Raspberry, Rape, Thyme, Sunflower and Mint Honeys Authentication Using Voltammetric Tongue
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Melissopalynological Analysis
2.2.2. Electrochemical Measurement
2.2.3. Statistical Analysis
3. Results
3.1. Melissopalynological Analysis
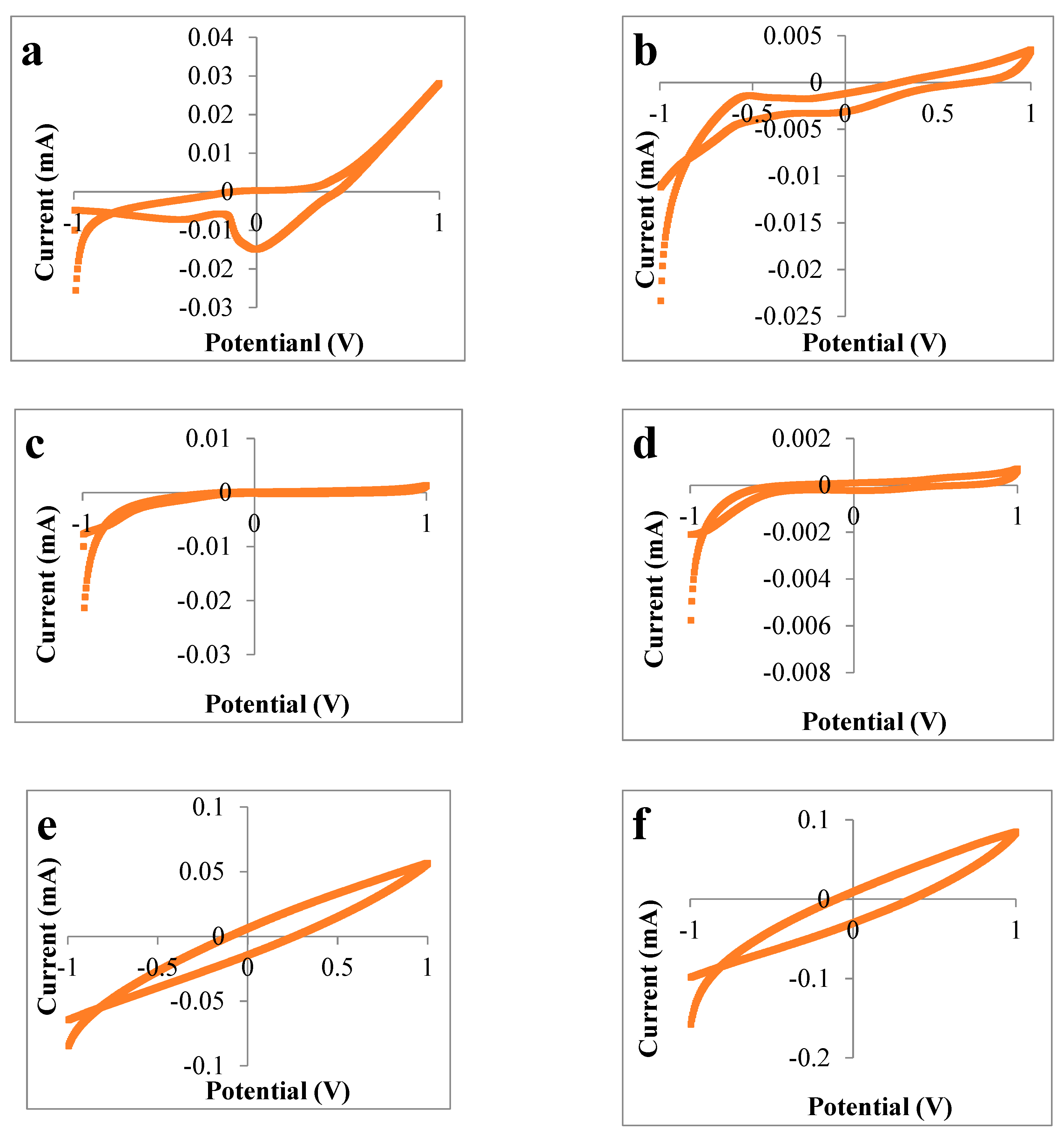
3.2. Voltammetric Electronic Tongue
3.3. Multivariate Analysis
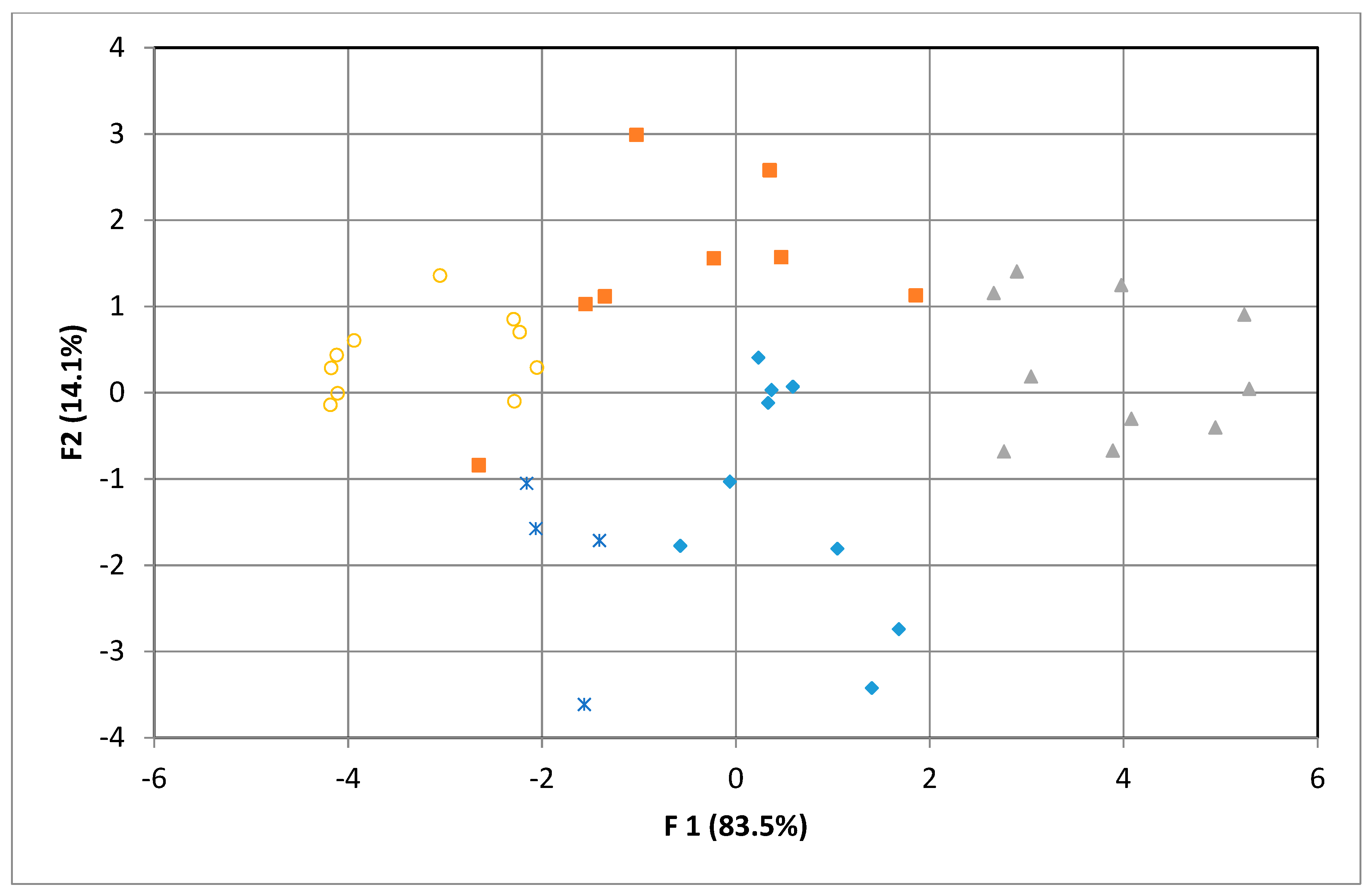
3.3.1. Principal Component Analysis (PCA)
3.3.2. Classification of Results with LDA
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Oroian, M.; Amariei, S.; Rosu, A.; Gutt, G. Classification of unifloral honeys using multivariate analysis. J. Essent. Oil Res. 2015, 27. [Google Scholar] [CrossRef]
- Ball, D.W. The chemical composition of honey. J. Chem. Educ. 2007, 84. [Google Scholar] [CrossRef]
- Terrab, A.; Recamales, A.F.; Hernanz, D.; Heredia, F.J. Characterisation of Spanish thyme honeys by their physicochemical characteristics and mineral contents. Food Chem. 2004. [Google Scholar] [CrossRef]
- Soares, S.; Amaral, J.S.; Oliveira, M.B.P.P.; Mafra, I. A Comprehensive Review on the Main Honey Authentication Issues: Production and Origin. Compr. Rev. Food Sci. Food Saf. 2017, 16. [Google Scholar] [CrossRef]
- Escriche, I.; Tanleque-Alberto, F.; Visquert, M.; Oroian, M. Physicochemical and rheological characterization of honey from Mozambique. LWT-Food Sci. Technol. 2017, 86. [Google Scholar] [CrossRef]
- White, J.W. Isotope ratio testing of honey: Demystifying the internal standard test. Am. Bee J. 2000, 140, 318–321. [Google Scholar]
- Dias, L.G.; Veloso, A.C.A.; Sousa, M.E.B.C.; Estevinho, L.; Machado, A.A.S.C.; Peres, A.M. A novel approach for honey pollen profile assessment using an electronic tongue and chemometric tools. Anal. Chim. Acta 2015, 900. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S. Honey authentication based on physicochemical parameters and phenolic compounds. Comput. Electron. Agric. 2017, 138. [Google Scholar] [CrossRef]
