Using an Active Sensor to Develop New Critical Nitrogen Dilution Curve for Winter Wheat
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Design
2.2. Spectral Data Collection
2.3. Plant Sampling and Measurements
2.4. Data Processing and Analysis
3. Results
3.1. Plant N Concentrations, Dry Matter and NDRE Under Different N Levels
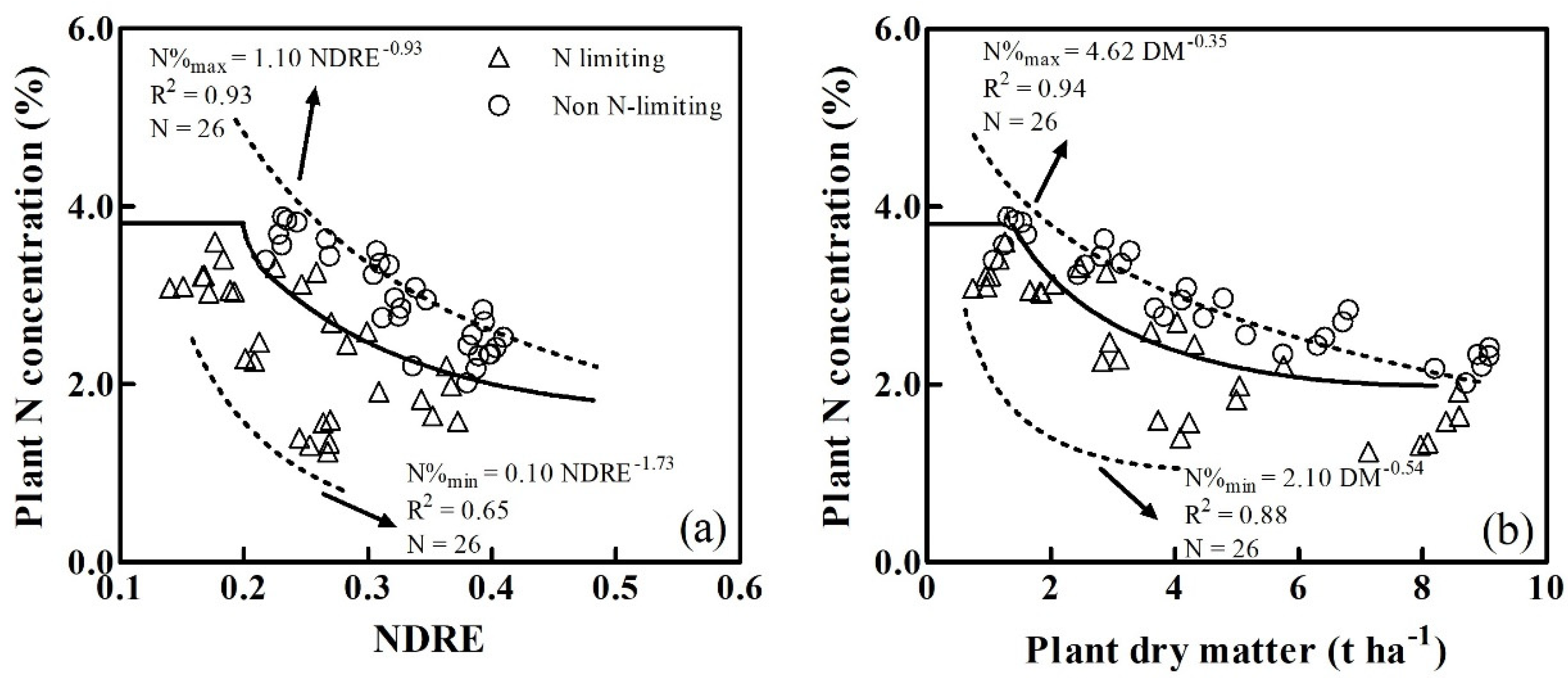
3.2. Determination of the Critical N Dilution Curves
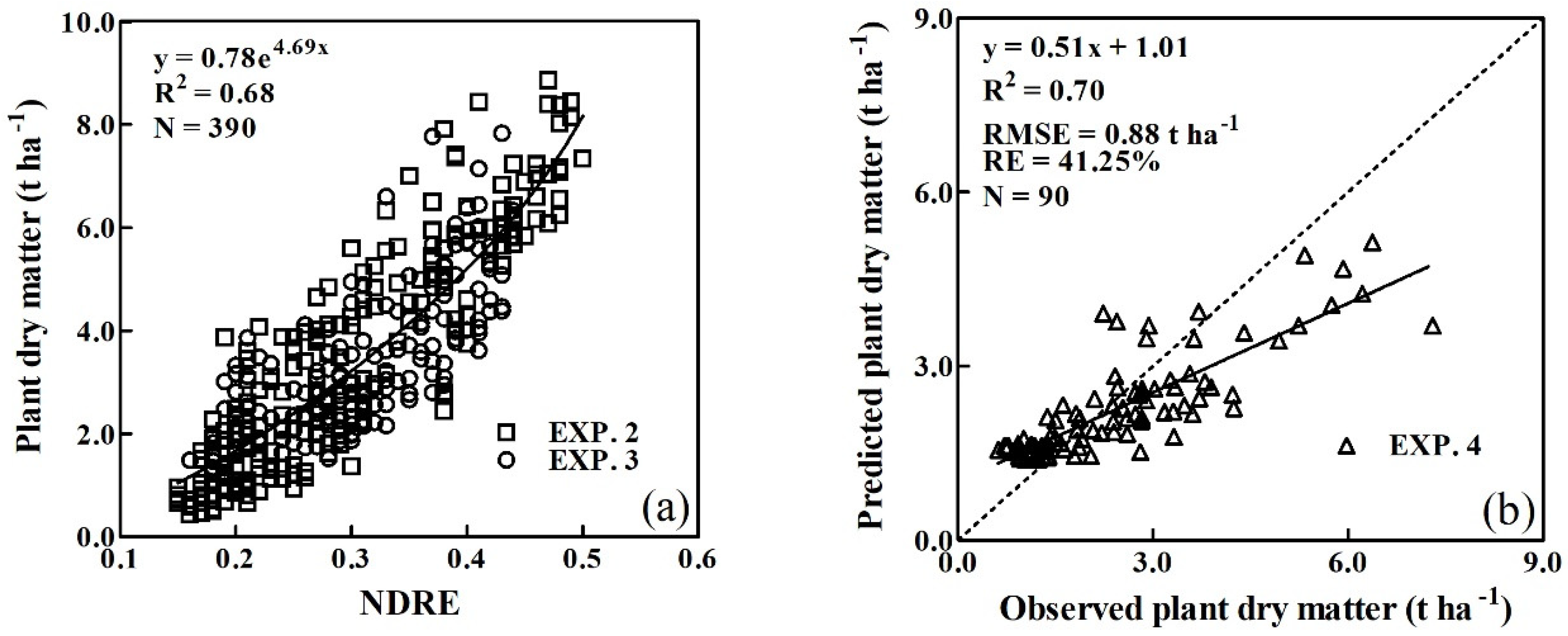
3.3. Validation of the Critical N Dilution Curves
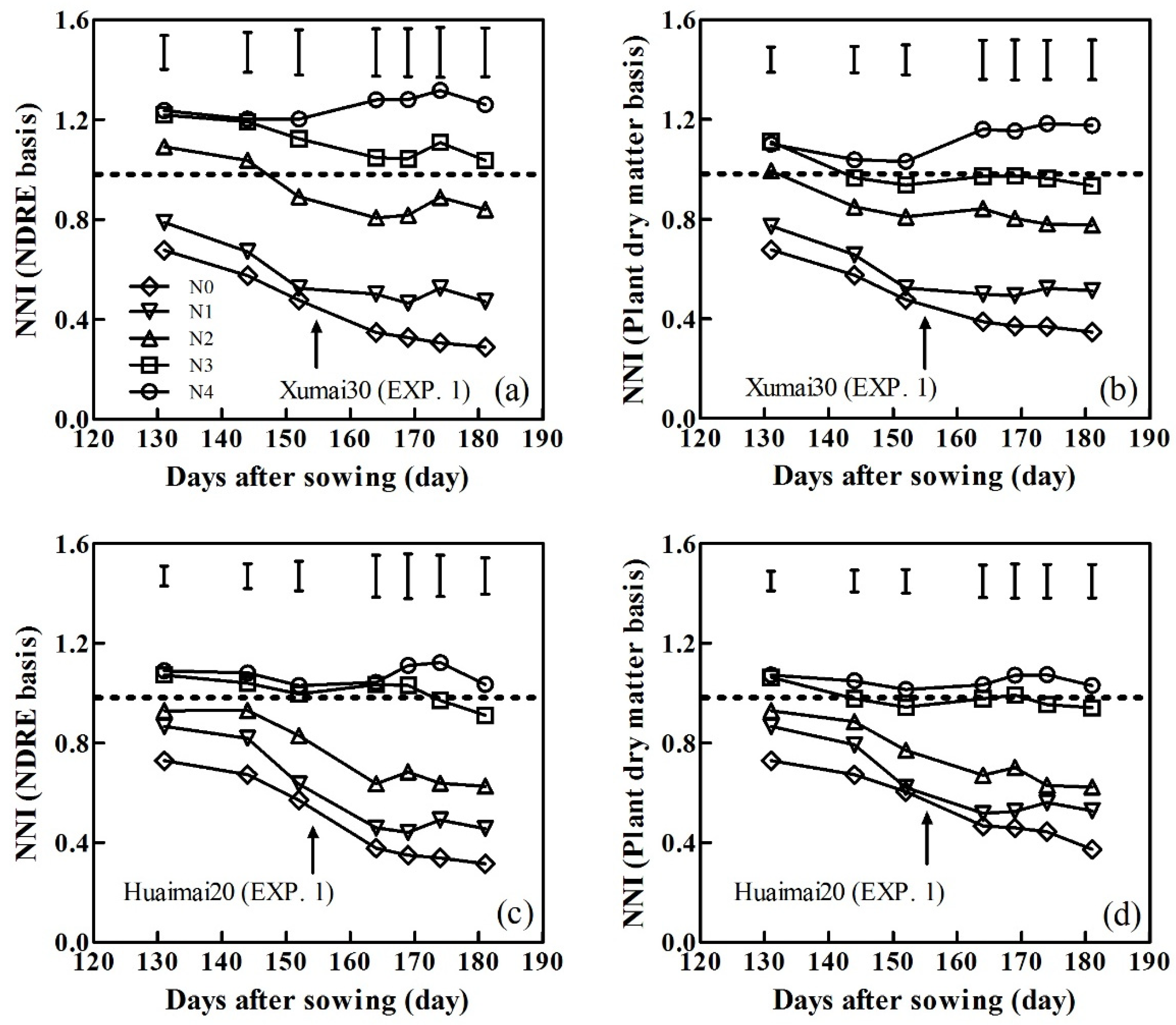
3.4. Variability of NNI During Winter Wheat Development
3.5. The Relationship Between Grain Yield and CNDC
4. Discussion
4.1. Variability in Plant Nitrogen Concentrations and NDRE
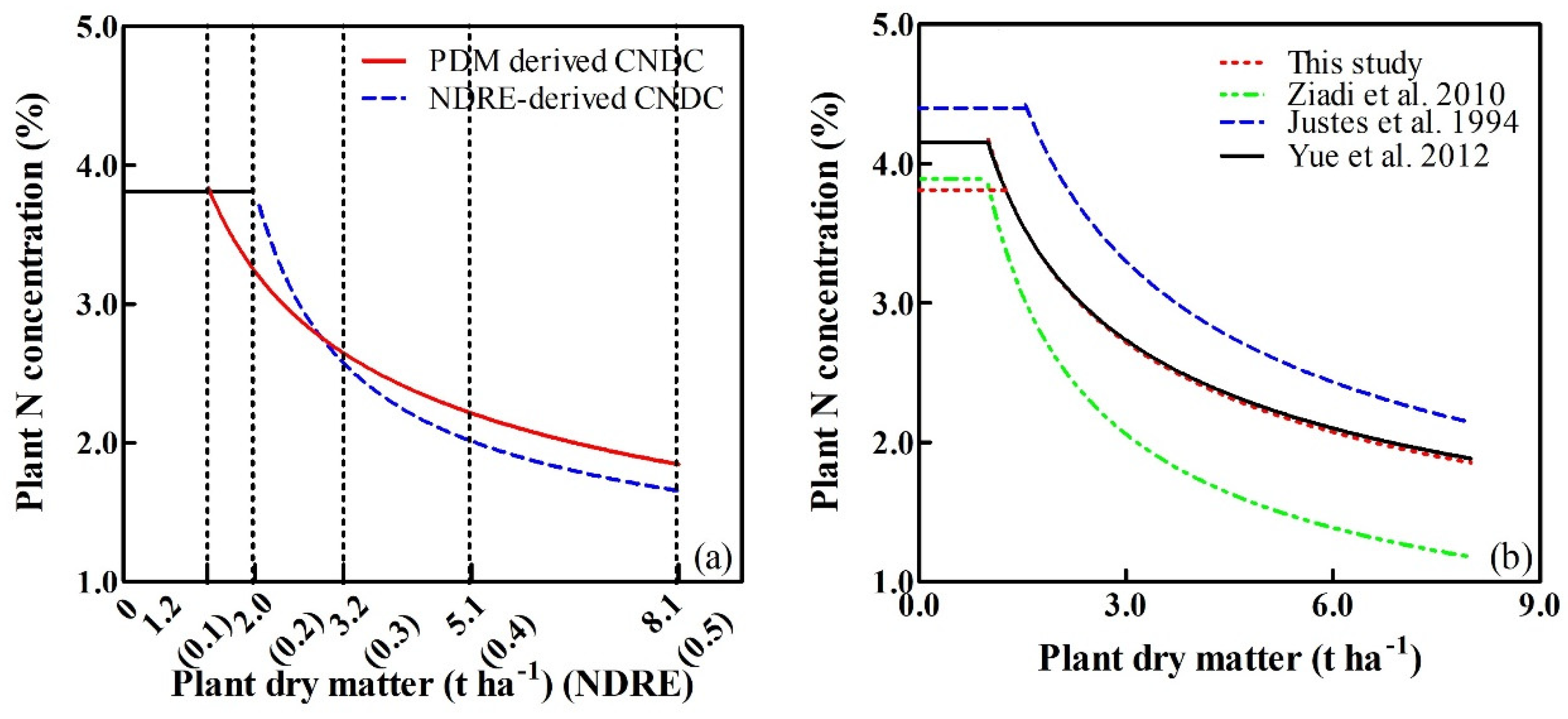
4.2. Comparison of the CNDCs based on the NDRE and Plant Dry Matter
4.3. Comparison of the CNDCs based on the PDM
4.4. Application of CNDC for in-Season Yield Estimation
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Miao, Y.; Zhang, F. Long-term experiments for sustainable nutrient management in China. A review. Agron. Sustain. Dev. 2011, 31, 397–414. [Google Scholar] [CrossRef]
- Diacono, M.; Montemurro, F. Precision nitrogen management of wheat. A review. Agron. Sustain. Dev. 2013, 33, 219–241. [Google Scholar] [CrossRef]
- Yuan, Z.; Ata-Ul-Karim, S.T.; Cao, Q.; Lu, Z.; Cao, W.; Zhu, Y.; Liu, X. Indicators for diagnosing nitrogen status of rice based on chlorophyll meter readings. Field Crop. Res. 2016, 185, 12–20. [Google Scholar] [CrossRef]
- Hansen, P.M.; Schjoerring, J.K. Reflectance measurement of canopy biomass and nitrogen status in wheat crops using normalized difference vegetation indices and partial least squares regression. Remote Sens. Environ. 2003, 86, 542–553. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Scharf, P.C.; Vories, E.D.; Drummond, S.T.; Dunn, D.; Stevens, W.G.; Bronson, K.; Benson, N.R.; Hubbard, V.C.; Jones, A.S. Calibrating canopy reflectance sensors to predict optimal mid-season nitrogen rate for cotton. Soil Sci. Soc. Am. J. 2013, 77, 173. [Google Scholar] [CrossRef]
- Peng, S.; Buresh, R.J.; Huang, J.; Zhong, X.; Zou, Y.; Yang, J.; Wang, G.; Liu, Y.; Hu, R.; Tang, Q.; et al. Improving nitrogen fertilization in rice by site-specific N management. Agron. Sustain. Dev. 2011, 30, 649–656. [Google Scholar] [CrossRef]
