Application of Modern Multi-Sensor Holter in Diagnosis and Treatment
Abstract
1. Introduction
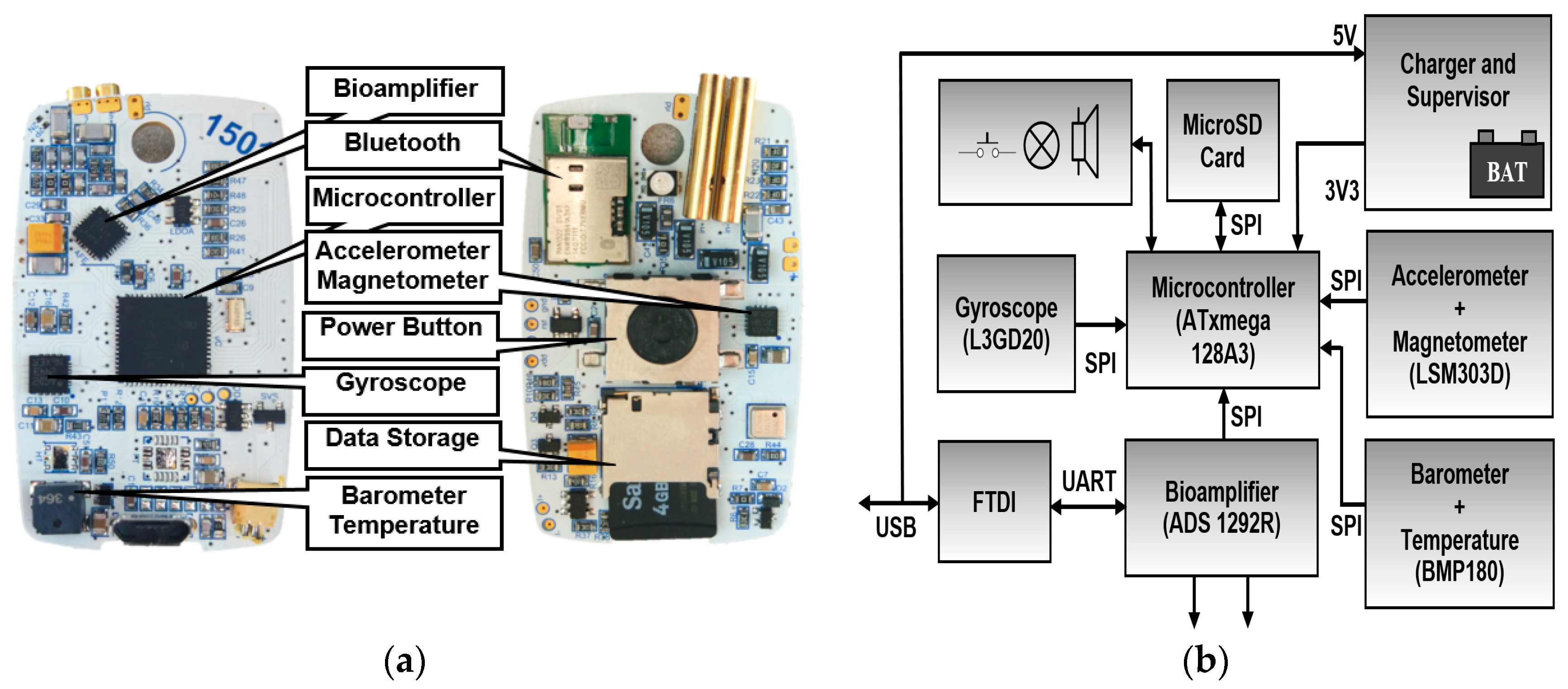
2. Capability of Modern Multi-Sensor Holters
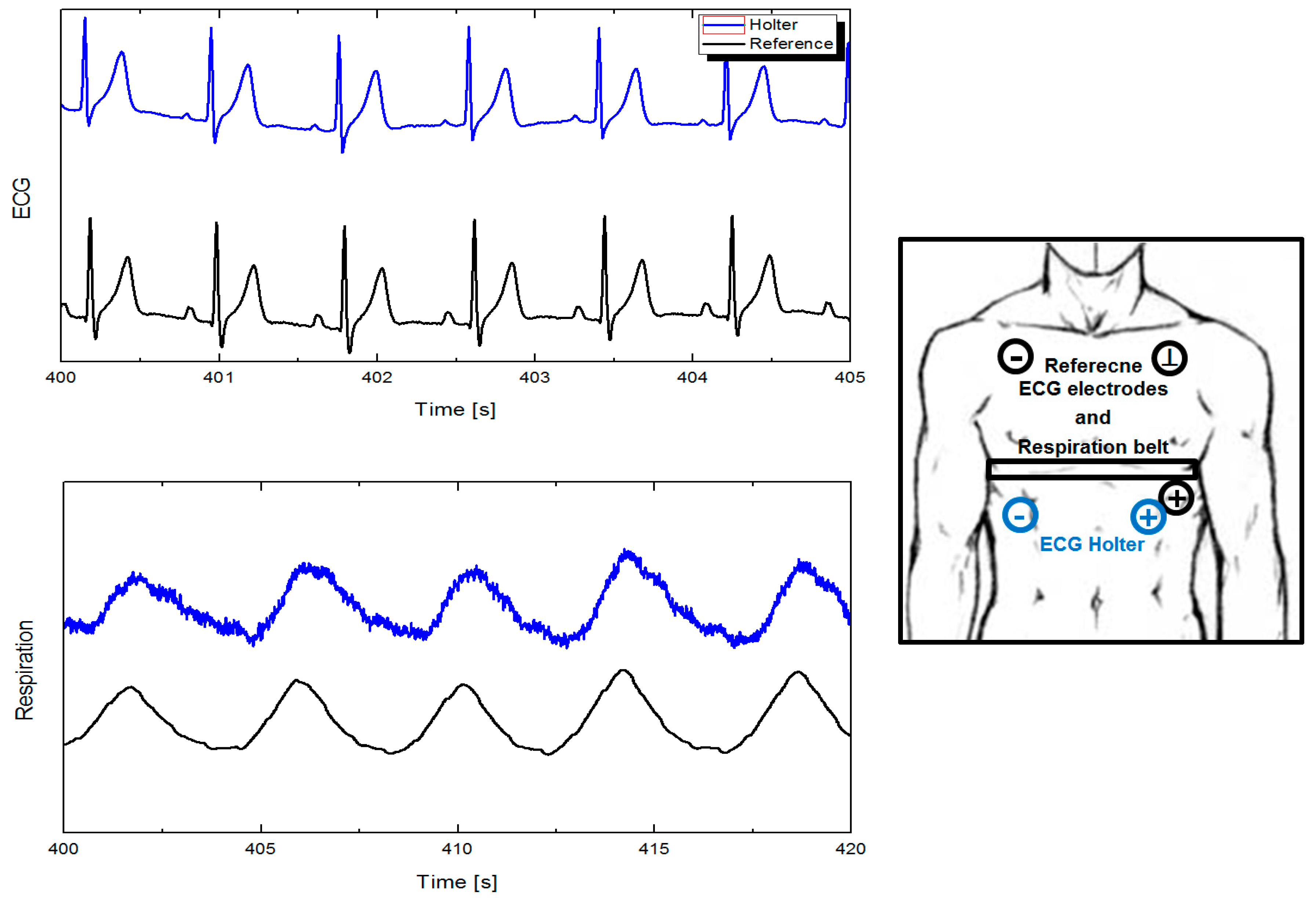
2.1. Electrocardiography and Respiration
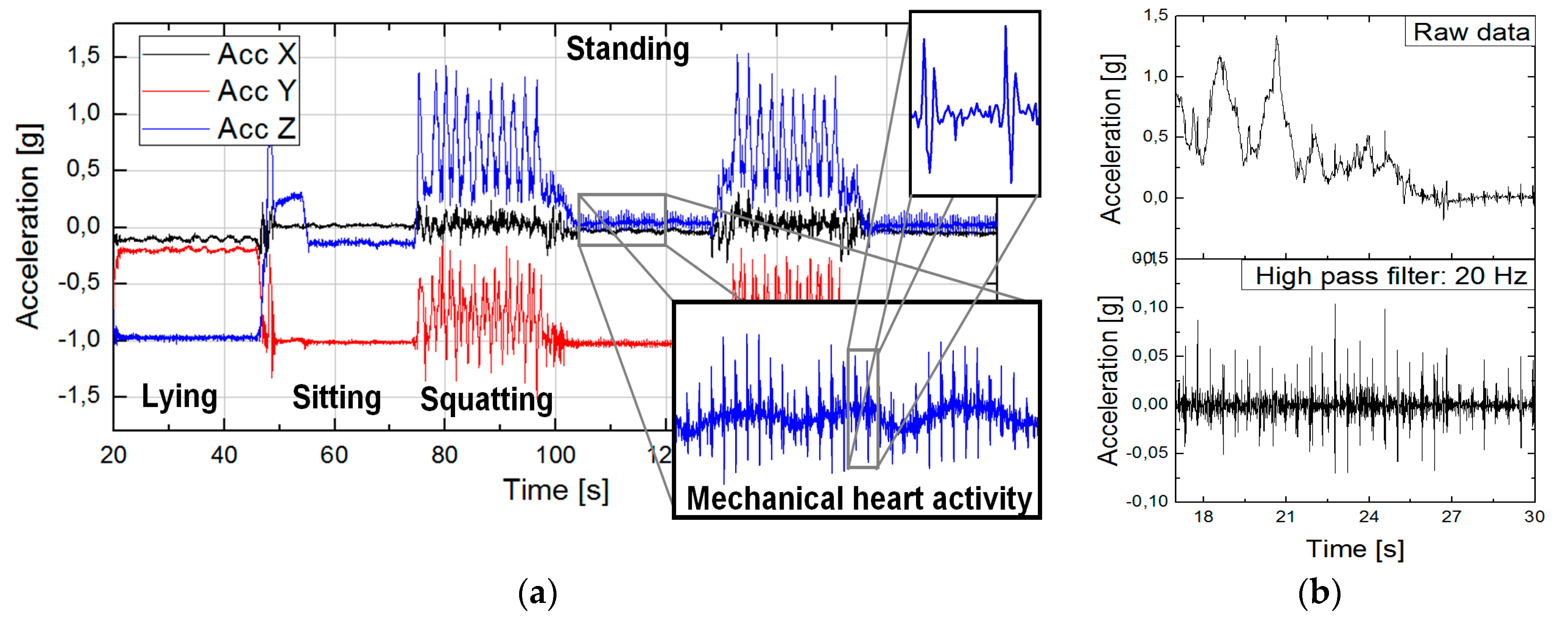
2.2. Inertial Measurement Units and Seismocardiography
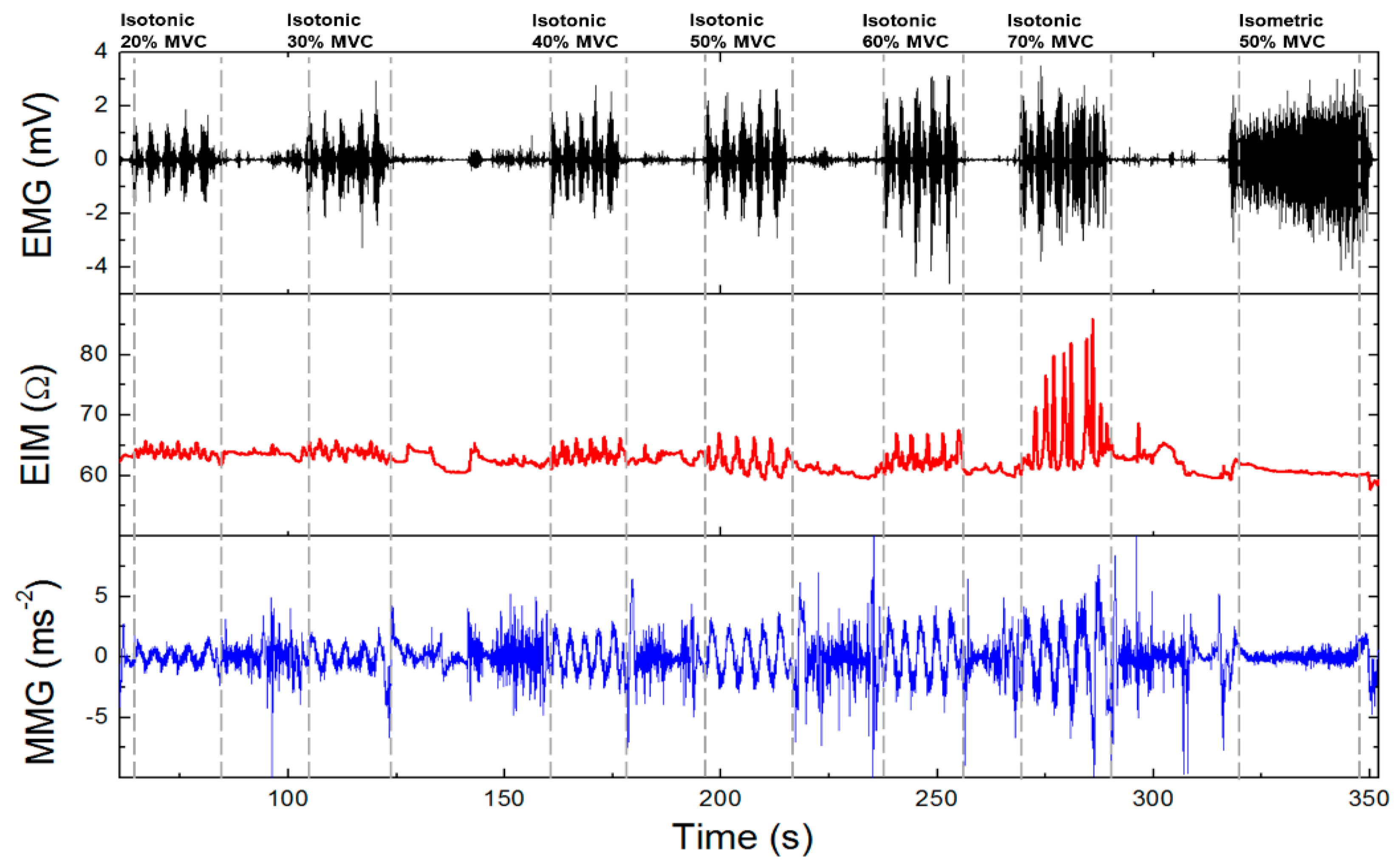
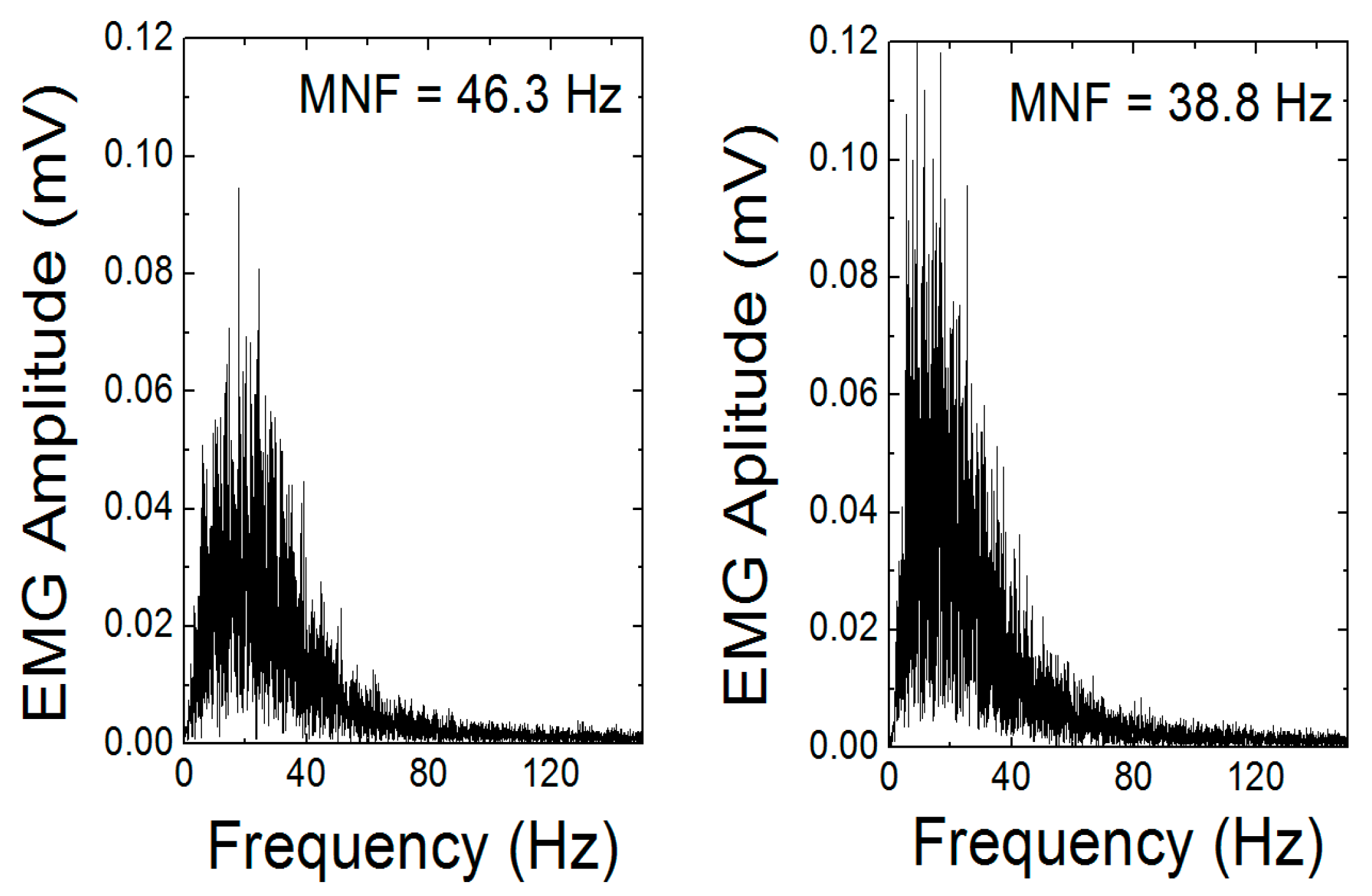
2.3. Muscle Activity
2.4. Electrodermal Activity (EDA)
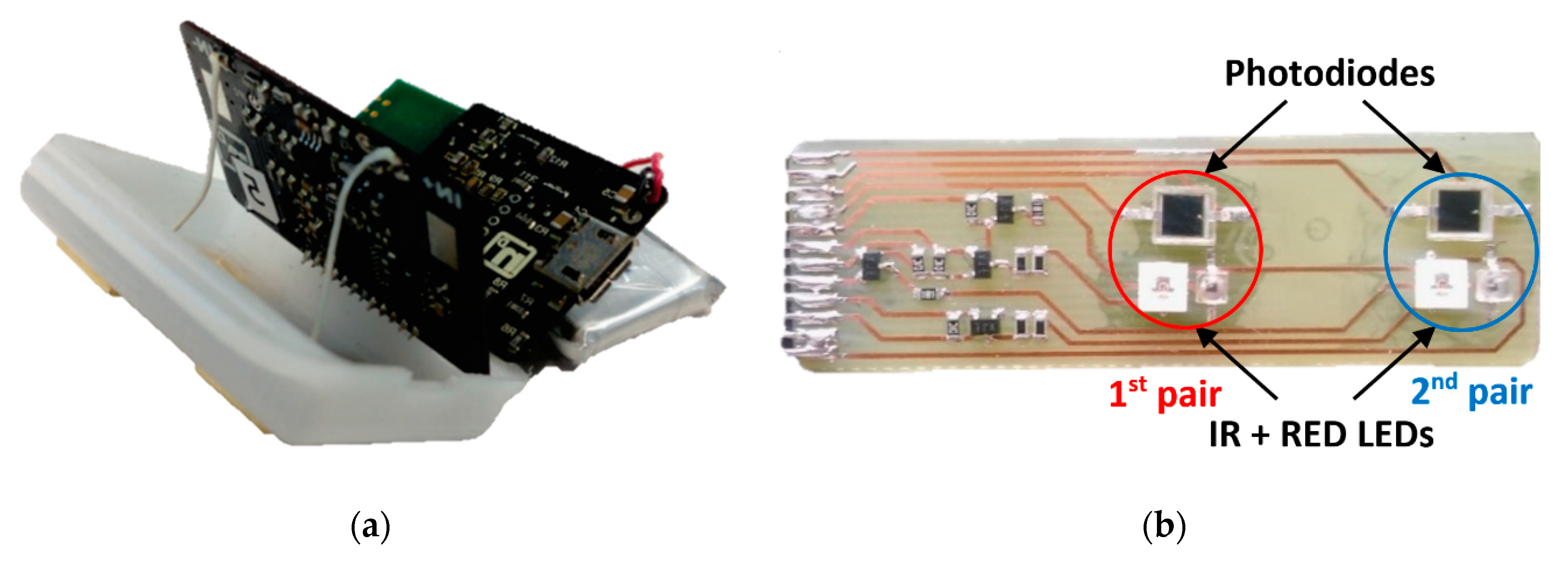
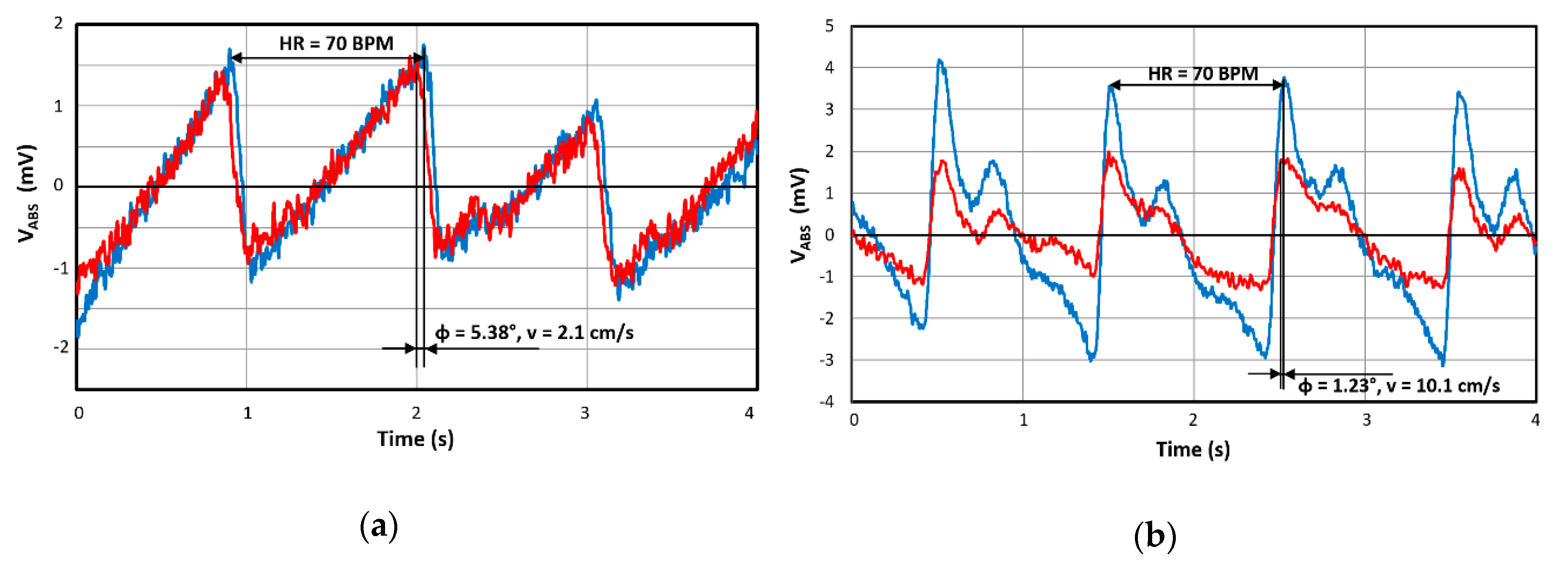
2.5. Pulse-Oximetry–Photoplethysmogram
2.6. Technolgies of Wearable Devices and Smart Clothing
3. Conclusions
Funding
Acknowledgments
Conflicts of Interest
References
- Kennedy, H.L. The evolution of ambulatory ECG monitoring. Prog. Cardiovasc. Dis. 2013, 56, 127–132. [Google Scholar] [CrossRef] [PubMed]
- Roberts, W.C.; Silver, M.A. Norman Jefferis Holter and ambulatory ECG monitoring. Am. J. Cardiol. 1983, 52, 903–906. [Google Scholar] [CrossRef]
- Makarov, L.M. To the centennial of Norman Holter (1914–1983). Kardiologiia 2015, 55, 59–61. [Google Scholar] [CrossRef] [PubMed]
- Nguyen, A.; Ansari, S.; Hooshmand, M.; Lin, K.; Ghanbari, H.; Gryak, J.; Najarian, K. Comparative Study on Heart Rate Variability Analysis for Atrial Fibrillation Detection in Short Single-Lead ECG Recordings. In Proceedings of the 2018 40th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Honolulu, HI, USA, 18–21 July 2018; pp. 526–529. [Google Scholar]
- Penders, J.; Altini, M.; de Molengraft, J.; van Romero, I.; Yazicioglu, F.; Van Hoof, C. A low-power wireless ECG necklace for reliable cardiac activity monitoring on the move. In Proceedings of the 33rd Annual International IEEE EMBS Conference, Boston, MA, USA, 30 August–3 September 2011. [Google Scholar]
- Ruangsuwana, R.; Velikic, G.; Bocko, M. Methods to extract respiration information from ECG signals. In Proceedings of the 2010 IEEE International Conference on Acoustics, Speech and Signal Processing, Dallas, TX, USA, 14–19 March 2010; pp. 570–573. [Google Scholar]
- Marco, T. De Novel Wearable Seismocardiography and Clinical Status of Heart Failure Patients. Circ. Hear. Fail. 2018, 11, 1–11. [Google Scholar]
- Fernández-mariño, A.I.; Harpole, T.; Oelstrom, K.; Delemotte, L.; Chanda, B. Gating interaction maps reveal a noncanonical electromechanical coupling mode in the Shaker K+ channel. Nat. Struct. Mol. Biol. 2018, 25, 320–326. [Google Scholar] [CrossRef]
- Gao, Y.; Yan, H.; Xu, Z.; Xiao, M.; Song, J. A principal component analysis based data fusion method for ECG-derived respiration from single-lead ECG. Australas. Phys. Eng. Sci. Med. 2018, 41, 59–67. [Google Scholar] [CrossRef]
- Kimura, T.; Aizawa, Y.; Kurata, N.; Nakajima, K.; Kashimura, S.; Kunitomi, A.; Nishiyama, T.; Katsumata, Y.; Nishiyama, N.; Fukumoto, K.; et al. Assessment of atrial fibrillation ablation outcomes with clinic ECG, monthly 24-h Holter ECG, and twice-daily telemonitoring. Heart Vessel. 2017, 32, 317–325. [Google Scholar] [CrossRef]
- Behar, J.A. Non-invasive fetal electrocardiography for the detection of fetal arrhythmias. Prenat. Diagn. 2019, 3, 178–187. [Google Scholar] [CrossRef]
- Abreu, R.; Nunes, S.; Leal, A.; Figueiredo, P. Physiological noise correction using ECG-derived respiratory signals for enhanced mapping of spontaneous neuronal activity with simultaneous EEG-fMRI. Neuroimage 2017, 154, 115–127. [Google Scholar] [CrossRef]
- Przystup, P.; Polinski, A.; Bujnowski, A.; Kocejko, T.; Wtorek, J. A body position influence on ECG derived respiration. In Proceedings of the 2017 39th Annual International Conference of the IEEE Engineering in Medicine and Biology Society (EMBC), Jeju Island, Korea, 11–15 July 2017; pp. 3513–3516. [Google Scholar]
- Boyle, J.; Bidargaddi, N.; Sarela, A.; Karunanithi, M. Automatic Detection of Respiration Rate from Ambulatory Single-Lead ECG. IEEE Trans. Inf. Technol. Biomed. 2009, 13, 890–896. [Google Scholar] [CrossRef]
- Norman, S.E.; Eager, R.A.; Waran, N.K.; Jeffery, L.; Schroter, R.C.; Marlin, D.J. Recording of ECG signals on a portable MiniDisc recorder for time and frequency domain heart rate variability analysis. Physiol. Behav. 2005, 83, 729–738. [Google Scholar] [CrossRef] [PubMed]
- Ryu, S.; Rochelle, N.; Mary, N.Y.; Liebert, A. History of Telemedicine: Evolution, Context, and Transformation. Healthc. Inform. Res. 2010, 16, 65–66. [Google Scholar] [CrossRef]
- Chaudhari, B.S.; Zennaro, M.; Borkar, S. LPWAN technologies: Emerging application characteristics, requirements, and design considerations. Futur. Internet 2020, 12, 46. [Google Scholar] [CrossRef]
- Rubio-Aparicio, J.; Cerdan-Cartagena, F.; Suardiaz-Muro, J.; Ybarra-Moreno, J. Design and Implementation of a Mixed IoT LPWAN Network Architecture. Sensors 2019, 19, 675. [Google Scholar] [CrossRef]
- Zemrane, H.; Baddi, Y.; Hasbi, A. Ehealth smart application of WSN on WWAN. ACM Int. Conf. Proc. Ser. 2019, 1–8. [Google Scholar]
- Cilfone, A.; Davoli, L.; Belli, L.; Ferrari, G. Wireless mesh networking: An IoT-oriented perspective survey on relevant technologies. Futur. Internet 2019, 11, 99. [Google Scholar] [CrossRef]
