Monitoring the Heavy Metal Lead Inside Living Drosophila with a FRET-Based Biosensor
Abstract
1. Introduction
1.1. Biosensors for Monitoring Lead in Living Organisms
1.2. Systematic Model for Lead Biosensing
2. Materials and Methods
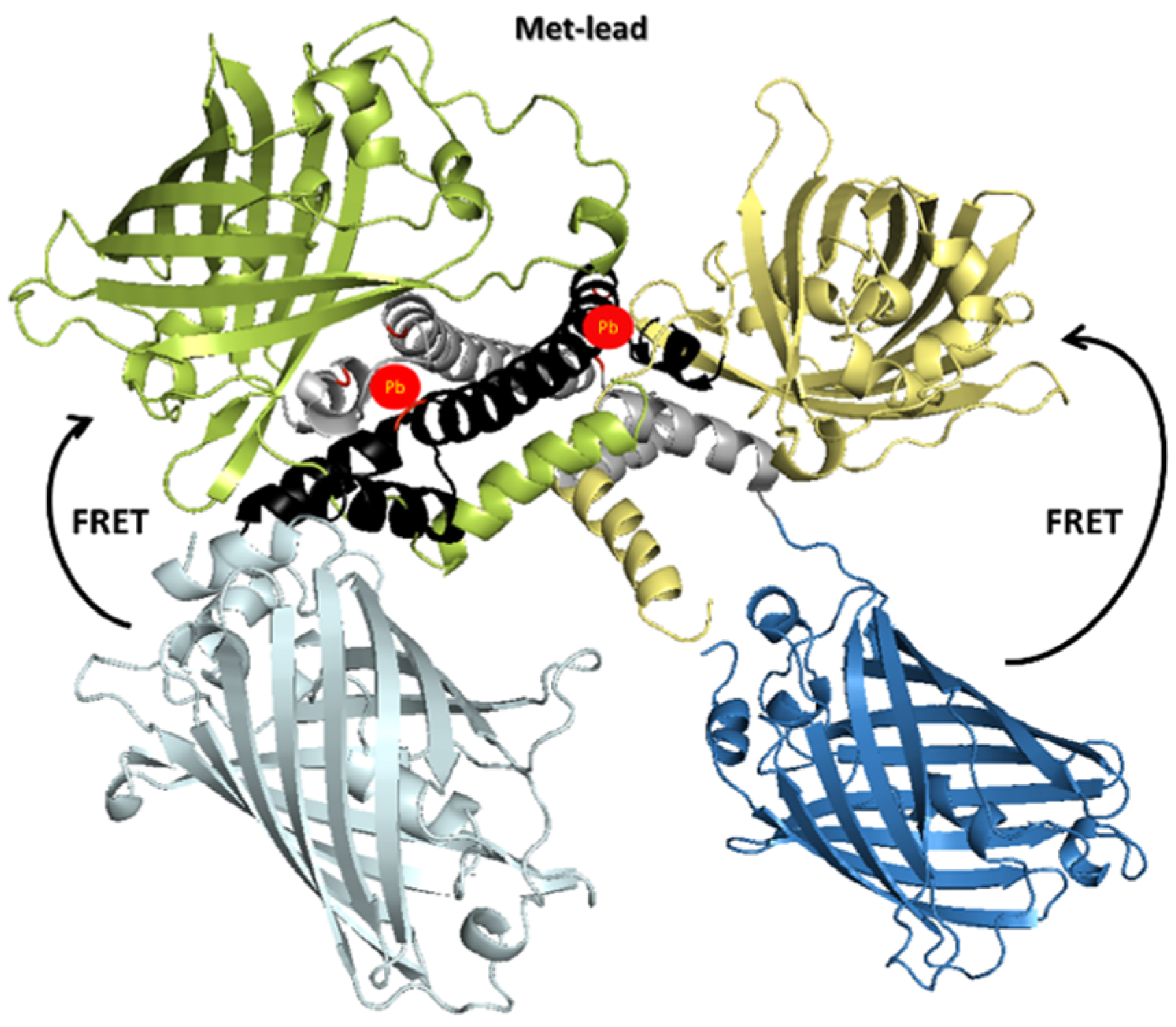
2.1. Construction and Molecular Simulation of Met-Lead
2.2. Sample Preparation
2.3. FRET Ratio Imaging
2.4. Data Analysis
3. Results
3.1. Structural Design of Met-Lead
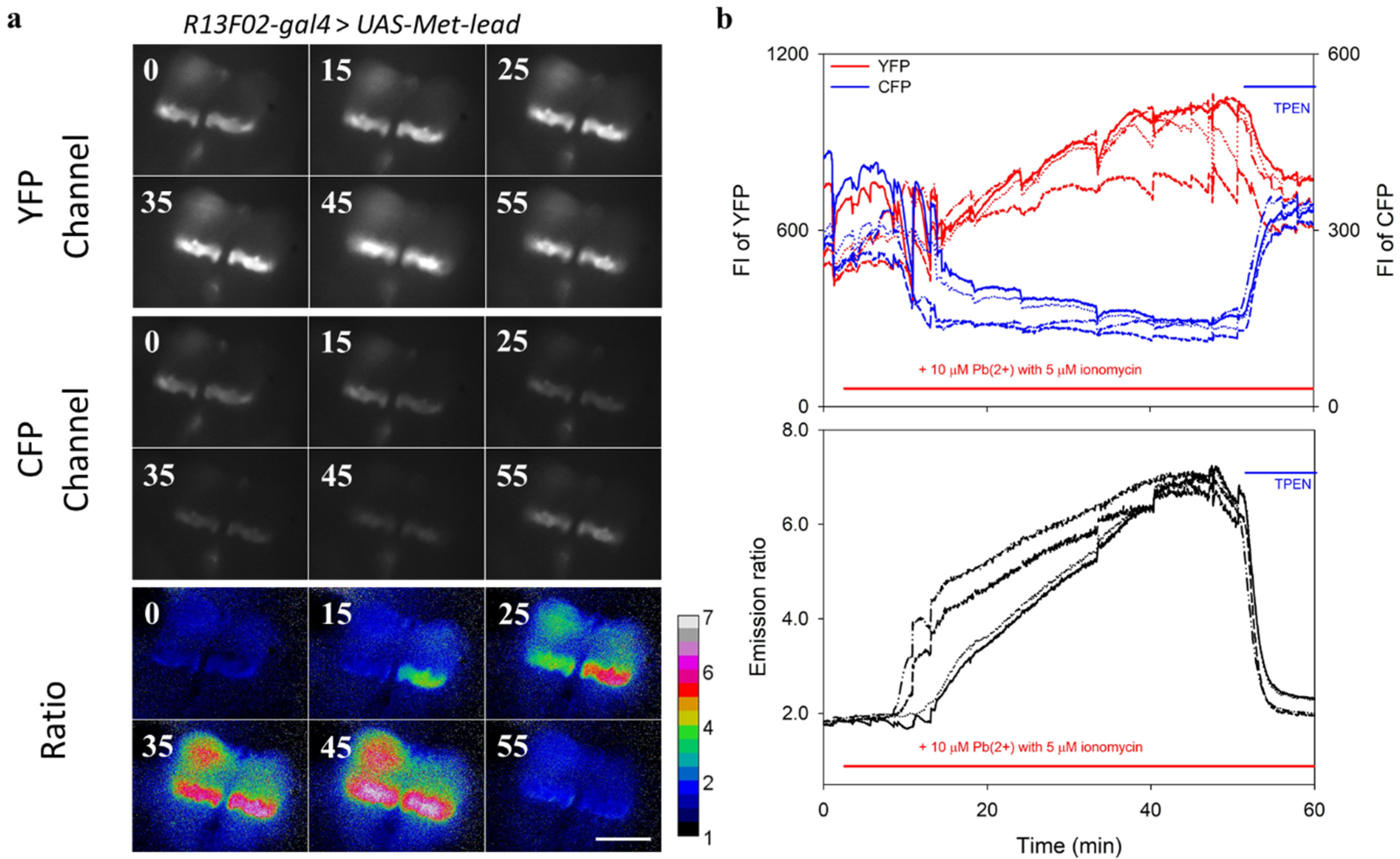
3.2. Real-Time In-Cell Biosensing of Lead
3.3. Fly Brains as Models for Lead Biosensing
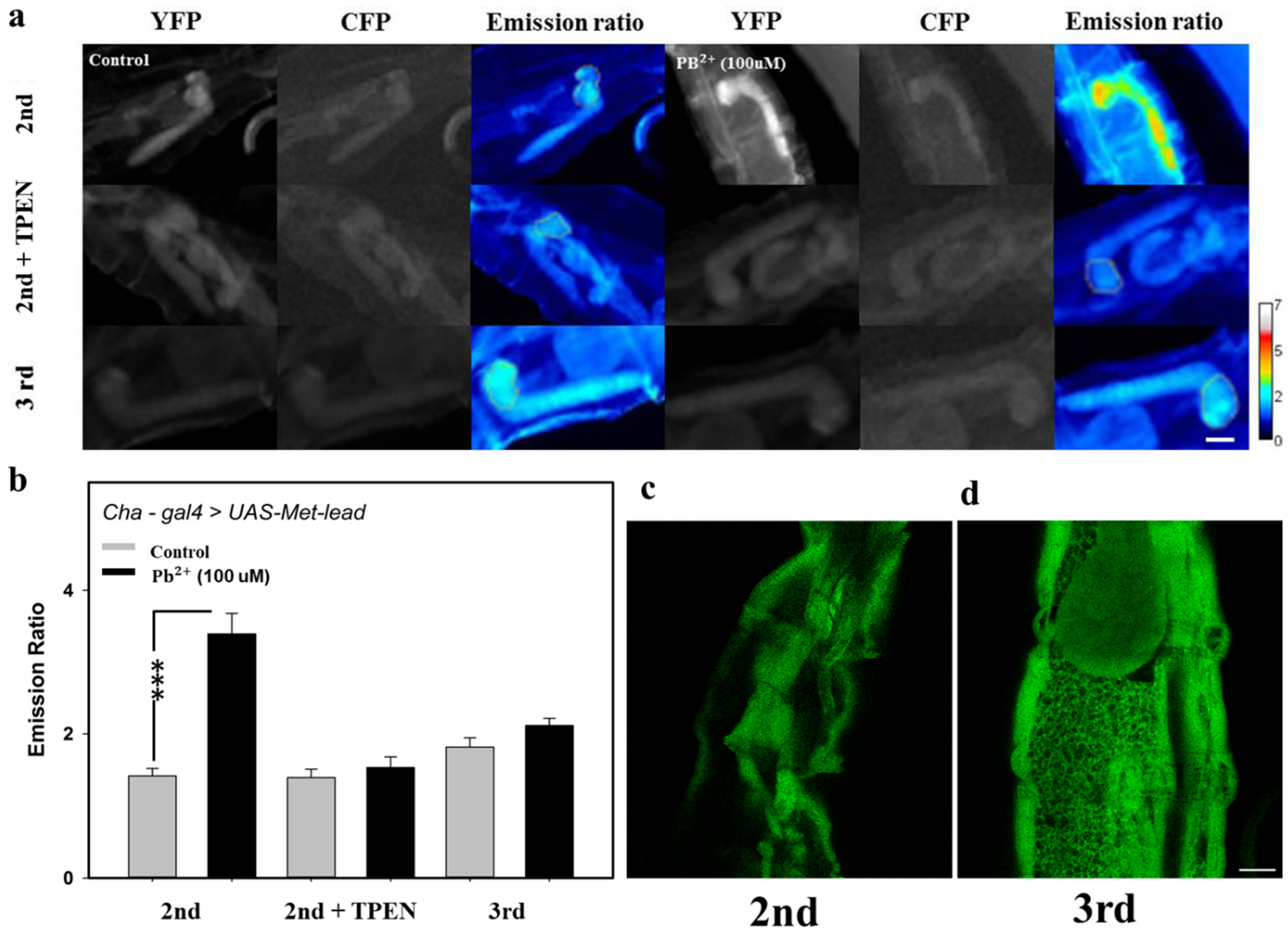