- Tremblay, N.; Wang, Z.; Cerovic, Z.G. Sensing crop nitrogen status with fluorescence indicators. A review. Agron. Sustain. Dev. 2012, 32, 451–464. [Google Scholar] [CrossRef]
- Muñoz-Huerta, R.; Guevara-Gonzalez, R.; Contreras-Medina, L.; Pacheco, I.; Prado-Olivarez, J.; Ocampo-Velázquez, R. A review of methods for sensing the nitrogen status in plants: Advantages, disadvantages and recent advances. Sensors 2013, 13, 10823–10843. [Google Scholar] [CrossRef]
- Greenwood, D.J.; Neeteson, J.J.; Draycott, A. Quantitative relationships for the dependence of growth rate of arable crops on their nitrogen content, dry weight and aerial environment. Plant Soil 1986, 91, 281–301. [Google Scholar] [CrossRef]
- Justes, E.; Mary, B.; Meynard, J.M.; Machet, J.M.; Thelier-Huche, L. Determination of a critical nitrogen dilution curve for winter wheat crops. Ann. Bot. 1994, 74, 397–407. [Google Scholar] [CrossRef]
- Greenwood, D.J.; Lemaire, G.; Gosse, G.; Cruz, P.; Draycott, A.; Neeteson, J.J. Decline in percentage N of C3 and C4 crops with increasing plant mass. Ann. Bot. 1990, 66, 425–436. [Google Scholar] [CrossRef]
- Greenwood, D.J.; Gastal, F.; Lemaire, G.; Draycott, A.; Millard, P.; Neeteson, J.J. Growth rate and % N of field grown crops: Theory and experiments. Ann. Bot. 1991, 67, 181–190. [Google Scholar] [CrossRef]
- Colnenne, C.; Meynard, J.M.; Reau, R.; Justes, E.; Merrien, A. Determination of a critical nitrogen dilution curve for winter oilseed rape. Ann. Bot. 1998, 81, 311–317. [Google Scholar] [CrossRef]
- Giletto, C.; Echeverría, H. Critical nitrogen dilution curve for processing potato in Argentinean humid pampas. Am. J. Potato Res. 2011, 89, 102–110. [Google Scholar] [CrossRef]
- Xue, X.; Wang, J.; Wang, Z.; Guo, W.; Zhou, Z. Determination of a critical dilution curve for nitrogen concentration in cotton. J. Plant Nutr. Soil Sci. 2010, 170, 811–817. [Google Scholar]
- Li, W.; He, P.; Jin, J. Critical nitrogen curve and nitrogen nutrition index for spring maize in north-east China. J. Plant Nutr. 2012, 35, 1747–1761. [Google Scholar] [CrossRef]
