A Review on Electrochemical Sensors and Biosensors Used in Phenylalanine Electroanalysis
Abstract
1. Introduction
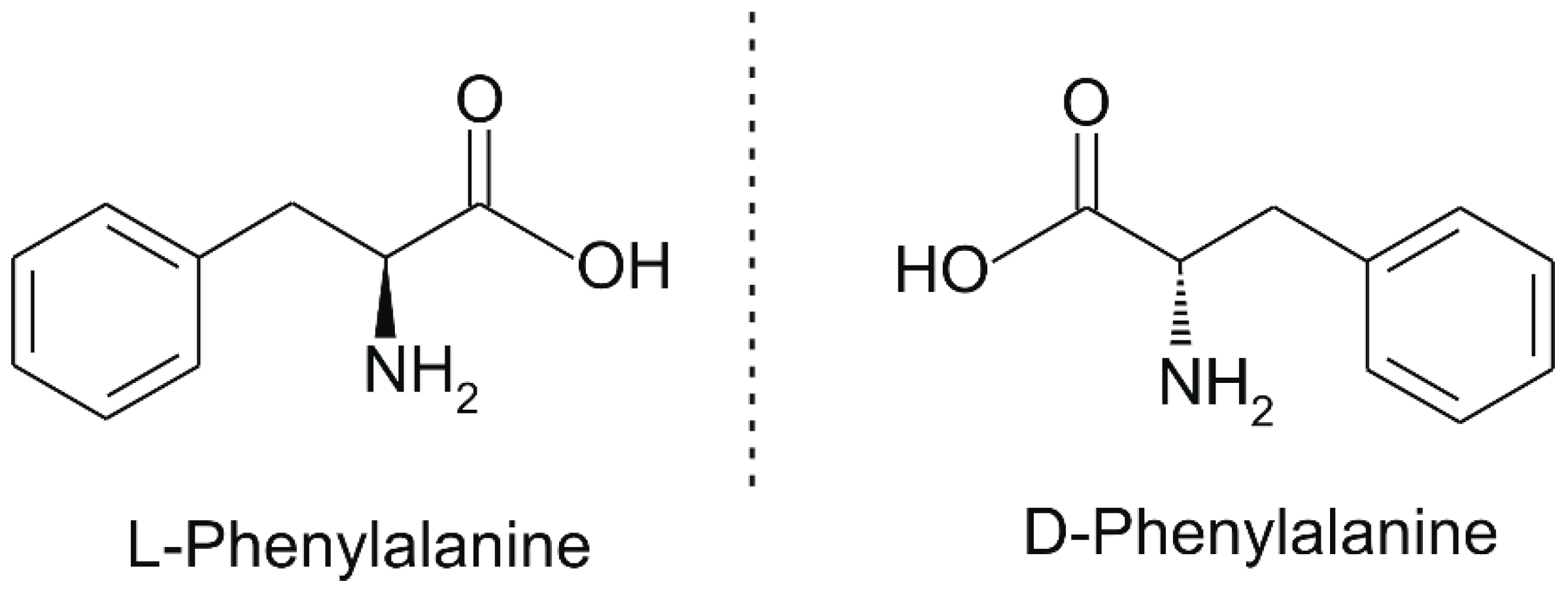
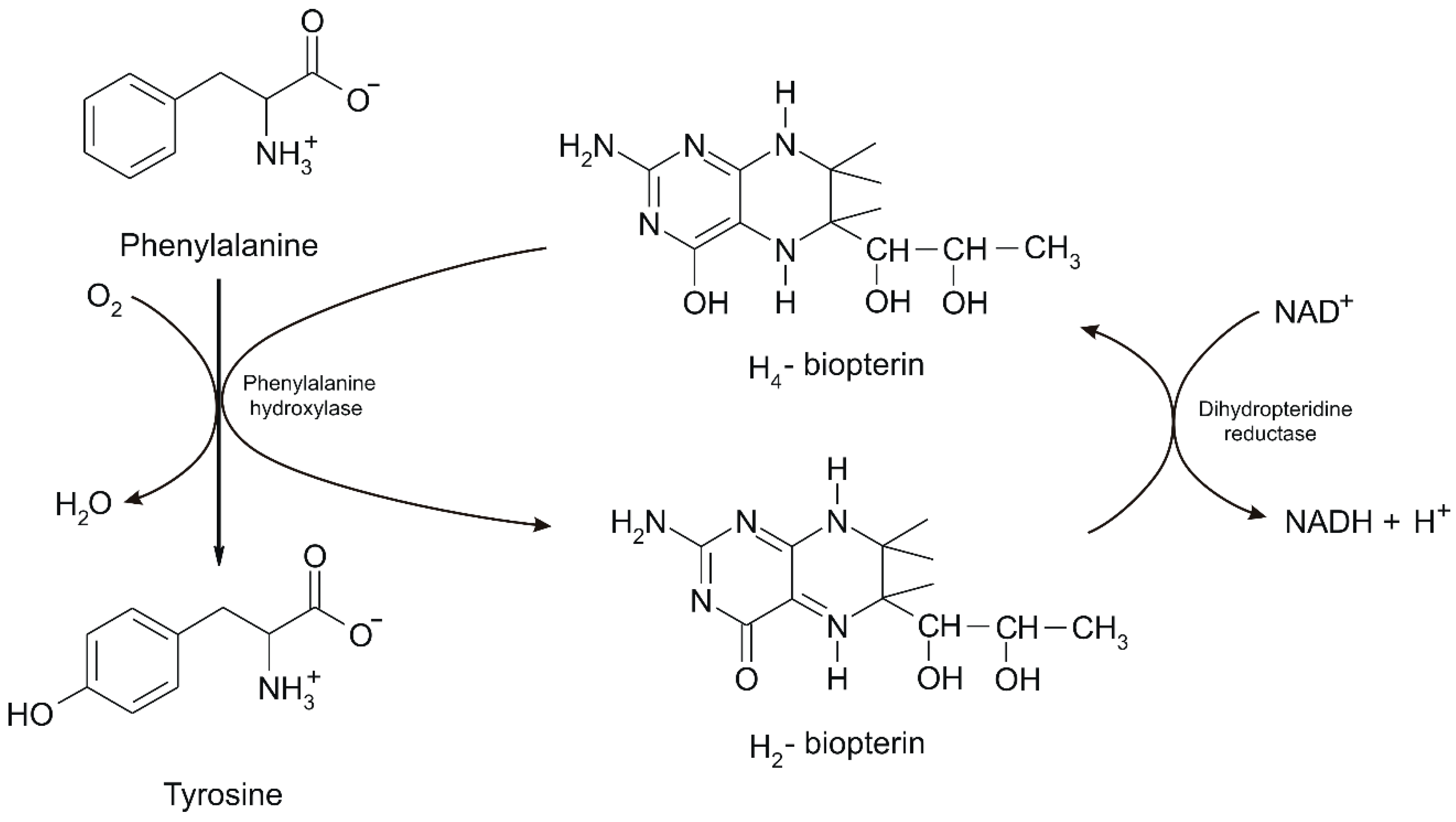
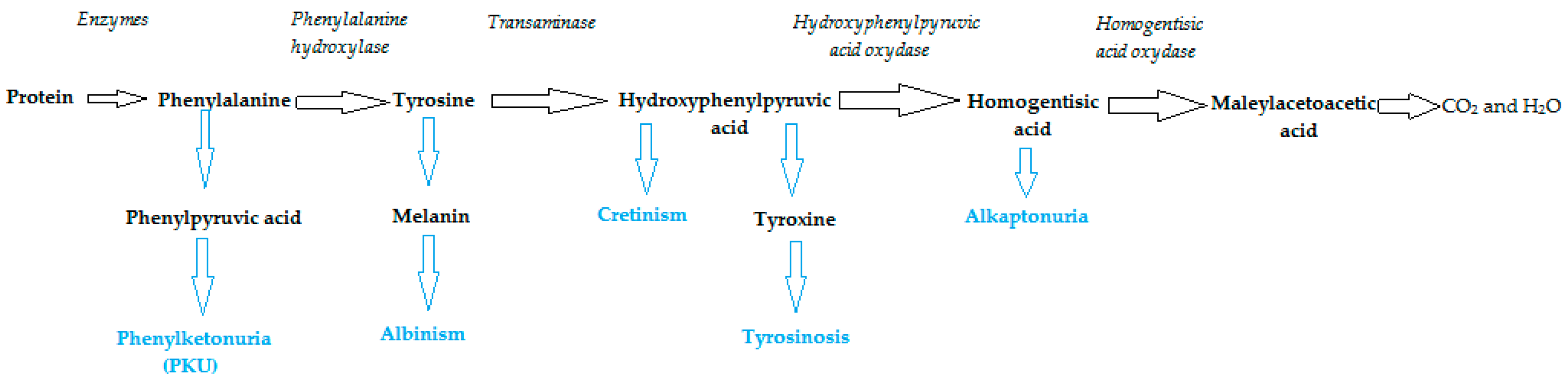
2. Phenylalanine. Properties and Importance for the Human Body
3. Analytical Methods for Phe Determination
3.1. Chemical Methods
3.2. Instrumental Methods
3.3. Sensors and Biosensors
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Nelson, D.L.; Cox, M.M. Lehninger Principles of Biochemistry; WH Freeman: New York, NY, USA, 2009; p. 680. [Google Scholar]
- Rodwell, V.W.; Bender, D.A.; Botham, K.M.; Kennelly, P.J.; Weil, P.A. Harper’s Illustrated Biochemistry, 30th ed.; McGraw-Hill Education: New York, NY, USA, 2015; pp. 281–323. [Google Scholar]
- Xu, X.; Ji, D.; Zhang, Y.; Gao, X.; Xu, P.; Li, X.; Liu, C.-C.; Wen, W. Detection of Phenylketonuria Markers Using a ZIF-67 Encapsulated PtPd Alloy Nanoparticle (PtPd@ ZIF-67)-Based Disposable Electrochemical Microsensor. ACS Appl. Mater. Interfaces 2019, 11, 20734–20742. [Google Scholar] [CrossRef] [PubMed]
- Pelley, J.W.; Goljan, E.F. Rapid Review Biochemistry E-Book, 3rd ed.; Elsevier Health Sciences: Amsterdam, The Netherlands, 2010. [Google Scholar]
- Komoda, T.; Matsunaga, T. Chapter 4-Metabolic Pathways in the Human Body. In Biochemistry for Medical Professionals; Academic Press: Amsterdam, The Netherlands, 2015; p. 58. [Google Scholar]
- Van Wegberg, A.; MacDonald, A.; Ahring, K.; Belanger-Quintana, A.; Blau, N.; Bosch, A.M.; Burlina, A.; Campistol, J.; Feillet, F.; Gizewska, M. The complete European guidelines on phenylketonuria: Diagnosis and treatment. Orphanet J. Rare Dis. 2017, 12, 162. [Google Scholar] [CrossRef] [PubMed]
- Bangaleh, Z.; Sadeghi, H.-B.; Ebrahimi, S.-A.; Najafizadeh, P. A New Potentiometric Sensor for Determination and Screening Phenylalanine in Blood Serum Based on Molecularly Imprinted Polymer. Iran. J. Pharm. Res. IJPR 2019, 18, 61–71. [Google Scholar] [PubMed]
- Lieberman, M.; Marks, A.D. Marks’ Basic Medical Biochemistry: A Clinical Approach; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2009; pp. 731–751. [Google Scholar]
- Online Microbiology Notes by Sagar Aryal. Available online: https://microbenotes.com/amino-acids-properties-structure-classification-and-functions.html (accessed on 10 April 2020).
- Council of Europe. European Pharmacopoeia 7.0; Council of Europe: Strasbourg, France, 2011; p. 2711. [Google Scholar]
- Ermiș, N.; Uzun, L.; Denizli, A. Preparation of molecularly imprinted electrochemical sensor for L-phenylalanine detection and its application. J. Electroanal. Chem. 2017, 807, 244–252. [Google Scholar] [CrossRef]
- Niu, X.; Yang, X.; Mo, Z.; Guo, R.; Liu, N.; Zhao, P.; Liu, Z. Perylene-functionalized graphene sheets modified with β-cyclodextrin for the voltammetric discrimination of phenylalanine enantiomers. Bioelectrochemistry 2019, 129, 189–198. [Google Scholar] [CrossRef]