- Kuś, P.M.; Congiu, F.; Teper, D.; Sroka, Z.; Jerković, I.; Tuberoso, C.I.G. Antioxidant activity, color characteristics, total phenol content and general HPLC fingerprints of six Polish unifloral honey types. LWT-Food Sci. Technol. 2014, 55. [Google Scholar] [CrossRef]
- Pisani, A.; Protano, G.; Riccobono, F. Minor and trace elements in different honey types produced in Siena County (Italy). Food Chem. 2008, 107. [Google Scholar] [CrossRef]
- Gan, Z.; Yang, Y.; Li, J.; Wen, X.; Zhu, M.; Jiang, Y.; Ni, Y. Using sensor and spectral analysis to classify botanical origin and determine adulteration of raw honey. J. Food Eng. 2015, 178. [Google Scholar] [CrossRef]
- Jiang, T.F.; Chong, L.; Yue, M.E.; Wang, Y.H.; Lv, Z.H. Separation and Determination of Carbohydrates in Food Samples by Capillary Electrophoresis Using Dynamically Coating the Capillary with Indirect UV Detection. Food Anal. Methods 2015, 8. [Google Scholar] [CrossRef]
- Soto, V.C.; Maldonado, I.B.; Jofré, V.P.; Galmarini, C.R.; Silva, M.F. Direct analysis of nectar and floral volatile organic compounds in hybrid onions by HS-SPME/GC-MS: Relationship with pollination and seed production. Microchem. J. 2015, 122. [Google Scholar] [CrossRef]
- Sergiel, I.; Pohl, P.; Biesaga, M. Characterisation of honeys according to their content of phenolic compounds using high performance liquid chromatography/tandem mass spectrometry. Food Chem. 2014. [Google Scholar] [CrossRef] [PubMed]
- Cavazza, A.; Corradini, C.; Musci, M.; Salvadeo, P. High-performance liquid chromatographic phenolic compound fingerprint for authenticity assessment of honey. J. Sci. Food Agric. 2013, 93. [Google Scholar] [CrossRef]
- Dinca, O.R.; Ionete, R.E.; Popescu, R.; Costinel, D.; Radu, G.L. Geographical and Botanical Origin Discrimination of Romanian Honey Using Complex Stable Isotope Data and Chemometrics. Food Anal. Methods 2015, 8. [Google Scholar] [CrossRef]
- Woodcock, T.; Downey, G.; Kelly, J.D.; O’Donnell, C. Geographical classification of honey samples by near-infrared spectroscopy: A feasibility study. J. Agric. Food Chem. 2007, 55. [Google Scholar] [CrossRef]