- Sheehy, J.E.; Dionora, M.J.A.; Mitchell, P.L.; Peng, S.; Cassman, K.G.; Lemaire, G.; Williams, R.L. Critical nitrogen concentrations: Implications for high-yielding rice (Oryza sativa L.) cultivars in the tropics. Field Crop. Res. 1998, 59, 41. [Google Scholar] [CrossRef]
- Yao, X.; Zhao, B.; Tian, Y.; Liu, X.; Ni, J.; Cao, W.; Zhu, Y. Using leaf dry matter to quantify the critical nitrogen dilution curve for winter wheat cultivated in eastern China. Field Crop. Res. 2014, 159, 33–42. [Google Scholar] [CrossRef]
- Zhao, B.; Yao, X.; Tian, Y.; Liu, X.; Ata-Ul-Karim, S.; Ni, J.; Cao, W.; Zhu, Y. New critical nitrogen curve based on leaf area index for winter wheat. Agron. J. 2014, 106, 379. [Google Scholar] [CrossRef]
- Tremblay, N.; Fallon, E.; Ziadi, N. Sensing of crop nitrogen status: Opportunities, tools, limitations, and supporting information requirements. HortTechnology 2011, 21, 274–281. [Google Scholar] [CrossRef]
- Wang, Y.; Shi, P.; Gang, Z.; Jing, R.; Shi, W.; Wang, D. A critical nitrogen dilution curve for japonica rice based on canopy images. Field Crop. Res. 2016, 198, 93–100. [Google Scholar] [CrossRef]
- Sripada, R.P.; White, J.G.; Crozier, C.R.; Heiniger, R.W.; Weisz, R.; Burleson, J. Aerial color infrared photography for determining in-season nitrogen requirements for corn. Agron. J. 2006, 97, 1511–1514. [Google Scholar] [CrossRef]
- Jannoura, R.; Brinkmann, K.; Uteau, D.; Bruns, C.; Joergensen, R.G. Monitoring of crop biomass using true colour aerial photographs taken from a remote controlled hexacopter. Biosyst. Eng. 2015, 129, 341–351. [Google Scholar] [CrossRef]
- Huete, A. A soil adjusted vegetation index (SAVI). Remote Sens. Environ. 1988, 17, 37–53. [Google Scholar] [CrossRef]
- Padilla, M.; Gallardo, M.; Peña-Fleitas, T.; De Souza, R.; Thompson, B. Proximal optical sensors for nitrogen management of vegetable crops: A review. Sensors 2018, 18, 2083. [Google Scholar] [CrossRef]
- Duan, T.; Chapman, S.; Guo, Y.; Zheng, B. Dynamic monitoring of NDVI in wheat agronomy and breeding trials using an unmanned aerial vehicle. Field Crop. Res. 2017, 210, 71–80. [Google Scholar] [CrossRef]
