim, Y.H. Flexible hydrogen sensors using graphene with palladium nanoparticle decoration. Sens. Actuator B Chem. 2012, 169, 387–392. [Google Scholar] [CrossRef]
- Cuong, T.V.; Pham, V.H.; Chung, J.S.; Shin, E.W.; Yoo, D.H.; Hahn, S.H.; Huh, J.S.; Rue, G.H.; Kim, E.J.; Hur, S.H.; et al. Solution-processed ZnO-chemically converted graphene gas sensor. Mater. Lett. 2010, 64, 2479–2482. [Google Scholar] [CrossRef]
- Choi, S.J.; Choi, C.; Kim, S.J.; Cho, H.J.; Hakim, M.; Jeon, S.; Kim, I.D. Highly efficient electronic sensitization of non-oxidized graphene flakes on controlled pore-loaded WO3 nanofibers for selective detection of H2S molecules. Sci. Rep. 2015, 5, 8067. [Google Scholar] [CrossRef] [PubMed]
- Gautam, M.; Jayatissa, A.H. Ammonia gas sensing behavior of graphene surface decorated with gold nanoparticles. Solid State Electron. 2012, 78, 159–165. [Google Scholar] [CrossRef]
- Jiang, L.; Fan, Z. Design of advanced porous graphene materials: From graphene nanomesh to 3D architectures. Nanoscale 2014, 6, 1922–1945. [Google Scholar] [CrossRef] [PubMed]
- Duy, L.T.; Kim, D.J.; Trung, T.Q.; Dang, V.Q.; Kim, B.Y.; Moon, H.K.; Lee, N.E. High performance three-dimensional chemical sensor platform using reduced graphene oxide formed on high aspect-ratio micropillars. Adv. Funct. Mater 2015, 25, 883–890. [Google Scholar] [CrossRef]
- Meng, F.-L.; Guo, Z.; Huang, X.-J. Graphene-based hybrids for chemiresistive gas sensors. Trends Anal. Chem. 2015, 68, 37–47. [Google Scholar] [CrossRef]
- Xia, Y.; Li, R.; Chen, R.; Wang, J.; Xiang, L. 3D Architectured graphene/metal oxide hybrids for gas sensors: A review. Sensors 2018, 18, 1456. [Google Scholar] [CrossRef]
- Ilnicka, A.; Lukaszewicz, J.P. Graphene-based hydrogen gas sensors: A review. Processes 2020, 8, 633. [Google Scholar] [CrossRef]
- Chen, T.; Yan, W.; Xu, J.; Li, J.; Zhang, G.; Ho, D. Highly sensitive and selective NO2 sensor based on 3D MoS2/rGO composites prepared by a low temperature self-assembly method. J. Alloys Compd. 2019, 793, 541–551. [Google Scholar] [CrossRef]
- Xu, Y.; Sheng, K.; Li, C.; Shi, G. Self-Assembled graphene hydrogel via a one-step hydrothermal process. ACS Nano 2010, 4, 4324–4330. [Google Scholar] [CrossRef]
- Yin, S.; Niu, Z.; Chen, X. Assembly of graphene sheets into 3D macroscopic structures. Small 2012, 8, 2458–2463. [Google Scholar] [CrossRef]
- Li, C.; Shi, G. Functional gels based on chemically modified graphenes. Adv. Mater. 2014, 26, 3992–4012. [Google Scholar] [CrossRef]
- Chen, J.; Sheng, K.; Luo, P.; Li, C.; Shi, G. Graphene hydrogels deposited in nickel foams for high-rate electrochemical capacitors. Adv. Mater. 2012, 24, 4569–4573. [Google Scholar] [CrossRef]
- Li, C.; Shi, G. Three-dimensional graphene architectures. Nanoscale 2012, 4, 5549–5563. [Google Scholar] [CrossRef]
- Sui, Z.; Zhang, X.; Lei, Y.; Luo, Y. Easy and green synthesis of reduced graphite oxide-based hydrogels. Carbon 2011, 49, 4314–4321. [Google Scholar] [CrossRef]
- Zhang, X.; Sui, Z.; Xu, B.; Yue, S.; Luo, Y.; Zhan, W.; Liu, B. Mechanically strong and highly conductive graphene aerogel and its use as electrodes for electrochemical power sources. J. Mater. Chem. 2011, 21, 6494–6497. [Google Scholar] [CrossRef]