3.4. In Vivo Lead Biosensing within Adult Fly Brain Neurons
3.5. Detecting Lead In Vivo within the CNS of Fly Larvae
4. Discussion
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | blood brain barrier |
| BLL | blood lead level |
| CNS | central nervous system |
| CMOS | complementary metal–oxide–semiconductor |
| cp | circular permutation |
| CFP | cyan fluorescent protein |
| DR | dynamic range |
| FRET | fluorescence resonance energy transfer |
| FIs | fluorescent intensities |
| GE | genetically encoded |
| GFP | green fluorescent protein |
| GPCR | G protein-coupled receptor |
| IQ | intelligence quotient |
| LOD | limit of detection |
| LUT | look up table |
| NA | numeric aperture |
| PBS | phosphate-buffered saline |
| SPG | subperineurialglial |
| TPEN | N,N,N′,N′-tetrakis-(2-pyridylmethyl)ethylenediamine |
| YFP | yellow fluorescent protein |
References
- Patterson, C.C. Contaminated and natural lead environments of man. Arch. Environ. Health 1965, 11, 344–360. [Google Scholar] [CrossRef] [PubMed]
- Centers for Disease Control and Prevention (CDC). Preventing Lead Poisoning in Young Children; U.S. Department of Health and Human Services: Atlanta, GA, USA, 1991.
