Gait Characteristics Based on Shoe-Type Inertial Measurement Units in Healthy Young Adults during Treadmill Walking
Abstract
1. Introduction
2. Methods
2.1. Study Population
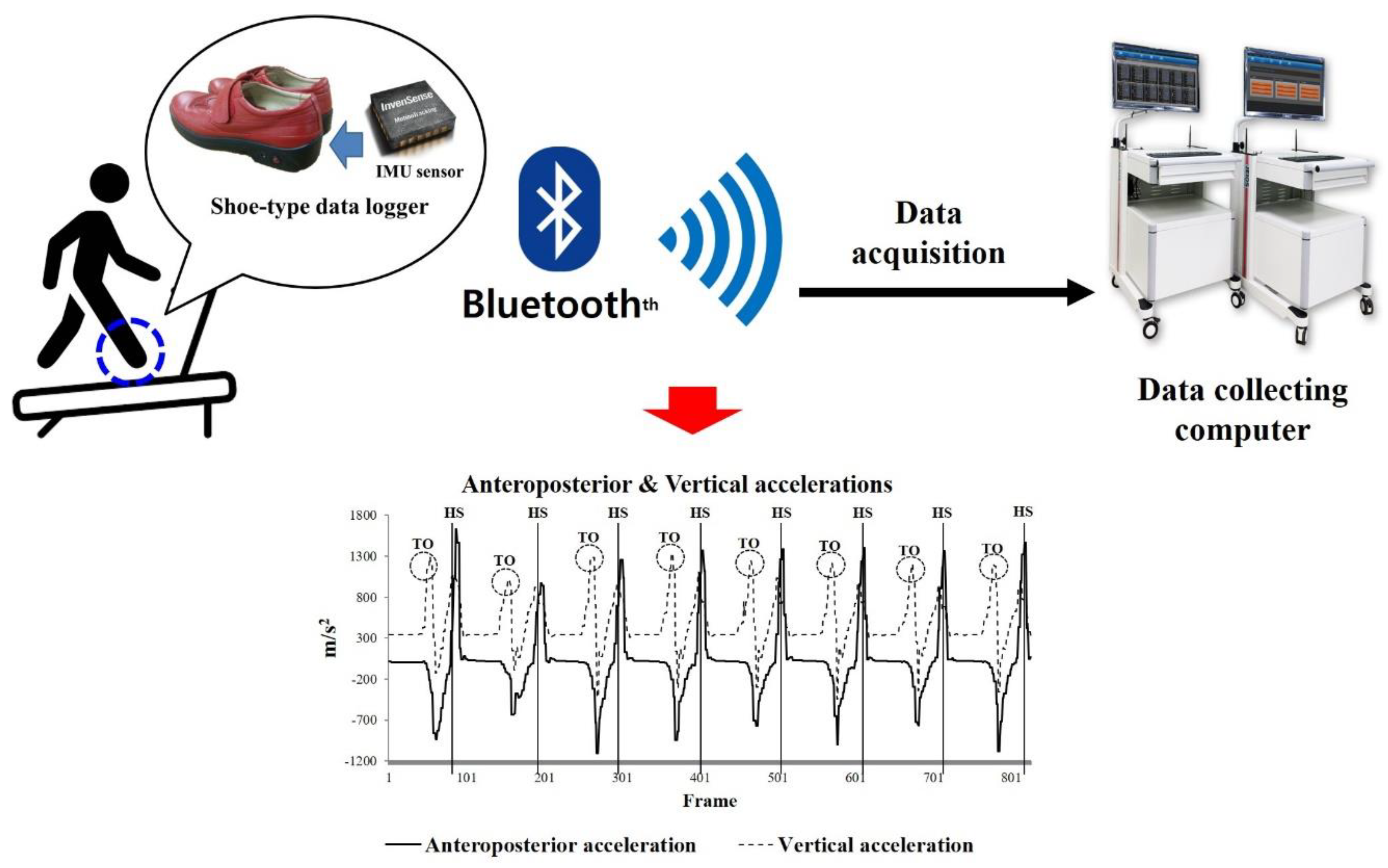
2.2. Instrumentation
2.3. Test Procedures
2.4. Data Analyses
2.5. Statistical Analyses
3. Results
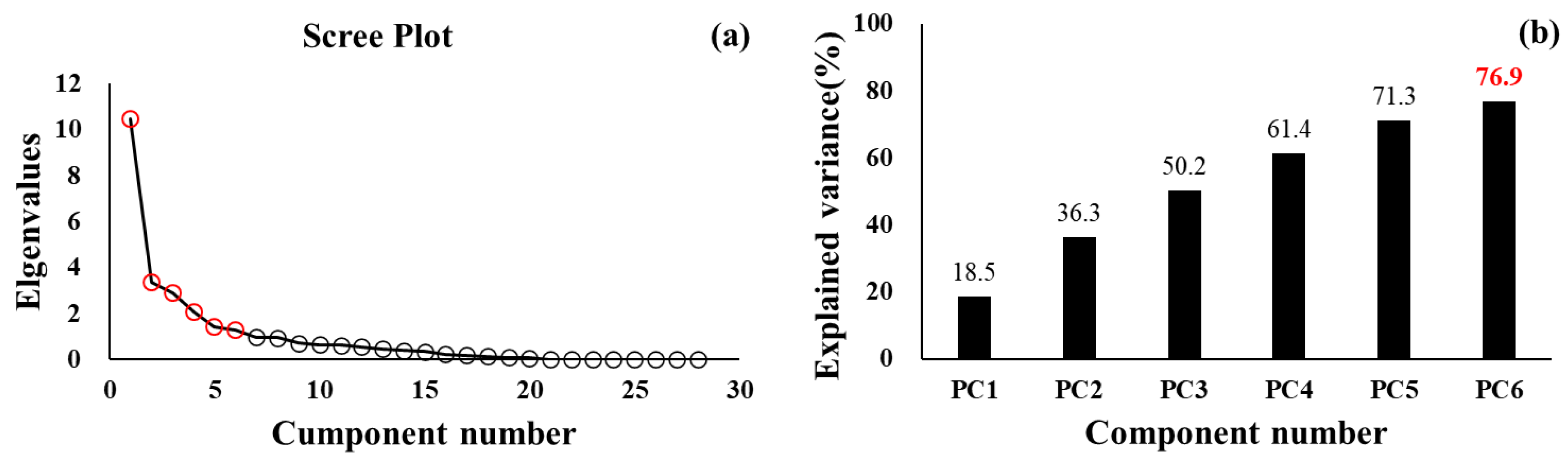
3.1. PCA for Healthy Young Adults
3.2. Gait-Related Variables for All Participants
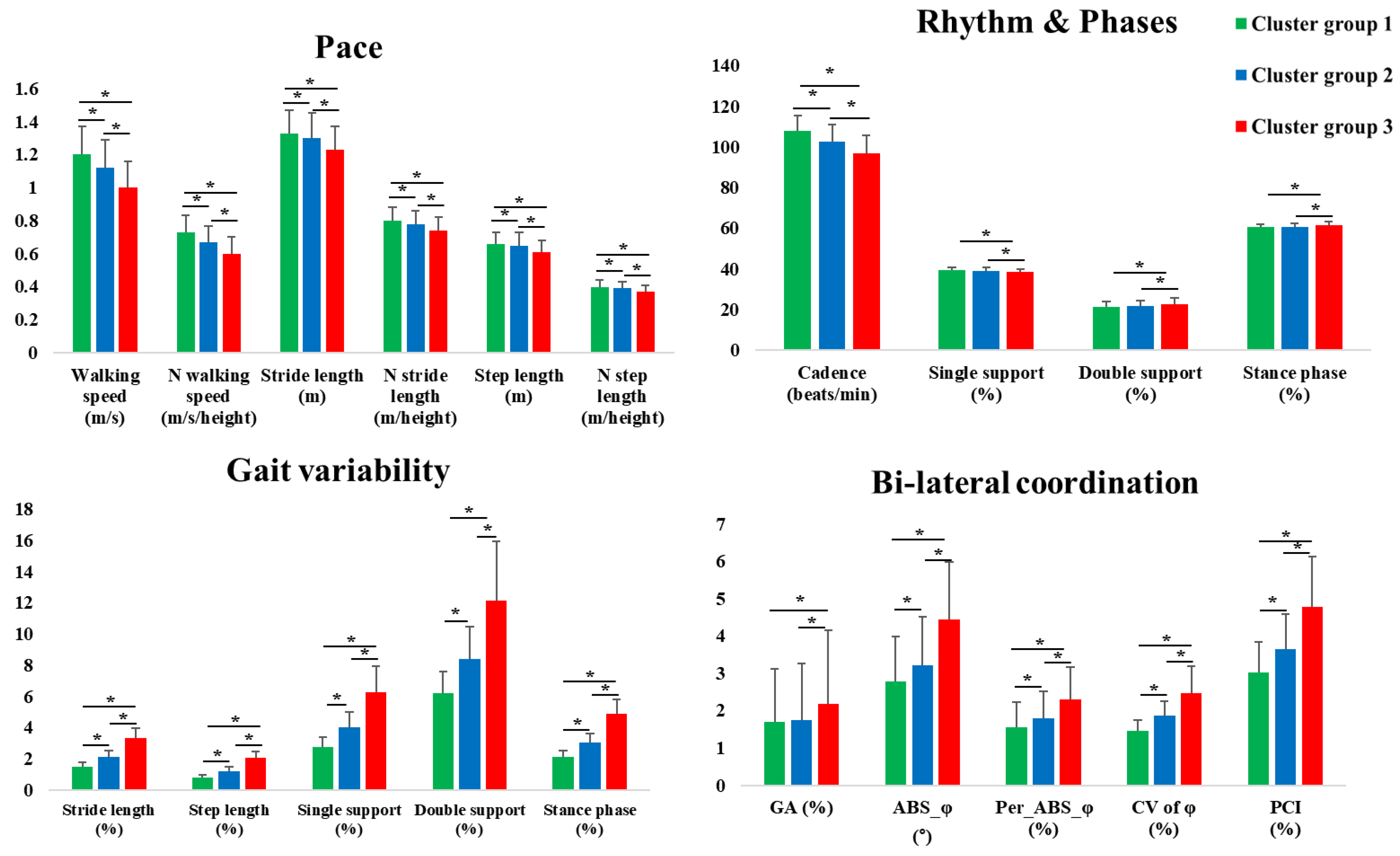
3.3. Comparisons of the Gait Characteristics According to the Cluster Groups
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Data Availability
References
- Hollman, J.H.; McDade, E.M.; Petersen, R.C. Normative spatiotemporal gait parameters in older adults. Gait Posture 2011, 34, 111–118. [Google Scholar] [CrossRef]
- Herssens, N.; Verbecque, E.; Hallemans, A.; Vereeck, L.; Van Rompaey, V.; Saeys, W. Do spatiotemporal parameters and gait variability differ across the lifespan of healthy adults? A systematic review. Gait Posture 2018, 64, 181–190. [Google Scholar] [CrossRef]
- McKay, M.J.; Baldwin, J.N.; Ferreira, P.; Simic, M.; Vanicek, N.; Wojciechowski, E.; Mudge, A.; Burns, J.; 1000 Norms Project Consortium. Spatiotemporal and plantar pressure patterns of 1000 healthy individuals aged 3–101 years. Gait Posture 2017, 58, 78–87. [Google Scholar] [CrossRef]
- Aboutorabi, A.; Arazpour, M.; Bahramizadeh, M.; Hutchins, S.W.; Fadayevatan, R. The effect of aging on gait parameters in able-bodied older subjects: A literature review. Aging Clin. Exp. Res. 2016, 28, 393–405. [Google Scholar] [CrossRef] [PubMed]
- Oberg, T.; Karsznia, A.; Oberg, K. Basic gait parameters: Reference data for normal subjects, 10–79 years of age. J. Rehabil. Res. Dev. 1993, 30, 210. [Google Scholar]
- Oh-Park, M.; Holtzer, R.; Xue, X.; Verghese, J. Conventional and robust quantitative gait norms in community-dwelling older adults. J. Am. Geriatr. Soc. 2010, 58, 1512–1518. [Google Scholar] [CrossRef] [PubMed]
- Almarwani, M.; VanSwearingen, J.M.; Perera, S.; Sparto, P.J.; Brach, J.S. Challenging the motor control of walking: Gait variability during slower and faster pace walking conditions in younger and older adults. Arch. Gerontol. Geriatr. 2016, 66, 54–61. [Google Scholar] [CrossRef] [PubMed]
- Mirelman, A.; Bernad-Elazari, H.; Nobel, T.; Thaler, A.; Peruzzi, A.; Plotnik, M.; Giladi, N.; Hausdorff, J.M. Effects of aging on arm swing during gait: The role of gait speed and dual tasking. PLoS ONE 2015, 10, e0136043. [Google Scholar] [CrossRef]
