A Radiolucent Electromagnetic Tracking System for Use with Intraoperative X-ray Imaging
Abstract
1. Introduction
2. Materials and Methods
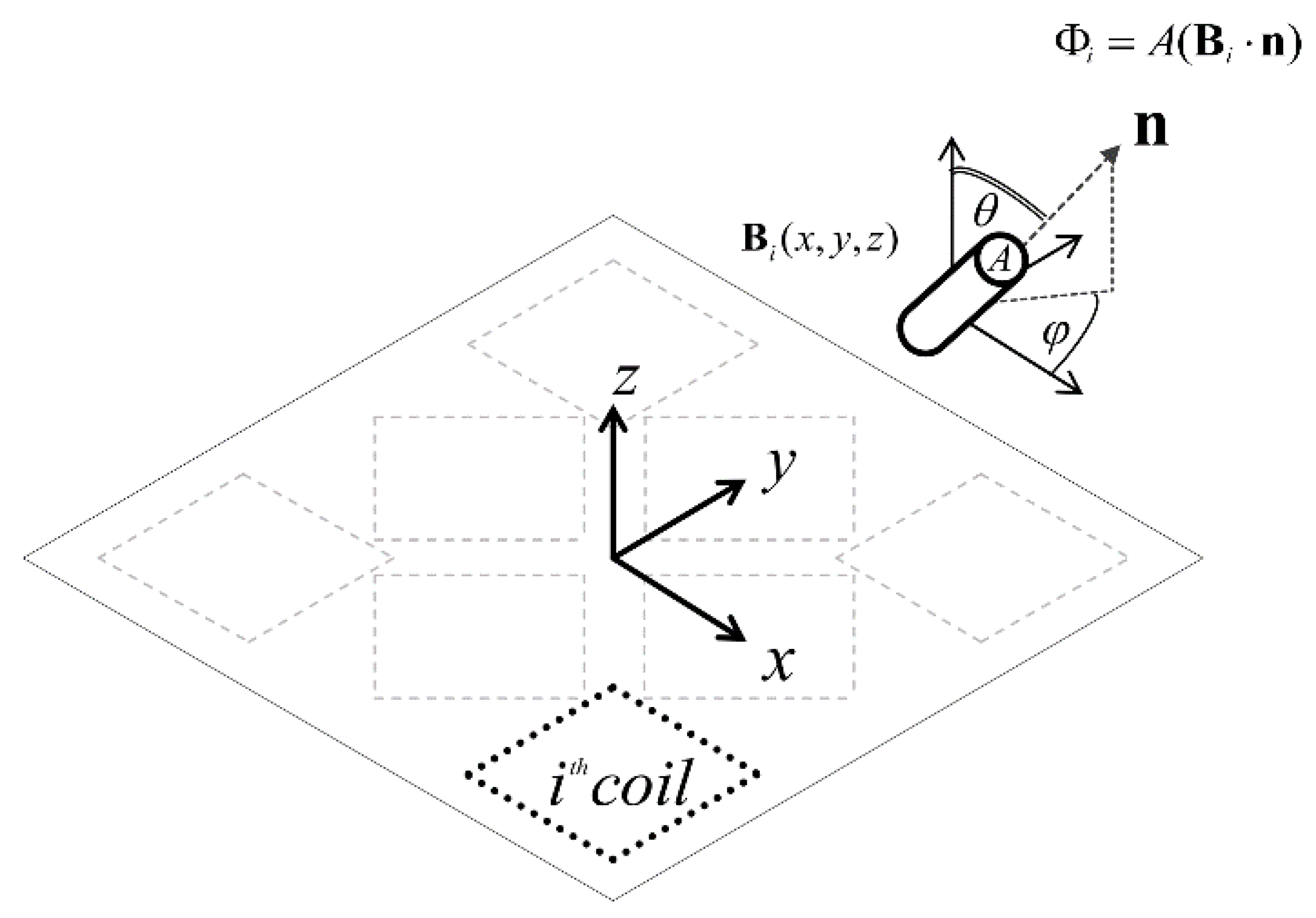
2.1. EM Tracking
2.2. Radiolucent Materials
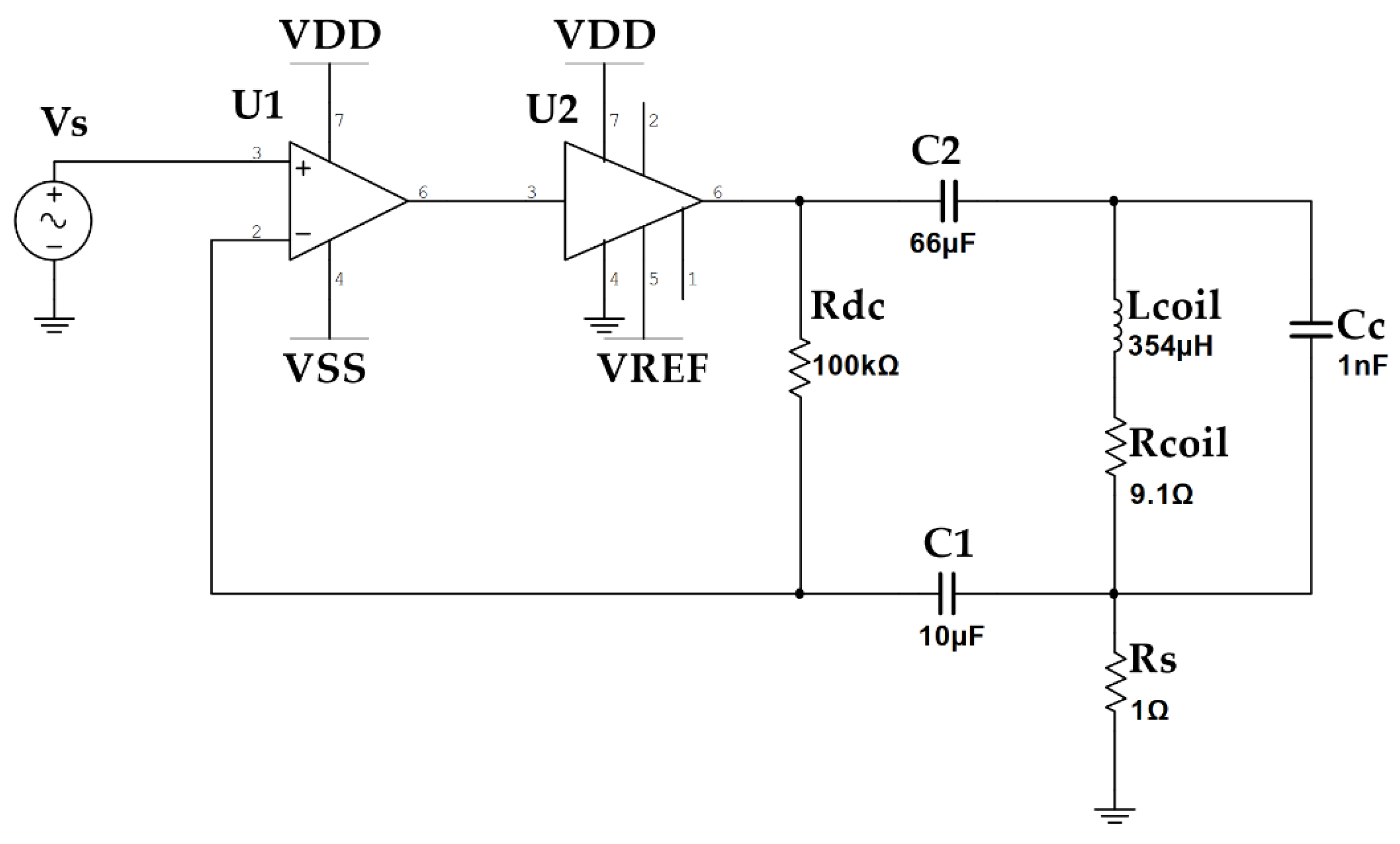
2.3. Transmitter Circuit
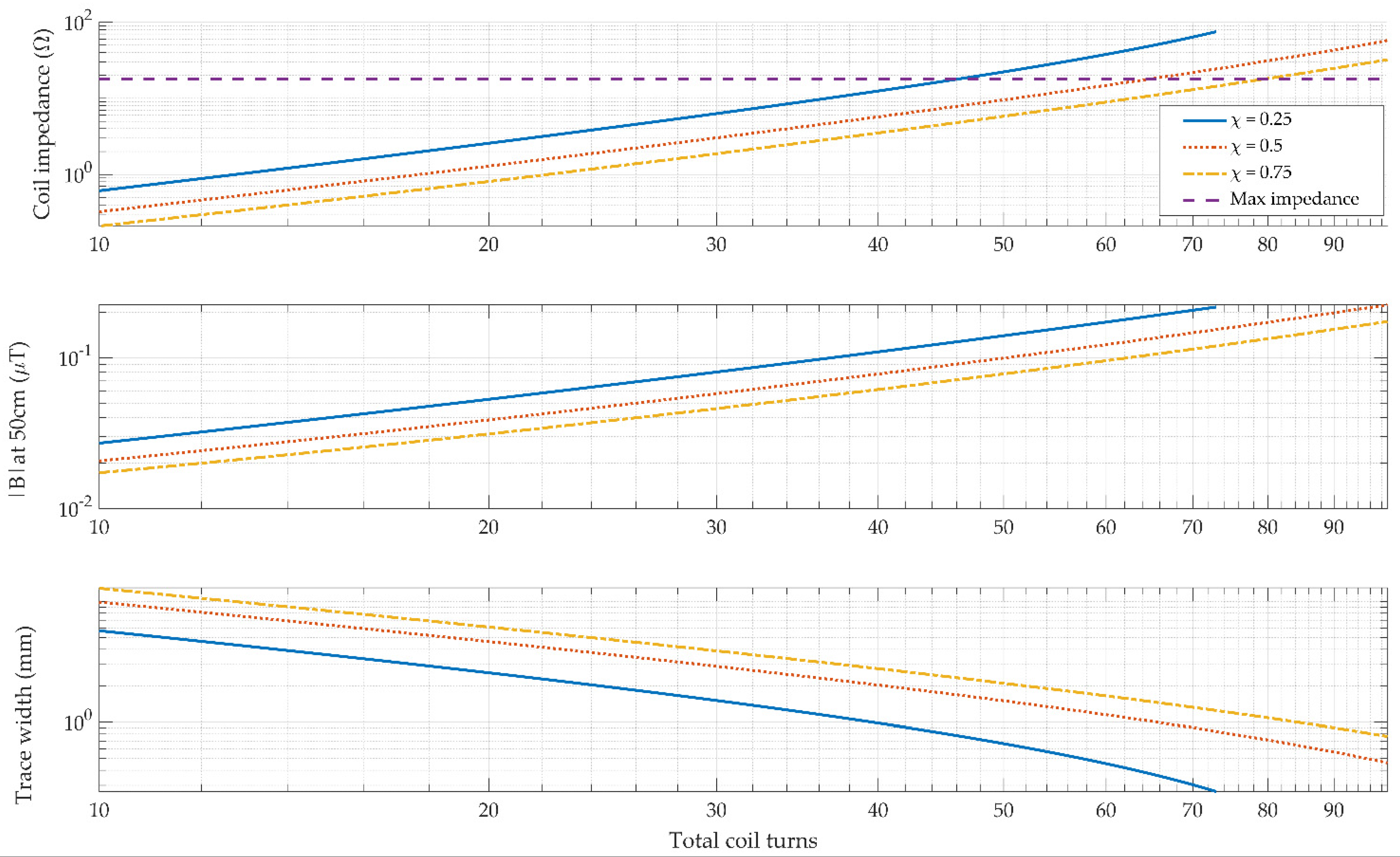
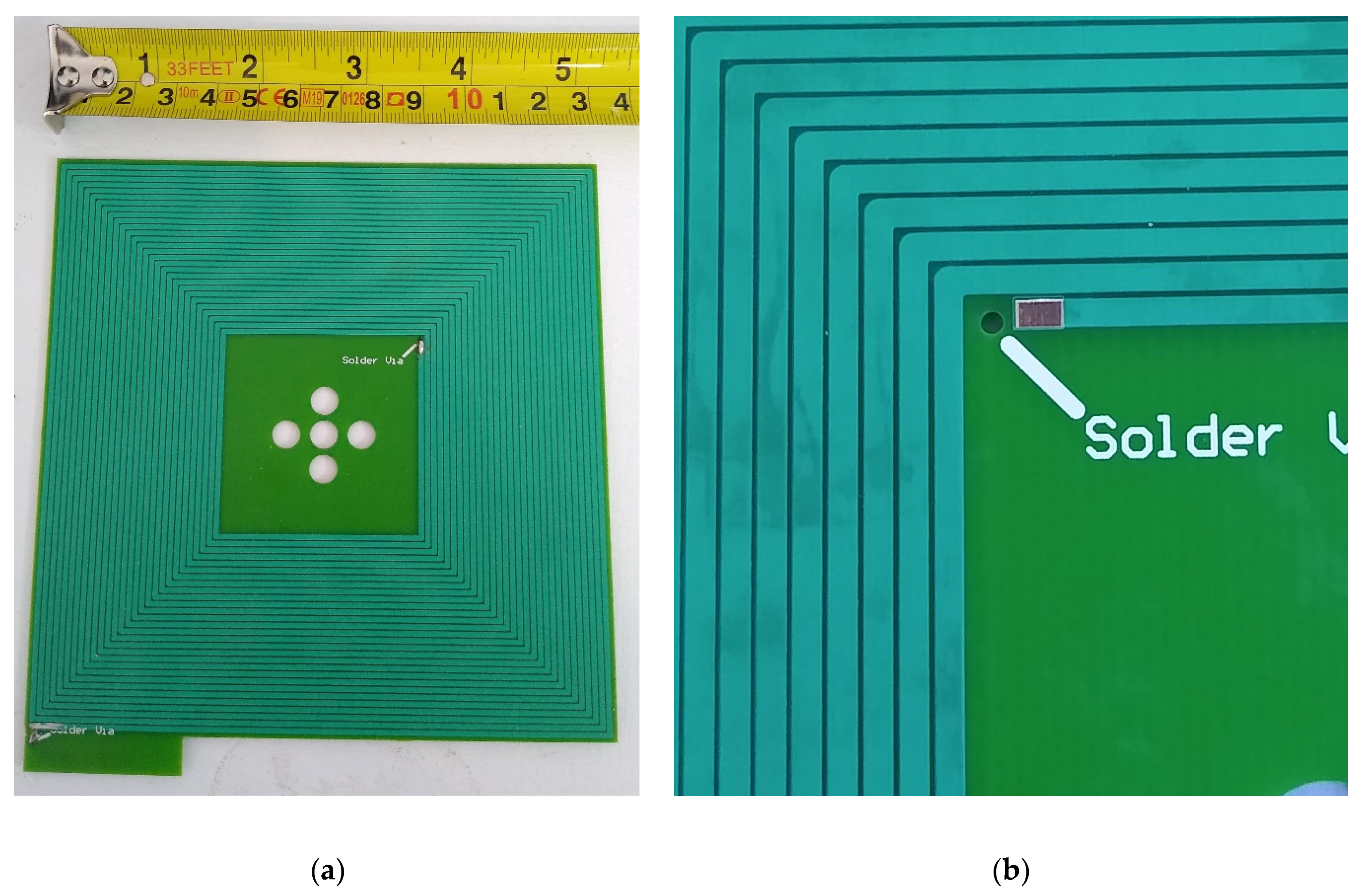
2.4. Planar Printed Circuit Board (PCB) Coils
2.5. Sensors
2.6. Sensor Interface
2.7. Aluminium Coils
2.8. Coil Arrays and Enclosure Design
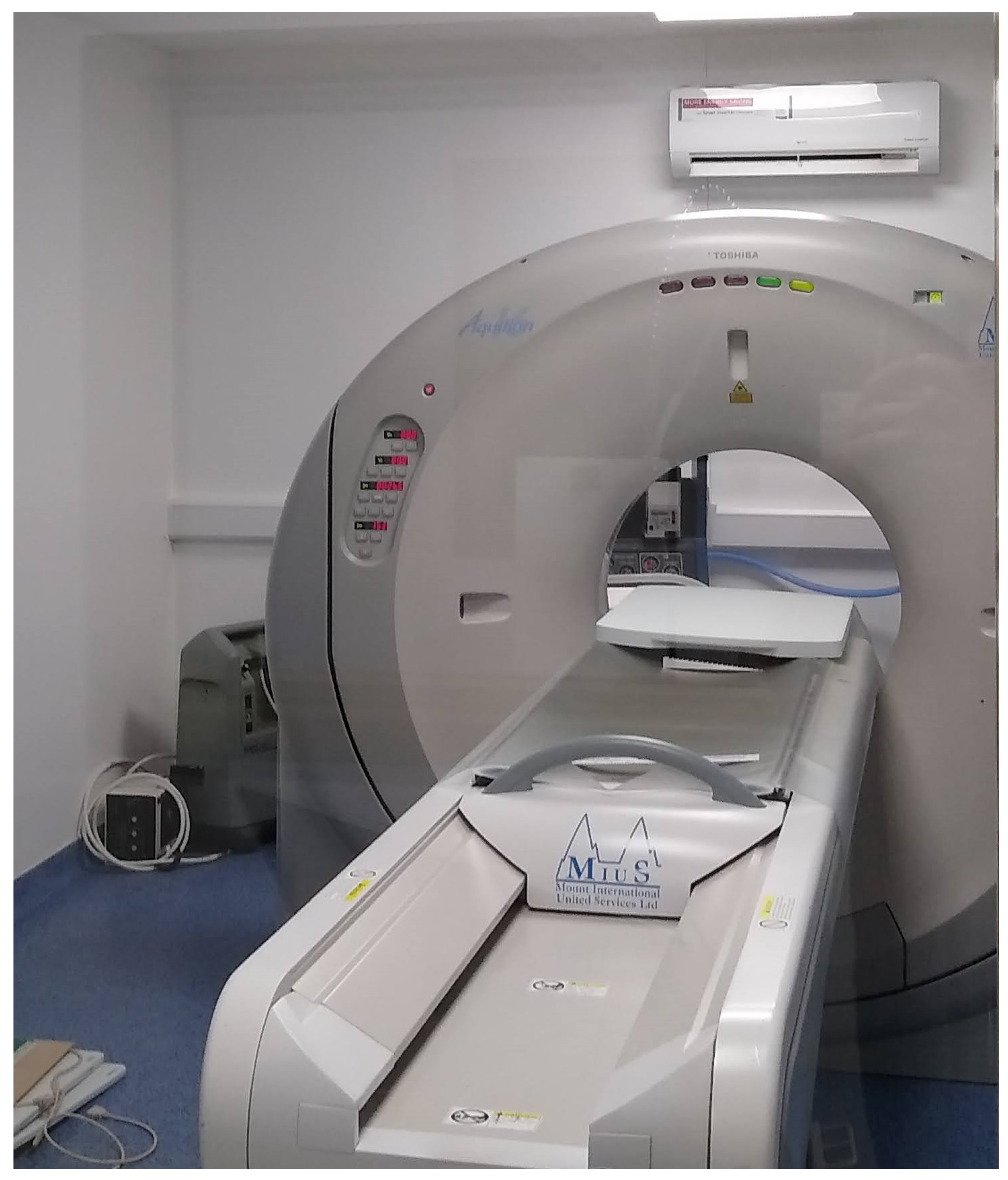
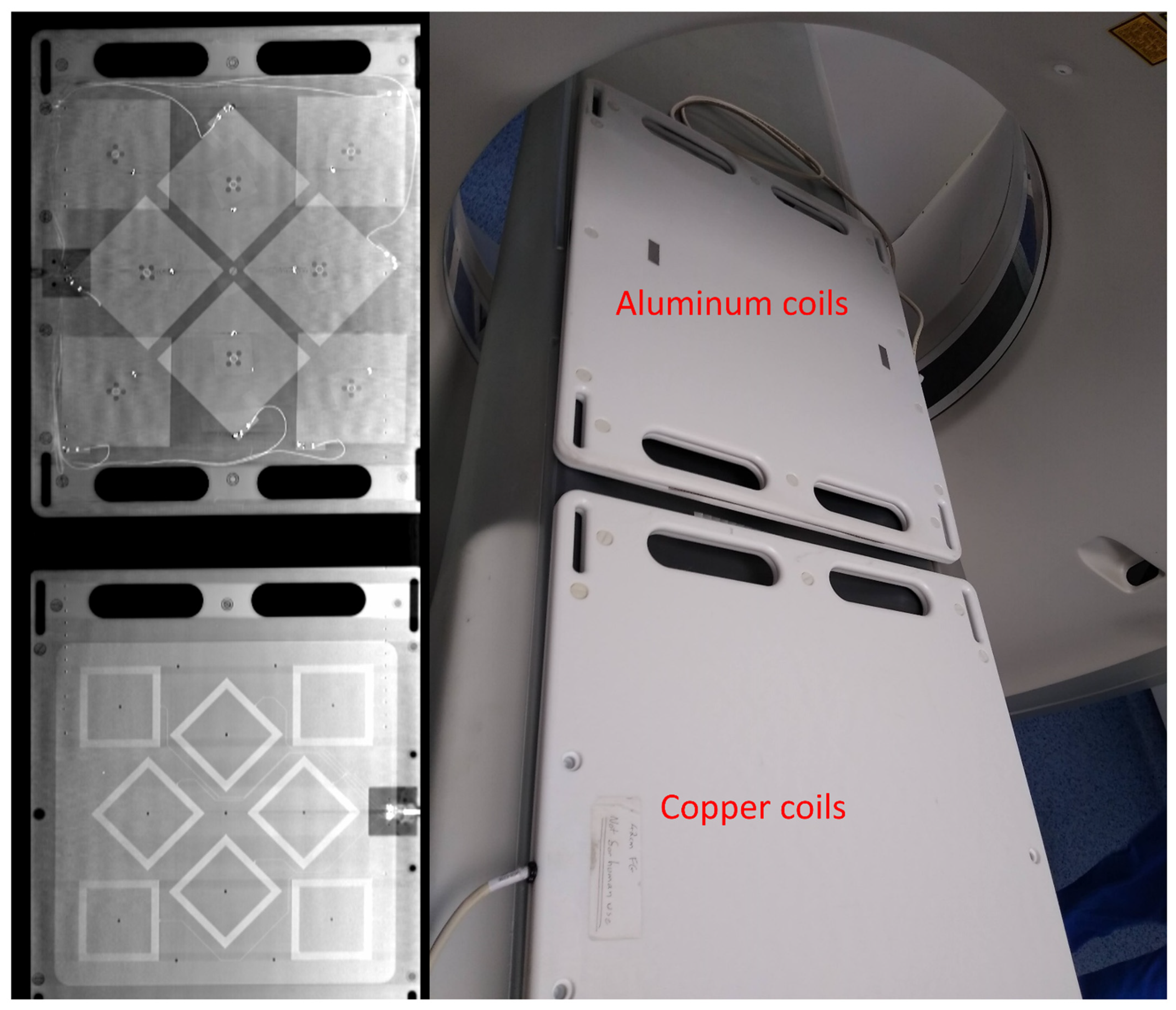
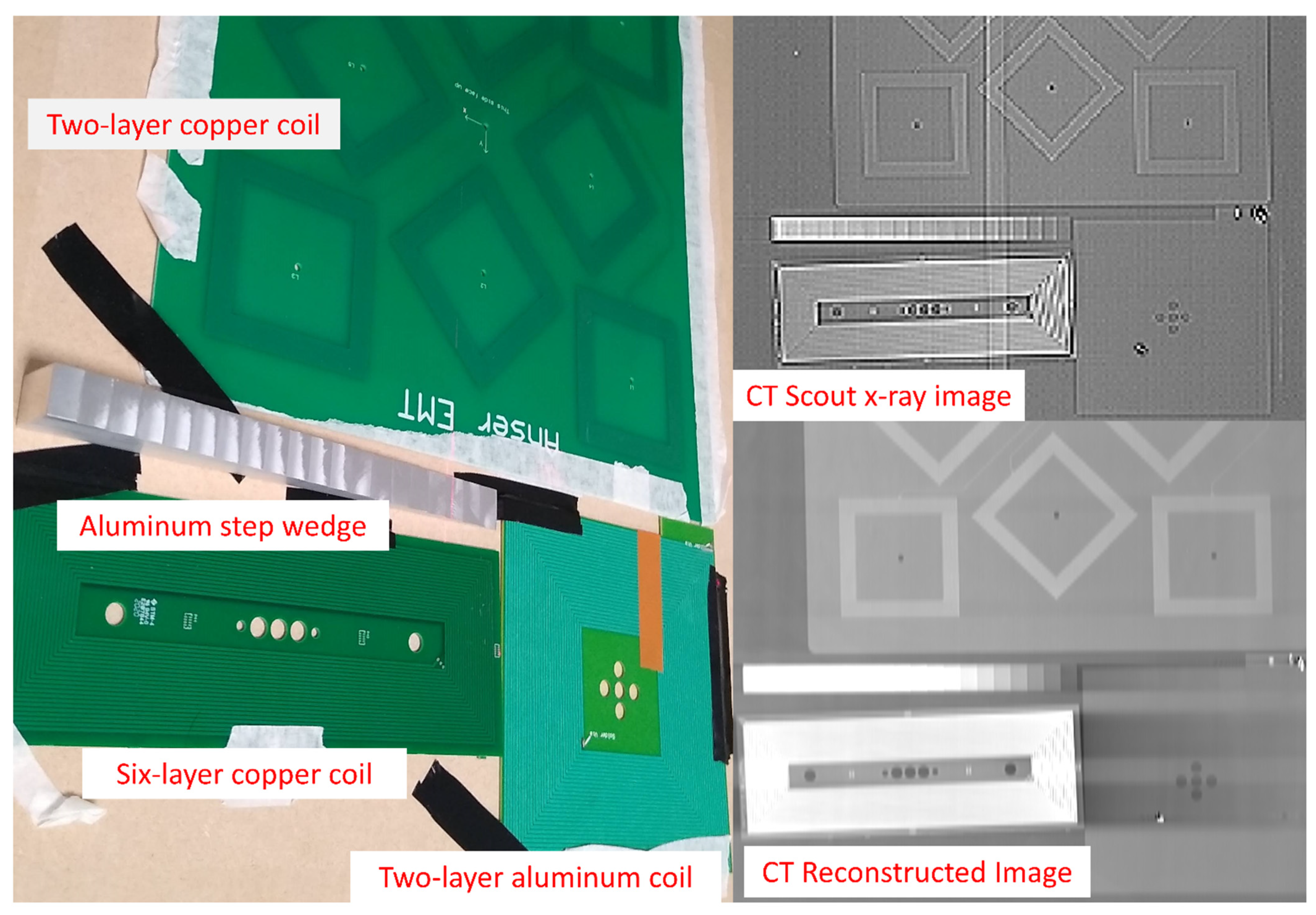
2.9. X-ray Imaging Testing
3. Results
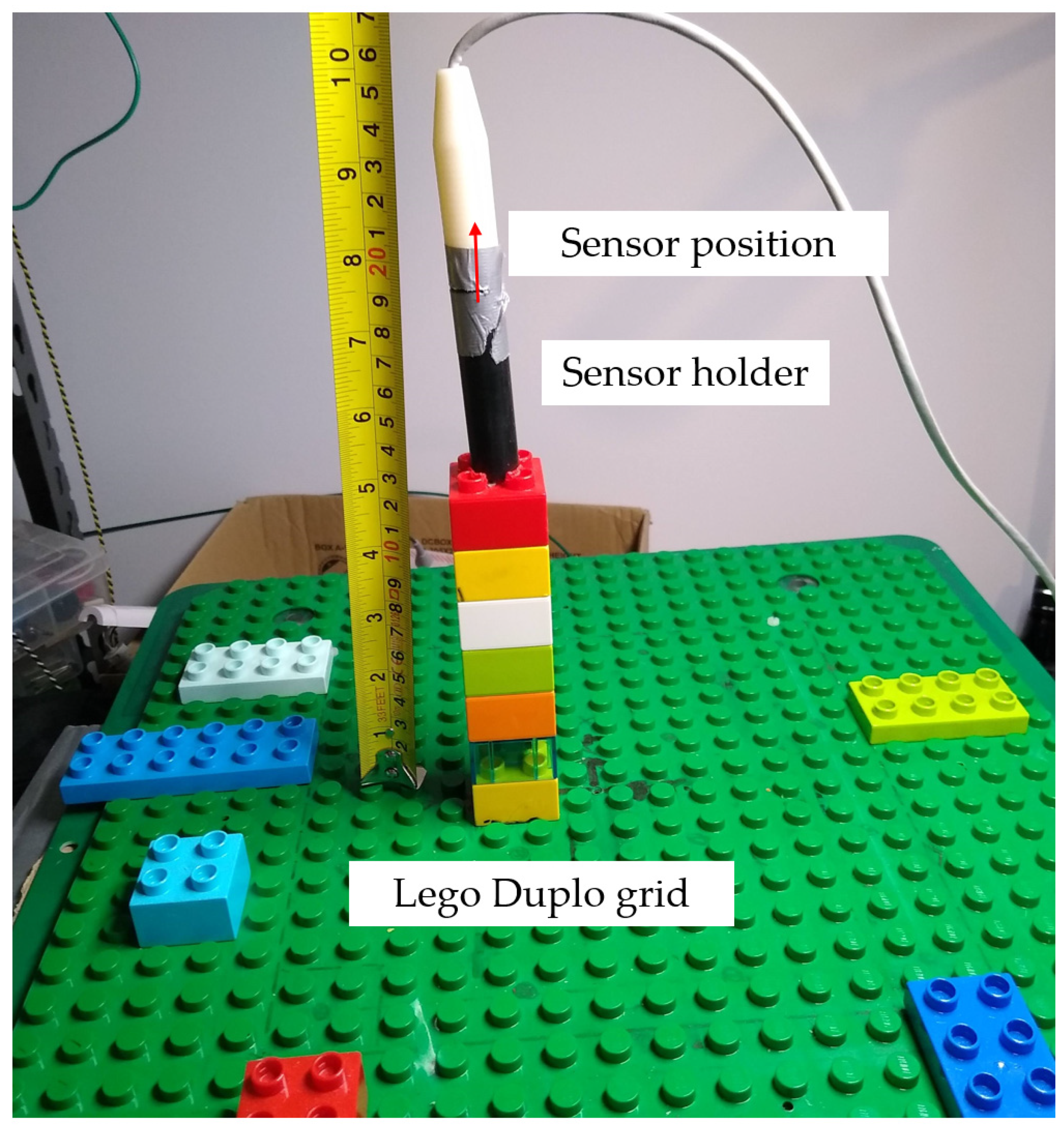
3.1. Position Accuracy
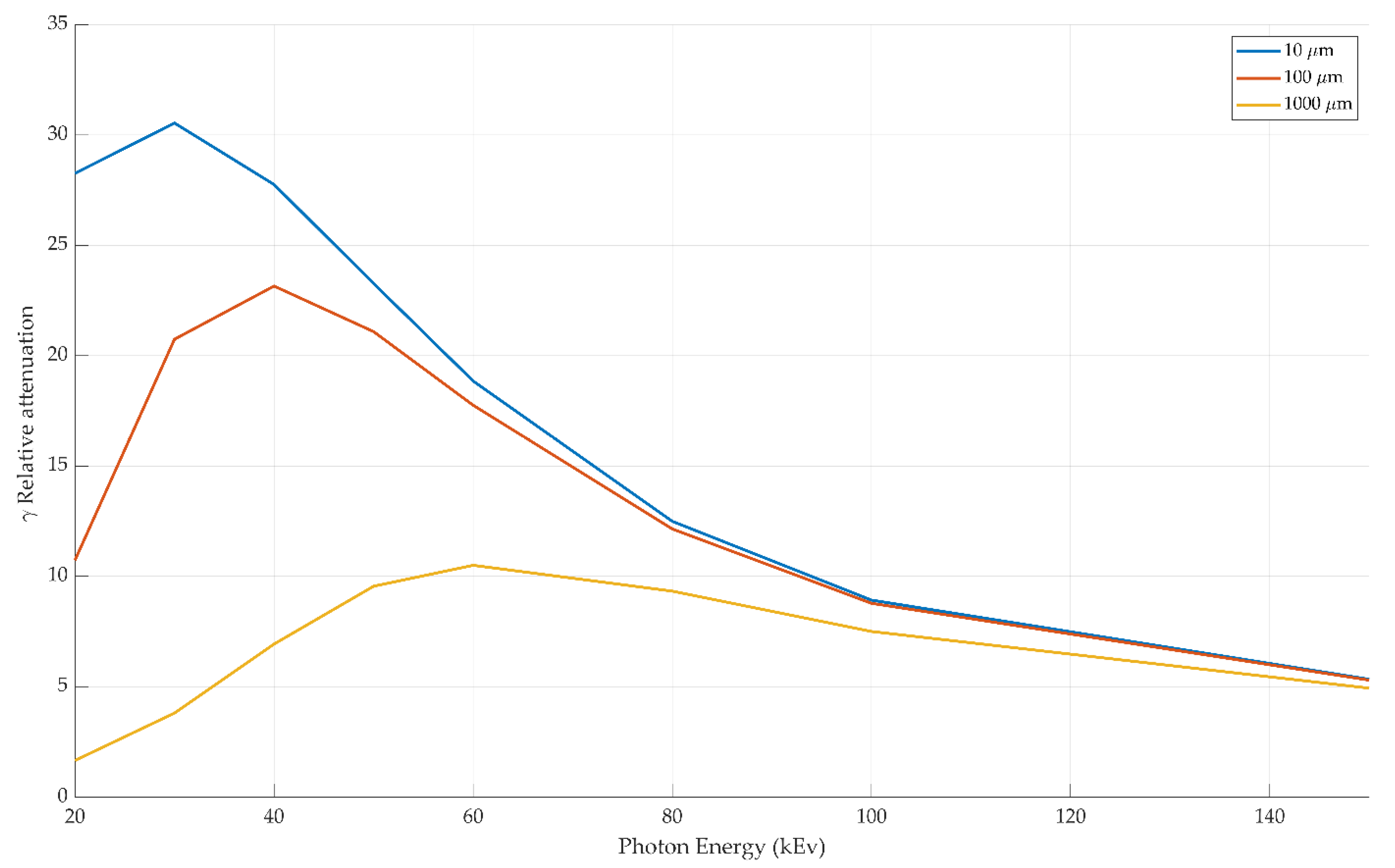
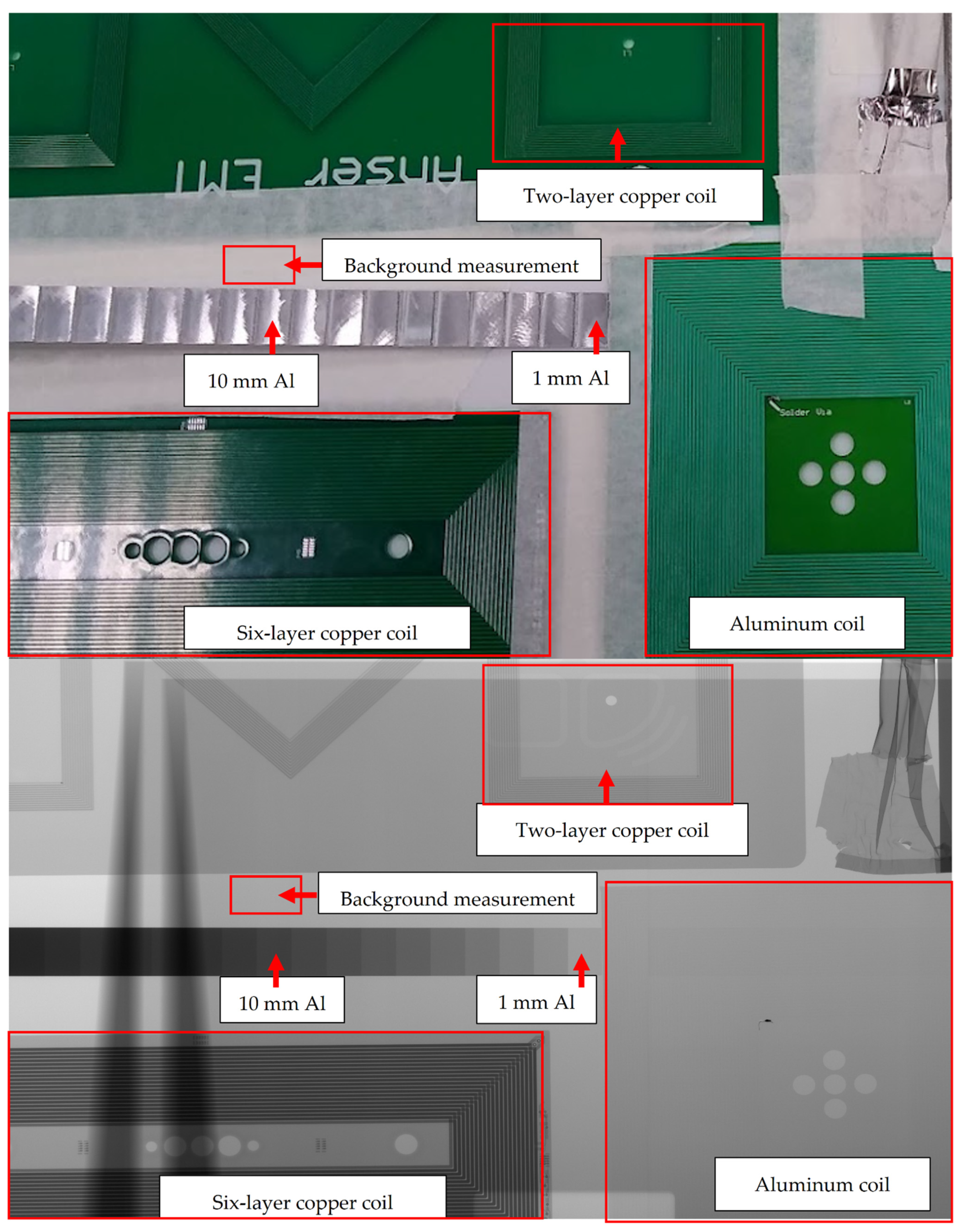
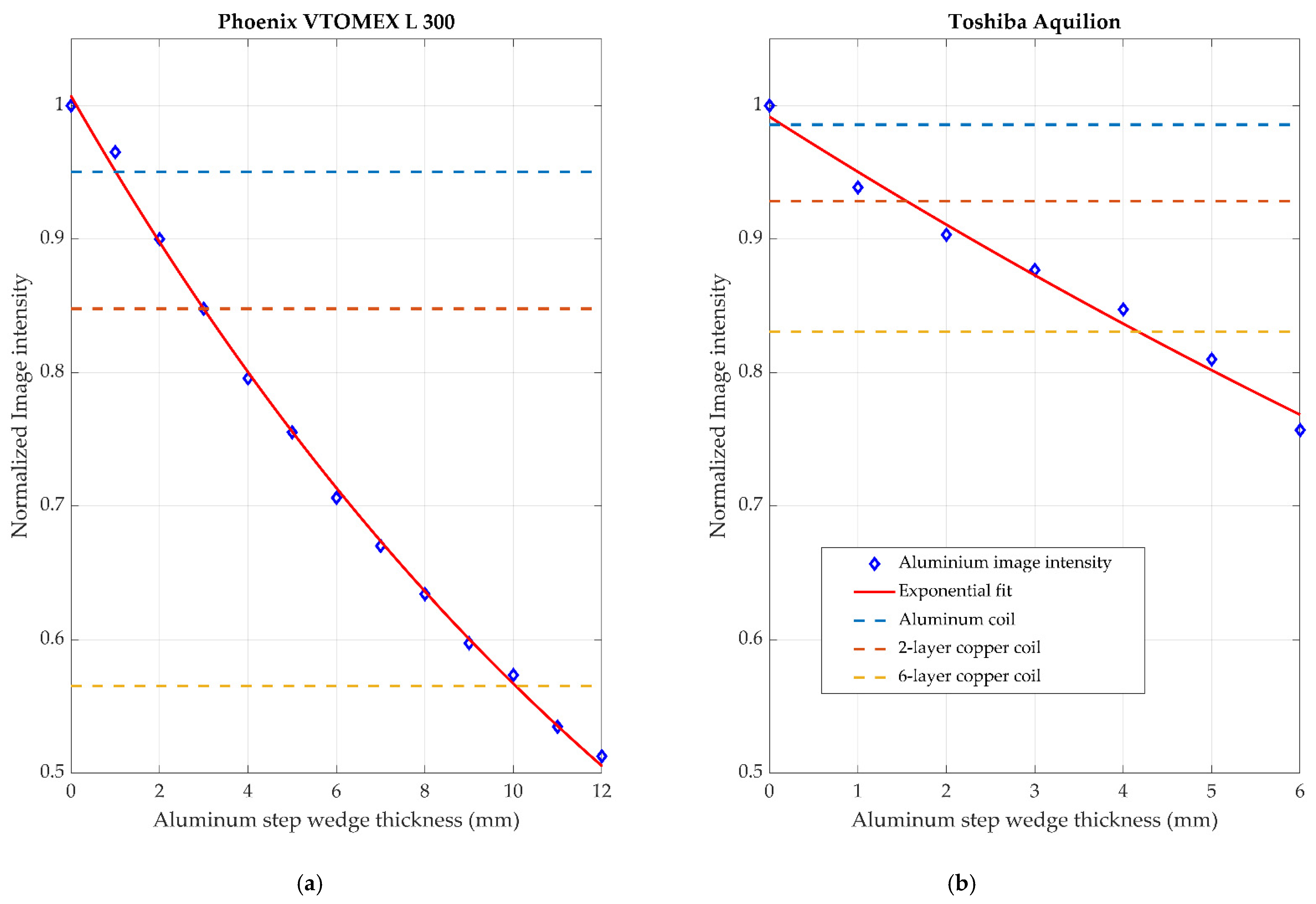
3.2. Radiolucency Analysis
- Two-layer, 70 μm thick copper coil
- Six-layer, 105 μm copper coil
- Two-layer, 50 μm aluminum coil
4. Discussion
4.1. Position Accuracy
4.2. Radiolucency
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hummel, J.B.; Bax, M.R.; Figl, M.L.; Kang, Y.; Maurer, C.; Birkfellner, W.W.; Bergmann, H.; Shahidi, R. Design and application of an assessment protocol for electromagnetic tracking systems. Med. Phys. 2005, 32, 2371–2379. [Google Scholar] [CrossRef] [PubMed]