- Wu, Q.; Sun, Y.; Bai, H.; Shi, G. High-performance supercapacitor electrodes based on graphene hydrogels modified with 2-aminoanthraquinone moieties. Phys. Chem. Chem. Phys 2011, 13, 11193–11198. [Google Scholar] [CrossRef]
- Chen, W.; Li, S.; Chen, C.; Yan, L. Self-assembly and embedding of nanoparticles by in situ reduced graphene for preparation of a 3D graphene/nanoparticle aerogel. Adv. Mater. 2011, 23, 5679–5683. [Google Scholar] [CrossRef]
- Pham, H.D.; Pham, V.H.; Cuong, T.V.; Nguyen-Phan, T.D.; Chung, J.S.; Shin, E.W.; Kim, S. Synthesis of the chemically converted graphene xerogel with superior electrical conductivity. Chem. Commun. 2011, 47, 9672–9674. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Zhang, C.; Li, X.; Zhang, L.; Ma, Y.; Zhang, L.; Xu, X.; Xia, F.; Wang, W.; Gao, J. A one-step method for reduction and self-assembling of graphene oxide into reduced graphene oxide aerogels. J. Mater. Chem. A 2013, 1, 2869–2877. [Google Scholar] [CrossRef]
- Luan, V.H.; Tien, H.N.; Le, T.H.; Hien, N.T.M.; Hur, S.H. Synthesis of a highly conductive and large surface area graphene oxide hydrogel and its use in a supercapacitor. J. Mater. Chem. A 2012, 1, 208–211. [Google Scholar] [CrossRef]
- Sheng, K.X.; Xu, Y.X.; Li, C.; Shi, Q.G. High-performance self-assembled graphene hydrogels prepared by chemical reduction of graphene oxide. New Carbon Mater. 2011, 26, 9–15. [Google Scholar] [CrossRef]
- Chen, K.; Chen, L.; Chen, Y.; Bai, H.; Li, L. Three-dimensional porous graphene-based composite materials: Electrochemical synthesis and application. J. Mater. Chem. 2012, 22, 20968–20976. [Google Scholar] [CrossRef]
- Shadkam, R.; Naderi, M.; Ghazitabar, A.; Asghari-Alamdari, A.; Shateri, S. Enhanced electrochemical performance of graphene aerogels by using combined reducing agents based on mild chemical reduction method. Ceram. Int. 2020, 46, 22197–22207. [Google Scholar] [CrossRef]
- Li, Y.; Sheng, K.; Yuan, W.; Shi, G. A high-performance flexible fibre-shaped electrochemical capacitor based on electrochemically reduced graphene oxide. Chem. Commun. 2012, 49, 291–293. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Qiu, L.; Cheng, C.; Wu, Y.; Ma, Z.F.; Li, D. Ordered gelation of chemically converted graphene for next-generation electroconductive hydrogel films. Angew. Chem. Int. Ed. 2011, 50, 7325–7328. [Google Scholar] [CrossRef]
- Yang, X.; Zhu, J.; Qiu, L.; Li, D. Bioinspired effective prevention of restacking in multilayered graphene films: Towards the next generation of high-performance supercapacitors. Adv. Mater. 2011, 23, 2833–2838. [Google Scholar] [CrossRef]
- Sun, Y.; Wu, Q.; Shi, G. Supercapacitors based on self-assembled graphene organogel. Phys. Chem. Chem. Phys. 2011, 13, 17249–17254. [Google Scholar] [CrossRef]
- Zhou, Q.; Gao, J.; Li, C.; Chen, J.; Shi, G. Composite organogels of graphene and activated carbon for electrochemical capacitors. J. Mater. Chem. A 2013, 1, 9196–9201. [Google Scholar] [CrossRef]
- Gun, J.; Kulkarni, S.A.; Xiu, W.; Batabyal, S.K.; Sladkevich, S.; Prikhodchenko, P.V.; Gutkin, V.; Lev, O. Graphene oxide organogel electrolyte for quasi solid dye sensitized solar cells. Electrochem. Commun. 2012, 19, 108–110. [Google Scholar] [CrossRef]
- Nardecchia, S.; Carriazo, D.; Ferrer, M.L.; Gutiérrez, M.C.; Monte, F.D. Three dimensional macroporous architectures and aerogels built of carbon nanotubes and/or graphene: Synthesis and applications. Chem. Soc. Rev. 2013, 42, 794–830. [Google Scholar] [CrossRef]