- Chuo, Y.; Marzencki, M.; Hung, B.; Jaggernauth, C.; Tavakolian, K.; Lin, P.; Kaminska, B. Mechanically Flexible Wireless Multisensor Platform for Human Physical Activity and Vitals Monitoring. IEEE Trans. Biomed. Circuits Syst. 2010, 4, 281–294. [Google Scholar] [CrossRef]
- Boquete, L.; Ascariz, J.M.R.; Cantos, J.; Barea, R.; Miguel, J.M.; Ortega, S.; Peixoto, N. A portable wireless biometric multi-channel system. Measurement 2012, 45, 1587–1598. [Google Scholar] [CrossRef]
- Carmo, J.P.; Correia, J.H. RF CMOS transceiver at 2.4 GHz in wearables for measuring the cardio-respiratory function. Measurement 2011, 44, 65–73. [Google Scholar] [CrossRef]
- Waller, M.; Stotler, C. Telemedicine: A Primer. Curr. Allergy Asthma Rep. 2018, 18, 54. [Google Scholar] [CrossRef]
- Belardinelli, A.; Muratori, L.; Corazza, I.; Magnalardo, M.; Marangoni, F.; Zannoli, R. Multi-functional device for cardiologic telemedicine and diagnostic holter Multi-Functional Device for Cardiologic Telemedicine and Diagnostic Holter. Comput. Cardiol. 2008, 985–987. [Google Scholar]
- Passler, M. Senner In-Ear Pulse Rate Measurement: A Valid Alternative to Heart Rate Derived from Electrocardiography? Sensors 2019, 19, 3641. [Google Scholar] [CrossRef] [PubMed]
- Fereniec, M.; Stix, G.; Kania, M.; Mroczka, T.; Janusek, D.; Maniewski, R. Risk assessment of ventricular arrhythmia using new parameters based on high resolution body surface potential mapping. Med. Sci. Monit. 2011, 17, 20. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Imam, M.H.; Karmakar, C.K.; Khandoker, A.H.; Palaniswami, M. Effect of ECG-derived respiration (EDR) on modeling ventricular repolarization dynamics in different physiological and psychological conditions. Med. Biol. Eng. Comput. 2014, 52, 851–860. [Google Scholar] [CrossRef]
- Lacko, A.; Hruboň, A.; Bestvina, D.; Mokáň, M. Diagnostika kardiálnej autonómnej dysfunkcie. Súčasná Klinická Prax. 2005, 3, 19–24. [Google Scholar]
- Siecinski, S.; Tkacz, E.J.; Kostka, P.S. Comparison of HRV indices obtained from ECG and SCG signals from CEBS database. Biomed. Eng. Online 2019, 18, 69. [Google Scholar] [CrossRef]
- Thakur, R.K.; Anoop, C.S. A non-contact capacitance based electrocardiograph and associated heart-rate detection using enhanced Fourier interpolation method. In Proceedings of the Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Milan, Italy, 25–29 August 2015; Volume 2015, pp. 849–852. [Google Scholar]
- Horton, J.F.; Stergiou, P.; Fung, T.S.; Katz, L. Comparison of Polar M600 Optical Heart Rate and ECG Heart Rate during Exercise. Med. Sci. Sports Exerc. 2017, 49, 2600–2607. [Google Scholar] [CrossRef]
- Her, L.; Care, I.; Her, E.L.; Guyen, Q.T.N.; Pateau, V.; Bodenes, L.; Lellouche, F. Photoplethysmographic determination of the respiratory rate in acutely ill patients: Validation of a new algorithm and implementation into a biomedical device. Ann. Intensive Care 2019, 9, 11. [Google Scholar]
- Li, K.H.C.; White, F.A.; Tipoe, T.; Liu, T.; Wong, M.C.S.; Jesuthasan, A.; Baranchuk, A.; Tse, G.; Yan, B.P. The current state of mobile phone apps for monitoring heart rate, heart rate variability, and atrial fibrillation: Narrative review. J. Med. Internet Res. 2019, 21, 1–23. [Google Scholar] [CrossRef]
- Tadi, M.J.; Lehtonen, E.; Hurnanen, T.; Koskinen, J.; Eriksson, J.; Pänkälä, M.; Teräs, M.; Koivisto, T. A real-time approach for heart rate monitoring using a Hilbert transform in seismocardiograms. Physiol. Meas. 2016, 37, 1885–1909. [Google Scholar] [CrossRef]
- Tadi, M.J.; Lehtonen, E.; Koivisto, T.; Pankaala, M.; Paasio, A.; Teras, M. Seismocardiography: Toward heart rate variability (HRV) estimation. In Proceedings of the 2015 IEEE International Symposium on Medical Measurements and Applications (MeMeA) Proceedings, Torino, Italy, 7–9 May 2015; pp. 261–266. [Google Scholar]
- Taebi, A.; Solar, B.; Bomar, A.; Sandler, R.; Mansy, H. Recent Advances in Seismocardiography. Vibration 2019, 2, 64–86. [Google Scholar] [CrossRef]
- Lee, H.; Lee, H.; Whang, M. An Enhanced Method to Estimate Heart Rate from Seismocardiography via Ensemble Averaging of Body Movements at Six Degrees of Freedom. Sensors 2018, 18, 238. [Google Scholar] [CrossRef] [PubMed]
- D’Mello, Y.; Skoric, J.; Xu, S.; Roche, P.J.; Lortie, M.; Gagnon, S.; Plant, D.V. Real-Time Cardiac Beat Detection and Heart Rate Monitoring from Combined Seismocardiography and Gyrocardiography. Sensors 2019, 19, 3472. [Google Scholar] [CrossRef] [PubMed]
- Mukhopadhyay, S.C. Wearable Sensors for Human Activity Monitoring: A Review. IEEE Sens. J. 2015, 15, 1321–1330. [Google Scholar] [CrossRef]
- Drachen, A.; Nacke, L.E.; Yannakakis, G.; Pedersen, A.L. Correlation between heart rate, electrodermal activity and player experience in first-person shooter games. In Sandbox ′10: Proceedings of the 5th ACM SIGGRAPH Symposium on Video Games; ACM Press: New York, NY, USA, 2010; pp. 49–54. [Google Scholar]
- Batista, D.; da Silva, H.P.; Fred, A.; Moreira, C.; Reis, M.; Ferreira, H.A. Benchmarking of the BITalino biomedical toolkit against an established gold standard. Healthc. Technol. Lett. 2019, 6, 32–36. [Google Scholar] [CrossRef]
