Trunk Range of Motion Is Related to Axial Rigidity, Functional Mobility and Quality of Life in Parkinson’s Disease: An Exploratory Study
Abstract
1. Introduction
2. Materials and Methods
2.1. Study Design
2.2. Participants
2.3. Procedure
2.3.1. Trunk ROM by Conventional Assessment
2.3.2. Trunk Rigidity by Technological Assessment
2.3.3. Functional Mobility Assessment
2.3.4. Health-Related Quality of Life (HRQoL) Assessment
2.4. Data Analysis
3. Results
4. Discussion
4.1. Range of Motion of the Trunk and Axial Rigidity
4.2. Trunk Range of Motion and Functional Mobility
4.3. Axial Impairments and Health Related Quality of Life
4.4. Study Limitations
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Schilder, J.C.M.; Overmars, S.S.; Marinus, J.; van Hilten, J.J.; Koehler, P.J. The Terminology of Akinesia, Bradykinesia and Hypokinesia: Past, Present and Future. Parkinsonism Relat. Disord. 2017, 37, 27–35. [Google Scholar]
- Ling, H.; Massey, L.A.; Lees, A.J.; Brown, P.; Day, B.L. Hypokinesia Without Decrement Distinguishes Progressive Supranuclear Palsy From Parkinson’s Disease. Brain 2012, 135, 1141–1153. [Google Scholar] [PubMed]
- Van Emmerik, R.E.; Wagenaar, R.C.; Winogrodzka, A.; Wolters, E.C. Identification of Axial Rigidity during Locomotion in Parkinson Disease. Arch. Phys. Med. Rehabil. 1999, 80, 186–191. [Google Scholar] [PubMed]
- Mak, M.K.; Wong, E.C.; Hui-Chan, C.W. Quantitative Measurement of Trunk Rigidity in Parkinsonian Patients. J. Neurol. 2007, 254, 202–209. [Google Scholar] [PubMed]
- Wright, W.; Gurfinkel, V.; Nutt, J.; Horak, F.; Cordo, P. Axial Hypertonicity in Parkinson’s Disease: Direct Measurements of Trunk and Hip Torque. Exp. Neurol. 2007, 208, 38–46. [Google Scholar] [PubMed]
- Bridgewater, K.J.; Sharpe, M.H. Trunk Muscle Performance in Early Parkinson’s Disease. Phys. Ther. 1998, 78, 566–576. [Google Scholar]
- Koh, S.B.; Park, Y.M.; Kim, M.J.; Kim, W.S. Influences of Elbow, Shoulder, Trunk Motion and Temporospatial Parameters on Arm Swing Asymmetry of Parkinson’s Disease during Walking. Hum. Mov. Sci. 2019, 68, 102527. [Google Scholar]