- Niu, J.-L.; Chai, K.-K.; Zeng, M.-X.; Wang, T.-T.; Zhang, C.-Y.; Chen, S.; Xu, J.-K.; Duan, X.-M. Boc-phenylalanine grafted poly (3, 4-propylenedioxythiophene) film for electrochemically chiral recognition of 3, 4-dihydroxyphenylalanine enantiomers. Chin. J. Polym. Sci. 2019, 37, 451–461. [Google Scholar] [CrossRef]
- Kapalka, G.-M. Chapter 6-Depression. In Nutritional and Herbal Therapies for Children and Adolescents: A Handbook for Mental Health Clinicians; Academic Press: Amsterdam, The Netherlands, 2010; pp. 141–187. [Google Scholar]
- Wood, D.R.; Reimherr, F.W.; Wender, P.H. Treatment of attention deficit disorder with DL-phenylalanine. Psychiatry Res. 1985, 16, 21–26. [Google Scholar] [CrossRef]
- Litwack, G. Chapter 13-Metabolism of Amino Acids. In Human Biochemistry; Academic Press: Amsterdam, The Netherlands, 2017; pp. 375–380. [Google Scholar]
- Voet, D.; Voet, J.G.; Pratt, C.W. Chapter 21-Syntesis and Degradation of Amino Acids. In Fundamentals of Biochemistry: Life at the Molecular Level; John Wiley & Sons: Cambridge, UK, 2016; pp. 724–758. [Google Scholar]
- Broadley, K.-J. The vascular effects of trace amines and amphetamines. Pharmacol. Ther. 2010, 125, 363–375. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Dong, G.; Yue, J. The endogenous substrates of brain CYP2D. Eur. J. Pharmacol. 2014, 724, 211–218. [Google Scholar] [CrossRef]
- Bhagavan, N.V.; Ha, C.-E. Chapter 15—Protein and Amino Acid Metabolism, In Essential of Medical Biochemistry, 2nd ed.; Academic Press: Amsterdam, The Netherlands, 2015; pp. 227–268. [Google Scholar]
- Jäntschi, L.; Nașcu, H.-I. Chapter 4-Metode electrochimice. In Chimie Analitică şi Instrumentală; Academic Press & Academic Direct: Cluj-Napoca, Romania, 2009; pp. 47–67. [Google Scholar]
- De Silva, V.; Oldham, C.-D.; May, S.-W. L-Phenylalanine concentration in blood of phenylketonuria patients: A modified enzyme colorimetric assay compared with amino acid analysis, tandem mass spectrometry, and HPLC methods. Clin. Chem. Lab. Med. 2010, 48, 1271–1279. [Google Scholar] [CrossRef]
- Shokrollahi, A.; Refahi, M. Development of cloud point extraction-scanometry, for the preconcentration and determination of colorless species: Application for the determination of phenylalanine. Quím. Nova 2019, 42, 36–41. [Google Scholar]
- Quintelas, C.; Braga, A.; Mesquita, D.-P.; Amaral, A.-L.; Ferreira, E.-C.; Belo, I. NIR spectroscopy applied to the determination of 2-phenylethanol and l-phenylalanine concentrations in culture medium of Yarrowia lipolytica. J. Chem. Technol. Biotechnol. 2019, 94, 812–818. [Google Scholar] [CrossRef]
- Li, C.-F.; Du, L.-M.; Wu, H.; Chang, Y.-X. Determination of L-phenylalanine by cucurbit [7] uril sensitized fluorescence quenching method. Chin. Chem. Lett. 2011, 22, 851–854. [Google Scholar] [CrossRef]