- Cleary, K.; Peters, T.M. Image-Guided Interventions: Technology Review and Clinical Applications. Annu. Rev. Biomed. Eng. 2010, 12, 119–142. [Google Scholar] [CrossRef]
- Jaeger, H.A.; Trauzettel, F.; Nardelli, P.; Daverieux, F.; Hofstad, E.F.; Leira, H.O.; Kennedy, M.P.; Langø, T.; Cantillon-Murphy, P. Peripheral tumour targeting using open-source virtual bronchoscopy with electromagnetic tracking: A multi-user pre-clinical study. Minim. Invasive Ther. Allied Technol. 2018, 28, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Khan, K.A.; Nardelli, P.; Jaeger, A.; O’Shea, C.; Cantillon-Murphy, P.; Kennedy, M.P. Navigational Bronchoscopy for Early Lung Cancer: A Road to Therapy. Adv. Ther. 2016, 33, 580–596. [Google Scholar] [CrossRef] [PubMed]
- Yaniv, Z.; Wilson, E.; Lindisch, D.; Cleary, K. Electromagnetic tracking in the clinical environment. Med. Phys. 2009, 36, 876–892. [Google Scholar] [CrossRef] [PubMed]
- Sorriento, A.; Porfido, M.B.; Mazzoleni, S.; Calvosa, G.; Tenucci, M.; Ciuti, G.; Dario, P. Optical and Electromagnetic Tracking Systems for Biomedical Applications: A Critical Review on Potentialities and Limitations. IEEE Rev. Biomed. Eng. 2020, 13, 212–232. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Hansen, C.; Rose, G. A software solution to dynamically reduce metallic distortions of electromagnetic tracking systems for image-guided surgery. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 1621–1633. [Google Scholar] [CrossRef]