- Krishnamurthi, N.; Murphey, C.; Driver-Dunckley, E. A Comprehensive Movement and Motion Training Program Improves Mobility in Parkinson’s Disease. Aging Clin. Exp. Res. 2019, 32, 633–643. [Google Scholar]
- Blanch, P. Conservative Management of Shoulder Pain in Swimming. Phys. Ther. Sport 2004, 5, 109–124. [Google Scholar]
- Jolicoeur, F.B.; Rivest, R.; Drumheller, A. Hypokinesia, Rigidity, and Tremor Induced by Hypothalamic 6-OHDA Lesions in the Rat. Brain Res. Bull. 1991, 26, 317–320. [Google Scholar]
- Pikstra, A.R.A.; Van Der Hoorn, A.; Leenders, K.L.; De Jong, B.M. Relation of 18-F-Dopa PET with Hypokinesia-Rigidity, Tremor and Freezing in Parkinson’s Disease. NeuroImage Clin. 2016, 11, 68–72. [Google Scholar] [PubMed]
- Berardelli, A.; Sabra, A.F.; Hallett, M. Physiological Mechanisms of Rigidity in Parkinson’s Disease. J. Neurol. Neurosurg. Psychiatry 1983, 46, 45–53. [Google Scholar] [PubMed]
- Mutch, W.; Strudwick, A.; Roy, S.; Downie, A. Parkinson’s Disease: Disability, Review, and Management. Br. Med. J. (Clin. Res. Ed.) 1986, 293, 675–677. [Google Scholar]
- Powell, D.; Threlkeld, A.J.; Fang, X.; Muthumani, A.; Xia, R. Amplitude- and Velocity-Dependency of Rigidity Measured at the Wrist in Parkinson’s Disease. Clin. Neurophysiol. 2012, 123, 764–773. [Google Scholar]
- Cano-De-La-Cuerda, R.; Vela-Desojo, L.; Miangolarra-Page, J.C.; Macías-Macías, Y. Isokinetic Dynamometry as a Technologic Assessment Tool for Trunk Rigidity in Parkinson’s Disease Patients. NeuroRehabilitation 2014, 35, 493–501. [Google Scholar]
- Xia, R.; Muthumani, A.; Mao, Z.-H.; Powell, D.W. Quantification of Neural Reflex and Muscular Intrinsic Contributions to Parkinsonian Rigidity. Exp. Brain Res. 2016, 234, 3587–3595. [Google Scholar]
- Cano-De-La-Cuerda, R.; Vela-Desojo, L.; Miangolarra-Page, J.C.; MacÍas-Macías, Y. Axial Rigidity Is Related to the Risk of Falls in Patients with Parkinson’s Disease. NeuroRehabilitation 2017, 40, 569–577. [Google Scholar] [PubMed]