- Rennie, L.; Löfgren, N.; Moe-Nilssen, R.; Opheim, A.; Dietrichs, E.; Franzén, E. The reliability of gait variability measures for individuals with Parkinson’s disease and healthy older adults–the effect of gait speed. Gait Posture 2018, 62, 505–509. [Google Scholar] [CrossRef]
- Plotnik, M.; Giladi, N.; Hausdorff, J.M. A new measure for quantifying the bilateral coordination of human gait: Effects of aging and Parkinson’s disease. Exp. Brain Res. 2007, 181, 561–570. [Google Scholar] [CrossRef]
- Plotnik, M.; Bartsch, R.P.; Zeev, A.; Giladi, N.; Hausdorff, J.M. Effects of walking speed on asymmetry and bilateral coordination of gait. Gait Posture 2013, 38, 864–869. [Google Scholar] [CrossRef] [PubMed]
- Williams, A.J.; Peterson, D.S.; Earhart, G.M. Gait coordination in Parkinson disease: Effects of step length and cadence manipulations. Gait Posture 2013, 38, 340–344. [Google Scholar] [CrossRef] [PubMed]
- Peterson, D.S.; Plotnik, M.; Hausdorff, J.M.; Earhart, G.M. Evidence for a relationship between bilateral coordination during complex gait tasks and freezing of gait in Parkinson’s disease. Parkinsonism Relat. Disord. 2012, 18, 1022–1026. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Moreno-Hernández, A.; Rodríguez-Reyes, G.; Quiñones-Urióstegui, I.; Núñez-Carrera, L.; Pérez-SanPablo, A.I. Temporal and spatial gait parameters analysis in non-pathological Mexican children. Gait Posture 2010, 32, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Al-Obaidi, S.; Wall, J.C.; Al-Yaqoub, A.; Al-Ghanim, M. Basic gait parameters: A comparison of reference data for normal subjects 20 to 29 years of age from Kuwait and Scandinavia. J. Rehabil. Res. Dev. 2003, 40, 361. [Google Scholar] [CrossRef]
- Hartmann, A.; Luzi, S.; Murer, K.; de Bie, R.A.; de Bruin, E.D. Concurrent validity of a trunk tri-axial accelerometer system for gait analysis in older adults. Gait Posture 2009, 29, 444–448. [Google Scholar] [CrossRef]
- Hollman, J.H.; Watkins, M.K.; Imhoff, A.C.; Braun, C.E.; Akervik, K.A.; Ness, D.K. A comparison of variability in spatiotemporal gait parameters between treadmill and overground walking conditions. Gait Posture 2016, 43, 204–209. [Google Scholar] [CrossRef]
- Highsmith, M.J.; Andrews, C.R.; Millman, C.; Fuller, A.; Kahle, J.T.; Klenow, T.D.; Orriola, J.J. Gait training interventions for lower extremity amputees: A systematic literature review. Technol. Innov. 2016, 18, 99. [Google Scholar] [CrossRef]