- Hu, H.; Zhao, Z.; Wu, W.; Gogotsi, Y.; Qiu, J. Ultralight and highly compressible graphene aerogels. Adv. Mater. 2012, 25, 2219–2223. [Google Scholar] [CrossRef] [PubMed]
- Li, W.L.; Lu, K.; Walz, J.Y. Freeze casting of porous materials: Review of critical factors in microstructure evolution. Int. Mater. Rev. 2012, 57, 37–60. [Google Scholar] [CrossRef]
- Qiu, L.; Liu, J.Z.; Chang, S.L.Y.; Wu, Y.; Li, D. Biomimetic superelastic graphene-based cellular monoliths. Nat. Commun. 2012, 3, 1241. [Google Scholar] [CrossRef] [PubMed]
- Donarelli, M.; Prezioso, S.; Perrozzi, F.; Bisti, F.; Nardone, M.; Giancaterini, L.; Ottaviano, L.; Cantalini, C. Response to NO2 and other gases of resistive chemically exfoliated MoS2-based gas sensors. Sens. Actuator B Chem. 2015, 207, 602–613. [Google Scholar] [CrossRef]
- Ratiu, I.A.; Ligor, T.; Bocos-Bintintan, V.; Mayhew, C.A.; Buszewski, B. Volatile organic compounds in exhaled breath as fingerprints of lung cancer, asthma and COPD. J. Clin. Med. 2020, 10, 32. [Google Scholar] [CrossRef]
- Wu, J.; Tao, K.; Miao, J.; Norford, L.K. Improved selectivity and sensitivity of gas sensing using a 3D reduced graphene oxide hydrogel with an integrated microheater. ACS Appl. Mater. Interfaces 2015, 7, 27502–27510. [Google Scholar] [CrossRef] [PubMed]
- Yuan, W.; Huang, L.; Zhou, Q.; Shi, G. Ultrasensitive and selective nitrogen dioxide sensor based on self-assembled graphene/polymer composite nanofibers. ACS Appl. Mater. Interfaces 2014, 6, 17003–17008. [Google Scholar] [CrossRef]
- Wu, J.; Li, Z.; Xie, X.; Tao, K.; Liu, C.; Khor, K.A.; Miao, J.; Norford, L.K. 3D superhydrophobic reduced graphene oxide for activated NO2 sensing with enhanced immunity to humidity. J. Mater. Chem. A 2018, 6, 478–488. [Google Scholar] [CrossRef]
- Wu, J.; Tao, K.; Guo, Y.; Li, Z.; Wang, X.; Luo, Z.; Feng, S.; Du, C.; Chen, D.; Miao, J.; et al. A 3D chemically modified graphene hydrogel for fast, highly sensitive, and selective gas sensor. Adv. Sci. 2017, 4, 1600319. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; He, S.; Liu, M.; Zhang, C.; Chen, W. Three-dimensional mesoporous graphene aerogel-supported SnO2 nanocrystals for high-performance NO2 gas sensing at low temperature. Anal. Chem. 2015, 87, 1638–1645. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Wu, Z.; Ding, H.; Wei, Y.; Huang, W.; Yang, X.; Li, Z.; Qiu, L.; Wang, X. Three-dimensional graphene hydrogel decorated with SnO2 for high-performance NO2 sensing with enhanced immunity to humidity. ACS Appl. Mater. Interfaces 2020, 12, 2634–2643. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Ding, H.; Wei, Y.; Huang, W.; Yang, X.; Li, Z.; Qiu, L.; Wang, X. Flexible, 3D SnS2/reduced graphene oxide heterostructured NO2 sensor. Sens. Actuator B Chem. 2020, 305, 127445. [Google Scholar] [CrossRef]
- Wu, J.; Wu, Z.; Ding, H.; Yang, X.; Wei, Y.; Xiao, M.; Yang, Z.; Yang, B.-R.; Liu, C.; Lu, X.; et al. Three-dimensional-structured boron- and nitrogen-doped graphene hydrogel enabling high-sensitivity NO2 detection at room temperature. ACS Sens. 2019, 4, 1889–1898. [Google Scholar] [CrossRef]