- Fujita, H.; Takeuchi, I.T.; Ohe, K. A novel cloud-based mobile 12-lead ECG significantly improved onset-to-balloon time for STEMI patients. Eur. Heart J. 2013, 34, 2771. [Google Scholar] [CrossRef][Green Version]
- Yufu, K.; Shimomura, T.; Fujinami, M.; Nakashima, T.; Saito, S.; Ayabe, R.; Kawano, K.; Ishii, Y.; Okada, N.; Akioka, H.; et al. Impact of Mobile Cloud Electrocardiography System on Door-to-Balloon Time in Patients with Acute Coronary Syndrome in Oita Prefecture. Circ. Rep. 2019, 1, 241–247. [Google Scholar] [CrossRef]
- Frigy, A.; Varga, I.; Fogarasi, Z.; Belényi, B.; Kocsis, I. The Influence of Sleep Apnea on 24-Hour and Nocturnal ECG and Blood Pressure Parameters in Patients with Acute Heart Failure. Med. Princ. Pract. 2019, 28, 150–157. [Google Scholar] [CrossRef]
- Shakhmatova, K.; Zalzman, A.; Antropova, O.; Osipova, I.; Lobanova, N. Electric heart instability in men of stress professions with arterial hypertension. J. Hypertens. 2011, 29, e534. [Google Scholar] [CrossRef]
- O’Brien, E.; Dolan, E. Ambulatory Blood Pressure Measurement in the Elderly. Hypertension 2019, 73, 961–964. [Google Scholar] [CrossRef]
- Wu, W.H.; Bui, A.A.T.; Batalin, M.A.; Au, L.K.; Binney, J.D.; Kaiser, W.J. MEDIC: Medical embedded device for individualized care. Artif. Intell. Med. 2008, 42, 137–152. [Google Scholar] [CrossRef]
- Alemdar, H.; Ersoy, C. Wireless sensor networks for healthcare: A survey. Comput. Netw. 2010, 54, 2688–2710. [Google Scholar] [CrossRef]
- Hamida, S.; Hamida, E.; Ahmed, B. A New mHealth Communication Framework for Use in Wearable WBANs and Mobile Technologies. Sensors 2015, 15, 3379–3408. [Google Scholar] [CrossRef]
- Mond, H.G. The Spectrum of Ambulatory Electrocardiographic Monitoring. Hear. Lung Circ. 2017, 26, 1–15. [Google Scholar] [CrossRef]
- Sanders, D.; Ungar, L.; Eskander, M.A.; Seto, A.H. Ambulatory ECG monitoring in the age of smartphones. Clevel. Clin. J. Med. 2019, 86, 483–493. [Google Scholar] [CrossRef]
- Deserno, T.M.; Marx, N. Computational electrocardiography: Revisiting Holter ECG monitoring. Methods Inf. Med. 2016, 55, 305–311. [Google Scholar]
- Vavrinsky, E.; Daricek, M.; Moskalova, D.; Horinek, F.; Donoval, M. Design of Very Precise and Miniature Low Power ECG Holter. In Proceedings of the 41st International Congress on Electrocardiology, Bratislava, Slovakia, 4–7 June 2014; pp. 257–260. [Google Scholar]
- Reinvuo, T.; Hannula, M.; Sorvoja, H.; Alasaarela, E.; Myllylä, R. Measurement of respiratory rate with high-resolution accelerometer and EMFit pressure sensor. In Proceedings of the 2006 IEEE Sensors Applications Symposium, Houston, TX, USA, 7–9 February 2006; pp. 192–195. [Google Scholar]
- Brady, S.; Dunne, L.E.; Tynan, R.; Diamond, D.; Smyth, B.; O’Hare, G.M.P. Garment-based monitoring of respiration rate using a foam pressure sensor. In Proceedings of the International Symposium on Wearable Computers (ISWC), Osaka, Japan, 18–21 October 2005; Volume 2005, pp. 214–215. [Google Scholar]
- Widjaja, D.; Taelman, J.; Vandeput, S.; Braeken, M.A.; Otte, R.A.; Van Den Bergh, B.R.; Van Huffel, S. ECG-Derived Respiration: Comparison and New Measures for Respiratory Variability. Comput. Cardiol. 2010, 37, 149–152. [Google Scholar]
- Chan, H.-L.; Lin, S.-H.; Wang, F.-T.; Hsu, W.-Y.; Wang, C.-L. ECG-derived respirations based on phase-space reconstruction of single-lead ECG: Validations over various physical activities based on parallel recordings of ECG, respiration, and body accelerations. In Proceedings of the 2014 36th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Chicago, IL, USA, 26–30 August 2014; Volume 2014, pp. 2282–2285. [Google Scholar]
- Ansari, S.; Ward, K.R.; Najarian, K. Motion Artifact Suppression in Impedance Pneumography Signal for Portable Monitoring of Respiration: An Adaptive Approach. IEEE J. Biomed. Heal. Inform. 2017, 21, 387–398. [Google Scholar] [CrossRef]
- Wang, F.-T.; Chan, H.-L.; Wang, C.-L.; Jian, H.-M.; Lin, S.-H. Instantaneous Respiratory Estimation from Thoracic Impedance by Empirical Mode Decomposition. Sensors 2015, 15, 16372–16387. [Google Scholar] [CrossRef]
- McGrath, T.; Fineman, R.; Stirling, L. An Auto-Calibrating Knee Flexion-Extension Axis Estimator Using Principal Component Analysis with Inertial Sensors. Sensors 2018, 18, 1882. [Google Scholar] [CrossRef]
- Du, S.; Sun, W.; Gao, Y. Improving Observability of an Inertial System by Rotary Motions of an IMU. Sensors 2017, 17, 698. [Google Scholar] [CrossRef]
- Xiao, Y.; Ruan, X.; Chai, J.; Zhang, X.; Zhu, X. Online IMU Self-Calibration for Visual-Inertial Systems. Sensors 2019, 19, 1624. [Google Scholar] [CrossRef]
- Zimmermann, T.; Taetz, B.; Bleser, G. IMU-to-Segment Assignment and Orientation Alignment for the Lower Body Using Deep Learning. Sensors 2018, 18, 302. [Google Scholar] [CrossRef]
- Niu, X.; Wang, Q.; Li, Y.; Zhang, Q.; Jiang, P. An IMU Evaluation Method Using a Signal Grafting Scheme. Sensors 2016, 16, 854. [Google Scholar] [CrossRef]
- Vargas-Valencia, L.; Elias, A.; Rocon, E.; Bastos-Filho, T.; Frizera, A. An IMU-to-Body Alignment Method Applied to Human Gait Analysis. Sensors 2016, 16, 2090. [Google Scholar] [CrossRef]
- Rahim, K.N.K.A.; Elamvazuthi, I.; Izhar, L.; Capi, G. Classification of Human Daily Activities Using Ensemble Methods Based on Smartphone Inertial Sensors. Sensors 2018, 18, 4132. [Google Scholar] [CrossRef]