- Booth, A.T.; Buizer, A.I.; Meyns, P.; Oude Lansink, I.L.; Steenbrink, F.; van der Krogt, M.M. The efficacy of functional gait training in children and young adults with cerebral palsy: A systematic review and meta-analysis. Dev. Med. Child Neurol. 2018, 60, 866–883. [Google Scholar] [CrossRef]
- Berra, E.; De Icco, R.; Avenali, M.; Dagna, C.; Cristina, S.; Pacchetti, C.; Tassorelli, C. Body weight support combined with treadmill in the rehabilitation of parkinsonian gait: A review of literature and new data from a controlled study. Front. Neurol. 2019, 9, 1066. [Google Scholar] [CrossRef]
- Galli, M.; Cimolin, V.; De Pandis, M.F.; Le Pera, D.; Sova, I.; Albertini, G.; Franceschini, M. Robot-assisted gait training versus treadmill training in patients with Parkinson’s disease: A kinematic evaluation with gait profile score. Funct. Neurol. 2016, 31, 163. [Google Scholar]
- Kang, M.G.; Yun, S.J.; Shin, H.I.; Kim, E.; Lee, H.H.; Oh, B.M.; Seo, H.G. Effects of robot-assisted gait training in patients with Parkinson’s disease: Study protocol for a randomized controlled trial. Trials 2019, 20, 15. [Google Scholar] [CrossRef] [PubMed]
- Lo, A.C.; Chang, V.C.; Gianfrancesco, M.A.; Friedman, J.H.; Patterson, T.S.; Benedicto, D.F. Reduction of freezing of gait in Parkinson’s disease by repetitive robot-assisted treadmill training: A pilot study. J. Neuroeng. Rehabil. 2010, 7, 51. [Google Scholar] [CrossRef]
- Paker, N.; Bugdayci, D.; Goksenoglu, G.; Sen, A.; Kesiktas, N. Effects of robotic treadmill training on functional mobility, walking capacity, motor symptoms and quality of life in ambulatory patients with Parkinson’s disease: A preliminary prospective longitudinal study. NeuroRehabilitation 2013, 33, 323–328. [Google Scholar] [CrossRef]
- Pilleri, M.; Weis, L.; Zabeo, L.; Koutsikos, K.; Biundo, R.; Facchini, S.; Antonini, A. Overground robot assisted gait trainer for the treatment of drug-resistant freezing of gait in Parkinson disease. J. Neurol. Sci. 2015, 355, 75–78. [Google Scholar] [CrossRef]
- Nam, K.Y.; Kim, H.J.; Kwon, B.S.; Park, J.W.; Lee, H.J.; Yoo, A. Robot-assisted gait training (Lokomat) improves walking function and activity in people with spinal cord injury: A systematic review. J. Neuroeng. Rehabil. 2017, 14, 24. [Google Scholar] [CrossRef]
- Del Din, S.; Godfrey, A.; Rochester, L. Validation of an accelerometer to quantify a comprehensive battery of gait characteristics in healthy older adults and Parkinson’s disease: Toward clinical and at home use. IEEE J. Biomed. Health Inf. 2016, 20, 838–847. [Google Scholar] [CrossRef] [PubMed]