- Naghashian-Haghig, A.; Hemmateenejad, B.; Shamsipur, M. Determination of enantiomeric excess of some amino acids by second-order calibration of kinetic-fluorescence data. Anal. Biochem. 2018, 550, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Kang, C.; Wu, H.-L.; Xiang, S.-X.; Xie, L.-X.; Liu, Y.-J.; Yu, Y.-J.; Sun, J.-J.; Yu, R.-Q. Simultaneous determination of aromatic amino acids in different systems using three-way calibration based on the PARAFAC-ALS algorithm coupled with EEM fluorescence: Exploration of second-order advantages. Anal. Methods 2014, 6, 6358–6368. [Google Scholar] [CrossRef]
- Thiessen, G.; Robinson, R.; De Los Reyes, K.; Monnat, R.-J.; Fu, E. Conversion of a laboratory-based test for phenylalanine detection to a simple paper-based format and implications for PKU screening in low-resource settings. Analyst 2015, 140, 609–615. [Google Scholar] [CrossRef]
- Huo, J.-Z.; Li, X.-S.; An, J.-D.; Li, Y.; Du, G.-X.; Wu, X.-X.; Liu, Y.-Y.; Ding, B. Photo-luminescent chiral carbon-dot@ Eu (D-cam) nanocomposites for selectively luminescence sensing of L-phenylalanine. J. Mol. Struct. 2020, 1201, 127–214. [Google Scholar] [CrossRef]
- Shan, P.-H.; Zhao, J.; Deng, X.Y.; Lin, R.L.; Bian, B.; Tao, Z.; Xiao, X.; Liu, J.X. Selective recognition and determination of phenylalanine by a fluorescent probe based on cucurbit [8] uril and palmatine. Anal. Chim. Acta 2020, 1104, 164–171. [Google Scholar] [CrossRef]
- Kamruzzaman, M.; Alam, A.-M.; Kim, K.-M.; Lee, S.-H.; Kim, Y.-H.; Kim, G.-M.; Dang, T.-D. Microfluidic chip based chemiluminescence detection of L-phenylalanine in pharmaceutical and soft drinks. Food Chem. 2012, 135, 57–62. [Google Scholar] [CrossRef]
- Qiu, H.; Xi, Y.; Lu, F.; Fan, L.; Luo, C. Determination of L-phenylalanine on-line based on molecularly imprinted polymeric microspheres and flow injection chemiluminescence. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2012, 86, 456–460. [Google Scholar]
- Borghei, Y.-S.; Hosseini, M.; Khoobi, M.; Ganjali, M.-R. Copper nanocluster-enhanced luminol chemiluminescence for high-selectivity sensing of tryptophan and phenylalanine. Luminescence 2017, 32, 1045–1050. [Google Scholar] [CrossRef] [PubMed]
- Kawana, S.; Nakagawa, K.; Hasegawa, Y.; Yamaguchi, S. Simple and rapid analytical method for detection of amino acids in blood using blood spot on filter paper, fast-GC/MS and isotope dilution technique. J. Chromatogr. B 2010, 878, 3113–3118. [Google Scholar] [CrossRef] [PubMed]
- Dick, L.; Dooley, N.; Elliot, M.A.; Ford, S.J.; Gordon, M.R.; Halbert, G.W.; Kerr, W.J. Boron phenylalanine and related impurities: HPLC analysis, stability profile and degradation pathways. J. Pharm. Biomed. Anal. 2011, 56, 633–636. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhang, M.; Liu, Y.; Yang, X.; Luo, L.; Yao, S. Preparation of L-phenylalanine imprinted polymer based on monodisperse hybrid silica microsphere and its application on chiral separation of phenylalanine racemates as HPLC stationary phase. Sep. Purif. Technol. 2012, 87, 142–148. [Google Scholar] [CrossRef]