- Pagani, A.; Echeverría, E.; Andrade, H.; Sainz Rozas, R. Characterization of corn nitrogen status with a greenness index under different availability of sulfur. Agron. J. 2009, 101, 315–322. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Wang, H.; Huang, S.; Cheng, S.; Khosla, R.; Jiang, R. Non-destructive estimation of rice plant nitrogen status with Crop Circle multispectral active canopy sensor. Field Crop. Res. 2013, 154, 133–144. [Google Scholar] [CrossRef]
- Xia, T.; Miao, Y.; Wu, D.; Hui, S.; Khosla, R.; Mi, G. Active optical sensing of spring maize for in-season diagnosis of nitrogen status based on nitrogen nutrition index. Remote Sens. 2016, 8, 605. [Google Scholar] [CrossRef]
- Miller, J.J.; Schepers, J.S.; Shapiro, C.A.; Arneson, N.J.; Eskridge, K.M.; Oliveira, M.C.; Giesler, L.J. Characterizing soybean vigor and productivity using multiple crop canopy sensor readings. Field Crop. Res. 2018, 216, 22–31. [Google Scholar] [CrossRef]
- Zhang, J.; Liu, X.; Liang, Y.; Cao, Q.; Tian, Y.; Zhu, Y.; Cao, Q.; Liu, X. Using a portable active sensor to monitor growth parameters and predict grain yield of winter wheat. Sensors 2019, 19, 1108. [Google Scholar] [CrossRef] [PubMed]
- Lukina, E.V.; Freeman, K.W.; Wynn, K.J.; Thomason, W.E.; Mullen, R.W.; Stone, M.L. Nitrogen fertilization optimization algorithm based on in-season estimates of yield and plant nitrogen uptake. J. Plant Nutr. 2001, 24, 885–898. [Google Scholar] [CrossRef]
- Gaydon, D.S.; Singh, B.; Wang, E.; Poulton, P.L.; Ahmad, B.; Ahmed, F. Evaluation of the APSIM model in cropping systems of Asia. Field Crop. Res. 2017, 204, 52–75. [Google Scholar] [CrossRef]
- Ziadi, N.; Bélanger, G.; Claessens, A.; Lefebvre, L.; Cambouris, A.N.; Tremblay, N.; Nolin, M.; Parent, L.E. Determination of a critical nitrogen dilution curve for spring wheat. Agron. J. 2010, 102, 241–250. [Google Scholar] [CrossRef]
- Tahir Ata-Ul-Karim, S.; Liu, X.; Lu, Z.; Yuan, Z.; Zhu, Y.; Cao, W. In-season estimation of rice grain yield using critical nitrogen dilution curve. Field Crop. Res. 2016, 195, 1–8. [Google Scholar] [CrossRef]
- Tucker, C.J. Red and photographic infrared linear combinations for monitoring vegetation. Remote Sens. Environ. 1979, 8, 127–150. [Google Scholar] [CrossRef]
- Barnes, E.M.; Clarke, T.R.; Richards, S.E.; Colaizzi, P.D.; Haberland, J.; Kostrzewski, M.; Waller, P.; Choi, C.; Riley, E.; Thompson, T.; et al. Coincident detection of crop water stress, nitrogen status and canopy density using ground-based multispectral data. In Proceedings of the Fifth International Conference on Precision Agriculture, Bloomington, MN, USA, 16–19 July 2000. [Google Scholar]