- Esser, P.; Dawes, H.; Collett, J.; Feltham, M.G.; Howells, K. Validity and inter-rater reliability of inertial gait measurements in Parkinson’s disease: A pilot study. J. Neurosci. Meth. 2012, 205, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Trojaniello, D.; Cereatti, A.; Pelosin, E.; Avanzino, L.; Mirelman, A.; Hausdorff, J.M.; Della, C.U. Estimation of step-by-step spatiotemporal parameters of normal and impaired gait using shank-mounted magneto-inertial sensors: Application to elderly, hemiparetic, parkinsonian and choreic gait. J. Neuroeng. Rehabil. 2014, 11, 152. [Google Scholar] [CrossRef] [PubMed]
- Mariani, B.; Hoskovec, C.; Rochat, S.; Büla, C.; Penders, J.; Aminian, K. 3D gait assessment in young and elderly subjects using foot-worn inertial sensors. J. Biomech. 2010, 43, 2999–3006. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.Y.; Kim, Y.K.; Park, J.Y. Reliability of 3D-inertia measurement unit based shoes in gait analysis. Korean J. Sport Biomech. 2015, 25, 123–130. [Google Scholar] [CrossRef][Green Version]
- Kim, Y.K.; Joo, J.Y.; Jeong, S.H.; Jeon, J.H.; Jung, D.Y. Effects of walking speed and age on the directional stride regularity and gait variability in treadmill walking. J. Mech. Sci. Technol. 2016, 30, 2899–2906. [Google Scholar] [CrossRef]
- Lee, M.; Youm, C.; Jeon, J.; Cheon, S.M.; Park, H. Validity of shoe-type inertial measurement units for Parkinson’s disease patients during treadmill walking. J. Neuroeng. Rehabil. 2018, 15, 38. [Google Scholar] [CrossRef] [PubMed]
- Preece, S.J.; Kenney, L.P.; Major, M.J.; Dias, T.; Lay, E.; Fernandes, B.T. Automatic identification of gait events using an instrumented sock. J. Neuroeng. Rehabil. 2011, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Oyeyemi, A.L.; Umar, M.; Oguche, F.; Aliyu, S.U.; Oyeyemi, A.Y. Accelerometer-determined physical activity and its comparison with the international physical activity questionnaire in a sample of Nigerian adults. PLoS ONE 2014, 9, e87233. [Google Scholar] [CrossRef] [PubMed]
- Lord, S.; Galna, B.; Verghese, J.; Coleman, S.; Burn, D.; Rochester, L. Independent domains of gait in older adults and associated motor and nonmotor attributes: Validation of a factor analysis approach. J. Gerontol. Series A 2012, 68, 820–827. [Google Scholar] [CrossRef] [PubMed]
- Verlinden, V.J.; van der Geest, J.N.; Hofman, A.; Ikram, M.A. Cognition and gait show a distinct pattern of association in the general population. Alzheimer Dement. 2014, 10, 328–335. [Google Scholar] [CrossRef]
- Wilkinson, L.; Engelman, L.; Corter, J.; Coward, M. Chapter 4: Cluster analysis. Systat 2009, 13. [Google Scholar]