- Mo, X.-M.; Li, Y.; Tang, A.-G.; Ren, Y.-P. Simultaneous determination of phenylalanine and tyrosine in peripheral capillary blood by HPLC with ultraviolet detection. Clin. Biochem. 2013, 46, 1074–1078. [Google Scholar] [CrossRef]
- Neurauter, G.; Scholl-Bürgi, S.; Haara, A.; Geisler, S.; Mayersbach, P.; Schennach, H.; Fuchs, D. Simultaneous measurement of phenylalanine and tyrosine by high performance liquid chromatography (HPLC) with fluorescence detection. Clin. Biochem. 2013, 46, 1848–1851. [Google Scholar] [CrossRef]
- Pecce, R.; Scolamiero, E.; Ingenito, L.; Parenti, G.; Ruoppolo, M. Optimization of an HPLC method for phenylalanine and tyrosine quantization in dried blood spot. Clin. Biochem. 2013, 46, 1892–1895. [Google Scholar] [CrossRef]
- Ciolacu-Ladașiu, F.C.; Ardelean, A.; Țurcuș, V.; Mândruțiu, I.; Belengeanu, A.-D.; Bechet, D.; Frențescu, L. A simple, sensitive and highly accurate procedure for plasma phenylalanine determination by HPLC. Acta Endocrinol. BUC 2015, 11, 143–146. [Google Scholar]
- Peat, J.; Garg, U. Determination of phenylalanine and tyrosine by high performance liquid chromatography-tandem mass spectrometry. Methods Mol. Biol. 2016, 1378, 219–225. [Google Scholar]
- Hroboňová, K.; Lomenova, A. Molecularly imprinted polymer as stationary phase for HPLC separation of phenylalanine enantiomers. Monatshefte Für Chem.-Chem. Mon. 2018, 149, 939–946. [Google Scholar] [CrossRef]
- Hroboňová, K.; Lomenova, A. Comparison of HPLC separation of phenylalanine enantiomers on different types of chiral stationary phases. Food Anal. Methods 2018, 11, 3314–3323. [Google Scholar]
- Kazan, R.-M.; Seddik, H.-A.; Marstani, Z.-M.; Elsutohy, M.-M.; Yasri, N.-G. Determination of amino acids content in tea species using liquid chromatography via pre-column fluorescence derivatization. Microchem. J. 2019, 150, 104103. [Google Scholar] [CrossRef]
- Pereira-Da Sila, K.; Ptak, M.; Pizani, P.S.; Mendes-Filho, J.; Melo, F.E.A.; Freire, P.T.C. Raman spectroscopy of L-phenylalanine nitric acid submitted to high pressure. Vib. Spectrosc. 2016, 85, 97–103. [Google Scholar]
- Sereda, V.; Ralbovsky, N.-M.; Vasudev, M.-C.; Naik, R.-R.; Lednev, I.-K. Polarized raman spectroscopy for determining the orientation of di-d-phenylalanine molecules in a nanotube. J. Raman Spectrosc. 2016, 47, 1056–1062. [Google Scholar] [CrossRef]
- Oztekin, E.-K.; Hahn, D.-W. Differential Laser-Induced Perturbation Spectroscopy for Analysis of Mixtures of the Fluorophores l-Phenylalanine, l-Tyrosine and l-Tryptophan Using a Fluorescence Probe. Photochem. Photobiol. 2016, 92, 658–666. [Google Scholar] [CrossRef]
- Li, Q.-Q.; Duan, J.; Wu, L.-J.; Huang, Y.; Tang, G.; Min, S.-G. Sucrose as chiral selector for determining enantiomeric composition of phenylalanine by UV–vis spectroscopy and chemometrics. Chin. Chem. Lett. 2012, 23, 1055–1058. [Google Scholar] [CrossRef]