- Sripada, R.; Heiniger, R.; White, J.; Weisz, R. Aerial color infrared photography for determining late-season nitrogen requirements in corn. Agron. J. 2005, 97, 1443–1451. [Google Scholar] [CrossRef]
- Richardson, A.; Wiegand, C. Distinguishing vegetation from soil background information. Photogramm. Eng. Remote Sens. 1978, 43, 1541–1552. [Google Scholar]
- Jasper, J.; Reusch, S.; Link, A. Active sensing of the N status of wheat using optimized wavelength combination: Impact of seed rate, variety and growth stage. In Proceedings of the 7th European Conference on Precision Agriculture, Wageningen, The Netherlands, 6–8 July 2009; Van Henten, E.J., Goense, D., Lokhorst, C., Eds.; pp. 23–30. [Google Scholar]
- Pearson, R.; Miller, L. Remote mapping of standing crop biomass for estimation of productivity of the shortgrass prairie. Remote Sens. Environ. 1972, 8, 1355. [Google Scholar]
- Gitelson, A. Wide dynamic range vegetation index for remote quantification of biophysical characteristics of vegetation. J. Plant Physiol. 2004, 161, 165–173. [Google Scholar] [CrossRef]
- Perry, C.; Lautenschlager, L. Functional equivalence of spectral vegetation indices. Remote Sens. Environ. 1984, 14, 169–182. [Google Scholar] [CrossRef]
- Rondeaux, G.; Steven, M.; Frederic, B. Optimization of soil-adjusted vegetation indices. Remote Sens. Environ. 1996, 55, 95–107. [Google Scholar] [CrossRef]
- Guyot, G.; Frederic, B. Utilisation de la haute resolution spectrale pour suivre l’etat des couverts vegetaux. Spectr. Signat. Objects Remote Sens. 1988, 287, 279. [Google Scholar]
- Bremner, J.M.; Mulvaney, C.S. Nitrogen-Total. In Methods of Soil Analysis; Page, A.L., Miller, R.H., Keeney, D.R., Eds.; American Society of Agronomy: Madison, WI, USA, 1982; pp. 595–624. [Google Scholar]
- Lemaire, G.; Jeuffroy, M.H.; Gastal, F. Diagnosis tool for plant and crop N status in vegetative stage: Theory and practices for crop N management. Eur. J. Agron. 2008, 28, 614–624. [Google Scholar] [CrossRef]