- Holt, K.G.; Jeng, S.F.; Ratcliffe, R.; Hamill, J. Energetic cost and stability during human walking at the preferred stride frequency. J. Motor Behav. 1995, 27, 164–178. [Google Scholar] [CrossRef]
- Wuehr, M.; Schniepp, R.; Pradhan, C.; Ilmberger, J.; Strupp, M.; Brandt, T.; Jahn, K. Differential effects of absent visual feedback control on gait variability during different locomotion speeds. Exp. Brain Res. 2013, 224, 287–294. [Google Scholar] [CrossRef]
- Nanhoe-Mahabier, W.; Snijders, A.H.; Delval, A.; Weerdesteyn, V.; Duysens, J.; Overeem, S.; Bloem, B.R. Walking patterns in Parkinson’s disease with and without freezing of gait. Neuroscience 2011, 182, 217–224. [Google Scholar] [CrossRef] [PubMed]
- Cristopoliski, F.; Barela, J.A.; Leite, N.; Fowler, N.E.; Rodacki, A.L.F. Stretching exercise program improves gait in the elderly. Gerontology 2009, 55, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Newell, D.; Shead, V.; Sloane, L. Changes in gait and balance parameters in elderly subjects attending an 8-week supervised Pilates programme. J. Bodyw. Mov. Ther. 2012, 16, 549–554. [Google Scholar] [CrossRef] [PubMed]
- Oh-Park, M.; Holtzer, R.; Mahoney, J.; Wang, C.; Verghese, J. Effect of treadmill training on specific gait parameters in older adults with frailty: Case series. J. Geriatr. Phys. Ther. 2011, 34, 184–188. [Google Scholar] [CrossRef] [PubMed]
- Pau, M.; Leban, B.; Collu, G.; Migliaccio, G.M. Effect of light and vigorous physical activity on balance and gait of older adults. Arch. Gerontol. Geriatr. 2014, 59, 568–573. [Google Scholar] [CrossRef] [PubMed]
- Persch, L.N.; Ugrinowitsch, C.; Pereira, G.; Rodacki, A.L. Strength training improves fall-related gait kinematics in the elderly: A randomized controlled trial. Clin. Biomechan. 2009, 24, 819–825. [Google Scholar] [CrossRef] [PubMed]
- Nasirzade, A.; Sadeghi, H.; Mokhtarinia, H.R.; Rahimi, A. A review of selected factors affecting gait symmetry. Phys. Treat. Specific Phys. Ther. J. 2017, 7, 3–12. [Google Scholar] [CrossRef]
- Perry, J.; Davids, J.R. Gait analysis: Normal and pathological function. J. Pediatr. Orthop. 1992, 12, 815. [Google Scholar] [CrossRef]
- Beauchet, O.; Allali, G.; Sekhon, H.; Verghese, J.; Guilain, S.; Steinmetz, J.P.; Kressig, R.W.; Barden, J.M.; Szturm, T.; Launay, C.P.; et al. Guidelines for assessment of gait and reference values for spatiotemporal gait parameters in older adults: The biomathics and Canadian gait consortiums initiative. Front. Hum. Neurosci. 2017, 11, 353. [Google Scholar] [CrossRef]