- Du, C.; Chen, X.; Wang, Y.; Li, J.; Yu, D. An Adaptive 6-DOF Tracking Method by Hybrid Sensing for Ultrasonic Endoscopes. Sensors 2014, 14, 9961–9983. [Google Scholar] [CrossRef]
- Pickering, E.M.; Kalchiem-Dekel, O.; Sachdeva, A. Electromagnetic navigation bronchoscopy: A comprehensive review. AME Med. J. 2018, 3, 117. [Google Scholar] [CrossRef]
- Krumb, H.; Das, D.; Chadda, R.; Mukhopadhyay, A. CycleGAN for interpretable online EMT compensation. Int. J. Comput. Assist. Radiol. Surg. 2021, 1–9. [Google Scholar] [CrossRef]
- Kügler, D.; Krumb, H.; Bredemann, J.; Stenin, I.; Kristin, J.; Klenzner, T.; Schipper, J.; Schmitt, R.; Sakas, G.; Mukhopadhyay, A. High-precision evaluation of electromagnetic tracking. Int. J. Comput. Assist. Radiol. Surg. 2019, 14, 1127–1135. [Google Scholar] [CrossRef] [PubMed]
- Bhattacharji, P.; Moore, W. Application of Real-Time 3D Navigation System in CT-Guided Percutaneous Interventional Procedures: A Feasibility Study. Radiol. Res. Pract. 2017, 2017, 1–7. [Google Scholar] [CrossRef]
- Kellermeier, M.; Herbolzheimer, J.; Kreppner, S.; Lotter, M.; Strnad, V.; Bert, C. Electromagnetic tracking (EMT) technology for improved treatment quality assurance in interstitial brachytherapy. J. Appl. Clin. Med. Phys. 2017, 18, 211–222. [Google Scholar] [CrossRef]