- Vavrinský, E.; Moskal’vá, D.; Darříček, M.; Donoval, M.; Horínek, F.; Popovič, M.; Miklovič, P. Application of Acceleration Sensors in Physiological Experiments. J. Electr. Eng. 2014, 65, 304–308. [Google Scholar] [CrossRef][Green Version]
- Robertson, W.; Stewart-Brown, S.; Wilcock, E.; Oldfield, M.; Thorogood, M. Utility of accelerometers to measure physical activity in children attending an obesity treatment intervention. J. Obes. 2011, 2011, 1–8. [Google Scholar] [CrossRef]
- Elmesmari, R.; Martin, A.; Reilly, J.J.; Paton, J.Y. Comparison of accelerometer measured levels of physical activity and sedentary time between obese and non-obese children and adolescents: A systematic review. BMC Pediatr. 2018, 18, 106. [Google Scholar] [CrossRef]
- Manchanda, S.; Ehsanullah, M. Suspected cardiac syncope in elderly patients: Use of the 12-lead electrocardiogram to select patients for Holter monitoring. Gerontology 2001, 47, 195–197. [Google Scholar] [CrossRef]
- Gokalp, H.; Clarke, M. Monitoring activities of daily living of the elderly and the potential for its use in telecare and telehealth: A review. Telemed. e-Health 2013, 19, 910–923. [Google Scholar] [CrossRef]
- Pandia, K.; Inan, O.T.; Kovacs, G.T.A.; Giovangrandi, L. Extracting respiratory information from seismocardiogram signals acquired on the chest using a miniature accelerometer. Physiol. Meas. 2012, 33, 1643–1660. [Google Scholar] [CrossRef]
- Han, D.K.; Hong, J.H.; Shin, J.Y.; Lee, T.S. Accelerometer based motion noise analysis of ECG signal. In Proceedings of the World Congress on Medical Physics and Biomedical Engineering, Munich, Germany, 7–12 September 2009; pp. 198–201. [Google Scholar]
- Marcelli, E.; Capucci, A.; Minardi, G.; Cercenelli, L. Multi-Sense CardioPatch. ASAIO J. 2017, 63, 73–79. [Google Scholar] [CrossRef]
- Yu, S.; Liu, S. A Novel Adaptive Recursive Least Squares Filter to Remove the Motion Artifact in Seismocardiography. Sensors 2020, 20, 1596. [Google Scholar] [CrossRef]
- Taebi, A.; Mansy, H.A. Time-frequency distribution of seismocardiographic signals: A comparative study. Bioengineering 2017, 4, 32. [Google Scholar] [CrossRef]
- Luu, L.; Dinh, A. Artifact Noise Removal Techniques on Seismocardiogram Using Two Tri-Axial Accelerometers. Sensors 2018, 18, 1067. [Google Scholar]
- Mora, N.; Cocconcelli, F.; Matrella, G.; Ciampolini, P. Detection and Analysis of Heartbeats in Seismocardiogram Signals. Sensors 2020, 20, 1670. [Google Scholar] [CrossRef]
- Sahoo, P.; Thakkar, H.; Lin, W.-Y.; Chang, P.-C.; Lee, M.-Y. On the Design of an Efficient Cardiac Health Monitoring System through Combined Analysis of ECG and SCG Signals. Sensors 2018, 18, 379. [Google Scholar] [CrossRef]
- Sahoo, P.; Thakkar, H.; Lee, M.-Y. A Cardiac Early Warning System with Multi Channel SCG and ECG Monitoring for Mobile Health. Sensors 2017, 17, 711. [Google Scholar] [CrossRef]
- Lee, K.H.; Ni, X.; Lee, J.Y.; Arafa, H.; Pe, D.J.; Xu, S.; Avila, R.; Irie, M.; Lee, J.H.; Easterlin, R.L.; et al. Mechano-acoustic sensing of physiological processes and body motions via a soft wireless device placed at the suprasternal notch. Nat. Biomed. Eng. 2020, 4, 148–158. [Google Scholar] [CrossRef]
- Kimoto, A.; Yamada, Y. A new layered sensor for simultaneous measurement of EMG, MMG and oxygen consumption at the same position. Med. Biol. Eng. Comput. 2015, 53, 15–22. [Google Scholar] [CrossRef] [PubMed]
- González-Izal, M.; Malanda, A.; Navarro-Amézqueta, I.; Gorostiaga, E.M.; Mallor, F.; Ibañez, J.; Izquierdo, M. EMG spectral indices and muscle power fatigue during dynamic contractions. J. Electromyogr. Kinesiol. 2010, 20, 233–240. [Google Scholar] [CrossRef] [PubMed]
- Cifrek, M.; Medved, V.; Tonković, S.; Ostojić, S. Surface EMG based muscle fatigue evaluation in biomechanics. Clin. Biomech. 2009, 24, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Konrad, P. The ABC of EMG; Noraxon Inc.: Scottsdale, AZ, USA, 2006; ISBN 0977162214. [Google Scholar]
- De Luca, C.J. The use of surface electromyography in biomechanics. J. Appl. Biomech. 1997, 13, 135–163. [Google Scholar] [CrossRef]
- De Luca, C.J. Electromyography. In Encyclopedia of Medical Devices and Instrumentation; Webster, J.G., Ed.; John Wiley Publisher: Hoboken, NJ, USA, 2006; pp. 98–109. [Google Scholar]
- Zhang, X.; Li, X.; Samuel, O.W.; Huang, Z.; Fang, P.; Li, G. Improving the robustness of electromyogram-pattern recognition for prosthetic control by a postprocessing strategy. Front. Neurorobotics 2017, 11, 1–15. [Google Scholar] [CrossRef]
- Lee, S.; Yoon, J.; Lee, D.; Seong, D.; Lee, S.; Jang, M.; Choi, J.; Yu, K.J.; Kim, J.; Lee, S.; et al. Wireless Epidermal Electromyogram Sensing System. Electronics 2020, 9, 269. [Google Scholar] [CrossRef]
- Pylatiuk, C.; Muller-Riederer, M.; Kargov, A.; Schulz, S.; Schill, O.; Reischl, M.; Bretthauer, G. Comparison of surface EMG monitoring electrodes for long-term use in rehabilitation device control. In Proceedings of the 2009 IEEE International Conference on Rehabilitation Robotics, Kyoto, Japan, 23–26 June 2009; pp. 300–304. [Google Scholar]