- Cano-de-la-Cuerda, R.; Vela, L.; Miangolarra-Page, J.C.; Macías-Macías, Y.; Muñoz-Hellín, E. Quantitative Measurement of Axial Rigidity, Functional Status and Health-Related Quality of Life in Patients with Parkinson’s Disease. Rev. Neurol. 2010, 51, 193–200. [Google Scholar] [PubMed]
- Thurman, D.J.; Stevens, J.A.; Rao, J.K. Practice Parameter: Assessing Patients in a Neurology Practice for Risk of Falls (an Evidence-Based Review): Report of the Quality Standards Subcommittee of the American Academy of Neurology. Neurology 2008, 70, 473–479. [Google Scholar] [PubMed]
- Cano-De-La-Cuerda, R.; Pérez-De-Heredia, M.; Miangolarra-Page, J.C.; Muñoz-Hellín, E.; Fernández-De-Las-Peñas, C. Is There Muscular Weakness in Parkinson’s Disease? Am. J. Phys. Med. Rehabil. 2010, 89, 70–76. [Google Scholar]
- Prochazka, A.; Bennett, D.J.; Stephens, M.J.; Patrick, S.K.; Sears-Duru, R.; Roberts, T.; Jhamandas, J.H. Measurement of Rigidity in Parkinson’s Disease. Mov. Disord. 1997, 12, 24–32. [Google Scholar] [PubMed]
- Caligiuri, M.P. Portable Device for Quantifying Parkinsonian Wrist Rigidity. Mov. Disord. 1994, 9, 57–63. [Google Scholar] [PubMed]
- Nuyens, G.; De Weerdt, W.; Dom, R.; Nieuwboer, A.; Spaepen, A. Torque Variations during Repeated Passive Isokinetic Movements of the Knee in Subjects with Parkinson’s Disease and Healthy Control Subjects. Parkinsonism Relat. Disord. 2000, 6, 87–93. [Google Scholar] [PubMed]
- Cano-De-La-Cuerda, R.; Vela-Desojo, L.; Miangolarra-Page, J.C.; MacÍas-Macías, Y.; Muñoz-Hellín, E. Axial Rigidity and Quality of Life in Patients with Parkinson’s Disease: A Preliminary Study. Qual. Life Res. 2011, 20, 817–823. [Google Scholar] [PubMed]
- Ferreira-Sánchez, M.D.R.; Moreno-Verdú, M.; Cano-De-La-Cuerda, R.; Rey, U.; Carlos, J. Quantitative Measurement of Rigidity in Parkinson’s Disease: A Systematic Review. Sensors 2020, 20, 880. [Google Scholar]
- Norkin, C.C.; White, D.J. Measuring Joint Motion: A Guide to Goniometry, 4th ed.; F.A. Davis Company: Philadelphia, PA, USA, 2016. [Google Scholar]