- O’Donoghue, K.; Corvò, A.; Nardelli, P.; Shea, C.O.; Khan, K.A.; Kennedy, M.; Cantillon-Murphy, P. Evaluation of a novel tracking system in a breathing lung model. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; Volume 2014, pp. 4046–4049. [Google Scholar]
- Gergel, I.; Gaa, J.; Muller, M.; Meinzer, H.-P.; Wegner, I. A novel fully automatic system for the evaluation of electromagnetic tracker. In Proceedings of the Medical Imaging 2012: Image-Guided Procedures, Robotic Interventions, and Modeling, San Diego, CA, USA, 16 February 2012; Volume 8316, p. 831608. [Google Scholar] [CrossRef]
- Katsura, M.; Sato, J.; Akahane, M.; Kunimatsu, A.; Abe, O. Current and Novel Techniques for Metal Artifact Reduction at CT: Practical Guide for Radiologists. Radiographics 2018, 38, 450–461. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, K.; Eustace, D.; Griffiths, J.; O’Shea, M.; Power, T.; Mansfield, H.; Cantillon-Murphy, P. Catheter Position Tracking System Using Planar Magnetics and Closed Loop Current Control. IEEE Trans. Magn. 2014, 50, 1–9. [Google Scholar] [CrossRef]
- O’Donoghue, K.; Cantillon-Murphy, P. Planar Magnetic Shielding for Use with Electromagnetic Tracking Systems. IEEE Trans. Magn. 2015, 51, 8500. [Google Scholar] [CrossRef]