- Zheng, X.; Zhao, Y.; Wu, H.; Dong, J.; Feng, J. Origin Identification and Quantitative Analysis of Honeys by Nuclear Magnetic Resonance and Chemometric Techniques. Food Anal. Methods 2016, 9. [Google Scholar] [CrossRef]
- Wei, Z.; Wang, J.; Liao, W. Technique potential for classification of honey by electronic tongue. J. Food Eng. 2009, 94. [Google Scholar] [CrossRef]
- Major, N.; Marković, K.; Krpan, M.; Šarić, G.; Hruškar, M.; Vahčić, N. Rapid honey characterization and botanical classification by an electronic tongue. Talanta 2011, 85. [Google Scholar] [CrossRef]
- Tian, S.Y.; Deng, S.P.; Chen, Z.X. Multifrequency large amplitude pulse voltammetry: A novel electrochemical method for electronic tongue. Sens. Actuators B Chem. 2007, 123. [Google Scholar] [CrossRef]
- Gil, L.; Barat, J.M.; Escriche, I.; Garcia-Breijo, E.; Martínez-Máñez, R.; Soto, J. An electronic tongue for fish freshness analysis using a thick-film array of electrodes. Microchim. Acta 2008, 163. [Google Scholar] [CrossRef]
- Ghasemi-Varnamkhasti, M.; Mohtasebi, S.S.; Rodriguez-Mendez, M.L.; Lozano, J.; Razavi, S.H.; Ahmadi, H. Potential application of electronic nose technology in brewery. Trends Food Sci. Technol. 2011, 22. [Google Scholar] [CrossRef]
- Escuder-Gilabert, L.; Peris, M. Review: Highlights in recent applications of electronic tongues in food analysis. Anal. Chim. Acta 2010, 665. [Google Scholar] [CrossRef] [PubMed]
- Wei, Z.; Wang, J.; Ye, L. Classification and prediction of rice wines with different marked ages by using a voltammetric electronic tongue. Biosens. Bioelectron. 2011, 26. [Google Scholar] [CrossRef]
- Riul, A.; Santos, D.S.D.; Wohnrath, K.; Di Tommazo, R.; Carvalho, A.C.P.L.F.; Fonseca, F.J.; Oliveira, O.N.; Taylor, D.M.; Mattoso, L.H.C. Artificial taste sensor: Efficient combination of sensors made from Langmuir-Blodgett films of conducting polymers and a ruthenium complex and self-assembled films of an azobenzene-containing polymer. Langmuir 2002, 18. [Google Scholar] [CrossRef]