- Murugesan, B.; Yang, J. Tunable Coffee Ring Formation on Polycarbonate Nanofiber Film for Sensitive SERS Detection of Phenylalanine in Urine. ACS Omega 2019, 4, 14928–14936. [Google Scholar] [CrossRef]
- Prakash, M.; Geetha, D.; Caroline, M.-L.; Ramesh, P.S. Crystal growth, structural, optical, dielectric and thermal studies of an amino acid based organic NLO material: L-Phenylalanine L-phenylalaninium malonate. Spectrochim. Acta A. Mol. Biomol. Spectrosc. 2011, 83, 461–466. [Google Scholar] [CrossRef]
- Hong, A.; Jang, H.; Jeong, C.; Choi, M.-C.; Heo, J.; Kim, N.-J. Electronic Circular Dichroism Spectroscopy of Jet-Cooled Phenylalanine and Its Hydrated Clusters. J. Phys. Chem. Lett. 2016, 7, 4385–4390. [Google Scholar] [CrossRef]
- Prinsen, H.-C.M.T.; Holwerda-Loof, N.-E.; Visser, G.; Verhoeven-Duif, N.-M. Reliable analysis of phenylalanine and tyrosine in a minimal volume of blood. Clin. Biochem. 2013, 46, 1272–1275. [Google Scholar] [CrossRef]
- Rigobellor-Masini, M.; Masini, J.-C. Sequential injection chromatography for fluorimetric determination of intracellular amino acids in marine microalgae. In Amino Acid Analysis; Springer: Berlin/Heidelberg, Germany, 2012; pp. 305–315. [Google Scholar]
- Ghasemi-Varnamkhasti, M.; Apetrei, C.; Lozano, J.; Anyogu, A. Potential use of electronic noses, electronic tongues and biosensors as multisensor systems for spoilage examination in foods. Trends Food Sci. Technol. 2018, 80, 71–92. [Google Scholar] [CrossRef]
- Apetrei, I.-M.; Apetrei, C. Application of voltammetric e-tongue for the detection of ammonia and putrescine in beef products. Sens. Actuators B Chem. 2016, 234, 371–379. [Google Scholar] [CrossRef]
- Narayan, R.J. Part One-Fundamentals of medical biosensors for POC applications, In Medical Biosensors for Point of Care (POC) Applications; Woodhead Publishing: Sawston, UK, 2016; pp. 27–42. [Google Scholar]
- Apetrei, I.; Apetrei, C. A modified nanostructured graphene-gold nanoparticle carbon screen-printed electrode for the sensitive voltammetric detection of rutin. Measurement 2018, 114, 37–43. [Google Scholar] [CrossRef]
- Settle, F.A. Chapter 37-Voltammetric Techniques, In Handbook of Instrumental Techniques for Analytical Chemistry; Prentice Hall PTR: Upper Saddle River, NJ, USA, 1997; pp. 709–725. [Google Scholar]
- Scholz, F. Voltammetric techniques of analysis: The essentials. ChemTexts 2015, 1, 17. [Google Scholar] [CrossRef]
- Marsili, E.; Baron, D.B.; Shikhare, I.D.; Coursolle, D.; Gralnick, J.A.; Bond, D.R. Shewanella secretes flavins that mediate extracellular electron transfer. Proc. Natl. Acad. Sci. USA 2008, 105, 3968–3973. [Google Scholar] [CrossRef]
- Hu, Y.-F.; Zhang, Z.-H.; Zhang, H.-B.; Luo, L.-J.; Yao, S.-Z. Electrochemical determination of L-phenylalanine at polyaniline modified carbon electrode based on β-cyclodextrin incorporated carbon nanotube composite material and imprinted sol–gel film. Talanta 2011, 84, 305–313. [Google Scholar] [CrossRef]
