Hearing Loss Is Associated with Increased Variability in Double Support Period in the Elderly
Abstract
1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Audiometric Data
2.3. Instrumented Footwear for Gait Analysis
2.4. Experimental Protocol for Measurement of Gait Parameters
2.5. Other Variables
2.6. Statistical Analysis
3. Results
3.1. Characteristics
3.2. Multiple Linear Regression
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lin, F.R.; Thorpe, R.; Gordon-Salant, S.; Ferrucci, L. Hearing loss prevalence and risk factors among older adults in the United States. J. Gerontol. A Biol. Sci. Med. Sci. 2011, 66, 582–590. [Google Scholar] [CrossRef] [PubMed]
- Viljanen, A.; Kaprio, J.; Pyykkö, I.; Sorri, M.; Koskenvuo, M.; Rantanen, T. Hearing acuity as a predictor of walking difficulties in older women. J. Am. Geriatr. Soc. 2009, 57, 2282–2286. [Google Scholar] [CrossRef] [PubMed]
- Lin, F.R.; Ferrucci, L. Hearing loss and falls among older adults in the United States. Arch. Intern. Med. 2012, 172, 369–371. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, E.; Basu, T.; Langa, K.; McKee, M.M.; Zazove, P.; Alexander, N.; Kamdar, N. Can Hearing Aids Delay Time to Diagnosis of Dementia, Depression, or Falls in Older Adults? J. Am. Geriatr. Soc. 2019, 67, 2362–2369. [Google Scholar] [CrossRef]
- Tiase, V.L.; Tang, K.; Vawdrey, D.K.; Raso, R.; Adelman, J.S.; Yu, S.P.; Applebaum, J.R.; Lalwani, A.K. Impact of Hearing Loss on Patient Falls in the Inpatient Setting. Am. J. Prev. Med. 2020, 58, 839–844. [Google Scholar] [CrossRef]
- Bergen, G.; Stevens, M.R.; Burns, E.R. Falls and Fall Injuries Among Adults Aged ≥65 Years—United States, 2014. MMWR Morb. Mortal. Wkly. Rep. 2016, 65, 993–998. [Google Scholar] [CrossRef]
- Ambrose, A.F.; Paul, G.; Hausdorff, J.M. Risk factors for falls among older adults: A review of the literature. Maturitas 2013, 75, 51–61. [Google Scholar] [CrossRef]
- Fletcher, P.C.; Hirdes, J.P. Restriction in activity associated with fear of falling among community-based seniors using home care services. Age Ageing 2004, 33, 273–279. [Google Scholar] [CrossRef]
- Burns, E.; Kakara, R. Deaths from Falls Among Persons Aged ≥65 Years—United States, 2007-2016. MMWR Morb. Mortal. Wkly. Rep. 2018, 67, 509–514. [Google Scholar] [CrossRef]
- Scheffer, A.C.; Schuurmans, M.J.; van Dijk, N.; van der Hooft, T.; de Rooij, S.E. Fear of falling: Measurement strategy, prevalence, risk factors and consequences among older persons. Age Ageing 2008, 37, 19–24. [Google Scholar] [CrossRef]
- Tinetti, M.E.; Speechley, M.; Ginter, S.F. Risk factors for falls among elderly persons living in the community. N. Engl. J. Med. 1988, 319, 1701–1707. [Google Scholar] [CrossRef] [PubMed]