- Zhu, P.; Li, S.; Zhao, C.; Zhang, Y.; Yu, J. 3D synergistical rGO/Eu(TPyP)(Pc) hybrid aerogel for high-performance NO2 gas sensor with enhanced immunity to humidity. J. Hazard. Mater. 2019, 384, 121426. [Google Scholar] [CrossRef]
- Wu, J.; Wei, Y.; Ding, H.; Wu, Z.; Yang, X.; Li, Z.; Huang, W.; Xie, X.; Tao, K.; Wang, X. Green synthesis of 3D chemically functionalized graphene hydrogel for high-performance NH3 and NO2 detection at room temperature. ACS Appl. Mater. Interfaces 2020, 12, 20623–20632. [Google Scholar] [CrossRef]
- Kumar, R.; Goel, N.; Kumar, M. UV-activated MoS2 based fast and reversible NO2 sensor at room temperature. ACS Sens. 2017, 2, 1744–1752. [Google Scholar] [CrossRef]
- Xu, T.; Pei, Y.; Liu, Y.; Wu, D.; Shi, Z.; Xu, J.; Tian, Y.; Li, X. High-response NO2 resistive gas sensor based on bilayer MoS2 grown by a new two-step chemical vapor deposition method. J. Alloys Compd. 2017, 725, 253–259. [Google Scholar] [CrossRef]
- Zhou, Y.; Zou, C.; Lin, X.; Guo, Y. UV light activated NO2 gas sensing based on Au nanoparticles decorated few-layer MoS2 thin film at room temperature. Appl. Phys. Lett. 2018, 113, 082103. [Google Scholar] [CrossRef]
- Zhou, Y.; Gao, C.; Guo, Y. UV assisted ultrasensitive trace NO2 gas sensing based on few-layer MoS2 nanosheet-ZnO nanowire heterojunctions at room temperature. J. Mater. Chem. A 2018, 6, 10286–10296. [Google Scholar] [CrossRef]
- Agrawal, A.V.; Kumar, R.; Venkatesan, S.; Zakhidov, A.; Yang, G.; Bao, J.; Kumar, M.; Kumar, M. Photoactivated mixed In-plane and edge-enriched p-Type MoS2 Flake-Based NO2 sensor working at room temperature. ACS Sens. 2018, 3, 998–1004. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Zhao, C.; Han, T.; Fei, T.; Liu, S.; Lu, G. Rational synthesis of molybdenum disulfide nanoparticles decorated reduced graphene oxide hybrids and their application for high-performance NO2 sensing. Sens. Actuator B Chem. 2018, 260, 508–518. [Google Scholar] [CrossRef]
- Zhou, Y.; Liu, G.; Zhu, X.; Guo, Y. Ultrasensitive NO2 gas sensing based on rGO/MoS2 nanocomposite film at low temperature. Sens. Actuator B Chem. 2017, 251, 280–290. [Google Scholar] [CrossRef]
- Yun, Y.J.; Hong, W.G.; Kim, D.Y.; Kim, H.J.; Jun, Y.; Lee, H.K. E-textile gas sensors composed of molybdenum disulfide and reduced graphene oxide for high response and reliability. Sens. Actuator B Chem. 2017, 248, 829–835. [Google Scholar] [CrossRef]
- Ou, J.Z.; Ge, W.; Carey, B.; Daeneke, T.; Rotbart, A.; Shan, W.; Wang, Y.; Fu, Z.; Chrimes, A.F.; Wlodarski, W.; et al. Physisorption-based charge transfer in two-dimensional SnS2 for selective and reversible NO2 gas sensing. ACS Nano 2015, 9, 10313–10323. [Google Scholar] [CrossRef]
- Huang, Y.; Jiao, W.; Chu, Z.; Ding, G.; Yang, M.; Zhong, X.; Wang, R. Ultrasensitive ppb-level NO2 gas sensors at room temperature based on SnS2/rGO nanohybrids with P-N transition property and optoelectronic visible light enhancement performance. J. Mater. Chem. C 2019, 7, 8616–8625. [Google Scholar] [CrossRef]
- Chen, H.; Chen, Y.; Zhang, H.; Zhang, D.W.; Zhou, P.; Huang, J. Suspended SnS2 layers by light assistance for ultrasensitive ammonia detection at room temperature. Adv. Funct. Mater. 2018, 28, 1801035. [Google Scholar] [CrossRef]
- Hsiao, M.C.; Liao, S.H.; Yen, M.Y.; Liu, P.I.; Pu, N.W.; Wang, C.A.; Ma, C.C.M. Preparation of covalently functionalized graphene using residual oxygen-containing functional groups. ACS Appl. Mater. Interfaces 2010, 2, 3092–3099. [Google Scholar] [CrossRef]