- Rieke, V.; Ganguly, A.; Daniel, B.L.; Scott, G.; Pauly, J.M.; Fahrig, R.; Pelc, N.J.; Butts, K. X-ray compatible radiofrequency coil for magnetic resonance imaging. Magn. Reson. Med. 2005, 53, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Wang, Y.; Zhou, D.; Hu, X.; Wu, J. Study on an experimental AC electromagnetic tracking system. In Proceedings of the World Congress on Intelligent Control and Automation (WCICA), Hangzhou, China, 15–19 June 2004; Volume 4, pp. 3692–3695. [Google Scholar] [CrossRef]
- Jaeger, H.A.; Cantillon-Murphy, P. Distorter Characterisation Using Mutual Inductance in Electromagnetic Tracking. Sensors 2018, 18, 3059. [Google Scholar] [CrossRef] [PubMed]
- Paperno, E.; Sasada, I.; Leonovich, E. A new method for magnetic position and orientation tracking. IEEE Trans. Magn. 2001, 37, 1938–1940. [Google Scholar] [CrossRef]
- Plotkin, A.; Paperno, E.; Vasserman, G.; Segev, R. Magnetic Tracking of Eye Motion in Small, Fast-Moving Animals. IEEE Trans. Magn. 2008, 44, 4492–4495. [Google Scholar] [CrossRef]
- Bien, T.; Li, M.; Salah, Z.; Rose, G. Electromagnetic tracking system with reduced distortion using quadratic excitation. Int. J. Comput. Assist. Radiol. Surg. 2014, 9, 323–332. [Google Scholar] [CrossRef] [PubMed]
- Bushberg, J.T.; Seibert, J.A.; Leidholdt, E.M.; Boone, J.M.; Goldschmidt, E.J. The Essential Physics of Medical Imaging, 3rd ed.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2013. [Google Scholar]