- Osoba, M.Y.; Rao, A.K.; Agrawal, S.K.; Lalwani, A.K. Balance and gait in the elderly: A contemporary review. Laryngoscope Investig. Otolaryngol. 2019, 4, 143–153. [Google Scholar] [CrossRef] [PubMed]
- Menz, H.B.; Lord, S.R.; Fitzpatrick, R.C. Age-related differences in walking stability. Age Ageing 2003, 32, 137–142. [Google Scholar] [CrossRef] [PubMed]
- Mortaza, N.; Abu Osman, N.A.; Mehdikhani, N. Are the spatio-temporal parameters of gait capable of distinguishing a faller from a non-faller elderly? Eur. J. Phys. Rehabil. Med. 2014, 50, 677–691. [Google Scholar]
- Zanotto, D.; Mamuyac, E.M.; Chambers, A.R.; Nemer, J.S.; Stafford, J.A.; Agrawal, S.K.; Lalwani, A.K. Dizziness Handicap Inventory Score Is Highly Correlated With Markers of Gait Disturbance. Otol. Neurotol. 2017, 38, 1490–1499. [Google Scholar] [CrossRef]
- Berge, J.E.; Nordahl, S.H.G.; Aarstad, H.J.; Goplen, F.K. Hearing as an Independent Predictor of Postural Balance in 1075 Patients Evaluated for Dizziness. Otolaryngol. Head Neck Surg. 2019, 161, 478–484. [Google Scholar] [CrossRef]
- Kennel, C.; Streese, L.; Pizzera, A.; Justen, C.; Hohmann, T.; Raab, M. Auditory reafferences: The influence of real-time feedback on movement control. Front. Psychol. 2015, 6, 69. [Google Scholar] [CrossRef]
- Sallard, E.; Spierer, L.; Ludwig, C.; Deiber, M.P.; Barral, J. Age-related changes in the bimanual advantage and in brain oscillatory activity during tapping movements suggest a decline in processing sensory reafference. Exp. Brain Res. 2014, 232, 469–479. [Google Scholar] [CrossRef]
- Cornwell, T.; Woodward, J.; Wu, M.M.; Jackson, B.; Souza, P.; Siegel, J.; Dhar, S.; Gordon, K.E. Walking with Ears: Altered Auditory Feedback Impacts Gait Step Length in Older Adults. Front. Sports Act. Living 2020, 2. [Google Scholar] [CrossRef]
- Bainbridge, K.E.; Wallhagen, M.I. Hearing loss in an aging American population: Extent, impact, and management. Annu. Rev. Public Health 2014, 35, 139–152. [Google Scholar] [CrossRef]
- Kohlberg, G.D.; Demmer, R.T.; Lalwani, A.K. Adolescent Obesity Is an Independent Risk Factor for Sensorineural Hearing Loss: Results From the National Health and Nutrition Examination Survey 2005 to 2010. Otol. Neurotol. 2018, 39, 1102–1108. [Google Scholar] [CrossRef] [PubMed]
- Lazard, D.S.; Collette, J.L.; Perrot, X. Speech processing: From peripheral to hemispheric asymmetry of the auditory system. Laryngoscope 2012, 122, 167–173. [Google Scholar] [CrossRef] [PubMed]
- Zanotto, D.; Turchet, L.; Boggs, E.M.; Agrawal, S.K. SoleSound: Towards a novel portable system for audio-tactile underfoot feedback. In Proceedings of the 5th IEEE RAS/EMBS International Conference on Biomedical Robotics and Biomechatronics, São Paulo, Brazil, 12–15 August 2014; pp. 193–198. [Google Scholar]