- Deng, S.; Tjoa, V.; Fan, H.M.; Tan, H.R.; Sayle, D.C.; Olivo, M.; Mhaisalkar, S.; Wei, J.; Sow, C.H. Reduced graphene oxide conjugated Cu2O nanowire mesocrystals for high-performance NO2 gas sensor. J. Am. Chem. Soc. 2012, 134, 4905–4917. [Google Scholar] [CrossRef] [PubMed]
- Xiao, Y.; Yang, Q.; Wang, Z.; Zhang, R.; Gao, Y.; Sun, P.; Sun, Y.; Lu, G. Improvement of NO2 gas sensing performance based on discoid tin oxide modified by reduced graphene oxide. Sens. Actuator B Chem. 2016, 227, 419–426. [Google Scholar] [CrossRef]
- Wang, Z.; Zhang, T.; Han, T.; Fei, T.; Liu, S.; Lu, G. Oxygen vacancy engineering for enhanced sensing performances: A case of SnO2 nanoparticles-reduced graphene oxide hybrids for ultrasensitive ppb-level room-temperature NO2 sensing. Sens. Actuator B Chem. 2018, 266, 812–822. [Google Scholar] [CrossRef]
- Lee, J.H.; Katoch, A.; Choi, S.W.; Kim, J.H.; Kim, H.W.; Kim, S.S. Extraordinary Improvement of Gas-sensing performances in SnO2 nanofibers due to creation of local p–n heterojunctions by loading reduced graphene oxide nanosheets. ACS Appl. Mater. Interfaces 2015, 7, 3101–3109. [Google Scholar] [CrossRef]
- Russo, P.A.; Donato, N.; Leonardi, S.G.; Baek, S.; Conte, D.E.; Neri, G.; Pinna, N. Room-temperature hydrogen sensing with heteronanostructures based on reduced graphene oxide and Tin oxide. Angew. Chem. Int. Ed. 2012, 124, 11215–11219. [Google Scholar] [CrossRef]
- Lv, R.; Chen, G.; Li, Q.; Mccreary, A.; Botello-Méndez, A.; Morozov, S.V.; Liang, L.; Declerck, X.; Perea-López, N.; Cullen, D.A.; et al. Ultrasensitive gas detection of large-area boron-doped graphene. Proc. Nat. Acad. Sci. USA 2015, 112, 14527–14532. [Google Scholar] [CrossRef] [PubMed]
- Dai, J.; Yuan, J.; Giannozzi, P. Gas adsorption on graphene doped with B, N, Al, and S. A theoretical study. Appl. Phys. Lett. 2009, 95, 232105. [Google Scholar] [CrossRef]
- Ma, C.; Shao, X.; Cao, D. Nitrogen-doped graphene as an excellent candidate for selective gas sensing. Sci. China Chem. 2014, 57, 911–917. [Google Scholar] [CrossRef]
- Shaik, M.; Rao, V.K.; Gupta, M.; Murthy, K.S.R.C.; Jain, R. Chemiresistive gas sensor for the sensitive detection of nitrogen dioxide based on nitrogen doped graphene nanosheets. RSC Adv. 2015, 6, 1527–1534. [Google Scholar] [CrossRef]
- Wang, H.; Maiyalagan, T.; Wang, X. Review on recent progress in nitrogen-doped graphene: Synthesis, characterization, and Its potential applications. ACS Catal. 2012, 2, 781–794. [Google Scholar] [CrossRef]
- Choudhuri, I.; Patra, N.; Mahata, A.; Ahuja, R.; Pathak, B. B–N@Graphene: Highly sensitive and selective gas sensor. J. Phys. Chem. C 2015, 119, 24827–24836. [Google Scholar] [CrossRef]
- Wei, X.L.; Chen, Y.P.; Liu, W.L.; Zhong, J.X. Enhanced gas sensor based on nitrogen-vacancy graphene nanoribbons. Phys. Lett. A 2012, 376, 559–562. [Google Scholar] [CrossRef]
- Agnoli, S.; Favaro, M. Doping graphene with boron: A review of synthesis methods, physicochemical characterization, and emerging applications. J. Mater. Chem. A 2016, 4, 5002–5025. [Google Scholar] [CrossRef]
- Li, H.; Xie, W.; Liu, B.; Wang, C.; Wang, Y.; Duan, X.; Li, Q.; Wang, T. Gas modulating effect in room temperature ammonia sensing. Sens. Actuator B Chem. 2017, 242, 404–411. [Google Scholar] [CrossRef]