- Macovski, A. Medical Imaging Systems; Prentice-Hall: Hoboken, NJ, USA, 1983. [Google Scholar]
- NIST. X-ray Mass Attenuation Coefficients. Available online: https://www.nist.gov/pml/x-ray-mass-attenuation-coefficients (accessed on 26 January 2021).
- Berger, M.; Yang, Q.; Maier, A. X-Ray Imaging. In Lecture Notes in Computer Science; (Including Subseries Lecture Notes in Artificial Intelligence and Lecture Notes in Bioinformatics); Springer: Berlin/Heidelberg, Germany, 2018; pp. 119–145. [Google Scholar]
- NIST. X-ray Mass Attenuation Coefficients—Aluminum. Available online: https://physics.nist.gov/PhysRefData/XrayMassCoef/ElemTab/z13.html (accessed on 26 January 2021).
- NIST. X-ray Mass Attenuation Coefficients—Copper. Available online: https://physics.nist.gov/PhysRefData/XrayMassCoef/ElemTab/z29.html (accessed on 26 January 2021).
- Horowitz, P. The Art of Electronics, 3rd ed.; Cambridge University Press: Cambridge, UK, 2015. [Google Scholar]
- Shamkhalichenar, H.; Bueche, C.; Choi, J.-W. Printed Circuit Board (PCB) Technology for Electrochemical Sensors and Sensing Platforms. Biosensors 2020, 10, 159. [Google Scholar] [CrossRef] [PubMed]