- Bellinger, D.C. Very low lead exposures and children’s neurodevelopment. Curr. Opin. Pediatr. 2008, 20, 172–177. [Google Scholar] [CrossRef] [PubMed]
- Reuben, A.; Caspi, A.; Belsky, D.W.; Broadbent, J.; Harrington, H.; Sugden, K.; Houts, R.M.; Ramrakha, S.; Poulton, R.; Moffitt, T.E. Association of childhood blood-lead levels with cognitive function and socioeconomic status at age 38 years and with IQ change and socioeconomic mobility between childhood and adulthood. JAMA 2017, 317, 1244–1251. [Google Scholar] [CrossRef] [PubMed]
- Rocha, A.; Trujillo, K. Neurotoxicity of low-level lead exposure: History, mechanisms of action, and behavioral effects in humans and preclinical models. Neurotoxicology 2019, 73, 58–80. [Google Scholar] [PubMed]
- Garza, A.; Vega, R.; Soto, E. Cellular mechanisms of lead neurotoxicity. Med. Sci. Monit. 2006, 12, RA57–RA65. [Google Scholar] [PubMed]
- Lanphear, B.P.; Rauch, P.; Allen, R.W.; Hornung, R.W. Low-level lead exposure and mortality in US adults: A population-based cohort study. Lancet Public Health 2018, 3, e177–e184. [Google Scholar]
- Carter, K.P.; Young, A.M.; Palmer, A.E. Fluorescent sensors for measuring metal ions in living system. Chem. Rev. 2014, 114, 4564–4601. [Google Scholar] [CrossRef]
- Chen, C.T.; Huang, W.P. A highly selective fluorescent chemosensor for lead ions. J. Am. Chem. Soc. 2002, 124, 6246–6247. [Google Scholar]
- Kerper, L.E.; Hinkle, P.M. Cellular uptake of lead is activated by depletion of intracellular calcium stores. J. Biol. Chem. 1997, 272, 8346–8352. [Google Scholar] [CrossRef]
- Chen, P.; Greenberg, B.; Taghavi, S.; Romano, C.; van der Lelie, D.; He, C. An exceptionally selective lead(II)-regulatory protein from Ralstonia metallidurans: Development of a fluorescent lead(II) probe. Angew. Chem. Int. Ed. 2005, 44, 2715–2719. [Google Scholar]
- Chiu, T.Y.; Yang, D.M. Intracellular Pb2+ content monitoring using a protein-based Pb2+ indicator. Toxicol. Sci. 2012, 126, 436–445. [Google Scholar] [CrossRef] [PubMed]
- Kuo, S.Y.; Li, H.H.; Wu, P.J.; Chen, C.P.; Huang, Y.C.; Chan, Y.H. Dual colorimetric and fluorescent sensor based on semiconducting polymer dots for ratiometric detection of lead ions in living cells. Anal. Chem. 2015, 87, 4765–4771. [Google Scholar] [CrossRef] [PubMed]
- Palmer, A.E.; Qin, Y.; Park, J.G.; McCombs, J.E. Design and application of genetically encoded biosensors. Trends Biotechnol. 2011, 29, 144–152. [Google Scholar] [CrossRef] [PubMed]
- Borremans, B.; Hobman, J.L.; Provoost, A.; Brown, N.L.; van Der Lelie, D. Cloning and functional analysis of the pbr lead resistance determinant of Ralstonia metallidurans CH34. J. Bacteriol. 2001, 183, 5651–5658. [Google Scholar] [CrossRef] [PubMed]
- Vijverberg, H.P.; Westerink, R.H. Sense in Pb2+ sensing. Toxicol. Sci. 2012, 130, 1–3. [Google Scholar] [CrossRef][Green Version]
- Yang, D.M.; Chiu, T.Y.; Fu, T.F.; Lin, C.S.; Huang, H.Y.; Chung, M.W.; Lin, Y.S.; Huang, C.C.; Manurung, R.V.; Chang, Y.F. High performance FRET biosensors for single-cell and whole-body lead detection. 2020, in press. [Google Scholar]
- Nagai, T.; Sawano, A.; Park, E.S.; Miyawaki, A. Circularly permuted green fluorescent proteins engineered to sense Ca2+. Proc. Natl. Acad. Sci. USA 2001, 98, 3197–3202. [Google Scholar] [CrossRef]