- Travlou, N.A.; Seredych, M.; Rodríguez-Castellón, E.; Bandosz, T.J. Insight into ammonia sensing on heterogeneous S- and N- co-doped nanoporous carbons. Carbon 2016, 96, 1014–1021. [Google Scholar] [CrossRef]
- Singh, E.; Meyyappan, M.; Nalwa, H.S. Flexible graphene-based wearable gas and chemical sensors. ACS Appl. Mater. Interfaces 2017, 9, 34544–34586. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Neri, G.; Pinna, N. Nanostructured materials for room-temperature gas sensors. Adv. Mater. 2016, 28, 795–831. [Google Scholar] [CrossRef]
- Gautam, M.; Jayatissa, A.H. Adsorption kinetics of ammonia sensing by graphene films decorated with platinum nanoparticles. J. App. Phys. 2012, 111, 094317. [Google Scholar] [CrossRef]
- Yang, F.; Taggart, D.K.; Penner, R.M. Joule heating a palladium nanowire sensor for accelerated response and recovery to hydrogen gas. Small 2010, 6, 1422–1429. [Google Scholar] [CrossRef]
- Wu, D.; Pen, Q.; Wu, S.; Wang, G.; Deng, L.; Tai, H.; Wang, L.; Yang, Y.; Dong, L.; Zhao, Y.; et al. A simple graphene NH3 gas sensor via laser direct writing. Sensors 2018, 18, 4405. [Google Scholar] [CrossRef]
- Bai, S.; Zhao, Y.; Sun, G.; Tian, Y.; Luo, R.; Li, D.; Chen, A. Ultrasensitive room temperature NH3 sensor based on a graphene–polyaniline hybrid loaded on PET thin film. Chem. Commun. 2012, 51, 7524–7527. [Google Scholar] [CrossRef]
- Basavaraja, C.; Kim, W.J.; Thinh, P.X.; Huh, D.S. Electrical conductivity studies on water-soluble polypyrrole–graphene oxide composites. Polym. Compos. 2011, 32, 2076–2083. [Google Scholar] [CrossRef]
- Bora, C.; Dolui, S.K. Fabrication of polypyrrole/graphene oxide nanocomposites by liquid/liquid interfacial polymerization and evaluation of their optical, electrical and electrochemical properties. Polymer 2012, 53, 923–932. [Google Scholar] [CrossRef]
- Joshi, A.; Gangal, S.A.; Gupta, S.K. Ammonia sensing properties of polypyrrole thin films at room temperature. Sens. Actuator B Chem. 2011, 156, 938–942. [Google Scholar] [CrossRef]
- Zhao, Y.; Li, X.; Yan, B.; Li, D.; Lawes, S.; Sun, X. Significant impact of 2D graphene nanosheets on large volume change tin-based anodes in lithium-ion batteries: A review. J. Power Sources 2015, 274, 869–884. [Google Scholar] [CrossRef]
- Chen, X.; Wong, C.K.Y.; Yuan, C.A.; Zhang, G. Nanowire-based gas sensors. Sens. Actuator B Chem. 2013, 117, 178–195. [Google Scholar] [CrossRef]
- Sun, J.; Shu, X.; Tian, Y.; Tong, Z.; Bai, S.; Luo, R.; Li, D.; Liu, C.C. Facile preparation of polypyrrole-reduced graphene oxide hybrid for enhancing NH3 sensing at room temperature. Sens. Actuator B Chem. 2017, 241, 658–664. [Google Scholar] [CrossRef]
- Qin, Y.; Zhang, B.; Zhang, Z. Combination of PPy with three-dimensional rGO to construct bioinspired nanocomposite for NH3-sensing enhancement. Org. Electron. 2019, 70, 240–245. [Google Scholar] [CrossRef]
- Bedi, R.K.; Singh, I. Room-temperature ammonia sensor based on cationic surfactant-assisted nanocrystalline CuO. ACS Appl. Mater. Interfaces 2010, 2, 1361–1368. [Google Scholar] [CrossRef]
- Singh, I.; Bedi, R.K. Surfactant-assisted synthesis, characterizations, and room temperature ammonia sensing mechanism of nanocrystalline CuO. Solid State Sci. 2011, 13, 2011–2018. [Google Scholar] [CrossRef]