- Ulloa, P.A.; Guerra, R.; Cavaco, A.M.; Da Costa, A.M.R.; Figueira, A.C.; Brigas, A.F. Determination of the botanical origin of honey by sensor fusion of impedance e-tongue and optical spectroscopy. Comput. Electron. Agric. 2013, 94. [Google Scholar] [CrossRef]
- Tiwari, K.; Tudu, B.; Bandyopadhyay, R.; Chatterjee, A.; Pramanik, P. Voltammetric sensor for electrochemical determination of the floral origin of honey based on a zinc oxide nanoparticle modified carbon paste electrode. J. Sens. Sens. Syst. 2018, 7. [Google Scholar] [CrossRef]
- Escriche, I.; Kadar, M.; Juan-Borrás, M.; Domenech, E. Suitability of antioxidant capacity, flavonoids and phenolic acids for floral authentication of honey. Impact of industrial thermal treatment. Food Chem. 2014, 142. [Google Scholar] [CrossRef]
- Winquist, F. Voltammetric electronic tongues—Basic principles and applications. Microchim. Acta 2008, 163. [Google Scholar] [CrossRef]
- Tiwari, K.; Tudu, B.; Bandyopadhyay, R.; Chatterjee, A. Identification of monofloral honey using voltammetric electronic tongue. J. Food Eng. 2013, 117. [Google Scholar] [CrossRef]
- Elamine, Y.; Inácio, P.M.C.; Lyoussi, B.; Anjos, O.; Estevinho, L.M.; Miguel, M.D.; Gomes, H.L. Insight into the sensing mechanism of an impedance based electronic tongue for honey botanic origin discrimination. Sens. Actuators B Chem. 2019, 285. [Google Scholar] [CrossRef]
- Veloso, A.C.A.; Sousa, M.E.B.C.; Estevinho, L.; Dias, L.G.; Peres, A.M. Honey evaluation using electronic tongues: An overview. Chemosensors 2018, 6, 28. [Google Scholar] [CrossRef]
- Louveaux, J.; Maurizio, A.; Vorwohl, G. Methods of Melissopalynology. Bee World 1978. [Google Scholar] [CrossRef]
- Gül, A.; Pehlivan, T. Antioxidant activities of some monofloral honey types produced across Turkey. Saudi J. Biol. Sci. 2018, 25. [Google Scholar] [CrossRef]
- El-Sofany, A.; Al Naggar, Y.; Naiem, E.; Giesy, J.P.; Seif, A. Authentication of the botanical and geographic origin of Egyptian honey using pollen analysis methods. J. Apic. Res. 2020. [Google Scholar] [CrossRef]
