1. Introduction
An electrocardiogram records a significant amount of useful information, including human heart health status. ECG is an important basis for the diagnosis of heart disease. It can also be used for diagnosing obstructive sleep apnea, evaluating therapeutic drugs, and for physiological monitoring [
1]. Long-term dynamic observations of the heart can capture transient non-sustained abnormal ECG changes which are essential for diagnosing heart disease, judging curative effects, and saving lives [
2]. Therefore, daily protection and long-term ECG monitoring are important methods to detect and control heart disease.
Generally, wearable ECG devices use dry electrodes that are gel-free and fabric-like, enabling long-term, dynamic, and unobstructed monitoring of ECG signals [
3]. These electrodes do not make their wearers uncomfortable. However, one of most critical problems of wearable ECG devices is how to suppress motion artifacts. The difficulty in removing motion artifacts lies in their dynamic characteristics, varying frequency ranges, and relatively large amplitudes [
4]. Thus, important biosignal information can be distorted or even buried.
The current research into solving this problem is roughly divided into three directions. The first direction focuses on electrode materials and structural studies. These studies aim to eliminate motion artifacts from their sources [
5,
6]. The second direction focuses on signal processing via software. Principal component analysis (PCA) and independent component analysis (ICA) are used to suppress motion artifacts as independent sources of signal variations [
7]. The third direction focuses on adaptive cancellation algorithms to reduce motion artifacts. This kind of algorithm uses the measurement signals of auxiliary sensors as reference input signals, including those from inertial [
8], pressure [
9], and photoelectric [
10] sensors.
There are several studies on suppressing motion artifacts with adaptive cancellation algorithms. Seo [
11] proposed a new noise cancellation method to suppress motion artifacts. Since an adaptive filter updates its weights as it adds impulsive noise, raw ECG signals at the R wave interval are considered to be impulsive noise. Tong et al. [
12] proposed an active adaptive filter for the reduction of motion artifacts. The authors designed two sensors for measuring electrode motion. These two motion sensors are a three-axis accelerometer sensor and a two-axis anisotropic magnetoresistive sensor. Pandey [
13] proposed an adaptive filtering method to suppress artifacts. Reference signals were acquired by increasing the absolute power spectrum of the 3-channel accelerometer output connected to an ECG electrode. Thakor et al. [
14] proposed an adaptive recurrent filter for obtaining impulse responses of the desired QRS complex. The main input signals of the adaptive filter were mixed ECG signals that contain noise. The reference input signals were an impulse train that coincided with the QRS complexes.
The adaptive noise cancellation algorithm is currently the most widely used algorithm for the suppression of motion artifacts. An adaptive filter requires the reference signal to be highly correlated with noise in the ECG signal but remain uncorrelated with the ECG information. If the correlation is weak, the filtered output signal may be erroneous. Indeed, motion artifacts are difficult to obtain due to their time-varying features, so they are not easy to estimate in practical applications. In addition, it is difficult to find highly correlated reference signals. Instead, uncorrelated noise signals that appear in reference signals have a significant influence on the performance of the adaptive cancellation algorithm. Therefore, traditional adaptive cancellation algorithms cannot suppress motion artifacts completely.