- Hughes, A.J.; Daniel, S.E.; Kilford, L.; Lees, A.J. Accuracy of Clinical Diagnosis of Idiopathic Parkinson’s Disease: A Clinico-Pathological Study of 100 Cases. J. Neurol. Neurosurg. Psychiatry 1992, 55, 181–184. [Google Scholar]
- Folstein, M.F.; Folstein, S.E.; McHugh, P.R. ‘Mini-Mental State’. A Practical Method for Grading the Cognitive State of Patients for the Clinician. J. Psychiatr. Res. 1975, 12, 189–198. [Google Scholar]
- Goetz, C.C. The Unified Parkinson’s Disease Rating Scale (UPDRS): Status and Recommendations. Mov. Disord. 2003, 18, 738–750. [Google Scholar]
- Schwab, R.; England, A.; Schwab, Z.J. Projection Technique for Evaluating Surgery in Parkinson’s Disease. In Third Symposium on Parkinson’s Disease; E & S Livingston: Edinburgh, UK, 1969; pp. 152–157. [Google Scholar]
- Chertman, C.; Campoy Dos Santos, H.M.; Pires, L.; Wajchenberg, M.; Martins, D.E.; Puertas, E.B. A Comparative Study of Lumbar Range of Movement in Healthy Athletes and Non-Athletes. Rev. Bras. Ortop. 2010, 45, 389–394. [Google Scholar]
- Lewis, J.S.; Valentine, R.E. Clinical Measurement of the Thoracic Kyphosis. A Study of the Intra-Rater Reliability in Subjects with and without Shoulder Pain. BMC Musculoskelet. Disord. 2010, 11, 39. [Google Scholar]
- Mathias, S.; Nayak, U.S.L.; Isaacs, B. Balance in Elderly Patients: The ‘get-up and Go’ Test. Arch. Phys. Med. Rehabil. 1986, 67, 387–389. [Google Scholar] [PubMed]
- Erola, T.; Karinen, P.; Heikkinen, E.; Tuominen, J.; Haapaniemi, T.; Koivukangas, J.; Myllylä, V. Bilateral Subthalamic Nucleus Stimulation Improves Health-Related Quality of Life in Parkinsonian Patients. Parkinsonism Relat. Disord. 2005, 11, 89–94. [Google Scholar] [PubMed]
- Badia, X.; Roset, M.; Montserrat, S.; Herdman, M.; Segura, A. The Spanish Version of EuroQol: A Description and Its Applications. European Quality of Life Scale. Med. Clin. (Barc.) 1999, 112, 79–85. [Google Scholar] [PubMed]
- Cohen, J. Statistical Power Analysis for the Behavioral Sciences, 2nd ed.; Lawrence Erlbaum Associates: Mahwah, NJ, USA, 1988. [Google Scholar]