- Sonntag, C.L.W.; Spree, M.; Lomonova, E.A.; Duarte, J.L.; Vandenput, A.J.A. Accurate magnetic field calculations for contactless energy transfer coils. In Proceedings of the 16th International Conference on the Computation of Electromagnetic Fields, Aachen, Germany, 25–28 June 2007; pp. 1–4. Available online: www.tue.nl/taverne (accessed on 5 March 2021).
- Griffiths, D.J. Introduction to Electrodynamics; Cambridge University Press: Cambridge, UK, 2017. [Google Scholar]
- Mohan, S.S.; Hershenson, M.D.M.; Boyd, S.P.; Lee, T.H. Simple accurate expressions for planar spiral inductances. IEEE J. Solid State Circuits 1999, 34, 1419. [Google Scholar] [CrossRef]
- ICNIRP. Guidelines for limiting exposure to time-varying electric and magnetic fields (1 Hz to 100 kHz). Health Phys. 2010, 99, 818–836. [Google Scholar] [CrossRef] [PubMed]
- O’Donoghue, K. Electromagnetic Tracking and Steering for Catheter Navigation. Ph.D. Thesis, University College Cork, Cork, Ireland, 2014. [Google Scholar]
- Jaeger, H.A.; Franz, A.M.; O’Donoghue, K.; Seitel, A.; Trauzettel, F.; Maier-Hein, L.; Cantillon-Murphy, P. Anser EMT: The first open-source electromagnetic tracking platform for image-guided interventions. Int. J. Comput. Assist. Radiol. Surg. 2017, 12, 1059–1067. [Google Scholar] [CrossRef] [PubMed]
- Mir, A.P.B.; Mir, M.P.B. Assessment of radiopacity of restorative composite resins with various target distances and exposure times and a modified aluminum step wedge. Imaging Sci. Dent. 2012, 42, 163–167. [Google Scholar] [CrossRef]