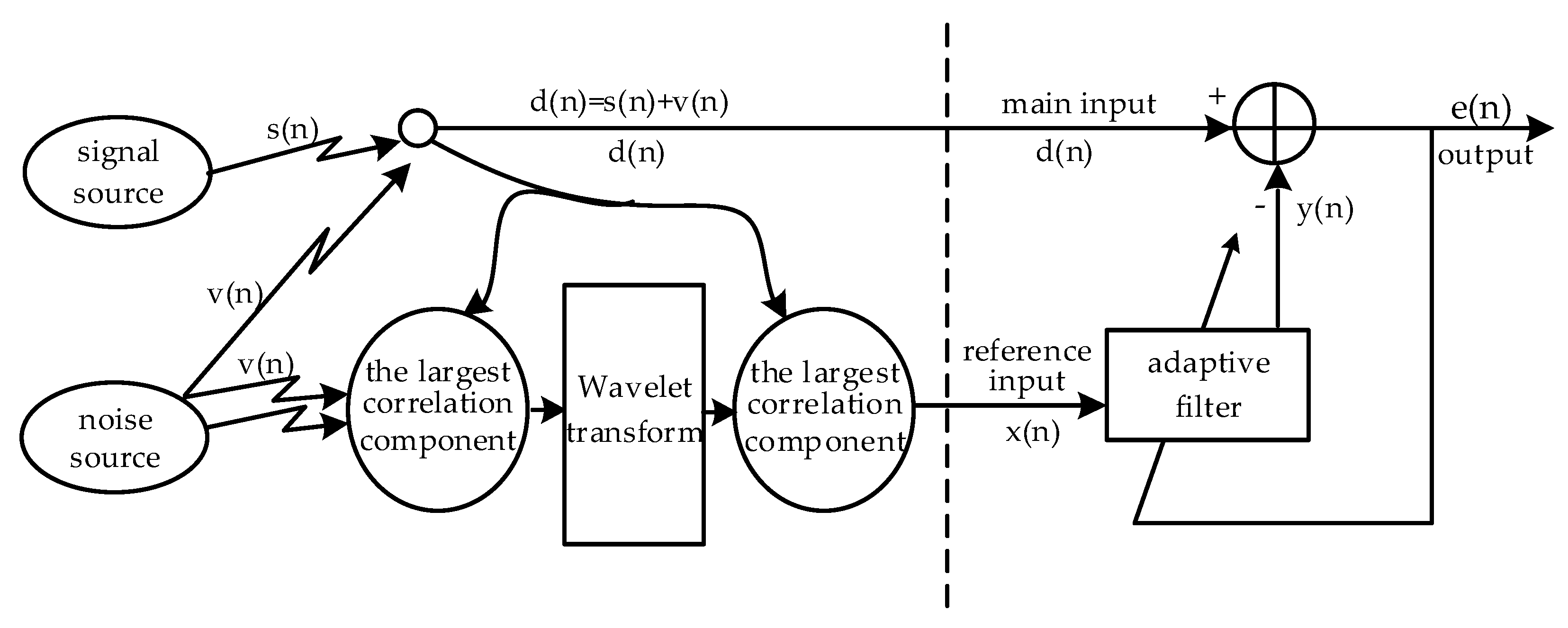
To solve the above problems, this paper combines the wavelet transform method with the concept of data fusion to study the adaptive filter in detail. It is possible to improve the correlation by using inertial sensors installed on different parts of the body to collect more comprehensive information related to the electrode motion under different actions. Then, the uncorrelated motion information is removed by wavelet preprocessing, and the correlation of the reference input signal is further improved. This paper is organized as follows. An introduction of the data acquisition, proposed methods, and data analysis is summarized in
Section 2. The results and discussion are presented in
Section 3, followed by the conclusions in
Section 4.
3. Results and Discussion
Under daily actions (walking, bending, chest expansion, and running), the correlation coefficient between the motion signal collected by multi-inertial sensors and the ECG signal collected by the electrode is shown in
Figure 4. X, Y, and Z represent the correlation coefficients between x, y, and z axis signals from the inertial sensor and ECG signals. Similarly, U, V, and W represent the correlation coefficients between the angular velocity and the ECG signal, respectively, when the gyroscope is rotated around the x, y, and z axes. R represents the correlation coefficient of the acceleration vector mode. Subscript r represents the right arm’s inertial sensors, and subscript l represents the left arm’s inertial sensors. In
Figure 4, when the subject is walking, there is a strong correlation between the ECG signal and the angular velocity of the left and right arm’s inertial sensors around the y and z axes, which may be caused by the chest strap stretching. When the subject is bending, there is also a strong correlation between the ECG signal and the angular velocity of the left and right arm inertial sensors around the x and z axes. When the chest is extended, the z-axis acceleration is higher. When the subject is running, the y and z axis accelerations are highly correlated. At the same time, they are less related in a walking state due to the difficulty in creating tension in the fabric chest strap. Conversely, the correlation is stronger under the other three kinds of movements due to the tension of the fabric chest strap.
Figure 5 shows the correlation coefficients under different states. In the walking state, if a single inertial module is used, the correlation coefficients of the three middle, right, and left positions are 0.193, 0.234, and 0.233, respectively. If multiple inertial modules are used, the correlation coefficient is 0.234. Compared to the method of using a single inertial module, the method using a multi-inertial module improved the correlation coefficient by 21%, 0%, and 0.4%, respectively.