- Siddiqui, A.J.; Musharraf, S.G.; Choudhary, M.I.; Rahman, A.U. Application of analytical methods in authentication and adulteration of honey. Food Chem. 2017, 217, 687–698. [Google Scholar] [CrossRef]
- Karabagias, I.K.; Badeka, A.; Kontakos, S.; Karabournioti, S.; Kontominas, M.G. Characterization and classification of Thymus capitatus (L.) honey according to geographical origin based on volatile compounds, physicochemical parameters and chemometrics. Food Res. Int. 2014. [Google Scholar] [CrossRef]
- Sobrino-Gregorio, L.; Bataller, R.; Soto, J.; Escriche, I. Monitoring honey adulteration with sugar syrups using an automatic pulse voltammetric electronic tongue. Food Control 2018, 91. [Google Scholar] [CrossRef]
- Oroian, M.; Ropciuc, S. Romanian honey authentication using voltammetric electronic tongue. Correlation of voltammetric data with physico-chemical parameters and phenolic compounds. Comput. Electron.Agric. 2019, 157. [Google Scholar] [CrossRef]
- Bougrini, M.; Tahri, K.; Saidi, T.; El Hassani, N.E.; Bouchikhi, B.; El Bari, N. Classification of Honey According to Geographical and Botanical Origins and Detection of Its Adulteration Using Voltammetric Electronic Tongue. Food Anal. Methods 2016, 9. [Google Scholar] [CrossRef]
- El Hassani, N.E.; Tahri, K.; Llobet, E.; Bouchikhi, B.; Errachid, A.; Zine, N.; El Bari, N. Emerging approach for analytical characterization and geographical classification of Moroccan and French honeys by means of a voltammetric electronic tongue. Food Chem. 2018, 243. [Google Scholar] [CrossRef] [PubMed]
- Sousa, M.E.B.C.; Dias, L.G.; Veloso, A.C.A.; Estevinho, L.; Peres, A.M.; Machado, A.A.S.C. Practical procedure for discriminating monofloral honey with a broad pollen profile variability using an electronic tongue. Talanta 2014, 128. [Google Scholar] [CrossRef] [PubMed]
- Escriche, I.; Kadar, M.; Domenech, E.; Gil-Sánchez, L. A potentiometric electronic tongue for the discrimination of honey according to the botanical origin. Comparison with traditional methodologies: Physicochemical parameters and volatile profile. J. Food Eng. 2012. [Google Scholar] [CrossRef]