- Zhang, H.; Tian, L.; Zhang, L.; Li, G. Using textile electrode EMG for prosthetic movement identification in transradial amputees. In Proceedings of the 2013 IEEE International Conference on Body Sensor Networks, Cambridge, MA, USA, 6–9 May 2013; pp. 1–5. [Google Scholar]
- Islam, M.A.; Sundaraj, K.; Ahmad, R.B.; Ahamed, N.U.; Ali, M.A. Mechanomyography Sensor Development, Related Signal Processing, and Applications: A Systematic Review. IEEE Sens. J. 2013, 13, 2499–2516. [Google Scholar] [CrossRef]
- Uchiyama, T.; Hashimoto, E. System identification of the mechanomyogram from single motor units during voluntary isometric contraction. Med. Biol. Eng. Comput. 2011, 49, 1035–1043. [Google Scholar] [CrossRef]
- Han, H.; Jo, S.; Kim, J. Comparative study of a muscle stiffness sensor and electromyography and mechanomyography under fatigue conditions. Med. Biol. Eng. Comput. 2015, 53, 577–588. [Google Scholar] [CrossRef]
- Casaccia, S.; Scalise, L.; Casacanditella, L.; Tomasini, E.P.; Rohrbaugh, J.W. Non-contact assessment of muscle contraction: Laser Doppler Myography. In Proceedings of the 2015 IEEE International Symposium on Medical Measurements and Applications (MeMeA) Proceedings, Torino, Italy, 7–9 May 2015; pp. 610–615. [Google Scholar]
- Al-Mulla, M.; Sepulveda, F. Novel Pseudo-Wavelet Function for MMG Signal Extraction during Dynamic Fatiguing Contractions. Sensors 2014, 14, 9489–9504. [Google Scholar] [CrossRef]
- Hill, E.C.; Housh, T.J.; Smith, C.M.; Cochrane, K.C.; Jenkins, N.D.M.; Cramer, J.T.; Schmidt, R.J.; Johnson, G.O. Effect of sex on torque, recovery, EMG, and MMG responses to fatigue. J. Musculoskelet. Neuronal Interact. 2016, 16, 310–317. [Google Scholar] [PubMed]
- Bilgin, G.; Hindistan, E.; Ozkaya, Y.G.; Koklukaya, E.; Polat, O.; Colak, O.H. Determination of Fatigue Following Maximal Loaded Treadmill Exercise by Using Wavelet Packet Transform Analysis and MLPNN from MMG-EMG Data Combinations. J. Med. Syst. 2015, 39, 108. [Google Scholar] [CrossRef] [PubMed]
- Woodward, R.B.; Stokes, M.J.; Shefelbine, S.J.; Vaidyanathan, R. Segmenting Mechanomyography Measures of Muscle Activity Phases Using Inertial Data. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Orizio, C.; Gobbo, M.; Diemont, B.; Esposito, F.; Veicsteinas, A. The surface mechanomyogram as a tool to describe the influence of fatigue on biceps brachii motor unit activation strategy. Historical basis and novel evidence. Eur. J. Appl. Physiol. 2003, 90, 326–336. [Google Scholar] [CrossRef] [PubMed]
- Cè, E.; Rampichini, S.; Monti, E.; Venturelli, M.; Limonta, E.; Esposito, F. Changes in the electromechanical delay components during a fatiguing stimulation in human skeletal muscle: An EMG, MMG and force combined approach. Eur. J. Appl. Physiol. 2017, 117, 95–107. [Google Scholar] [CrossRef]
- Smith, C.M.; Housh, T.J.; Hill, E.C.; Johnson, G.O.; Schmidt, R.J. Changes in electromechanical delay during fatiguing dynamic muscle actions. Muscle Nerve 2017, 56, 315–320. [Google Scholar] [CrossRef]
- Sanchez, B.; Rutkove, S.B. Present Uses, Future Applications, and Technical Underpinnings of Electrical Impedance Myography. Curr. Neurol. Neurosci. Rep. 2017, 17, 86. [Google Scholar] [CrossRef]
- Li, X.; Shin, H.; Li, L.; Magat, E.; Li, S.; Zhou, P. Assessing the immediate impact of botulinum toxin injection on impedance of spastic muscle. Med. Eng. Phys. 2017, 43, 97–102. [Google Scholar] [CrossRef][Green Version]
- Ma, C.Z.-H.; Ling, Y.T.; Shea, Q.T.K.; Wang, L.-K.; Wang, X.-Y.; Zheng, Y.-P. Towards Wearable Comprehensive Capture and Analysis of Skeletal Muscle Activity during Human Locomotion. Sensors 2019, 19, 195. [Google Scholar] [CrossRef]
- Vavrinsky, E.; Svobodova, H.; Donoval, M.; Daricek, M.; Kopani, M.; Miklovic, P.; Horinek, F.; Telek, P. Application of single wireless holter to simultaneous EMG, MMG and EIM measurement of human muscles activity. Lek. Tech. 2018, 48, 52–58. [Google Scholar]
- Samulski, B.; Prebor, J.; Armitano, C.; Morrison, S. Coupling of motor oscillators—What really happens when you chew gum and walk? Neurosci. Lett. 2019, 698, 90–96. [Google Scholar] [CrossRef]
- Harmon, K.K.; Girts, R.M.; Maclennan, R.J.; Stock, M.S. Is the motor unit mean firing rate versus recruitment threshold relationship linear? Physiol. Meas. 2019, 40, 095002. [Google Scholar] [CrossRef] [PubMed]
- Miller, J.D.; Marissa, C.J.L.; Kyle, D.G.; Mandy, L.S.; Trent, E.W. The effect of rate of torque development on motor unit recruitment and firing rates during isometric voluntary trapezoidal contractions. Exp. Brain Res. 2019, 237, 2653–2664. [Google Scholar] [CrossRef] [PubMed]
- Hsu, W.-C.; Chang, C.-C.; Lin, Y.-J.; Yang, F.-C.; Lin, L.-F.; Chou, K.-N. The Use of Wearable Sensors for the Movement Assessment on Muscle Contraction Sequences in Post-Stroke Patients during Sit-to-Stand. Sensors 2019, 19, 657. [Google Scholar] [CrossRef] [PubMed]
- Kordi, M.; Folland, J.; Goodall, S.; Barratt, P.; Howatson, G. Reliability of traditional and task specific reference tasks to assess peak muscle activation during two different sprint cycling tests. J. Electromyogr. Kinesiol. 2019, 46, 41–48. [Google Scholar] [CrossRef]