- Schenkman, M.L.; Clark, K.; Xie, T.; Kuchibhatla, M.; Shinberg, M.; Ray, L. Spinal Movement and Performance of a Standing Reach Task in Participants with and without Parkinson Disease. Phys. Ther. 2001, 81, 1400–1411. [Google Scholar] [PubMed]
- Yang, W.C.; Hsu, W.L.; Wu, R.M.; Lu, T.W.; Lin, K.H. Motion Analysis of Axial Rotation and Gait Stability during Turning in People with Parkinson’s Disease. Gait Posture 2016, 44, 83–88. [Google Scholar] [PubMed]
- Mitchell, T.; Conradsson, D.; Paquette, C. Gait and Trunk Kinematics during Prolonged Turning in Parkinson’s Disease with Freezing of Gait. Parkinsonism Relat. Disord. 2019, 64, 188–193. [Google Scholar]
- Jehu, D.; Nantel, J. Fallers with Parkinson’s Disease Exhibit Restrictive Trunk Control during Walking. Gait Posture 2018, 65, 246–250. [Google Scholar]
- Nikfekr, E.; Kerr, K.; Attfield, S.; Playford, D.E. Trunk Movement in Parkinson’s Disease during Rising from Seated Position. Mov. Disord. 2002, 17, 274–282. [Google Scholar]
- Fasano, A.; Aquino, C.C.; Krauss, J.K.; Honey, C.R.; Bloem, B.R. Axial Disability and Deep Brain Stimulation in Patients with Parkinson Disease. Nat. Rev. Neurol. 2015, 11, 98–110. [Google Scholar]
- Ferrarin, M.; Rizzone, M.; Lopiano, L.; Recalcati, M.; Pedotti, A. Effects of Subthalamic Nucleus Stimulation and L-Dopa in Trunk Kinematics of Patients with Parkinson’s Disease. Gait Posture 2004, 19, 164–171. [Google Scholar]
- Estrada-Barranco, C.; Cano-de-la-Cuerda, R.; Molina-Rueda, F. Construct Validity of the Wisconsin Gait Scale in Acute, Subacute and Chronic Stroke. Gait Posture 2019, 68, 363–368. [Google Scholar] [PubMed]
- Gor-García-Fogeda, M.D.; Cano-de-la-Cuerda, R.; Daly, J.J.; Molina-Rueda, F. Spanish Cross-Cultural Adaptation of the Gait Assessment and Intervention Tool. PM R 2019, 11, 954–962. [Google Scholar] [PubMed]
- Drootin, M. Summary of the Updated American Geriatrics Society/British Geriatrics Society Clinical Practice Guideline for Prevention of Falls in Older Persons. J. Am. Geriatr. Soc. 2011, 59, 148–157. [Google Scholar]
- National Institute for Health and Care Excellence. Falls in Older People: Assessing Risk and Prevention. Available online: https://www.nice.org.uk/guidance/cg161 (accessed on 27 April 2020).