In the bending state, if a single inertial module is used, the correlation coefficients of the three middle, right, and left positions are 0.358, 0.434, and 0.439, respectively. If multiple inertial modules are used, the correlation coefficient is 0.439. Compared with the method using a single inertial module, the method of adopting a multi-inertial module increased the correlation coefficient by 23%, 1%, and 0%, respectively.
In the chest expansion state, if a single inertial module is used, the correlation coefficients of the three middle, right, and left positions are 0.479, 0.371, and 0.414, respectively. If multiple inertial modules are used, the correlation coefficient is 0.479. Compared with the method of using a single inertial module, the method of adopting a multi-inertial module increased the mutual relationship by 0%, 11%, and 16%, respectively.
In the running state, if a single inertial module is used, the correlation coefficients of the three middle, right, and left positions are 0.262, 0.397, and 0.535, respectively. If multiple inertial modules are used, the correlation coefficient is 0.535. Compared with the method of using a single inertial module, the method of adopting a multi-inertial module increased the mutual relationship by 51%, 35%, and 0%, respectively.
Through the above analysis of daily movements, we calculated the correlation between the motion signals collected by single inertial sensors and multi-inertial sensors and the ECG signals collected by the electrodes. It can be concluded that the reference signal of the adaptive motion artifact cancellation algorithm based on the inertial sensors is not only related to the position where the inertial sensor is placed but is also related to the multiple inertial sensors distributed on different parts of the body. Using multiple inertial sensors is more efficient as they allow one to sense more correlations with motion artifacts in the ECG.
Under different actions, the measured components corresponding to the maximum correlation coefficient values of the three inertial sensors were selected. Multiscale wavelet decomposition is used to preserve the wavelet coefficients with large correlation coefficients to reconstruct the signals. The component corresponding to the maximum correlation coefficient is then selected as the reference input signal.
Figure 6a shows the correlation coefficient values (the absolute values) before and after the wavelet transform processing reference signal, and
Figure 6b shows the SNR under several different states. Obviously, the correlation coefficient and SNR are improved to different degrees under different actions.
The data from
Table 1 show that the correlation improvement is greater after wavelet preprocessing in the case of walking and chest expansions, increasing from 0.2340 to 0.6716 and from 0.4789 to 0.8437, respectively. Under different actions (walking, bending, chest expansion, and running), the SNR is also correspondingly improved to varying degrees after applying the LMS and NLMS adaptive cancellation algorithms, as shown in