- Kutyla-Olesiuk, A.; Jańczyk, M.; Cetó, X.; Del Valle, M.; Ciosek, P.; Wróblewski, W. The application of an array of sensors based on boronic acid derivative for the quantitative analysis of amino acids. Procedia Eng. 2012, 47, 522–525. [Google Scholar] [CrossRef][Green Version]
- Wang, Y.; Luo, L.; Ding, Y.; Zhang, X.; Xu, Y.; Liu, X. Perovskite nanoparticles LaNi0. 5Ti0. 5O3 modified carbon paste electrode as a sensor for electrooxidation and detection of amino acids. J. Electroanal. Chem. 2012, 667, 54–58. [Google Scholar] [CrossRef]
- Omidinia, E.; Shadjou, N.; Hasanzadeh, M. Electrochemical nanobiosensing of phenylalanine using phenylalanine dehydrogenase incorporated on amino-functionalized mobile crystalline material-41. IEEE Sens. J. 2013, 14, 1081–1088. [Google Scholar] [CrossRef]
- Omidinia, E.; Shadjou, N.; Hasanzadeh, M. Aptamer-based biosensor for detection of phenylalanine at physiological pH. Appl. Biochem. Biotechnol. 2014, 172, 2070–2080. [Google Scholar] [CrossRef]
- Yoshimi, Y.; Ishii, N. Improved gate effect enantioselectivity of phenylalanine-imprinted polymers in water by blending crosslinkers. Anal. Chim. Acta 2015, 862, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Bi, Q.; Dong, S.; Sun, Y.; Lu, X.; Zhao, L. An electrochemical sensor based on cellulose nanocrystal for the enantioselective discrimination of chiral amino acids. Anal. Biochem. 2016, 508, 50–57. [Google Scholar] [CrossRef] [PubMed]
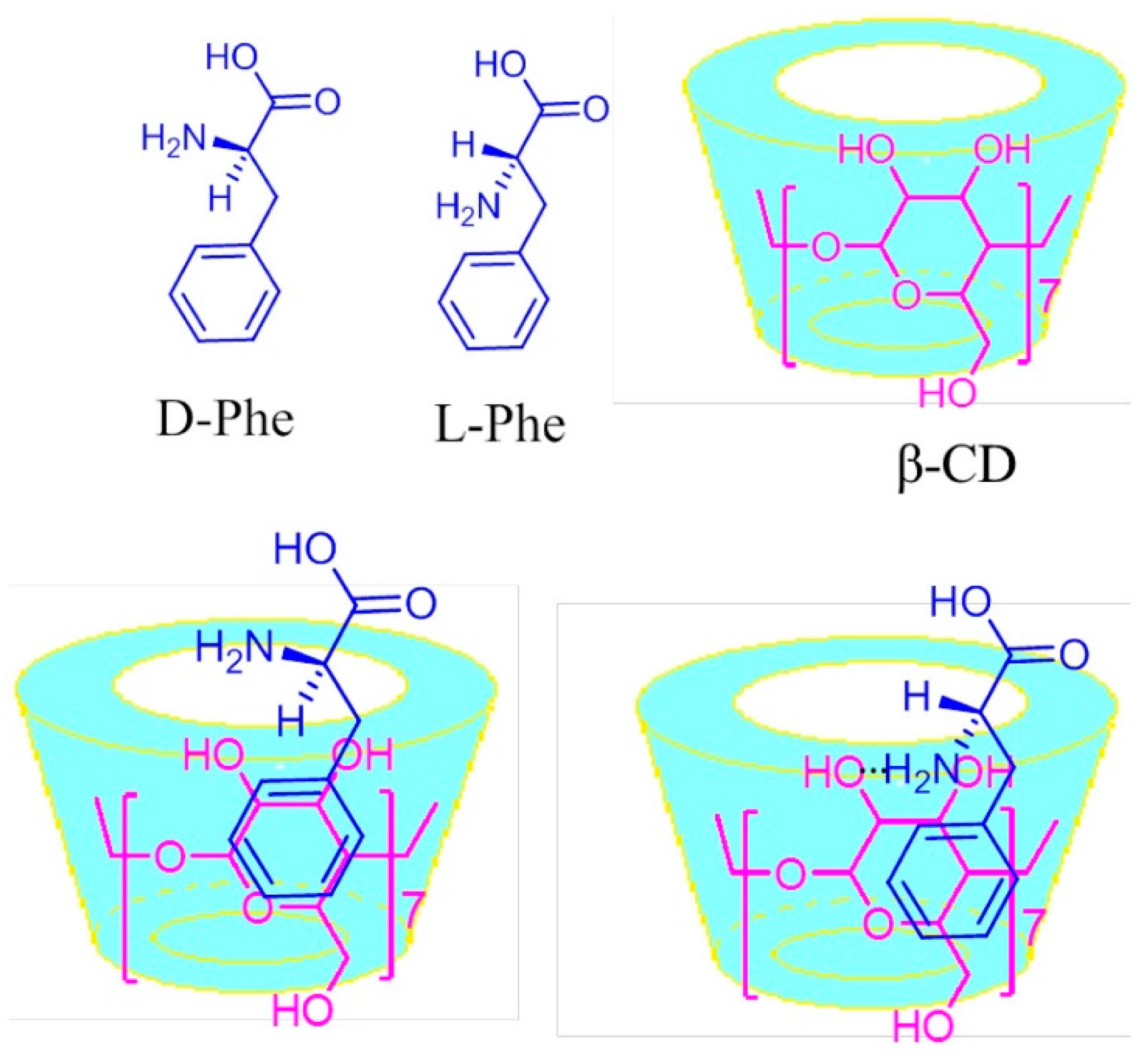
- Zaidi, S.-A. Facile and efficient electrochemical enantiomer recognition of phenylalanine using β-Cyclodextrin immobilized on reduced graphene oxide. Biosens. Bioelectron. 2017, 94, 714–718. [Google Scholar] [CrossRef] [PubMed]
- Aghaei, F.; Seifati, S.-M.; Nasirizadeh, N. Development of a DNA biosensor for the detection of phenylketonuria based on a screen-printed gold electrode and hematoxylin. Anal. Methods 2017, 9, 966–973. [Google Scholar] [CrossRef]
- Wu, T.; Wei, X.; Ma, X.; Li, J. Amperometric sensing of L-phenylalanine using a gold electrode modified with a metal organic framework, a molecularly imprinted polymer, and β-cyclodextrin-functionalized gold nanoparticles. Microchim. Acta 2017, 184, 2901–2907. [Google Scholar] [CrossRef]
- Seifati, S.-M.; Nasirizadeh, N.; Azimzadeh, M. Nano-biosensor based on reduced graphene oxide and gold nanoparticles, for detection of phenylketonuria-associated DNA mutation. IET Nanobiotechnol. 2017, 12, 417–422. [Google Scholar] [CrossRef]
- Lin, C.; Jair, Y.-C.; Chou, Y.-C.; Chen, P.-S.; Yeh, Y.-C. Transcription factor-based biosensor for detection of phenylalanine and tyrosine in urine for diagnosis of phenylketonuria. Anal. Chim. Acta 2018, 1041, 108–113. [Google Scholar] [CrossRef]
- Niu, X.; Mo, Z.; Yang, X.; Shuai, C.; Liu, N.; Guo, R. Graphene-ferrocene functionalized cyclodextrin composite with high electrochemical recognition capability for phenylalanine enantiomers. Bioelectrochemistry 2019, 128, 74–82. [Google Scholar] [CrossRef]