- Cole, M.H.; Silburn, P.A.; Wood, J.M.; Worringham, C.J.; Kerr, G.K. Falls in Parkinson’s Disease: Kinematic Evidence for Impaired Head and Trunk Control. Mov. Disord. 2010, 25, 2369–2378. [Google Scholar] [PubMed]
- Muslimović, D.; Post, B.; Speelman, J.D.; Schmand, B.; De Haan, R.J. Determinants of Disability and Quality of Life in Mild to Moderate Parkinson Disease. Neurology 2008, 70, 2241–2247. [Google Scholar]
- Bryant, M.S.; Hou, J.G.; Collins, R.L.; Protas, E.J. Contribution of Axial Motor Impairment to Physical Inactivity in Parkinson Disease. Am. J. Phys. Med. Rehabil. 2016, 95, 348–354. [Google Scholar]
- Youdas, J.W.; Arend, D.B.; Exstrom, J.M.; Helmus, T.J.; Rozeboom, J.D.; Hollman, J.H. Comparison of Muscle Activation Levels during Arm Abduction in the Plane of the Scapula vs. Proprioceptive Neuromuscular Facilitation Upper Extremity Patterns. J. Strength Cond. Res. 2012, 26, 1058–1065. [Google Scholar]
- Adler, S.S.; Beckers, D.; Buck, M. Propioceptive Neuromuscular Facilitation in the Practice, 3rd ed.; Editorial Médica Panamericana S.A.: Madrid, Spain, 2002. [Google Scholar]
- Greene, B.R.; Caulfield, B.; Lamichhane, D.; Bond, W.; Svendsen, J.; Zurski, C.; Pratt, D. Longitudinal Assessment of Falls in Patients with Parkinson’s Disease Using Inertial Sensors and the Timed Up and Go Test. J. Rehabil. Assist. Technol. Eng. 2018, 5, 205566831775081. [Google Scholar]
- Winser, S.J.; Kannan, P.; Bello, U.M.; Whitney, S.L. Measures of Balance and Falls Risk Prediction in People with Parkinson’s Disease: A Systematic Review of Psychometric Properties. Clin. Rehabil. 2019, 33, 1949–1962. [Google Scholar]
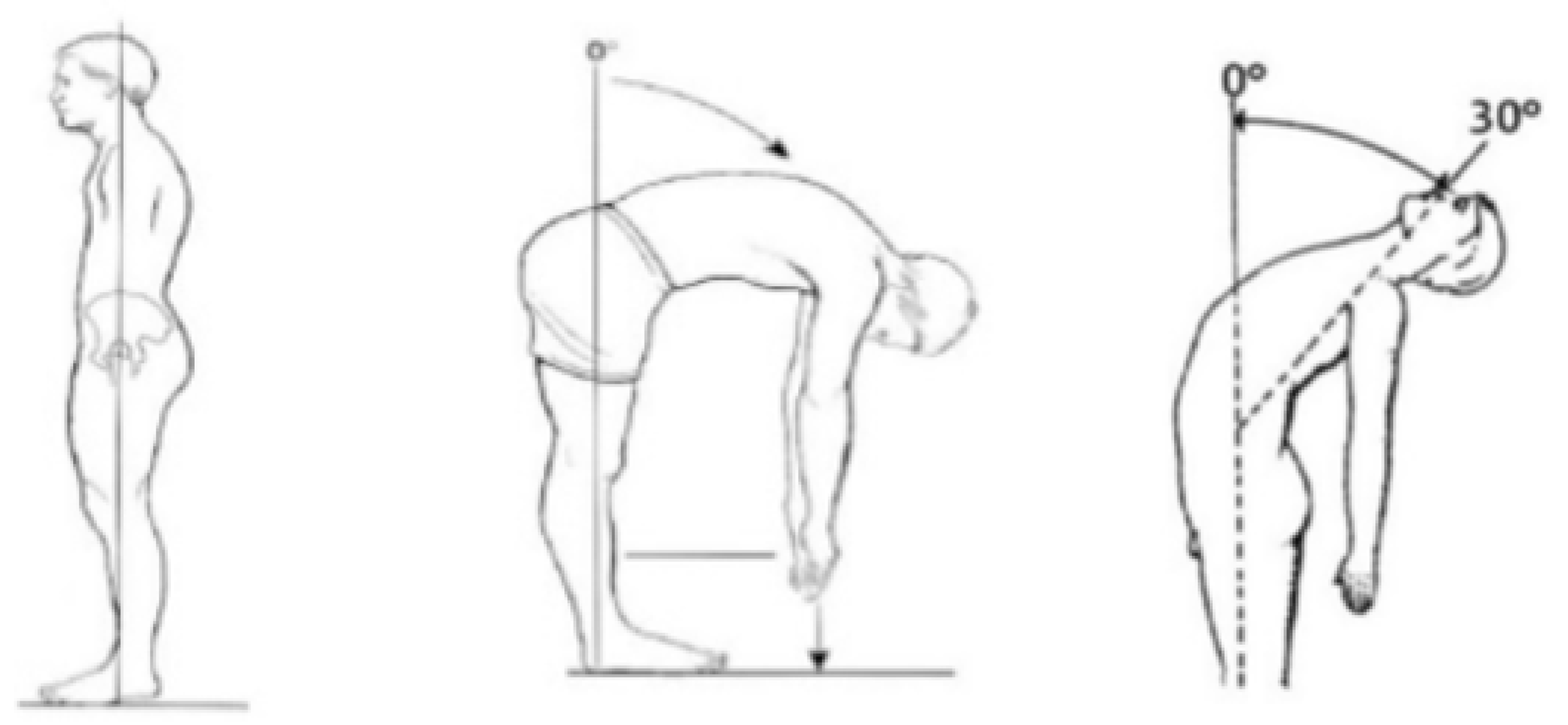
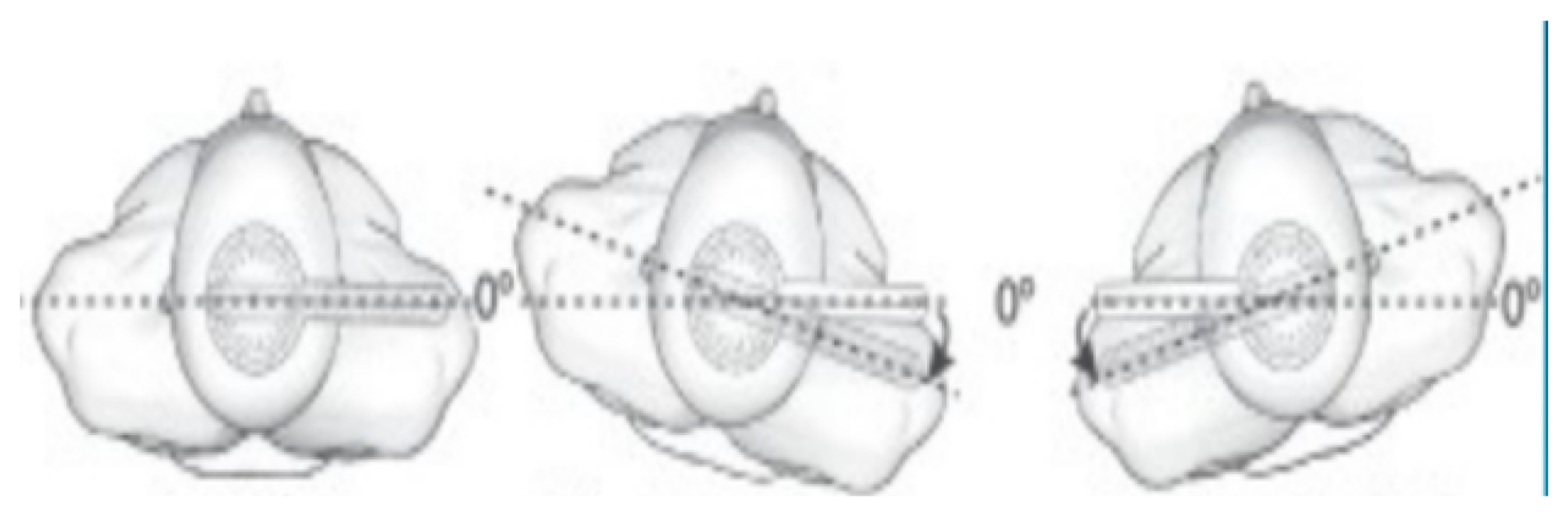
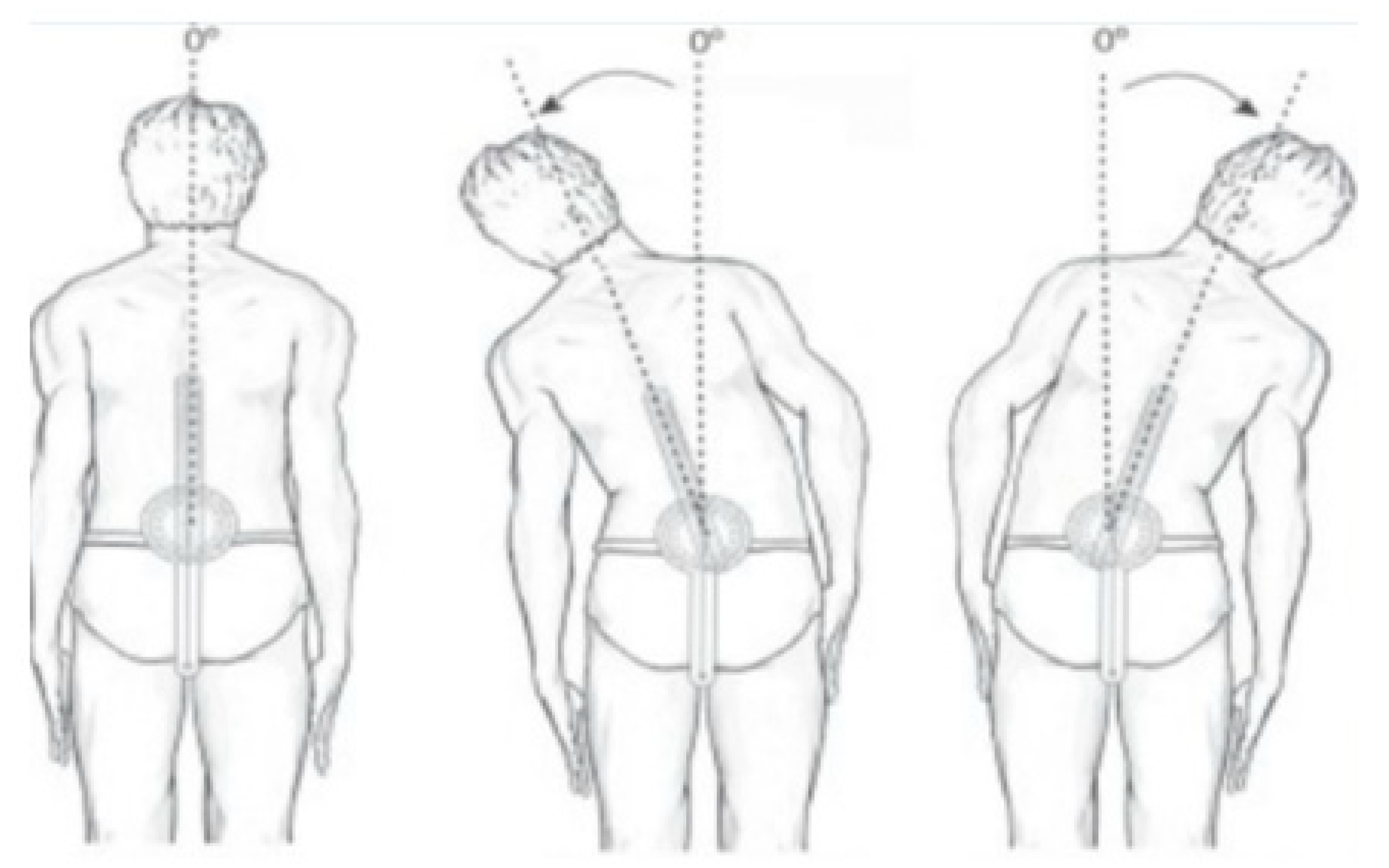
| Variable | Mean Score ± SD | Range |
|---|---|---|
| Disease duration (months) | 55.4 ± 14.3 | 30–75 |
| UPDRS-III * | 22 ± 8 | 10–32 |
| Schwab and England | 80–100 | |
| 80% | n = 26 | |
| 90% | n = 7 | |