Figure 6b. This can also be seen from the data in
Table 1; after applying the LMS algorithm under different actions, the SNR increased from 3.6346, 3.9511, 2.1802, and 4.0256 to 5.1084, 4.2186, 5.0617, and 4.2943. After applying the NLMS algorithm, the SNR under different actions increased from 4.0197, 5.2128, 3.6769, and 4.1449 to 6.2146, 5.4424, 5.9939, and 4.3650, respectively.
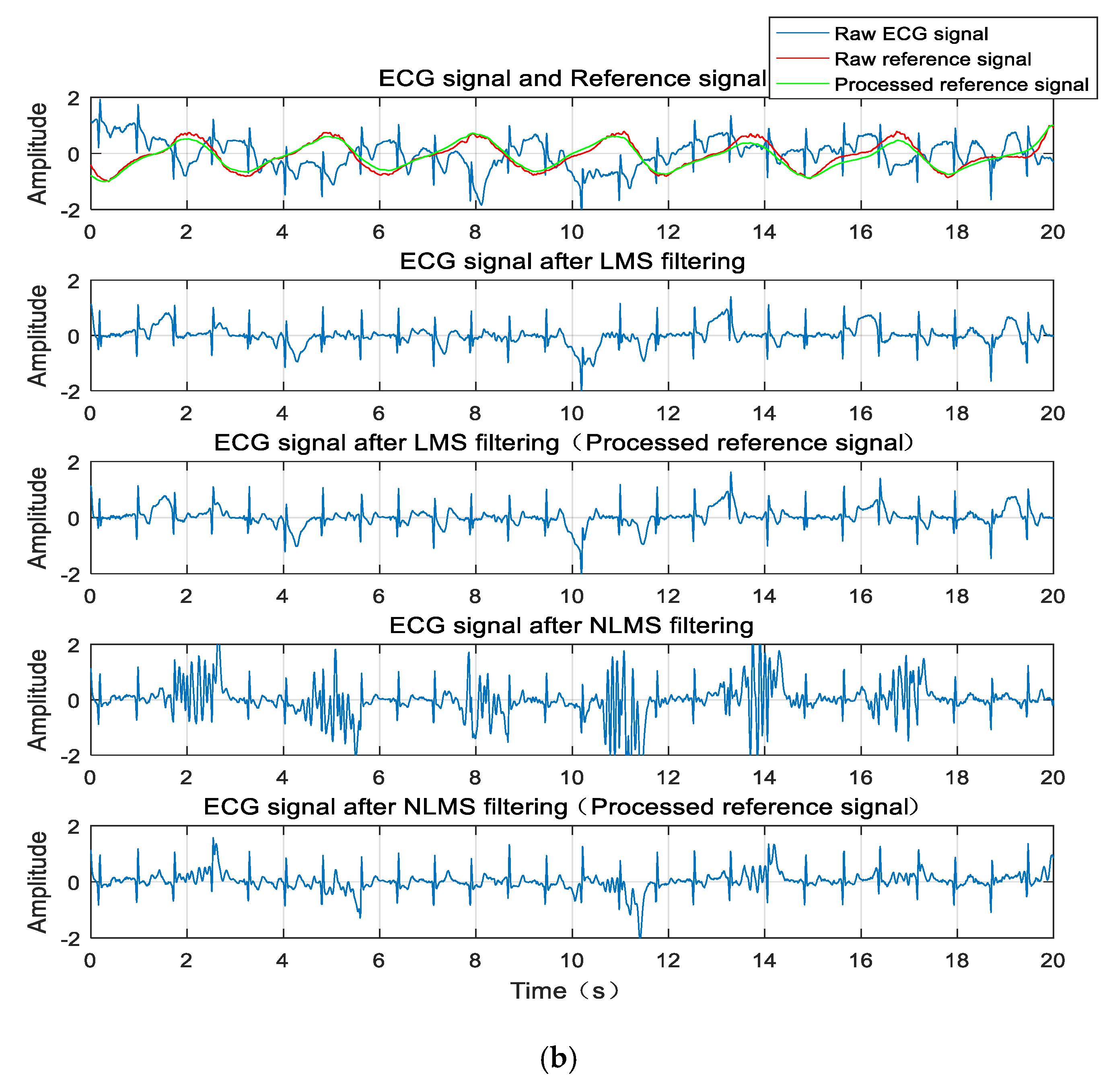
The waveform diagram is shown in
Figure 7. Compared to the reference signal (red line) before wavelet transform processing, the reference signal (green line) processed by the wavelet transform is more similar to the original ECG signal. Correspondingly, these two filtering methods are compared before and after. The ECG signals produced by the adaptive cancellation algorithm after wavelet transform processing all have different degrees of improvement. The waveform improvement is most obvious in the walking state of the subject, where the correlation is greatly improved. The QRS complex is also apparent. With the exception of a few waveforms, the P and T waves are also visible.
In summary, the data collected by the inertial sensors installed on different parts of the body are different under different actions. The multiple inertial values compensate for the lack of relative motion information from the single inertial sensors, and the correlation of the reference input signal is improved in the adaptive cancellation algorithm.
The reference signal is decomposed by the multiscale wavelet, removing the uncorrelated wavelet coefficients. The high wavelet correlations are retained to reconstruct the reference input signal. This method increases the correlation effectively. The SNR of the ECG signal is improved after the adaptive cancellation algorithm, and the ECG waveform is also improved. This occurs because the inertial sensors are mounted on the outside of the clothes, and the fabric electrodes are in close contact with the surface of the human skin. Therefore, when the human body moves, the inertial sensors inevitably create vibrations, and the electrodes may not be displaced or vibrated. At this time, the inertia generates motion information that is not related to the electrode artifacts. If such a motion signal is used as the reference input signal for the adaptive cancellation algorithm, additional noise will be introduced, resulting in signal distortion, while the wavelet decomposition can remove wavelet coefficients with little or no correlation. The signal is then reconstructed with the preserved wavelet coefficients, which is equivalent to removing a portion of the uncorrelated motion information. In this way, the correlation of the reference input signal is increased. The above results only show the data of one participant. The test results of the other five participants have similar rules and conclusions.