- Lemaire, G.; Salette, J. Relationship between growth and nitrogen uptake in a pure grass stand. 1. Environmental effects [nitrogen nutrition, nitrogen content, allometric relations, variability between years, tall fescue]. Agronomie 1984, 121, 316–321. [Google Scholar]
- Lemaire, G.; Onillon, B.; Gosse, G.; Chartier, M.; Allirand, M. Nitrogen distribution within a lucerne canopy during regrowth: Relation with light distribution. Ann. Bot. 1991, 68, 483–488. [Google Scholar] [CrossRef]
- Lemaire, G.; Khaity, M.; Onillon, B.; Allirand, M.; Chartier, M.; Gosse, G. Dynamics of accumulation and partitioning of N in leaves, stems and roots of lucerne (Medicago sativa L.) in a dense canopy. Ann. Bot. 1992, 70, 429–435. [Google Scholar] [CrossRef]
- Li, S.; Ding, X.; Kuang, Q.; Ata-Ul-Karim, S.; Cheng, T.; Liu, X.; Tian, Y.; Zhu, Y.; Cao, Q. Potential of UAV-based active sensing for monitoring rice leaf nitrogen status. Front. Plant Sci. 2018, 9, 1834. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Shen, J.; Yuan, F.; Cheng, S.; Cui, Z. Evaluating two Crop Circle active canopy sensors for in-season diagnosis of winter wheat nitrogen status. Agronomy 2018, 8, 201. [Google Scholar] [CrossRef]
- Ziadi, N.; Brassard, M.; Bélanger, G.; Cambouris, A.; Tremblay, N.; Nolin, M.; Claessens, A.; Parent, L.E. Critical nitrogen curve and nitrogen nutrition index for corn in eastern Canada. Agron. J. 2008, 100, 271–276. [Google Scholar] [CrossRef]
- Li, F.; Zhang, H.; Jia, L.; Bareth, G.; Miao, Y.; Chen, X. Estimating winter wheat biomass and nitrogen status using an active crop sensor. Intell. Autom. Soft Comput. 2010, 16, 1221–1230. [Google Scholar]
- Link, J.; Senner, D.; Claupein, W. Developing and evaluating an aerial sensor platform (ASP) to collect multispectral data for deriving management decisions in precision farming. Comput. Electron. Agric. 2013, 94, 20–28. [Google Scholar] [CrossRef]
- Lucieer, A.; Malenovský, Z.; Veness, T.; Wallace, L. HyperUAS-imaging spectroscopy from a multirotor unmanned aircraft system. J. Field Robot. 2014, 31, 571–590. [Google Scholar] [CrossRef]
- Fowler, D.B. Winter Cereal Production. Chapter 10: Growth Stages of Wheat; University of Saskatchewan: Saskatoon, SK, Canada, 2002; Available online: www.usask.ca/agriculture/plantsci/winter_cereals/Winterwheat/CHAPT10/cvchpt10.php (accessed on 23 November 2009).