- Minto, S.; Zanotto, D.; Boggs, E.M.; Rosati, G.; Agrawal, S.K. Validation of a Footwear-Based Gait Analysis System with Action-Related Feedback. IEEE Trans. Neural. Syst. Rehabil. Eng. 2016, 24, 971–980. [Google Scholar] [CrossRef] [PubMed]
- Montes, J.; Zanotto, D.; Dunaway Young, S.; Salazar, R.; De Vivo, D.C.; Agrawal, S. Gait assessment with solesound instrumented footwear in spinal muscular atrophy. Muscle Nerve 2017, 56, 230–236. [Google Scholar] [CrossRef] [PubMed]
- Hof, A.L. Scaling gait data to body size. Gait Posture 1996, 4, 222–223. [Google Scholar] [CrossRef]
- Hollman, J.H.; Childs, K.B.; McNeil, M.L.; Mueller, A.C.; Quilter, C.M.; Youdas, J.W. Number of strides required for reliable measurements of pace, rhythm and variability parameters of gait during normal and dual task walking in older individuals. Gait Posture 2010, 32, 23–28. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.; Howe, T.; Greenland, J.; Simpson, L.; Rochester, L. Gait variability in older adults: A structured review of testing protocol and clinimetric properties. Gait Posture 2011, 34, 443–450. [Google Scholar] [CrossRef]
- Jacobson, G.P.; Newman, C.W. The development of the Dizziness Handicap Inventory. Arch. Otolaryngol. Head Neck Surg. 1990, 116, 424–427. [Google Scholar] [CrossRef]
- Mutlu, B.; Serbetcioglu, B. Discussion of the dizziness handicap inventory. J. Vestib. Res. 2013, 23, 271–277. [Google Scholar] [CrossRef]
- Ardıç, F.N.; Tümkaya, F.; Akdağ, B.; Şenol, H. The subscales and short forms of the dizziness handicap inventory: Are they useful for comparison of the patient groups? Disabil. Rehabil. 2017, 39, 2119–2122. [Google Scholar] [CrossRef]
- Cook, R.D.; Weisberg, S. Residuals and Influence in Regression; Chapman and Hall: New York, NY, USA, 1982. [Google Scholar]
- Cook, R.D. Detection of Influential Observation in Linear Regression. Technometrics 1977, 19, 15–18. [Google Scholar] [CrossRef]
- Stevens, J.P. Outliers and influential data points in regression analysis. Psychol. Bull. 1984, 95, 334–344. [Google Scholar] [CrossRef]
- Hausdorff, J.M.; Rios, D.A.; Edelberg, H.K. Gait variability and fall risk in community-living older adults: A 1-year prospective study. Arch. Phys. Med. Rehabil. 2001, 82, 1050–1056. [Google Scholar] [CrossRef] [PubMed]
- Hausdorff, J.M. Gait variability: Methods, modeling and meaning. J. Neuroeng. Rehabil. 2005, 2, 19. [Google Scholar] [CrossRef] [PubMed]
- Reelick, M.F.; van Iersel, M.B.; Kessels, R.P.; Rikkert, M.G. The influence of fear of falling on gait and balance in older people. Age Ageing 2009, 38, 435–440. [Google Scholar] [CrossRef] [PubMed]