- Yang, C.; Su, X.; Xiao, F.; Jian, J.; Wang, J. Gas sensing properties of CuO nanorods synthesized by a microwave-assisted hydrothermal method. Sens. Actuator B Chem. 2011, 158, 299–303. [Google Scholar] [CrossRef]
- Hsu, Y.W.; Hsu, T.K.; Sun, C.L.; Nien, Y.T.; Pu, N.W.; Ger, M.D. Synthesis of CuO/graphene nanocomposites for nonenzymatic electrochemical glucose biosensor applications. Electrochim. Acta 2012, 82, 152–157. [Google Scholar] [CrossRef]
- Tabar, F.A.; Nikfarjam, A.; Tavakoli, N.; Gavgani, J.N.; Mahyari, M.; Hosseini, S.G. Chemical-resistant ammonia sensor based on polyaniline/CuO nanoparticles supported on three-dimensional nitrogen-doped graphene-based framework nanocomposites. Microchim. Acta 2020, 187, 293. [Google Scholar] [CrossRef]
- Acosta, C.A.; Pasquali, C.E.L.; Paniagua, G.; Garcinuño, R.M.; Hernando, P.F. Evaluation of total phenol pollution in water of San Martin Canal from Santiago del Estero, Argentina. Environ. Pollut. 2018, 236, 265–272. [Google Scholar] [CrossRef] [PubMed]
- Liu, F.; Piao, Y.; Choi, J.S.; Seo, T.S. Three-dimensional graphene micropillar based electrochemical sensor for phenol detection. Biosens. Bioelectron. 2013, 50, 387–392. [Google Scholar] [CrossRef] [PubMed]
- Guo, D.; Cai, P.; Sun, J.; He, W.; Wu, X.; Zhang, T.; Wang, X.; Zhang, X. Reduced-graphene-oxide/metal-oxide p-n heterojunction aerogels as efficient 3D sensing frameworks for phenol detection. Carbon 2016, 99, 571–578. [Google Scholar] [CrossRef]
- Gao, J.; Liu, M.; Song, H.; Zhang, S.; Qian, Y.; Li, A. Highly-sensitive electrocatalytic determination for toxic phenols based on coupled cMWCNT/cyclodextrin edge-functionalized graphene composite. J. Hazard. Mater. 2016, 318, 99–108. [Google Scholar] [CrossRef] [PubMed]
- Qi, Y.; Cao, Y.; Meng, X.; Cao, J.; Li, X.; Hao, Q.; Lei, W.; Li, Q.; Li, J.; Si, W. Facile synthesis of 3D sulfur/nitrogen co-doped graphene derived from graphene oxide hydrogel and the simultaneous determination of hydroquinone and catechol. Sens. Actuator B Chem. 2019, 279, 170–176. [Google Scholar] [CrossRef]
- Hunter, G.W.; Akbar, S.; Bhansali, S.; Daniele, M.; Erb, P.D.; Johnson, K.; Liu, C.C.; Miller, D.; Oralkan, O.; Hesketh, P.J.; et al. Editors’ Choice—Critical Review—A critical review of solid state gas sensors. J. Electrochem. Chem. Soc. 2020, 167, 037570. [Google Scholar] [CrossRef]
- Dey, A. Semiconductor metal oxide gas sensors: A review. Mater. Sci. Eng. B 2018, 229, 206–217. [Google Scholar] [CrossRef]
- Oros, C.; Horprathum, M.; Wisitsoraat, A.; Srichaiyaperk, T.; Samransuksamer, B.; Limwichean, S.; Eiamchai, P.; Phokharatkul, D.; Nuntawong, N.; Chananonnawathorn, C.; et al. Ultra-sensitive NO2 sensor based on vertically aligned SnO2 nanorods deposited by DC reactive magnetron sputtering with glancing angle deposition technique. Sens. Actuator B Chem. 2016, 223, 936–945. [Google Scholar] [CrossRef]
- Specification Sheet for Nitrogen Dioxide Gas Sensor Type NO2/S-100. Available online: http://www.membrapor.ch/sheet/Nitrogen-Dioxide-Gas-Sensor-NO2-S-100.pdf (accessed on 4 May 2021).
- Specification Sheet for Ammonia Gas Sensor Type NH3/SR-200. Available online: http://www.membrapor.ch/sheet/Ammonia-Gas-Sensor-NH3-SR-200.pdf (accessed on 4 May 2021).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).