- Sajini, T.; John, S.; Mathew, B. Tailoring of photo-responsive molecularly imprinted polymers on multiwalled carbon nanotube as an enantioselective sensor and sorbent for L-PABE. Compos. Sci. Technol. 2019, 181, 107676. [Google Scholar] [CrossRef]
- Chen, Y.; Chen, L.; Bi, R.; Xu, L.; Liu, Y. A potentiometric chiral sensor for L-Phenylalanine based on crosslinked polymethylacrylic acid–polycarbazole hybrid molecularly imprinted polymer. Anal. Chim. Acta 2012, 754, 83–90. [Google Scholar] [CrossRef]
- Yi, Y.; Zhang, D.; Ma, Y.; Wu, X.; Zhu, G. Dual-signal electrochemical enantiospecific recognition system via competitive supramolecular host–guest interactions: The case of phenylalanine. Anal. Chem. 2019, 91, 2908–2915. [Google Scholar] [CrossRef] [PubMed]
- Idili, A.; Parolo, C.; Ortega, G.; Plaxco, K.W. Calibration-free measurement of phenylalanine levels in blood using an electrochemical aptamer-based sensor suitable for point-of-care applications. ACS Sens. 2019, 4, 3227–3233. [Google Scholar] [CrossRef] [PubMed]
- Saha, M.; Das, S. Electrochemical detection of L-serine and L-phenylalanine at bamboo charcoal–carbon nanosphere electrode. J. Nanostructure Chem. 2014, 4, 102. [Google Scholar] [CrossRef]
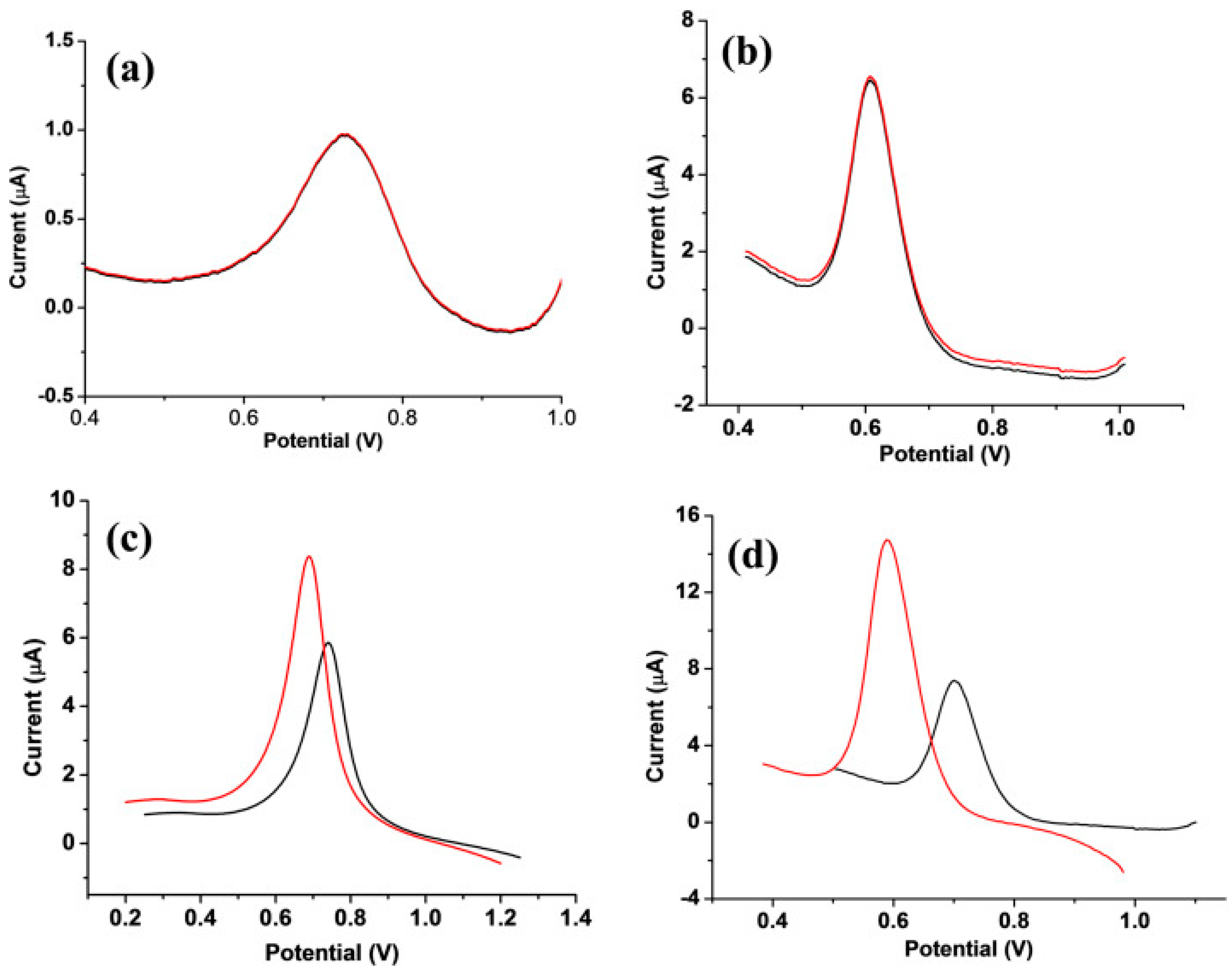
- Ou, S.-H.; Pan, L.-S.; Jow, J.-J.; Chen, H.-R.; Ling, T.-R. Molecularly imprinted electrochemical sensor, formed on Ag screen-printed electrodes, for the enantioselective recognition of d and l phenylalanine. Biosens. Bioelectron. 2018, 105, 143–150. [Google Scholar] [CrossRef]
- Bian, Y.; Zhang, G.; Zhong, X.; Tian, D.; Li, H. Enantioselective recognition of electrochemically inactive phenylalanine by thiolated-cyclodextrin/ferrocene-coated gold nanoparticles. Supramol. Chem. 2011, 23, 455–461. [Google Scholar] [CrossRef]



| Amino Acids | Structure | Oxidated Amino Acids | Linear Range (μM) | R | Sensitivity (nA/μM) | Detection Limit (μM) |
|---|---|---|---|---|---|---|
| L-Cys | 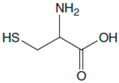 | 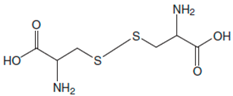 | 2.5–40 | 0.998 | 55 | 0.5 |
| L-Trp |  |  | 5–60 | 0.996 | 38.2 | 0.67 |
| L-Ala |  |  | 50–600 | 0.997 | 3.08 | 8 |
| L-Phe | 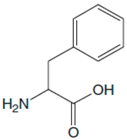 |  | 25–500 | 0.997 | 3.84 | 3 |
| Sensitive Material | Electroanalytical Technique | LOL (mol × L−1) | LOD (mol × L−1) | Ref. |
|---|---|---|---|---|
| ZIF-67 Encapsulated PtPd Alloy Nanoparticle (PtPd@ZIF-67) | CV, CA | 5–500 × 10−6 | 20 × 10−9 | [3] |
| MIP–Thiophen-3-carbonyl tryptophan | CV | 1.0 × 10−8–1.0 × 10−7 | 1.0 × 10−9 | [5] |
| MIP polyvinyl chloride | Potentiometry | 1 × 10−8–1 × 10−4 | 5 × 10−9 | [7] |
| Perylene-functionalized graphene/β-CD | CV, DPV | 0.01–5 × 10−3 | 0.08 × 10−9 for L-Phe 0.2 × 10−9 for D-Phe | [12] |
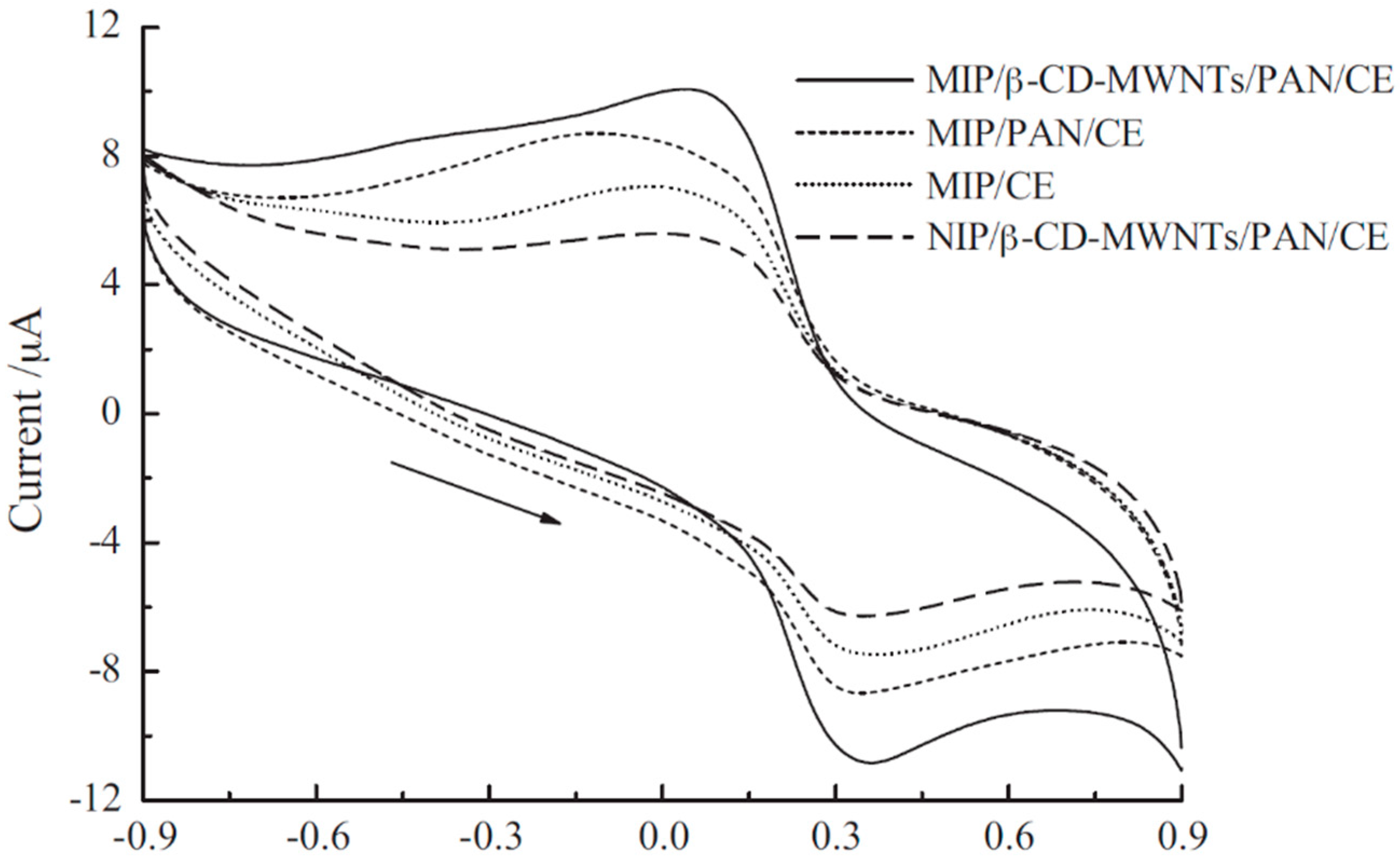