- Callisaya, M.L.; Blizzard, L.; Schmidt, M.D.; Martin, K.L.; McGinley, J.L.; Sanders, L.M.; Srikanth, V.K. Gait, gait variability and the risk of multiple incident falls in older people: A population-based study. Age Ageing 2011, 40, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Sterke, C.S.; van Beeck, E.F.; Looman, C.W.N.; Kressig, R.W.; van der Cammen, T.J.M. An electronic walkway can predict short-term fall risk in nursing home residents with dementia. Gait Posture 2012, 36, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Larsson, J.; Ekvall Hansson, E.; Miller, M. Increased double support variability in elderly female fallers with vestibular asymmetry. Gait Posture 2015, 41, 820–824. [Google Scholar] [CrossRef]
- Larsson, J.; Miller, M.; Hansson, E.E. Vestibular asymmetry increases double support time variability in a counter-balanced study on elderly fallers. Gait Posture 2016, 45, 31–34. [Google Scholar] [CrossRef]
- Perry, J.; Davids, J.R. Gait analysis: Normal and pathological function. J. Pediatric Orthop. 1992, 12, 815. [Google Scholar] [CrossRef]
- Gabell, A.; Nayak, U.S. The effect of age on variability in gait. J. Gerontol. 1984, 39, 662–666. [Google Scholar] [CrossRef] [PubMed]
- Serrien, D.J.; Swinnen, S.P.; Stelmach, G.E. Age-related deterioration of coordinated interlimb behavior. J. Gerontol. B Psychol. Sci. Soc. Sci. 2000, 55, P295–P303. [Google Scholar] [CrossRef] [PubMed]
- James, E.G.; Leveille, S.G.; Hausdorff, J.M.; Travison, T.; Kennedy, D.N.; Tucker, K.L.; Al Snih, S.; Markides, K.S.; Bean, J.F. Rhythmic Interlimb Coordination Impairments and the Risk for Developing Mobility Limitations. J. Gerontol. Ser. A Biol. Sci. Med. Sci. 2017, 72, 1143–1148. [Google Scholar] [CrossRef]
- Stepanchenko, N.; Hrybovska, I.; Danylevych, M.; Hryboskyy, R. Aspects of psychomotor development of primary school children with hearing loss from the standpoint of Bernstein’s theory of movement construction. Pedagog. Phys. Cult. Sports 2020, 24, 151–156. [Google Scholar] [CrossRef]
- Ronsse, R.; Puttemans, V.; Coxon, J.P.; Goble, D.J.; Wagemans, J.; Wenderoth, N.; Swinnen, S.P. Motor learning with augmented feedback: Modality-dependent behavioral and neural consequences. Cereb. Cortex 2011, 21, 1283–1294. [Google Scholar] [CrossRef]
- Zelic, G.; Mottet, D.; Lagarde, J. Audio-tactile events can improve the interlimb coordination in Juggling. BIO Web Conf. 2011, 1, 00102. [Google Scholar] [CrossRef]
- Keefe, D.H.; Gorga, M.P.; Jesteadt, W.; Smith, L.M. Ear asymmetries in middle-ear, cochlear, and brainstem responses in human infants. J. Acoust. Soc. Am. 2008, 123, 1504–1512. [Google Scholar] [CrossRef]
- Henkin, Y.; Swead, R.T.; Roth, D.A.; Kishon-Rabin, L.; Shapira, Y.; Migirov, L.; Hildesheimer, M.; Kaplan-Neeman, R. Evidence for a right cochlear implant advantage in simultaneous bilateral cochlear implantation. Laryngoscope 2014, 124, 1937–1941. [Google Scholar] [CrossRef]