- Matsas, A.; Taylor, N.; McBurney, H. Knee joint kinematics from familiarised treadmill walking can be generalised to overground walking in young unimpaired subjects. Gait Posture 2000, 11, 46–53. [Google Scholar] [CrossRef]
- Parvataneni, K.; Ploeg, L.; Olney, S.J.; Brouwer, B. Kinematic, kinetic and metabolic parameters of treadmill versus overground walking in healthy older adults. Clin. Biomech. 2009, 24, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Tulchin, K.; Orendurff, M.; Karol, L. A comparison of multi-segment foot kinematics during level overground and treadmill walking. Gait Posture 2010, 31, 104–108. [Google Scholar] [CrossRef] [PubMed]
- Alton, F.; Baldey, L.; Caplan, S.; Morrissey, M.C. A kinematic comparison of overground and treadmill walking. Clin. Biomech. 1998, 13, 434–440. [Google Scholar] [CrossRef]
- Murray, M.P.; Spurr, G.B.; Sepic, S.B.; Gardner, G.M.; Mollinger, L.A. Treadmill vs. floor walking: Kinematics, electromyogram, and heart rate. J. Appl. Physiol. 1985, 59, 87–91. [Google Scholar] [CrossRef]
| All Participants N = 1478 | Male N = 558 | Female N = 920 | P-Value * | |
|---|---|---|---|---|
| Age (years) | 21.70 ± 2.37 | 22.48 ± 2.41 | 21.23 ± 2.22 | <0.001 |
| Height (cm) | 165.84 ± 8.40 | 174.17 ± 5.66 | 160.79 ± 5.12 | <0.001 |
| Body weight (kg) | 63.06 ± 13.07 | 74.20 ± 11.75 | 56.30 ± 8.36 | <0.001 |
| Body mass index (kg/m2) | 22.75 ± 3.30 | 24.41 ± 3.30 | 21.75 ± 2.87 | <0.001 |
| Waist circumference (cm) | 66.84 ± 7.87 | 72.96 ± 6.69 | 63.12 ± 5.98 | <0.001 |
| Blood pressure (mmHg) | 116.06 ± 13.06 | 123.74 ± 12.04 | 111.41 ± 11.35 | <0.001 |
| Total PA (frequency/week) | 7.91 ± 4.26 | 9.26 ± 4.39 | 7.10 ± 3.96 | <0.001 |
| Total METs for PA (METs/min) | 1923.99 ± 3899.35 | 2533.86 ± 3588.24 | 1554.10 ± 4033.68 | <0.001 |
| Variables | Components | ||||||
|---|---|---|---|---|---|---|---|
| PC1 | PC2 | PC3 | PC4 | PC5 | PC6 | ||
| Gait variability | CV of step length | 0.895 | −0.149 | −0.247 | 0.115 | −0.057 | −0.031 |
| CV of stance phase | 0.886 | −0.197 | −0.268 | 0.124 | −0.058 | −0.029 | |
| CV of stride length | 0.882 | −0.171 | −0.100 | 0.099 | −0.075 | −0.044 | |
| CV of single support | 0.810 | −0.217 | −0.356 | 0.166 | −0.056 | −0.047 | |
| CV of double support | 0.764 | −0.066 | 0.055 | 0.082 | −0.073 | −0.015 | |
| CV of φ | 0.698 | −0.296 | −0.123 | 0.314 | −0.097 | −0.075 | |
| Pace | N step length | −0.205 | 0.928 | 0.244 | −0.074 | −0.042 | 0.001 |
| N stride length | −0.207 | 0.927 | 0.250 | −0.071 | −0.040 | 0.002 | |
| Step length | −0.162 | 0.894 | 0.234 | −0.080 | 0.280 | 0.060 | |