- Tian, L.; Zimmerman, B.; Akhtar, A.; Yu, K.J.; Moore, M.; Wu, J.; Larsen, R.J.; Lee, J.W.; Li, J.; Liu, Y.; et al. Large-area MRI-compatible epidermal electronic interfaces for prosthetic control and cognitive monitoring. Nat. Biomed. Eng. 2019, 3, 194–205. [Google Scholar] [CrossRef]
- Posada-Quintero, H.F.; Chon, K.H. Innovations in Electrodermal Activity Data Collection and Signal Processing: A Systematic Review. Sensors 2020, 20, 479. [Google Scholar] [CrossRef]
- Onorati, F.; Regalia, G.; Gaborni, C.; Picard, R. Improvement of a convulsive seizure detector relying on accelerometer and electrodermal activity collected continuously by a wristband. In Proceedings of the 2016 Epilepsy Pipeline Conference, San Francisco, CA, USA, 26 February 2016. [Google Scholar]
- Regalia, G.; Onorati, F.; Migliorini, M.; Picard, R.W. An improved wrist-worn convulsive seizure detector based on accelerometry and electrodermal activity sensors. Am. Epilepsy Soc. Annu. Meet. 2015, 2015, 1. [Google Scholar]
- Poh, M.Z.; Loddenkemper, T.; Reinsberger, C.; Swenson, N.C.; Goyal, S.; Sabtala, M.C.; Madsen, J.R.; Picard, R.W. Convulsive seizure detection using a wrist-worn electrodermal activity and accelerometry biosensor. Epilepsia 2012, 53, 93–97. [Google Scholar] [CrossRef]
- Caborni, C.; Regalia, G.; Onorati, F.; Picard, R.W. Clinical evaluation of the Embrace smart watch detection capability of generalized tonic-clonic seizures recorded at the ankles. In Proceedings of the American Epilepsy Society annual meeting, Houston, TX, USA, 30 November–4 December 2018. [Google Scholar]
- Zheng, D.; Chernyshov, G.; Kunze, K. Electrodermal activity sensing using smart eyewear. In Proceedings of the 2019 ACM International Joint Conference on Pervasive and Ubiquitous Computing and Proceedings of the 2019 ACM International Symposium on Wearable Computers—UbiComp/ISWC ′19, London, UK, 16 September 2019; pp. 653–656. [Google Scholar]
- Carroll, E.A.; Czerwinski, M.; Roseway, A.; Kapoor, A.; Johns, P.; Rowan, K.; Schraefel, M.C. Food and mood: Just-in-time support for emotional eating. In Proceedings of the 2013 Humaine Association Conference on Affective Computing and Intelligent Interaction, Geneva, Switzerland, 2–5 September 2013; pp. 252–257. [Google Scholar]
- Vavrinsky, E.; Stopjakova, V.; Majer, L.; Tvarozek, V.; Weis, M.; Marman, P. Monitoring of Psychosomatic Properties of Human Body by Skin Conductivity Measurements using Thin Film Microelectrode Arrays. In Proceedings of the 2006 International Conference on Advanced Semiconductor Devices and Microsystems, Smolenice, Slovakia, 16–18 October 2006; pp. 275–278. [Google Scholar]
- Aldosky, H.Y.Y.; Bari, D.S. Electrodermal Activity: Simultaneous Recordings. In Electrochemical Impedance Spectroscopy; IntechOpen: London, UK, 2019; Volume 395, pp. 116–124. [Google Scholar]
- Malmivuo, J.; Plonsey, R. The Electrodermal Response. In Biomagnetism. Principles and Applications of Bioelectric and Biomagnetic Fields; Oxford University Press: Oxford, UK, 1995. [Google Scholar]
- Vavrinsky, E.; Stopjakova, V.; Donoval, M.; Daricek, M.; Svobodova, H.; Mihalov, J.; Hanic, M.; Tvarozek, V. Design of sensor systems for long time electrodermal activity monitoring. Adv. Electr. Electron. Eng. 2017, 15, 184–191. [Google Scholar] [CrossRef]
- Tannheimer, M. The Use of Pulse Oximetry at High Altitude. Res. Inves. Sports. Med. 2020, 6, 10–13. [Google Scholar]
- Mannheimer, P.D. The light-tissue interaction of pulse oximetry. Anesth. Analg. 2007, 105, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Nitzan, M.; Romem, A.; Koppel, R. Pulse oximetry: Fundamentals and technology update. Med. Devices Evid. Res. 2014, 7, 231–239. [Google Scholar] [CrossRef]
- May, J.M.; Phillips, J.P.; Fitchat, T.; Ramaswamy, S.; Snidvongs, S.; Kyriacou, P.A. A novel photoplethysmography sensor for vital signs monitoring from the human trachea. Biosensors 2019, 9, 119. [Google Scholar] [CrossRef] [PubMed]
- Kumar, S.; Buckley, J.L.; Barton, J.; Pigeon, M.; Newberry, R.; Rodencal, M.; Hajzeraj, A.; Hannon, T.; Rogers, K.; Casey, D.; et al. A Wristwatch-Based Wireless Sensor Platform for IoT Health Monitoring Applications. Sensors 2020, 20, 1675. [Google Scholar] [CrossRef] [PubMed]
- Elsamnah, F.; Bilgaiyan, A.; Affiq, M.; Shim, C.H.; Ishidai, H.; Hattori, R. Reflectance-based organic pulse meter sensor for wireless monitoring of photoplethysmogram signal. Biosensors 2019, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Tedesco, S.; Sica, M.; Ancillao, A.; Timmons, S.; Barton, J.; O’Flynn, B. Accuracy of consumer-level and research-grade activity trackers in ambulatory settings in older adults. PLoS ONE 2019, 14, 1–17. [Google Scholar] [CrossRef]
- GARMIN. Foreruner 945 Owner’s Manual. Pulse Oximeter. Available online: https://www8.garmin.com/manuals/webhelp/forerunner945/EN-US/GUID-4D425925-D4EE-4C26-B974-5375D0670860.html (accessed on 27 March 2020).
- Husain, I.M. How Apple Watch’s Ability to Measure Blood Oxygen Saturation Can Be Used in Medicine. Available online: https://www.imedicalapps.com/2015/04/how-apple-watchs-ability-to-measure-blood-oxygen-saturation-can-be-used-in-medicine/ (accessed on 26 March 2020).