- Miyawaki, A.; Llopis, J.; Heim, R.; McCaffery, J.M.; Adams, J.A.; Ikura, M.; Tsien, R.Y. Fluorescent indicators for Ca2+ based on green fluorescent proteins and calmodulin. Nature 1997, 388, 882–887. [Google Scholar] [CrossRef]
- Nagai, T.; Yamada, S.; Tominaga, T.; Ichikawa, M.; Miyawaki, A. Expanded dynamic range of fluorescent indicators for Ca2+ by circularly permuted yellow fluorescent proteins. Proc. Natl. Acad. Sci. USA 2004, 101, 10554–10559. [Google Scholar] [CrossRef]
- Fiala, A.; Spall, T. In vivo calcium imaging of brain activity in Drosophila by transgenic cameleon expression. Sci. STKE 2003, 174, PL6. [Google Scholar] [CrossRef]
- Riemensperger, T.; Pech, U.; Dipt, S.; Fiala, A. Optical calcium imaging in the nervous system of Drosophila melanogaster. Biochim. Biophys. Acta. 2012, 1820, 1169–1178. [Google Scholar] [CrossRef] [PubMed]
- Akerboom, J.; Chen, T.W.; Wardill, T.J.; Tian, L.; Marvin, J.S.; Mutlu, S.; Calderon, N.C.; Esposti, F.; Borghuis, B.G.; Sun, X.R.; et al. Optimization of a GCaMP calcium indicator for neural activity imaging. J. Neurosci. 2012, 32, 13819–13840. [Google Scholar] [CrossRef]
- Chen, T.W.; Wardill, T.J.; Sun, Y.; Pulver, S.R.; Renninger, S.L.; Baohan, A.; Schreiter, E.R.; Kerr, R.A.; Orger, M.B.; Jayaraman, V.; et al. Ultrasensitive fluorescent proteins for imaging neuronal activity. Nature 2013, 499, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Ruden, D.M.; Chen, L.; Possidente, D.; Possidente, B.; Rasouli, P.; Wang, L.; Lu, X.; Garfinkel, M.D.; Hirsch, H.V.; Page, G.P. Genetical toxigenomics in Drosophila identifies master-modulatory loci that regulated by developmental exposure to lead. Neurotoxicology 2009, 30, 898–914. [Google Scholar] [CrossRef] [PubMed]
- Akins, J.M.; Schroeder, J.A.; Brower, D.L.; Aposhian, H.V. Evaluation of Drosophila melanogaster as an alternative animal for studying the neurotoxicity of heavy metals. Biometals 1992, 5, 11–20. [Google Scholar] [CrossRef] [PubMed]
- Stork, T.; Engelen, D.; Krudewig, A.; Silies, M.; Bainton, R.J.; Klämbt, C. Organization and function of the blood-brain barrier in Drosophila. J. Neurosci. 2008, 28, 587–597. [Google Scholar] [CrossRef]
- Hindle, S.J.; Bainton, R.J. Barrier mechanisms in the Drosophila blood-brain barrier. Front. Neurosci. 2014, 8, 414. [Google Scholar] [CrossRef]
- Hirsch, H.V.; Mercer, J.; Sambaziotis, H.; Huber, M.; Stark, D.T.; Torno-Morley, T.; Hollocher, K.; Ghiradella, H.; Ruden, D.M. Behavioral effects of chronic exposure to low levels of lead in Drosophila melanogaster. Neurotoxicology 2003, 24, 435–442. [Google Scholar] [CrossRef]
- Changela, A.; Chen, K.; Xue, Y.; Holschen, J.; Outten, C.E.; O’Halloran, T.V.; Mondragon, A. Molecular basis of metal-ion selectivity and zeptomolar sensitivity by CueR. Science 2003, 301, 1383–1387. [Google Scholar] [CrossRef]
- Chen, C.C.; Hwang, J.K.; Yang, J.M. (PS)2: Protein structure prediction server. Nucleic Acids Res. 2006, 34, W152–W157. [Google Scholar] [CrossRef]
- Huang, T.T.; Hwang, J.K.; Chen, C.H.; Chu, C.S.; Lee, C.W.; Chen, C.C. (PS)2: Protein structure prediction server version 3.0. Nucleic Acids Res. 2015, 43, W338–W342. [Google Scholar] [CrossRef] [PubMed]