| Stride length | −0.162 | 0.894 | 0.234 | −0.080 | 0.281 | 0.060 | |
| Walking speed | −0.343 | 0.677 | 0.553 | −0.100 | 0.181 | 0.031 | |
| N walking speed | −0.374 | 0.653 | 0.568 | −0.092 | −0.054 | −0.012 | |
| Phases and rhythm | Double support | 0.124 | −0.396 | −0.872 | 0.074 | 0.018 | −0.025 |
| Single support | −0.114 | 0.366 | 0.832 | −0.109 | 0.002 | 0.009 | |
| Stance phase | 0.121 | −0.386 | −0.822 | 0.032 | 0.037 | −0.038 | |
| Cadence | −0.441 | 0.041 | 0.738 | −0.085 | −0.045 | −0.028 | |
| Bilateral coordination and GA | Percentage_ABS_φ | 0.275 | −0.143 | −0.049 | 0.898 | −0.032 | −0.022 |
| ABS_φ | 0.275 | −0.143 | −0.049 | 0.898 | −0.032 | −0.022 | |
| PCI | 0.512 | −0.236 | −0.091 | 0.767 | −0.067 | −0.050 | |
| Φ | 0.094 | −0.044 | 0.042 | −0.675 | 0.024 | −0.034 | |
| GA | 0.060 | 0.003 | −0.045 | 0.490 | 0.028 | −0.027 | |
| Demographic characteristics | Waist circumference | −0.035 | 0.080 | −0.062 | −0.018 | 0.839 | 0.090 |
| Sex | −0.005 | −0.068 | −0.074 | 0.043 | −0.815 | −0.085 | |
| BMI | −0.116 | 0.056 | −0.159 | 0.002 | 0.738 | 0.046 | |
| Blood pressure | 0.014 | −0.009 | 0.011 | −0.012 | 0.691 | −0.031 | |
| Age | −0.104 | 0.073 | 0.054 | 0.004 | 0.298 | 0.112 | |
| Physical activities | Total METs for PA | −0.048 | −0.004 | −0.018 | −0.023 | 0.092 | 0.887 |
| Total PA frequency | −0.064 | 0.063 | 0.041 | −0.030 | 0.202 | 0.848 | |
| Explained Variance (%) | 18.5 | 17.8 | 13.9 | 11.2 | 9.9 | 5.6 | |
| Variables | All Participants (N = 1478) (min to max, 95% CI) | Male (N = 558) (min to max, 95% CI) | Female (N = 920) (min to max, 95% CI) |
|---|---|---|---|
| Walking speed (m/s) | 1.16 ± 0.18 (1.15 to 1.17) | 1.22 ± 0.18 (1.20 to 1.23) | 1.12 ± 0.17 (1.11 to 1.13) |
| N walking speed (m/s/height) | 0.70 ± 0.10 (0.69 to 0.70) | 0.70 ± 0.10 (0.69 to 0.71 | 0.70 ± 0.11 (0.69 to 0.71) |
| Stride length (m) | 1.31 ± 0.15 (1.30 to 1.32) | 1.38 ± 0.14 (1.37 to 1.39) | 1.27 ± 0.14 (1.26 to 1.28) |
| N stride length (m/height) | 0.79 ± 0.08 (0.79 to 0.79) | 0.79 ± 0.08 (0.79 to 0.80) | 0.79 ± 0.08 (0.78 to 0.79) |
| Step length (m) | 0.66 ± 0.07 (0.65 to 0.66) | 0.69 ± 0.07 (0.68 to 0.70) | 0.63 ± 0.07 (0.63 to 0.64) |
| N step length (m/height) | 0.39 ± 0.04 (0.39 to 0.40) | 0.40 ± 0.04 (0.39 to 0.40) | 0.39 ± 0.04 (0.39 to 0.40) |
| Cadence (beats/min) | 105.23 ± 8.62 (104.79 to 105.67) | 105.15 ± 7.97 (104.49 to 105.81) | 105.28 ± 8.99 (104.70 to 105.86) |
| Single support phase (%) | 39.21 ± 1.44 (39.14 to 39.28) | 39.35 ± 1.46 (39.23 to 39.47) | 39.13 ± 1.43 (39.03 to 39.22) |