- Wang, E.; Engel, T. Simulation of phenological development of wheat crops. Agric. Syst. 1998, 58, 1–24. [Google Scholar] [CrossRef]
- Yue, S.; Meng, Q.; Zhao, R.; Fei, L.; Chen, X.; Zhang, F.; Cui, Z. Critical nitrogen dilution curve for optimizing nitrogen management of winter wheat production in the north China plain. Agron. J. 2012, 104, 523. [Google Scholar] [CrossRef]
- Soomro, A.; Rahman, U.; Odhano, A.; Gul, S.; Tareen, Q. Effects of sowing method and seed rate on growth and yield of wheat (Triticum aestivum). World J. Agric. Sci. 2009, 5, 159–162. [Google Scholar]
- Chen, C.; Neill, K.; Wichman, D.; Westcott, M. Hard red spring wheat response to row spacing, seeding rate, and nitrogen. Agron. J. 2008, 100, 1296–1302. [Google Scholar] [CrossRef]
- Zhang, L.; Guo, C.; Zhao, L.; Zhu, Y.; Cao, W.; Tian, Y.; Cheng, T.; Wang, X. Estimating wheat yield by integrating the WheatGrow and PROSAIL models. Field Crop. Res. 2016, 192, 55–66. [Google Scholar] [CrossRef]
- Porter, J.R. AFRCWHEAT2: A model of the growth and development of wheat incorporating responses to water and nitrogen. Eur. J. Agron. 1993, 2, 69–82. [Google Scholar] [CrossRef]
- Cao, Q.; Miao, Y.; Feng, G.; Gao, X.; Li, F.; Liu, B.; Yue, S.; Cheng, S.; Ustin, S.; Khosla, R. Active canopy sensing of winter wheat nitrogen status: An evaluation of two sensor systems. Comput. Electron. Agric. 2015, 112, 54–67. [Google Scholar] [CrossRef]
- Krienke, B.; Ferguson, R.B.; Schlemmer, M.; Holland, K.; Marx, D.; Eskridge, K. Using an unmanned aerial vehicle to evaluate nitrogen variability and height effect with an active crop canopy sensor. Precis. Agric. 2017, 18, 1–16. [Google Scholar] [CrossRef]





| Experiment No. | Location | Plot Size (m2) | Cultivar | N Rate (kg ha−1) | Sampling Stage (date) | Soil Classification |
|---|---|---|---|---|---|---|
| Experiment 1 2014–2015 | Rugao (32.27° N, 120.75° E) | 30 (5 m × 6 m) | Ningmai13 Huaimai20 Yangfumai4 | 0 (N0) 120 (N1) 225 (N2) 330 (N3) | Spring re-growth (9 February) Pre-jointing (8 March) Mid-jointing (19 March) Post-jointing (28 March) Booting (8 April) | Soil type = Loam soil Soil pH = 6.40 OM = 23.55 g kg−1 Total N = 1.55 g kg−1 Available P = 44.80 mg g−1 Available K = 110.50 mg g−1 |
| Experiment 2 2015–2016 | Sihong (33.37° N, 118.26° E) | 30 (5 m × 6 m) | Xumai30 Huaimai20 | 0 (N0) 90 (N1) 180 (N2) 270 (N3) 360 (N4) | Spring re-growth (3 March) Spring re-growth (16 March) Pre-jointing (23 March) Mid-jointing (5 April) Post-jointing (10 April) Booting (15 April) Heading (22 April) Harvest (3 June) | Soil type = Clay soil Soil pH = 6.56 OM = 26.3 g kg−1 Total N = 2.91 g kg−1 Available P = 43.12 mg g−1 Available K = 89.23 mg g−1 |
| Experiment 3 2017–2018 | Xinghua (33.08° N, 119.98° E) | 63 (7 m × 9 m) | Zhenmai12 Yangmai23 Ningmai13 | 0 (N0) 90 (N1) 180 (N2) 270 (N3) 360 (N4) | Pre-jointing (22 March) Mid-jointing (1 April) Post-jointing (8 April) Booting (15 April) Harvest (2 June) | Soil type = Loam soil Soil pH = 6.61 OM = 21.26 g kg−1 Total N = 1.71 g kg−1 Available P = 41.06 mg g−1 Available K = 108.61 mg g−1 |