| Honey Type | Predicted Group Membership | Total | ||||||
|---|---|---|---|---|---|---|---|---|
| Rape | Sunflower | Mint | Raspberry | Thyme | ||||
| Original | Count | Raspberry | 9 | 0 | 0 | 0 | 0 | 9 |
| Sunflower | 0 | 6 | 1 | 0 | 1 | 8 | ||
| Mint | 0 | 0 | 10 | 0 | 0 | 10 | ||
| Rape | 0 | 0 | 0 | 10 | 0 | 10 | ||
| Thyme | 0 | 0 | 0 | 1 | 3 | 4 | ||
| % | Raspberry | 100 | 0 | 0 | 0 | 0 | 100 | |
| Sunflower | 0 | 75 | 12.5 | 0 | 12.5 | 100 | ||
| Mint | 0 | 0 | 100 | 0 | 0 | 100 | ||
| Rape | 0 | 0 | 0 | 100 | 0 | 100 | ||
| Thyme | 0 | 0 | 0 | 25 | 75 | 100 | ||
| Total correct classification | 92.7% | |||||||
| Cross validated | Count | Raspberry | 6 | 1 | 0 | 0 | 2 | 9 |
| Sunflower | 0 | 6 | 1 | 0 | 1 | 8 | ||
| Mint | 0 | 0 | 10 | 0 | 0 | 10 | ||
| Rape | 0 | 0 | 0 | 10 | 0 | 10 | ||
| Thyme | 0 | 0 | 0 | 1 | 3 | 4 | ||
| % | Raspberry | 66.7 | 11.1 | 0 | 0 | 22.2 | 100 | |
| Sunflower | 0 | 75.0 | 12.5 | 0 | 12.5 | 100 | ||
| Mint | 0 | 0 | 100 | 0 | 0 | 100 | ||
| Rape | 0 | 0 | 0 | 100 | 0 | 100 | ||
| Thyme | 0 | 0 | 0 | 25 | 75 | 100 | ||
| Total correct classification | 85.4% | |||||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pauliuc, D.; Dranca, F.; Oroian, M. Raspberry, Rape, Thyme, Sunflower and Mint Honeys Authentication Using Voltammetric Tongue. Sensors 2020, 20, 2565. https://doi.org/10.3390/s20092565
Pauliuc D, Dranca F, Oroian M. Raspberry, Rape, Thyme, Sunflower and Mint Honeys Authentication Using Voltammetric Tongue. Sensors. 2020; 20(9):2565. https://doi.org/10.3390/s20092565
Chicago/Turabian StylePauliuc, Daniela, Florina Dranca, and Mircea Oroian. 2020. "Raspberry, Rape, Thyme, Sunflower and Mint Honeys Authentication Using Voltammetric Tongue" Sensors 20, no. 9: 2565. https://doi.org/10.3390/s20092565
APA StylePauliuc, D., Dranca, F., & Oroian, M. (2020). Raspberry, Rape, Thyme, Sunflower and Mint Honeys Authentication Using Voltammetric Tongue. Sensors, 20(9), 2565. https://doi.org/10.3390/s20092565