| 100% | n = 3 | |
| Hoehn and Yahr | I-III | |
| IB | n = 8 | |
| II | n = 24 | |
| III | n = 4 |
| Variable | Mean Score ± SD | Range |
|---|---|---|
| Trunk flexion (°) | 80.9 ± 13.2 | 70–100 |
| Trunk extension (°) | 26.4 ± 7.4 | 15–35 |
| Trunk lateral flexion (right) (°) | 28.9 ± 8.1 | 20–40 |
| Trunk lateral flexion (left) (°) | 26.6 ± 7.6 | 20–40 |
| Trunk rotation (right) (°) | 32.2 ± 10.7 | 25–45 |
| Trunk rotation (left) (°) | 30.2 ± 18.7 | 25–45 |
| W/BW Extensors 30°/s | 20.2 ± 13.7 | 10–40 |
| W/BW Flexors 30°/s | 14.9 ± 7.9 | 10–25 |
| W/BW Extensors 45°/s | 17.2 ± 10.1 | 5–35 |
| W/BW Flexors 45°/s | 11.5 ± 5.5 | 5–25 |
| W/BW Extensors 60°/s | 19.0 ± 11.6 | 5–35 |
| W/BW Flexors 60°/s | 13.7 ± 5.7 | 5–25 |
| GUG | I–III | |
| I | n = 14 (38.89%) | |
| II | n = 17 (47.22%) | |
| III | n = 5 (13.89%) | |
| PDQ-39 | 25.2 ± 7.0 | 20–40 |
| EuroQoL-5D | 70.5 ± 10.0 | 50–100 |
| Trunk Flexion | Trunk Extension | Trunk Rotation (Right) | Trunk Rotation (Left) | Trunk Lateral Flexion (Right) | Trunk Lateral Flexion (Left) | |
|---|---|---|---|---|---|---|
| W/BW Extensors 30°/s | r = −0.687, p = 0.005 * | r = −0.534, p = 0.042 * | r = −0.032, p = 0.849 | r = −0.067, p = 0.692 | r = −0.156, p = 0.356 | r = −0.290, p = 0.082 |
| W/BW Flexors 30°/s | r = −0.189, p = 0.263 | r = −0.017, p = 0.918 | r = −0.082, p = 0.628 | r = −0.067, p = 0.694 | r = −0.174, p = 0.303 | r = −0.015, p = 0.931 |
| W/BW Extensors 45°/s | r = −0.510, p = 0.006 * | r = −0.410, p = 0.005 * | r = −0.030, − = 0.861 | r = −0.025, p = 0.885 | r = −0.082, p = 0.628 | r = −0.180, p = 0.285 |
| W/BW Flexors 45°/s | r = −0.103, p = 0.545 | r = −0.093, p = 0.586 | r = −0.267, p = 0.111 | r = −0.133, p = 0.433 | r = −0.062, p = 0.718 | r = −0.116, p = 0.495 |
| W/BW Extensors 60°/s | r = −0.210, p = 0.213 | r = −0.091, p = 0.594 | r = −0.035, p = 0.873 | r = −0.043, p = 0.802 | r = −0.027, p = 0.875 | r = −0.192, p = 0.256 |
| W/BW Flexors 60°/s | r = −0.027, p = 0.873 | r = −0.094, p = 0.579 | r = −0.168, p = 0.322 | r = −0.225, p = 0.181 | r = −0.153, p = 0.366 | r = −0.211, p = 0.210 |