- König, V.; Huch, R.; Huch, A. Reflectance pulse oximetry—Principles and obstetric application in the Zurich system. J. Clin. Monit. Comput. 1998, 14, 403–412. [Google Scholar] [CrossRef]
- Jørgensen, J.S.; Schmid, E.R.; König, V.; Faisst, K.; Huch, A.; Huch, R. Limitations of forehead pulse oximetry. J. Clin. Monit. 1995, 11, 253–256. [Google Scholar] [CrossRef]
- Mendelson, Y.; Ochs, B.D. Noninvasive Pulse Oximetry Utilizing Skin Reflectance Photoplethysmography. IEEE Trans. Biomed. Eng. 1988, 35, 798–805. [Google Scholar] [CrossRef]
- Lee, H.; Ko, H.; Lee, J. Reflectance pulse oximetry: Practical issues and limitations. ICT Express 2016, 2, 195–198. [Google Scholar] [CrossRef]
- Wójcikowski, M.; Pankiewicz, B. Photoplethysmographic Time-Domain Heart Rate Measurement Algorithm for Resource-Constrained Wearable Devices and its Implementation. Sensors 2020, 20, 1783. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.; Kim, M.; Park, H.-K.; Kim, I.Y. Motion Artifact Reduction in Wearable Photoplethysmography Based on Multi-Channel Sensors with Multiple Wavelengths. Sensors 2020, 20, 1493. [Google Scholar] [CrossRef] [PubMed]
- Perpetuini, D.; Chiarelli, A.M.; Vinciguerra, V.; Vitulli, P.; Rinella, S.; Cardone, D.; Bianco, F.; Perciavalle, V.; Gallina, S.; Fallica, G.; et al. Integrated Multi-channel PPG and ECG System for Cardiovascular Risk Assessment. Proceedings 2019, 27, 8. [Google Scholar] [CrossRef]
- Georgieva-Tsaneva, G.; Gospodinova, E.; Gospodinov, M.; Cheshmedzhiev, K. Portable Sensor System for Registration, Processing and Mathematical Analysis of PPG Signals. Appl. Sci. 2020, 10, 1051. [Google Scholar] [CrossRef]
- Millán, C.A.; Girón, N.A.; Lopez, D.M. Analysis of relevant features from photoplethysmographic signals for atrial fibrillation classification. Int. J. Environ. Res. Public Health 2020, 17, 498. [Google Scholar] [CrossRef]
- Rundo, F.; Petralia, S.; Fallica, G.; Conoci, S. A Nonlinear Pattern Recognition Pipeline for PPG/ECG Medical Assessments. In Convegno Nazionale Sensori; Andò, B., Baldini, F., Di Natale, C., Ferrari, V., Marletta, V., Marrazza, G., Eds.; Springer International Publishing: Cham, Switzerland, 2019; pp. 473–480. [Google Scholar]
- Jagelka, M.; Jeleň, M.; Vavrinský, E.; Daříček, M.; Donoval, M. Implementation of pulse oximetry measurement to wireless biosignals probe. Lek. Tech. 2014, 44, 37–40. [Google Scholar]
- Hosanee, M.; Chan, G.; Welykholowa, K.; Cooper, R.; Kyriacou, P.A.; Zheng, D.; Allen, J.; Abbott, D.; Menon, C.; Lovell, N.H.; et al. Cuffless Single-Site Photoplethysmography for Blood Pressure Monitoring. J. Clin. Med. 2020, 9, 723. [Google Scholar] [CrossRef]
- He, K.; Zhang, X.; Ren, S.; Sun, J. Deep residual learning for image recognition. Proc. IEEE Comput. Soc. Conf. Comput. Vis. Pattern Recognit. 2016, 2016, 770–778. [Google Scholar]
- Hardoon, D.R.; Szedmak, S.; Shawe-Taylor, J. Canonical correlation analysis: An overview with application to learning methods. Neural Comput. 2004, 16, 2639–2664. [Google Scholar] [CrossRef]
- Rundo, F.; Ortis, A.; Battiato, S.; Conoci, S. Advanced bio-inspired system for noninvasive cuff-less blood pressure estimation from physiological signal analysis. Computation 2018, 6, 46. [Google Scholar] [CrossRef]
- Liang, Y.; Chen, Z.; Ward, R.; Elgendi, M. Photoplethysmography and deep learning: Enhancing hypertension risk stratification. Biosensors 2018, 8, 101. [Google Scholar] [CrossRef] [PubMed]
- Slapničar, G.; Mlakar, N.; Luštrek, M. Blood Pressure Estimation from Photoplethysmogram Using a Spectro-Temporal Deep Neural Network. Sensors 2019, 19, 3420. [Google Scholar] [CrossRef]
- Welykholowa, K.; Hosanee, M.; Chan, G.; Cooper, R.; Kyriacou, P.A.; Zheng, D.; Allen, J.; Abbott, D.; Menon, C.; Lovell, N.H.; et al. Multimodal Photoplethysmography-Based Approaches for Improved Detection of Hypertension. J. Clin. Med. 2020, 9, 1203. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wen, C.; Tao, G.; Bi, M.; Li, G. Continuous and noninvasive blood pressure measurement: A novel modeling methodology of the relationship between blood pressure and pulse wave velocity. Ann. Biomed. Eng. 2009, 37, 2222–2233. [Google Scholar] [CrossRef] [PubMed]
- Padilla, J.M.; Berjano, E.J.; Sáiz, J.; Fácila, L.; Díaz, P.; Mercé, S. Assessment of relationships between blood pressure, pulse wave velocity and digital volume pulse. Comput. Cardiol. 2006, 33, 893–896. [Google Scholar]
- Yoon, Y.; Cho, J.H.; Yoon, G. Non-constrained blood pressure monitoring using ECG and PPG for personal healthcare. J. Med. Syst. 2009, 33, 261–266. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Fang, X.; Chen, Q.; Li, Y.; Li, T. Reliability analysis of an integrated device of ECG, PPG and pressure pulse wave for cardiovascular disease. Microelectron. Reliab. 2018, 87, 183–187. [Google Scholar] [CrossRef]
- Shin, W.; Cha, Y.D.; Yoon, G. ECG/PPG integer signal processing for a ubiquitous health monitoring system. J. Med. Syst. 2010, 34, 891–898. [Google Scholar] [CrossRef]
- Jeon, G.-R.; Jung, D.-K.; Kim, G.-R.; Shin, B.-J. The Development of Integrated Sensor System for Measuring Simultaneously ECG, PPG and PPW. J. Korea Acad. Coop. Soc. 2009, 10, 992–999. [Google Scholar]
- GlutracTM. Available online: https://www.add-care.net/ (accessed on 26 March 2020).