| MIP/β-CD-MWNTs/PAN | CV, DPV, CA | 5.0 × 10−7–1.0 × 10−4 | 1.0 × 10−9 | [61] |
| Phenylboronic acid/PVC | potentiometry | 0.1–3 × 10−3 | - | [62] |
| Perovskite nanoparticles (LaNi0.5Ti0.5O3) | CV, EIS | 25–500 × 10−6 | 3 × 10−6 | [63] |
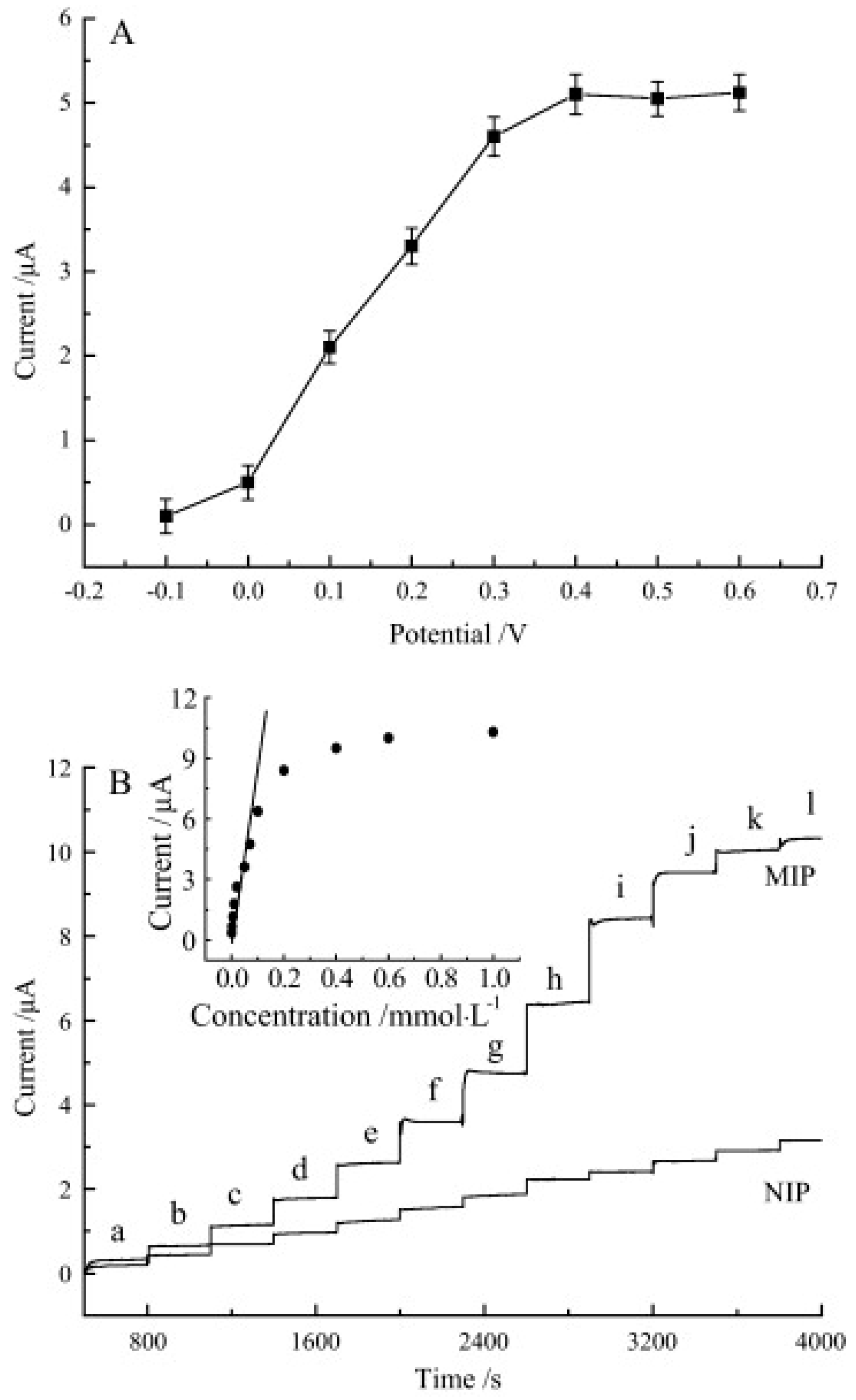
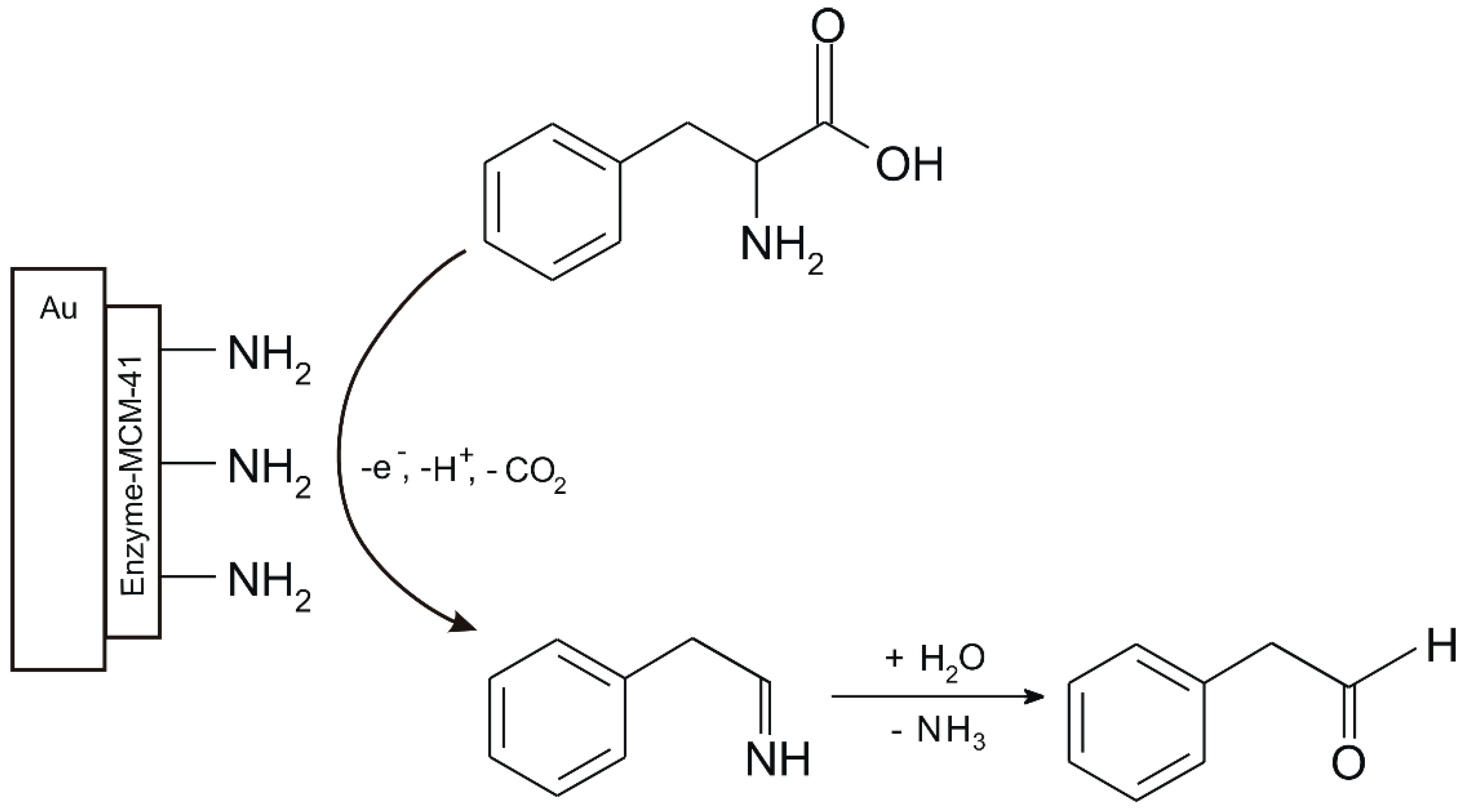
| Phenylalanine Dehydrogenase /Amino-Functionalized Mobile Crystalline Material-41 | CV, DPV, SWV, LSV | 0.01–0.15 × 10−6 | 0.006 × 10−6 | [64] |
| 5′ Thiolated L-Phe aptamer/Au | CV, DPV | 1–10 × 10−9 | 1 × 10−9 | [65] |
| MIP/ethyleneglycol dimethacrylate–methylene bisacrylamide | CV | 0.02–1 × 10−3 | 3–5 × 10−6 | [66] |
| 2,2,6,6-tetramethylpiperidine-1-oxyl-oxidized cellulose nanocrystals/L-cystines/ Au | CV, DPV | 0.05–5 × 10−3 | 5.6 × 10−6 for L-Phe 9.0 × 10−6 for D-Phe | [67] |
| β-CD/RGO/GCE | DPV | 0.4–40 × 10−6 | 0.10 × 10−6 for L-Phe 0.15 × 10−6 for D-Phe | [68] |
| DNA (thiol modified oligonucleotide probe)/hematoxylin | CV | 20 × 10−12–1.5 × 10−7 | 8.5 × 10−12 | [69] |
| MIP/thiolated β-CD/L-cysteine | CV, DPV | 2×10−12–6×10−10 | 0.33 × 10−12 | [70] |
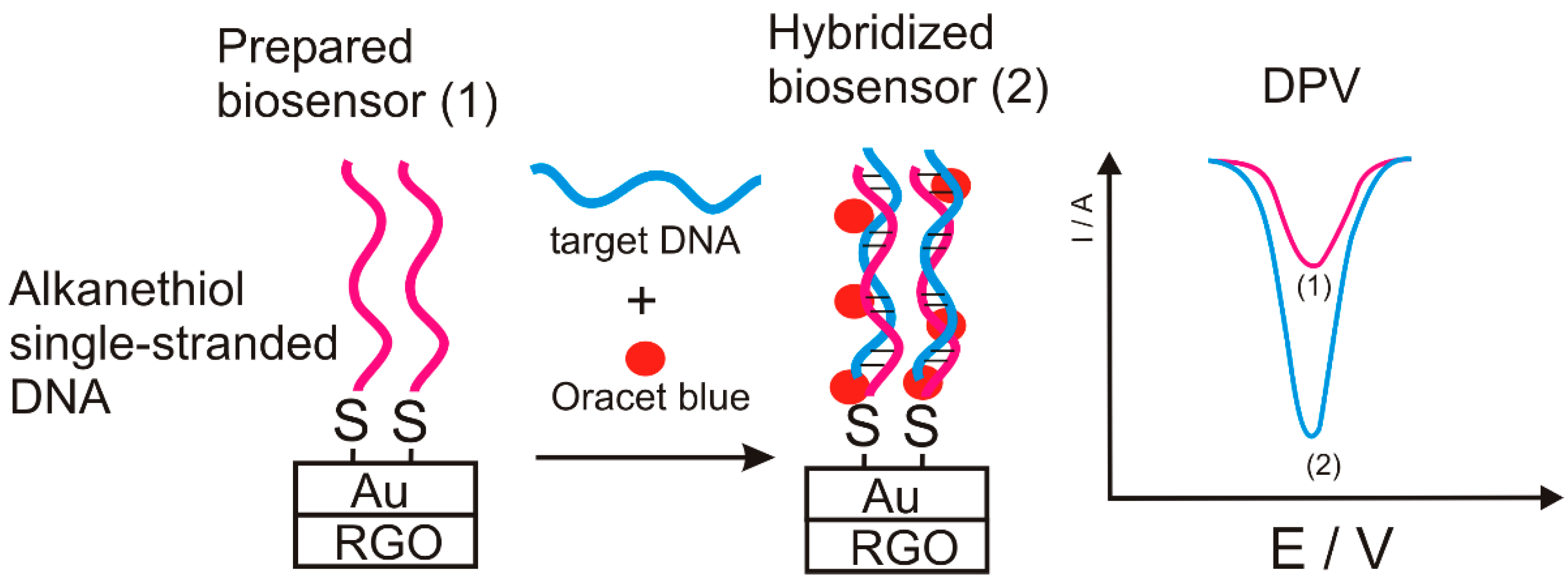
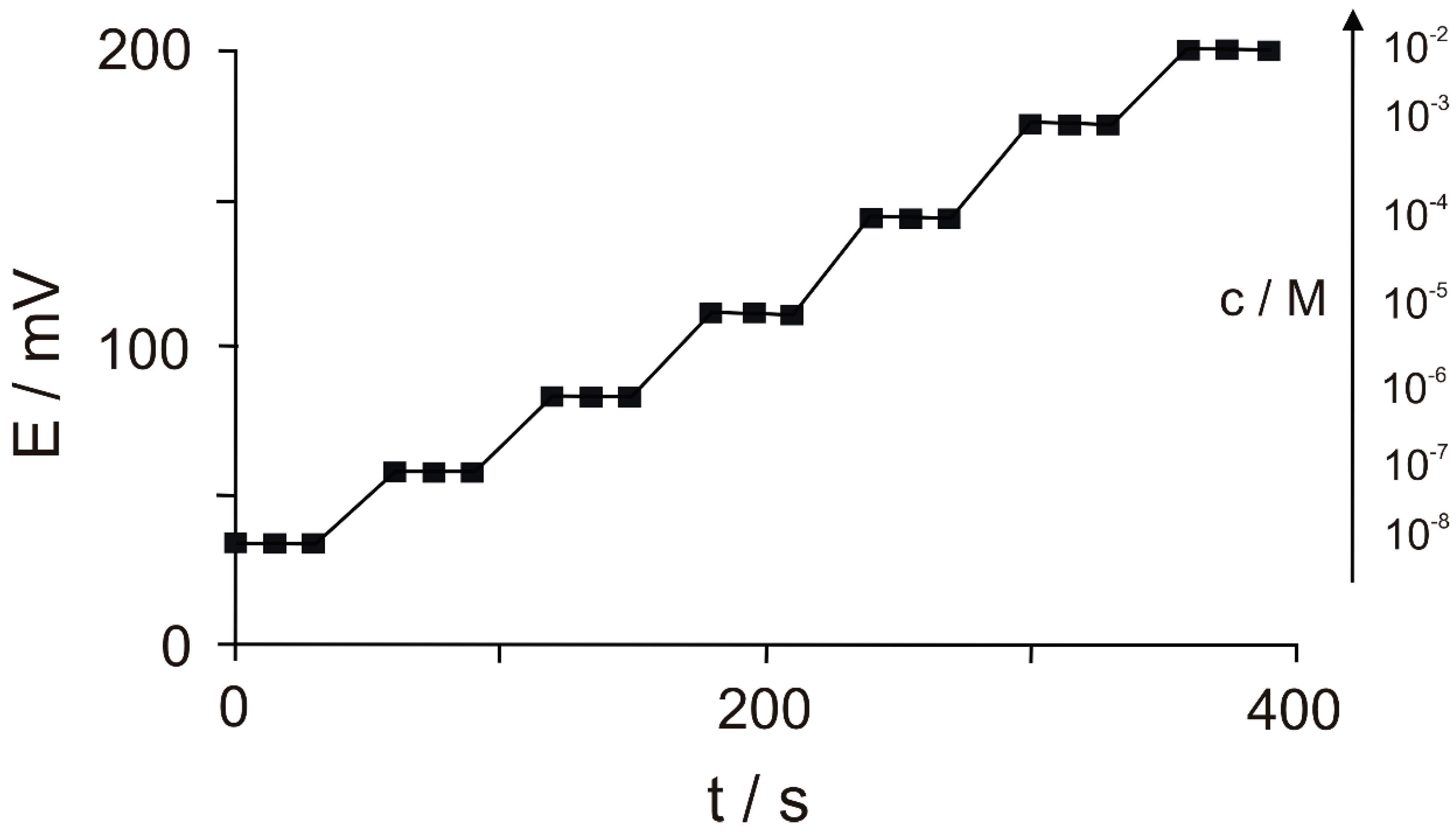
| gold nanoparticles/rGO/alkanethiol single-stranded DNA/Oracet blue | CV | 80 × 10−15–1200 × 10−15 | 21.3 × 10−15 | [71] |
| E. coli DH5a cells | FS | 5 × 10−6–100 × 10−6 | 3.7 × 10−6 | [72] |
| Graphene-ferrocene functionalized CD | DPV | 0.01–5.0 × 10−3 | 27 × 10−9 for L-Phe 52 × 10−9 for D-Phe | [73] |
| MIP (4-[ (4-methacryloyloxy) phenylazo] benzoic acid)/MWCNT | CV, DPV | 0.5–3 × 10−6 | 0.2086 × 10−6 | [74] |
| Crosslinked polymethylacrylic acid–polycarbazole hybrid MIP | Potentiometry | 2.5 × 10−6–2.5 × 10−2 | 1.37 × 10−6 | [75] |
| β-CD/CNTs@rGO | CV, DPV | 0.2–13.0 × 10−6 | 0.08 × 10−6 | [76] |
| DNA aptamer/methylene blue | SWV | 9.0 × 10−8–7.0 × 10−6 | 0.4 × 10−6 | [77] |
| Bamboo charcoal–carbon nanosphere | CV, DPV, SWV, LSV | 1–100 × 10−6 | 1× 10−6 | [78] |
| L-Phe-MIP PPy/Ag | CA | 0.1–50 × 10−3 | 1.39 × 10−3 for L-Phe 27.7 × 10−3 for D-Phe | [79] |
| Thiolated-CD/ferrocene-coated gold nanoparticles | CV | 0.1–1 × 10−6 | 8.4 × 10−8 | [80] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dinu, A.; Apetrei, C. A Review on Electrochemical Sensors and Biosensors Used in Phenylalanine Electroanalysis. Sensors 2020, 20, 2496. https://doi.org/10.3390/s20092496
Dinu A, Apetrei C. A Review on Electrochemical Sensors and Biosensors Used in Phenylalanine Electroanalysis. Sensors. 2020; 20(9):2496. https://doi.org/10.3390/s20092496
Chicago/Turabian StyleDinu, Ancuta, and Constantin Apetrei. 2020. "A Review on Electrochemical Sensors and Biosensors Used in Phenylalanine Electroanalysis" Sensors 20, no. 9: 2496. https://doi.org/10.3390/s20092496
APA StyleDinu, A., & Apetrei, C. (2020). A Review on Electrochemical Sensors and Biosensors Used in Phenylalanine Electroanalysis. Sensors, 20(9), 2496. https://doi.org/10.3390/s20092496