| Experiment 4 2018–2019 | Xinghua (33.08° N, 119.98° E) | 63 (7 m × 9 m) | Zhenmai12 Yangmai23 Ningmai13 | 0 (N0) 90 (N1) 180 (N2) 270 (N3) 360 (N4) | Jointing (22 March) Booting (4 April) Harvest (29 May) | Soil type = Loam soil Soil pH = 6.61 OM = 21.26 g kg−1 Total N = 1.71 g kg−1 Available P = 41.06 mg g−1 Available K = 108.61 mg g−1 |
| Index | Formula | Reference |
|---|---|---|
| Normalised difference vegetation index (NDVI) | (NIR – R)/(NIR + R) | [36] |
| Normalised difference red-edge (NDRE) | (NIR – RE)/(NIR + RE) | [37] |
| Red edge soil-adjusted vegetation index (RESAVI) | 1.5 × [(NIR – RE)/(NIR + RE + 0.5)] | [38] |
| Difference vegetation index (DVI) | NIR – R | [39] |
| Soil-adjusted vegetation index (SAVI) | (1 + L)(NIR – R)/(NIR + R + L); L = 0.5 | [24] |
| Red edge ratio vegetation index (RERVI) | NIR/RE | [40] |
| Perpendicular vegetation index (PVI) | (NIR + 1.05R – 0.03)/SQRT(1 + 1.052) | [39] |
| Red edge difference vegetation index (REDVI) | NIR – RE | [36] |
| Ratio vegetation index (RVI) | R/NIR | [41] |
| Red edge wide dynamic range vegetation index (REWDRVI) | (a × NIR – RE)/(a × NIR + RE); a = 0.12 | [42] |
| Optimised vegetation index 1 (VIopt1) | 100 × (lnNIR – lnRE) | [40] |
| Transformed vegetation index (TVI) | SQRT((NIR – R)/(NIR + R) + 0.5) | [43] |
| Optimised soil-adjusted vegetation index (OSAVI) | (NIR – R)/(NIR + R + 0.16) | [44] |
| Reflection in red-edge (RRE) | (NIR + R)/2 | [45] |
| Vegetation Indices | Calibration | Validation | |||
|---|---|---|---|---|---|
| Regression Equation | R² | R² | RMSE (t ha-1) | RE (%) | |
| NDRE | y = 0.78e4.69x | 0.68 | 0.70 | 0.88 | 41.25 |
| RESAVI | y = 0.78e3.14x | 0.68 | 0.70 | 0.88 | 41.30 |
| VIOPT1 | y = 0.86e0.02x | 0.67 | 0.69 | 0.88 | 43.28 |
| REWDRVI | y = 217.18e6.70x | 0.67 | 0.69 | 0.91 | 48.07 |
| REDVI | y = 1.07e0.06x | 0.67 | 0.69 | 0.91 | 48.03 |
| Growth Stage | Calibration | Validation | ||||
|---|---|---|---|---|---|---|
| Regression Equation | R² | R² | RMSE | RE (%) | ||
| Jointing stage | y = 0.96x + 0.01 | x < 0.94 | 0.79 | 0.76 | 0.09 | 12.50 |
| y = 0.91 | x ≥ 0.94 | |||||
| Booting stage | y = 0.81x + 0.19 | x < 0.90 | 0.87 | 0.80 | 0.07 | 9.56 |
| y = 0.92 | x ≥ 0.90 | |||||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jiang, J.; Wang, C.; Wang, Y.; Cao, Q.; Tian, Y.; Zhu, Y.; Cao, W.; Liu, X. Using an Active Sensor to Develop New Critical Nitrogen Dilution Curve for Winter Wheat. Sensors 2020, 20, 1577. https://doi.org/10.3390/s20061577
Jiang J, Wang C, Wang Y, Cao Q, Tian Y, Zhu Y, Cao W, Liu X. Using an Active Sensor to Develop New Critical Nitrogen Dilution Curve for Winter Wheat. Sensors. 2020; 20(6):1577. https://doi.org/10.3390/s20061577
Chicago/Turabian StyleJiang, Jie, Cuicun Wang, Yu Wang, Qiang Cao, Yongchao Tian, Yan Zhu, Weixing Cao, and Xiaojun Liu. 2020. "Using an Active Sensor to Develop New Critical Nitrogen Dilution Curve for Winter Wheat" Sensors 20, no. 6: 1577. https://doi.org/10.3390/s20061577
APA StyleJiang, J., Wang, C., Wang, Y., Cao, Q., Tian, Y., Zhu, Y., Cao, W., & Liu, X. (2020). Using an Active Sensor to Develop New Critical Nitrogen Dilution Curve for Winter Wheat. Sensors, 20(6), 1577. https://doi.org/10.3390/s20061577