| Field Generator | Mean Error (mm) | RMS Error (mm) | Standard Deviation Error (mm) | 90th Percentile Error (mm) |
|---|---|---|---|---|
| Standard copper | 0.99 | 1.51 | 0.76 | 2.39 |
| Aluminum | 1.23 | 1.25 | 0.88 | 1.95 |
| Material | Phoenix VTOMEX L 300 | Toshiba Aquilion |
|---|---|---|
| Two-layer aluminum | 0.95 | 0.98 |
| Two-layer copper | 0.85 | 0.93 |
| Six-layer copper | 0.57 | 0.83 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
O’Donoghue, K.; Jaeger, H.A.; Cantillon-Murphy, P. A Radiolucent Electromagnetic Tracking System for Use with Intraoperative X-ray Imaging. Sensors 2021, 21, 3357. https://doi.org/10.3390/s21103357
O’Donoghue K, Jaeger HA, Cantillon-Murphy P. A Radiolucent Electromagnetic Tracking System for Use with Intraoperative X-ray Imaging. Sensors. 2021; 21(10):3357. https://doi.org/10.3390/s21103357
Chicago/Turabian StyleO’Donoghue, Kilian, Herman Alexander Jaeger, and Padraig Cantillon-Murphy. 2021. "A Radiolucent Electromagnetic Tracking System for Use with Intraoperative X-ray Imaging" Sensors 21, no. 10: 3357. https://doi.org/10.3390/s21103357
APA StyleO’Donoghue, K., Jaeger, H. A., & Cantillon-Murphy, P. (2021). A Radiolucent Electromagnetic Tracking System for Use with Intraoperative X-ray Imaging. Sensors, 21(10), 3357. https://doi.org/10.3390/s21103357