| Double support phase (%) | 21.56 ± 2.74 (21.42 to 21.70) | 21.33 ± 2.64 (21.11 to 21.55) | 21.70 ± 2.80 (21.52 to 21.88) |
| Stance phase (%) | 60.77 ± 1.45 (60.70 to 60.85) | 60.68 ± 1.35 (60.57 to 60.79) | 60.83 ± 1.51 (60.73 to 60.93) |
| CV of stride length (%) | 1.84 ± 0.65 (1.80 to 1.87) | 1.76 ± 0.63 (1.70 to 1.81) | 1.89 ± 0.67 (1.85 to 1.93) |
| CV of step length (%) | 1.06 ± 0.43 (1.04 to 1.08) | 1.01 ± 0.41 (0.97 to 1.05) | 1.09 ± 0.44 (1.06 to 1.12) |
| CV of single support phase (%) | 3.48 ± 1.37 (3.41 to 3.55) | 3.30 ± 1.23 (3.20 to 3.40) | 3.59 ± 1.44 (3.50 to 3.68) |
| CV of double support phase (%) | 7.47 ± 2.64 (7.34 to 7.61) | 7.24 ± 2.70 (7.01 to 7.46) | 7.62 ± 2.59 (7.45 to 7.78) |
| CV of stance phase (%) | 2.66 ± 0.98 (2.61 to 2.71) | 2.55 ± 0.93 (2.47 to 2.63) | 2.72 ± 1.01 (2.66 to 2.79) |
| Variables | All Participants (N = 1478) (min to max, 95% CI) | Male (N = 558) (min to max, 95% CI) | Female (N = 920) (min to max, 95% CI) |
|---|---|---|---|
| GA (%) | 1.76 ± 1.51 (1.68 to 1.83) | 1.72 ± 1.62 (1.59 to 1.86) | 1.78 ± 1.45 (1.68 to 1.87) |
| φ (°) | 178.80 ± 2.23 (178.69 to 178.91) | 178.88 ± 2.26 (178.69 to 179.07) | 178.75 ± 2.21 (178.61 to 178.89) |
| ABS_φ (°) | 3.06 ± 1.32 (2.99 to 3.12) | 2.94 ± 1.32 (2.83 to 3.05) | 3.13 ± 1.32 (3.04 to 3.21) |
| Percentage_ABS_φ (%) | 1.70 ± 0.73 (1.66 to 1.74) | 1.63 ± 0.73 (1.57 to 1.69) | 1.74 ± 0.73 (1.69 to 1.79) |
| CV of φ (%) | 1.69 ± 0.49 (1.66 to 1.71) | 1.59 ± 0.46 (1.56 to 1.63) | 1.74 ± 0.49 (1.71 to 1.78) |
| PCI (%) | 3.39 ± 1.06 (3.33 to 3.44) | 3.23 ± 1.00 (3.14 to 3.31) | 3.48 ± 1.08 (3.41 to 3.55) |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.; Youm, C.; Noh, B.; Park, H. Gait Characteristics Based on Shoe-Type Inertial Measurement Units in Healthy Young Adults during Treadmill Walking. Sensors 2020, 20, 2095. https://doi.org/10.3390/s20072095
Lee M, Youm C, Noh B, Park H. Gait Characteristics Based on Shoe-Type Inertial Measurement Units in Healthy Young Adults during Treadmill Walking. Sensors. 2020; 20(7):2095. https://doi.org/10.3390/s20072095
Chicago/Turabian StyleLee, Myeounggon, Changhong Youm, Byungjoo Noh, and Hwayoung Park. 2020. "Gait Characteristics Based on Shoe-Type Inertial Measurement Units in Healthy Young Adults during Treadmill Walking" Sensors 20, no. 7: 2095. https://doi.org/10.3390/s20072095
APA StyleLee, M., Youm, C., Noh, B., & Park, H. (2020). Gait Characteristics Based on Shoe-Type Inertial Measurement Units in Healthy Young Adults during Treadmill Walking. Sensors, 20(7), 2095. https://doi.org/10.3390/s20072095