| GUG | r = −0.444, p = 0.004 * | r = −0.564, p = 0.042 * | r = −0.651, p = 0.033 * | r = −0.677, p = 0.022 * | r = −0.189, p = 0.263 | r = −0.062, p = 0.718 |
| Trunk Movement | PDQ-39 Total Score | EuroQoL-5D |
|---|---|---|
| Flexion | r = −0.492, p = 0.018 * | r = 0.245, p = 0.150 |
| Extension | r = −0.549, p = 0.049 * | r = 0.265, p = 0.119 |
| Rotation (right) | r = −0.482, p = 0.021 * | r = 0.324, p = 0.054 |
| Rotation (left) | r = −0.447, p = 0.038 * | r = 0.249, p = 0.142 |
| Lateral flexion (right) | r = −0.365, p = 0.448 | r = 0.015, p = 0.930 |
| Lateral flexion (left) | r = −0.476, p = 0.403 | r = 0.007, p = 0.969 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cano-de-la-Cuerda, R.; Vela-Desojo, L.; Moreno-Verdú, M.; Ferreira-Sánchez, M.d.R.; Macías-Macías, Y.; Miangolarra-Page, J.C. Trunk Range of Motion Is Related to Axial Rigidity, Functional Mobility and Quality of Life in Parkinson’s Disease: An Exploratory Study. Sensors 2020, 20, 2482. https://doi.org/10.3390/s20092482
Cano-de-la-Cuerda R, Vela-Desojo L, Moreno-Verdú M, Ferreira-Sánchez MdR, Macías-Macías Y, Miangolarra-Page JC. Trunk Range of Motion Is Related to Axial Rigidity, Functional Mobility and Quality of Life in Parkinson’s Disease: An Exploratory Study. Sensors. 2020; 20(9):2482. https://doi.org/10.3390/s20092482
Chicago/Turabian StyleCano-de-la-Cuerda, Roberto, Lydia Vela-Desojo, Marcos Moreno-Verdú, María del Rosario Ferreira-Sánchez, Yolanda Macías-Macías, and Juan Carlos Miangolarra-Page. 2020. "Trunk Range of Motion Is Related to Axial Rigidity, Functional Mobility and Quality of Life in Parkinson’s Disease: An Exploratory Study" Sensors 20, no. 9: 2482. https://doi.org/10.3390/s20092482
APA StyleCano-de-la-Cuerda, R., Vela-Desojo, L., Moreno-Verdú, M., Ferreira-Sánchez, M. d. R., Macías-Macías, Y., & Miangolarra-Page, J. C. (2020). Trunk Range of Motion Is Related to Axial Rigidity, Functional Mobility and Quality of Life in Parkinson’s Disease: An Exploratory Study. Sensors, 20(9), 2482. https://doi.org/10.3390/s20092482