- Zhang, H.; Guo, Y.; Zanotto, D. Accurate ambulatory gait analysis in walking and running using machine learning models. IEEE Trans. Neural Syst. Rehabil. Eng. 2019, 28, 191–202. [Google Scholar] [CrossRef]


| Characteristic | N = 80 |
|---|---|
| Age, mean ± SD | 73.7 ± 8.8 |
| Sex, N (%) | |
| Male | 37 (46.2) |
| Female | 43 (53.8) |
| Race/Ethnicity, N (%) | |
| Non-Hispanic White | 42 (52.5) |
| All others | 38 (47.5) |
| BMI, kg/m2, mean ± SD | 26.3 ± 4.0 |
| Height, m, mean ± SD | 1.67 ± 0.09 |
| Weight, kg, mean ± SD | 71.2 ± 15.2 |
| Hearing parameters, mean ± SD | |
| Low frequency PTA, poorer hearing ear | 44.4 ± 27.2 |
| Low frequency PTA, right ear | 37.4 ± 23.9 |
| Low frequency PTA, left ear | 37.4 ± 23.7 |
| High frequency PTA, poorer hearing ear | 61.1 ± 24.7 |
| High frequency PTA, right ear | 52.2 ± 23.5 |
| High frequency PTA, left ear | 55.9 ± 24.5 |
| Low frequency PTA, poorer hearing ear, N (%) | |
| 0 to 25 dB | 22 (27.5) |
| >25 dB to 40 dB | 22 (27.5) |
| >40 dB to 60 dB | 22 (27.5) |
| >60 dB to 80 dB | 6 (7.5) |
| >80 dB | 8 (10.0) |
| High frequency PTA, poorer hearing ear, N (%) | |
| 0 to 25 dB | 4 (5.0) |
| >25 dB to 40 dB | 14 (17.5) |
| >40 dB to 60 dB | 23 (28.8) |
| >60 dB to 80 dB | 21 (26.3) |
| >80 dB | 18 (22.5) |
| DHI-S score, mean ± SD | 6.3 ± 8.5 |
| Chief complaints | |
| Hearing loss | 70 (87.5) |
| Tinnitus | 34 (42.5) |
| Dizziness or Imbalance | 41 (51.3) |
| Other | 12 (15.2) |
| Gait Parameter | N | Mean | SD | Min | Max |
|---|---|---|---|---|---|
| Cadence | |||||
| Mean (stp/min) | 80 | 108 | 10.7 | 73.6 | 130.6 |
| CV (%) | 80 | 2.7 | 1 | 1.1 | 6.1 |
| Double supp. period | |||||
| Mean (%) | 74 | 10 | 2.6 | 4.8 | 15.5 |
| CV (%) | 74 | 10.4 | 6 | 4.1 | 40.9 |
| Stride Height | |||||
| Mean (m) | 80 | 0.15 | 0.026 | 0.091 | 0.257 |
| CV (%) | 80 | 9.5 | 9.9 | 2.5 | 45.5 |
| Normalized Stride Height | |||||
| Mean (%) | 80 | 9.1 | 1.4 | 5.6 | 14.5 |
| CV (%) | 80 | 9.5 | 9.9 | 2.5 | 45.5 |
| Stride Length | |||||
| Mean (m) | 80 | 1.223 | 0.21 | 0.583 | 1.592 |
| CV (%) | 80 | 4.8 | 2.8 | 1.9 | 16.6 |
| Normalized Stride Length | |||||
| Mean (%) | 80 | 73.7 | 11.7 | 35.9 | 93.6 |
| CV (%) | 80 | 4.8 | 2.8 | 1.9 | 16.6 |
| Stance-to-swing | |||||
| Mean | 80 | 1.52 | 0.16 | 1.22 | 1.9 |
| CV (%) | 80 | 5.7 | 3.3 | 2.3 | 22.6 |
| Swing period | |||||
| Mean (%) | 80 | 39.9 | 2.5 | 34.5 | 45.2 |
| CV (%) | 80 | 3.3 | 1.6 | 1.3 | 9.9 |
| Walking Speed | |||||
| Mean (m/s) | 80 | 1.111 | 0.243 | 0.475 | 1.54 |
| CV (%) | 80 | 5.9 | 3.3 | 2.4 | 20.6 |
| Normalized Walking Speed | |||||
| Mean (%) | 80 | 27.5 | 5.9 | 11.9 | 37.7 |
| CV (%) | 80 | 5.9 | 3.3 | 2.4 | 20.6 |
| Outcome | PTA | N | R2 | BPTA | p-Value | BDHI-S | p-Value | Bage | p-Value | βPTA | βDHI-S | βage |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
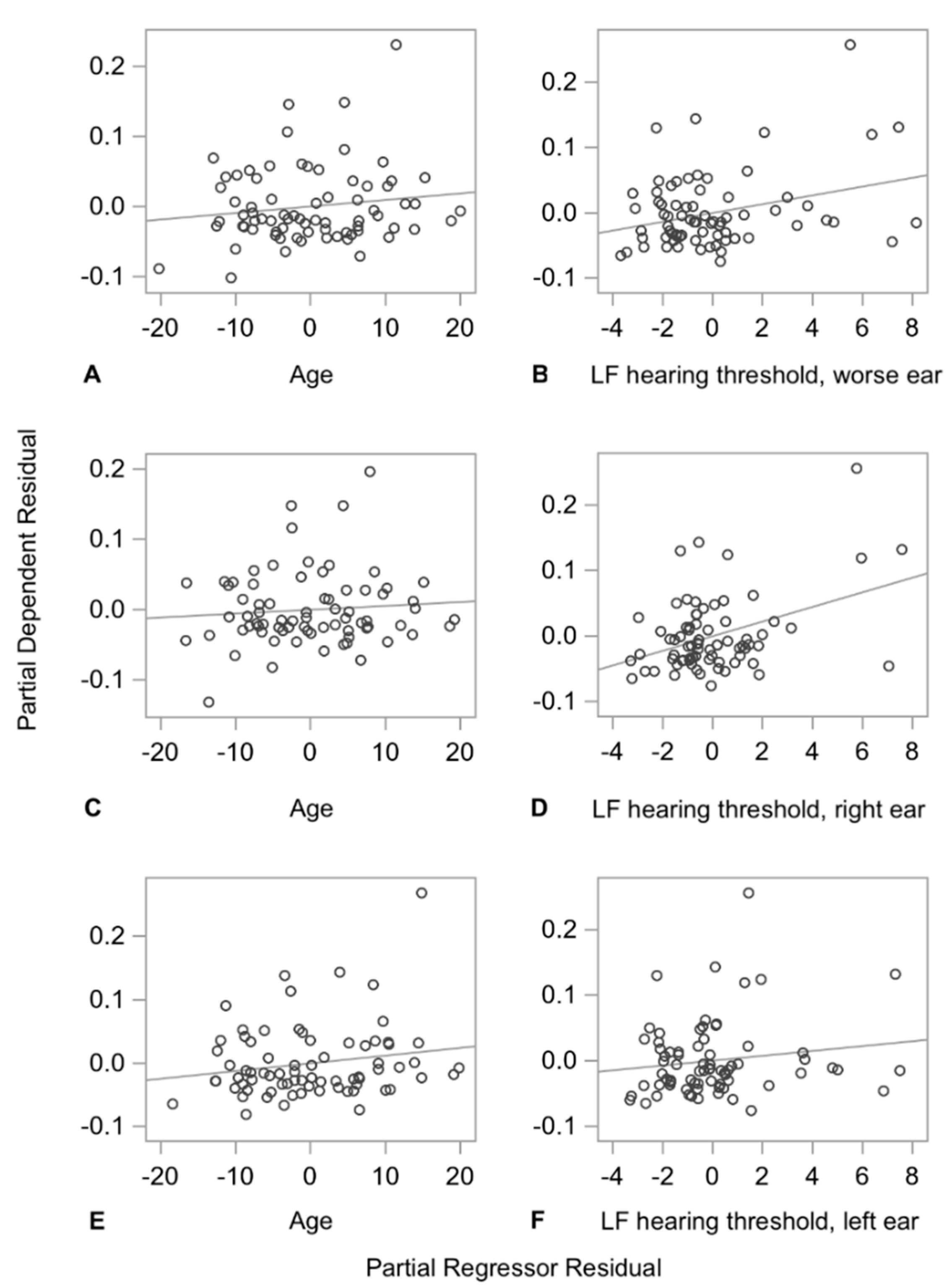