- CCGI, MOE. Molecular Operating Environment (MOE), 2013.08; Chemical Computing Group Inc.: Montreal, QC, Canada, 2017. [Google Scholar]
- Erdahl, W.L.; Chapman, C.J.; Taylor, R.W.; Pfeiffer, D.R. Ionomycin, a carboxylic acid ionophore, transports Pb2+ with high selectivity. J. Biol. Chem. 2000, 275, 7071–7079. [Google Scholar] [CrossRef]
- Hu, A.; Zhang, W.; Wang, Z. Functional feedback from mushroom bodies to antennal lobes in the Drosophila olfactory pathway. Proc. Natl. Acad. Sci. USA 2010, 107, 10262–10267. [Google Scholar] [CrossRef] [PubMed]
- Mao, Z.; Davis, R.L. Eight different types of dopaminergic neurons innervate the Drosophila mushroom body neuropil: Anatomical and physiological heterogeneity. Front. Neural Circuits 2009, 3, 5. [Google Scholar] [CrossRef] [PubMed]
- Chiang, A.S.; Lin, C.Y.; Chuang, C.C.; Chang, H.M.; Hsieh, C.H.; Yeh, C.W.; Wu, C.C. Three-dimensional reconstruction of brain-wide wiring networks in Drosophila at single-cell resolution. Curr. Biol. 2011, 21, 1–11. [Google Scholar] [CrossRef] [PubMed]
- DeSalvo, M.K.; Hindle, S.J.; Rusan, Z.M.; Orng, S.; Eddison, M.; Halliwill, K.; Bainton, R.J. The Drosophila surface glia transcriptome: Evolutionary conserved blood-brain barrier processes. Front. Neurosci. 2014, 8, 346. [Google Scholar] [CrossRef] [PubMed]
- Limmer, S.; Weiler, A.; Volkenhoff, A.; Babatz, F.; Klämbt, C. The Drosophila blood-brain barrier: Development and function of a glial endothelium. Front. Neurosci. 2014, 8, 365. [Google Scholar] [CrossRef]
- Bainton, R.J.; Tsai, L.T.; Schwabe, T.; DeSalvo, M.; Gaul, U.; Heberlein, U. Moody encodes two GPCRs that regulate cocaine behaviors and blood-brain barrier permeability in Drosophila. Cell 2005, 123, 145–156. [Google Scholar] [CrossRef]
- Daneman, R.; Barres, B.A. The blood-brain barrier--lessons from moody flies. Cell 2005, 123, 9–12. [Google Scholar] [CrossRef][Green Version]
- Schwabe, T.; Bainton, R.J.; Fetter, R.D.; Heberlein, U.; Gaul, U. GPCR signaling is required for blood-brain barrier formation in Drosophila. Cell 2005, 123, 133–144. [Google Scholar] [CrossRef]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yang, D.-M.; Manurung, R.V.; Lin, Y.-S.; Chiu, T.-Y.; Lai, W.-Q.; Chang, Y.-F.; Fu, T.-F. Monitoring the Heavy Metal Lead Inside Living Drosophila with a FRET-Based Biosensor. Sensors 2020, 20, 1712. https://doi.org/10.3390/s20061712
Yang D-M, Manurung RV, Lin Y-S, Chiu T-Y, Lai W-Q, Chang Y-F, Fu T-F. Monitoring the Heavy Metal Lead Inside Living Drosophila with a FRET-Based Biosensor. Sensors. 2020; 20(6):1712. https://doi.org/10.3390/s20061712
Chicago/Turabian StyleYang, De-Ming, Robeth Viktoria Manurung, Yu-Syuan Lin, Tai-Yu Chiu, Wei-Qun Lai, Yu-Fen Chang, and Tsai-Feng Fu. 2020. "Monitoring the Heavy Metal Lead Inside Living Drosophila with a FRET-Based Biosensor" Sensors 20, no. 6: 1712. https://doi.org/10.3390/s20061712
APA StyleYang, D.-M., Manurung, R. V., Lin, Y.-S., Chiu, T.-Y., Lai, W.-Q., Chang, Y.-F., & Fu, T.-F. (2020). Monitoring the Heavy Metal Lead Inside Living Drosophila with a FRET-Based Biosensor. Sensors, 20(6), 1712. https://doi.org/10.3390/s20061712