- Rundo, F.; Conoci, S.; Ortis, A.; Battiato, S. An Advanced Bio-Inspired PhotoPlethysmoGraphy (PPG) and ECG Pattern Recognition System for Medical Assessment. Sensors 2018, 18, 405. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.-H.; Liu, L.-J.; Pan, K.-L.; Chen, W.; Tan, T.-H. Using the Characteristics of Pulse Waveform to Enhance the Accuracy of Blood Pressure Measurement by a Multi-Dimension Regression Model. Appl. Sci. 2019, 9, 2922. [Google Scholar] [CrossRef]
- Di Rienzo, M.; Rizzo, G.; Işilay, Z.M.; Lombardi, P. SeisMote: A Multi-Sensor Wireless Platform for Cardiovascular Monitoring in Laboratory, Daily Life, and Telemedicine. Sensors 2020, 20, 680. [Google Scholar] [CrossRef] [PubMed]
- Lazazzera, R.; Belhaj, Y.; Carrault, G. A New Wearable Device for Blood Pressure Estimation Using Photoplethysmogram. Sensors 2019, 19, 2557. [Google Scholar] [CrossRef]
- OMRON HeartGuideTM. Available online: https://omronhealthcare.com/products/heartguide-wearable-blood-pressure-monitor-bp8000m/ (accessed on 26 March 2020).
- Niknejad, N.; Ismail, W.B.; Mardani, A.; Liao, H.; Ghani, I. A comprehensive overview of smart wearables: The state of the art literature, recent advances, and future challenges. Eng. Appl. Artif. Intell. 2020, 90, 103529. [Google Scholar] [CrossRef]
- Silva, M.C.; Amorim, V.J.P.; Ribeiro, S.P.; Oliveira, R.A.R. Field Research Cooperative Wearable Systems: Challenges in Requirements, Design and Validation. Sensors 2019, 19, 4417. [Google Scholar] [CrossRef]
- Williamson, J.; Liu, Q.; Lu, F.; Mohrman, W.; Li, K.; Dick, R.; Shang, L. Data sensing and analysis: Challenges for wearables. In Proceedings of the 20th Asia and South Pacific Design Automation Conference, Chiba, Japan, 19–22 January 2015; pp. 136–141. [Google Scholar]
- Orfanidis, C.; Dimitrakopoulos, K.; Fafoutis, X.; Jacobsson, M. No Batteries Needed: Providing Physical Context with Energy-Harvesting Beacons. In Proceedings of the 7th International Workshop on Energy Harvesting & Energy-Neutral Sensing Systems—ENSsys′19, New York, NY, USA, 10 November 2019; pp. 52–53. [Google Scholar]
- Steinberg, C.; Philippon, F.; Sanchez, M.; Fortier-Poisson, P.; O’Hara, G.; Molin, F.; Sarrazin, J.F.; Nault, I.; Blier, L.; Roy, K.; et al. A novelwearable device for continuous ambulatory ECG recording: Proof of concept and assessment of signal quality. Biosensors 2019, 9, 17. [Google Scholar] [CrossRef]
- Fouassier, D.; Roy, X.; Blanchard, A.; Hulot, J.S. Assessment of signal quality measured with a smart 12-lead ECG acquisition T-shirt. Ann. Noninvasive Electrocardiol. 2020, 25, 1–7. [Google Scholar] [CrossRef]
- Movesense Sports Bra. Available online: https://www.movesense.com/product/movesense-sports-bra/ (accessed on 26 March 2020).
- Soh, P.J.; Vandenbosch, G.A.E.; Mercuri, M.; Schreurs, D.M.M.P. Wearable wireless health monitoring: Current developments, challenges, and future trends. IEEE Microw. Mag. 2015, 16, 55–70. [Google Scholar] [CrossRef]
- Ortega, L.; Llorella, A.; Esquivel, J.P.; Sabaté, N. Self-powered smart patch for sweat conductivity monitoring. Microsyst. Nanoeng. 2019, 5, 3. [Google Scholar] [CrossRef]
- Ahmad, R.; Wolfbeis, O.S.; Alshareef, H.N.; Salama, K.N.; Surya, S.G.; Batista Sales, J.; Mkaouar, H.; Cavalcanti Catunda, S.Y.; Rodrigues Belfort, D.; Lei, Y.; et al. KAUSTat: A Wireless, Wearable, Open-Source Potentiostat for Electrochemical Measurements. Proc. IEEE Sensors 2019, 2019, 1–4. [Google Scholar]
- Svobodova, H.; Vavrinsky, E.; Turonova, D.; Donoval, M.; Daricek, M.; Telek, P.; Kopani, M. Optimization of the position of single-lead wireless sensor with low electrodes separation distance for ECG-derived respiration. Adv. Electr. Electron. Eng. 2018, 16, 528–537. [Google Scholar] [CrossRef]
- Kabir, M.M.; Perez-Alday, E.A.; Thomas, J.; Sedaghat, G.; Tereshchenko, L.G. Optimal configuration of adhesive ECG patches suitable for long-term monitoring of a vectorcardiogram. J. Electrocardiol. 2017, 50, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Dias, D.; Cunha, J.P.S. Wearable Health Devices—Vital Sign Monitoring, Systems and Technologies. Sensors 2018, 18, 2414. [Google Scholar] [CrossRef] [PubMed]
- Motti, V.G.; Caine, K. Users’ Privacy Concerns about Wearables. In Financial Cryptography and Data Security; Brenner, M., Christin, N., Johnson, B., Rohloff, K., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 231–244. [Google Scholar]
- Perez, A.J.; Zeadally, S. Privacy Issues and Solutions for Consumer Wearables. IT Prof. 2018, 20, 46–56. [Google Scholar] [CrossRef]
- Schaub, F.; Balebako, R.; Durity, A.L.; Cranor, L.F. A design space for effective privacy notices. In Proceedings of the Eleventh Symposium on Usable Privacy and Security (SOUPS), Santa Clara, CA, USA, 11–13 August 2019; pp. 1–17. [Google Scholar]
- Guler, S.D.; Gannon, M.; Sicchio, K. Crafting Wearables; Apress: Berkeley, CA, USA, 2016; ISBN 978-1-4842-1807-5. [Google Scholar]
- Kotradyova, V.; Vavrinsky, E.; Kalinakova, B.; Petro, D.; Jansakova, K.; Boles, M.; Svobodova, H. Wood and Its Impact on Humans and Environment Quality in Health Care Facilities. Int. J. Environ. Res. Public Health 2019, 16, 3496. [Google Scholar] [CrossRef] [PubMed]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vavrinsky, E.; Subjak, J.; Donoval, M.; Wagner, A.; Zavodnik, T.; Svobodova, H. Application of Modern Multi-Sensor Holter in Diagnosis and Treatment. Sensors 2020, 20, 2663. https://doi.org/10.3390/s20092663
Vavrinsky E, Subjak J, Donoval M, Wagner A, Zavodnik T, Svobodova H. Application of Modern Multi-Sensor Holter in Diagnosis and Treatment. Sensors. 2020; 20(9):2663. https://doi.org/10.3390/s20092663
Chicago/Turabian StyleVavrinsky, Erik, Jan Subjak, Martin Donoval, Alexandra Wagner, Tomas Zavodnik, and Helena Svobodova. 2020. "Application of Modern Multi-Sensor Holter in Diagnosis and Treatment" Sensors 20, no. 9: 2663. https://doi.org/10.3390/s20092663
APA StyleVavrinsky, E., Subjak, J., Donoval, M., Wagner, A., Zavodnik, T., & Svobodova, H. (2020). Application of Modern Multi-Sensor Holter in Diagnosis and Treatment. Sensors, 20(9), 2663. https://doi.org/10.3390/s20092663