| Double supp. period CV (%) | HF, poorer ear | 74 | 0.1895 | 0.814 | 0.0055 | 0.092 | 0.2300 | 0.051 | 0.5286 | 0.33449 | 0.13381 | 0.07512 |
| HF, right ear | 74 | 0.2277 | 1.022 | 0.0042 | 0.094 | 0.2143 | 0.020 | 0.8186 | 0.38664 | 0.13720 | 0.02889 | |
| HF, left ear | 74 | 0.2099 | 0.759 | 0.0096 | 0.105 | 0.1728 | 0.068 | 0.4011 | 0.31613 | 0.15209 | 0.10006 | |
| LF, poorer ear | 74 | 0.1750 | 0.671 | 0.0107 | 0.053 | 0.5055 | 0.092 | 0.2400 | 0.30466 | 0.07677 | 0.13561 | |
| LF, right ear | 74 | 0.2397 | 1.114 | 0.0006 | 0.043 | 0.5842 | 0.054 | 0.4819 | 0.43071 | 0.06183 | 0.07994 | |
| LF, left ear | 74 | 0.1118 | 0.372 | 0.2209 | 0.090 | 0.2792 | 0.121 | 0.1347 | 0.15138 | 0.13063 | 0.17860 |
| Outcome | PTA | N | R2 | BPTA | p-Value | BDHI-S | p-Value | Bage | p-Value | βPTA | βDHI-S | βage |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Normalized stride height, mean (%) | HF, poorer ear | 80 | 0.1535 | −0.130 | 0.0491 | 0.007 | 0.7059 | −0.019 | 0.3052 | −0.22689 | 0.04105 | −0.11807 |
| HF, right ear | 80 | 0.1514 | −0.136 | 0.0859 | 0.009 | 0.6160 | −0.016 | 0.4159 | −0.22588 | 0.05556 | −0.09887 | |
| HF, left ear | 80 | 0.1588 | −0.129 | 0.0581 | 0.009 | 0.6027 | −0.018 | 0.3391 | −0.22517 | 0.05744 | −0.11150 | |
| LF, poorer ear | 80 | 0.1293 | −0.079 | 0.1794 | 0.011 | 0.5625 | −0.027 | 0.1353 | −0.15257 | 0.06531 | −0.16786 | |
| LF, right ear | 80 | 0.1414 | −0.081 | 0.2555 | 0.015 | 0.4326 | −0.027 | 0.1438 | −0.13759 | 0.09062 | −0.16633 | |
| LF, left ear | 80 | 0.1401 | −0.075 | 0.2771 | 0.014 | 0.4673 | −0.029 | 0.1041 | −0.12682 | 0.08328 | −0.18171 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szeto, B.; Zanotto, D.; Lopez, E.M.; Stafford, J.A.; Nemer, J.S.; Chambers, A.R.; Agrawal, S.K.; Lalwani, A.K. Hearing Loss Is Associated with Increased Variability in Double Support Period in the Elderly. Sensors 2021, 21, 278. https://doi.org/10.3390/s21010278
Szeto B, Zanotto D, Lopez EM, Stafford JA, Nemer JS, Chambers AR, Agrawal SK, Lalwani AK. Hearing Loss Is Associated with Increased Variability in Double Support Period in the Elderly. Sensors. 2021; 21(1):278. https://doi.org/10.3390/s21010278
Chicago/Turabian StyleSzeto, Betsy, Damiano Zanotto, Erin M. Lopez, John A. Stafford, John S. Nemer, Adam R. Chambers, Sunil K. Agrawal, and Anil K. Lalwani. 2021. "Hearing Loss Is Associated with Increased Variability in Double Support Period in the Elderly" Sensors 21, no. 1: 278. https://doi.org/10.3390/s21010278
APA StyleSzeto, B., Zanotto, D., Lopez, E. M., Stafford, J. A., Nemer, J. S., Chambers, A. R., Agrawal, S. K., & Lalwani, A. K. (2021). Hearing Loss Is Associated with Increased Variability in Double Support Period in the Elderly. Sensors, 21(1), 278. https://doi.org/10.3390/s21010278






