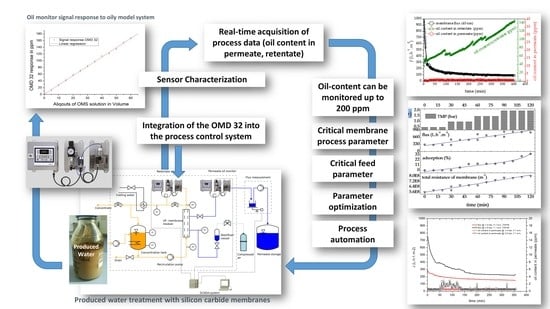
Innovative Optical-Sensing Technology for the Online Fouling Characterization of Silicon Carbide Membranes during the Treatment of Oily Water
Abstract
:1. Introduction
1.1. Produced Water (PW)
1.2. Ceramic Membrane Technology for PW Treatment
1.3. Oil-in-Water Sensors for PW Treatment Applications
2. Objectives
- To evaluate the application, performance and fouling behavior of SiC MF and UF membranes for the separation of oil during the treatment of TDPW and prepared OMSs.
- To characterize a novel online oil-in-water sensor based on light scattering for (i) the continuous measurement of oil content during the filtration of oily wastewater samples (permeate, concentrate, and retentate samples) and (ii) the evaluation of process parameters that affect membrane performance, specifically the crossflow velocity (CFV) and transmembrane pressure (TMP).
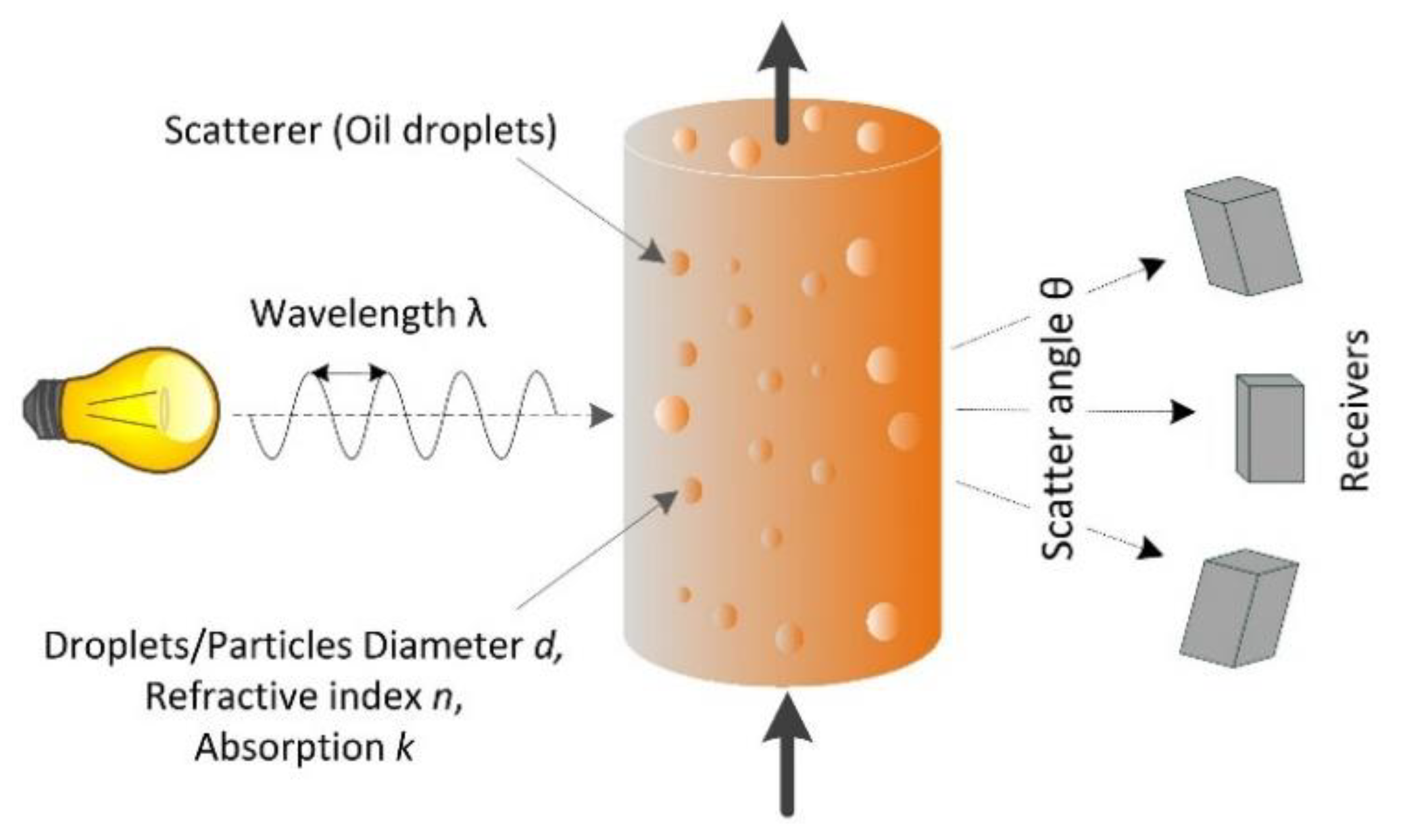
3. Theory of Light Scattering to Determine the Oil-in-Water Content
3.1. Overview
3.2. Light-Scattering Theory
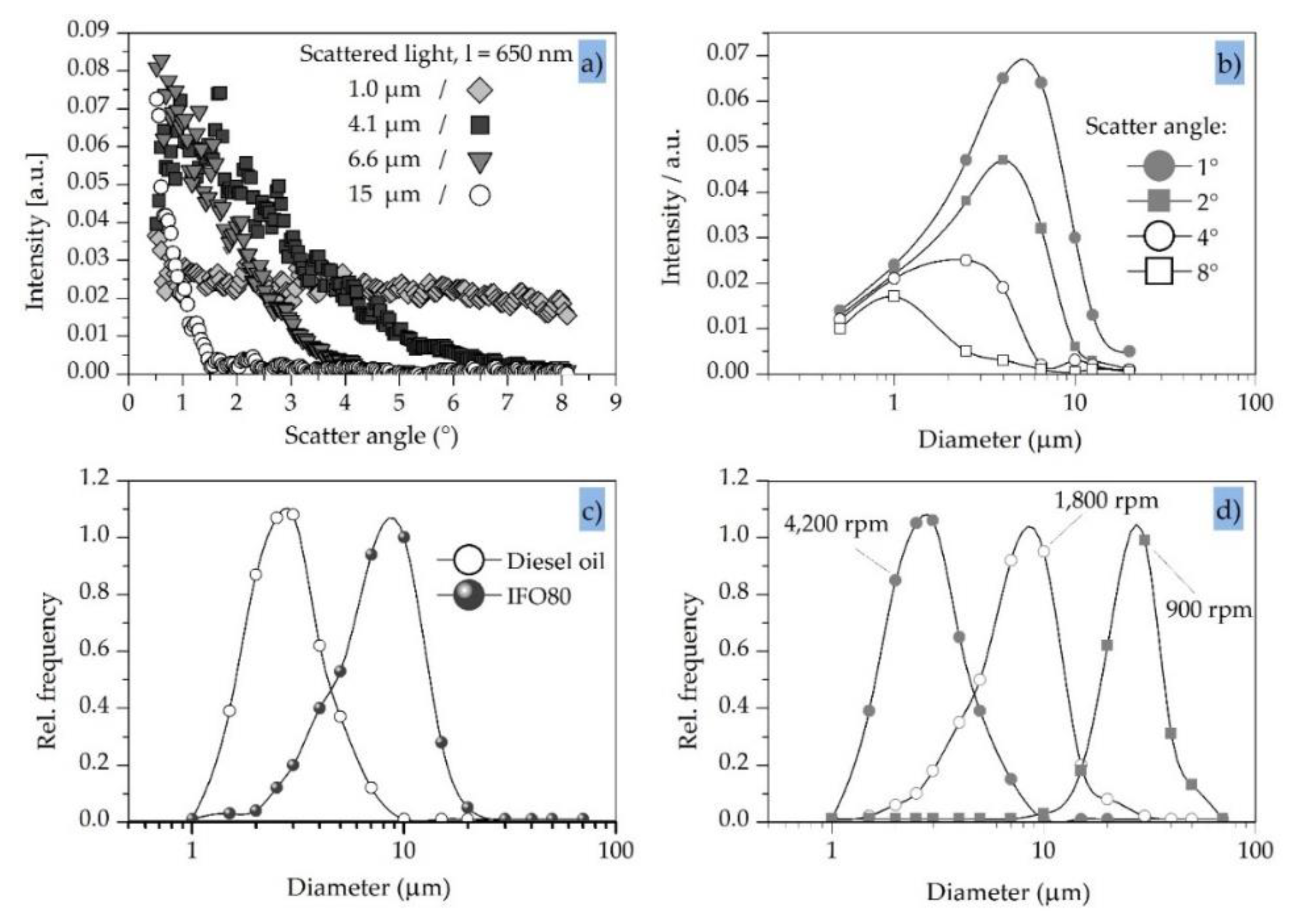
3.3. Typical Light-Scatter Pattern
3.4. Light-Scatter Sensitivity Versus Particle Diameter
3.5. Light Scattering and Oil Droplet Diameter
4. Materials and Methods
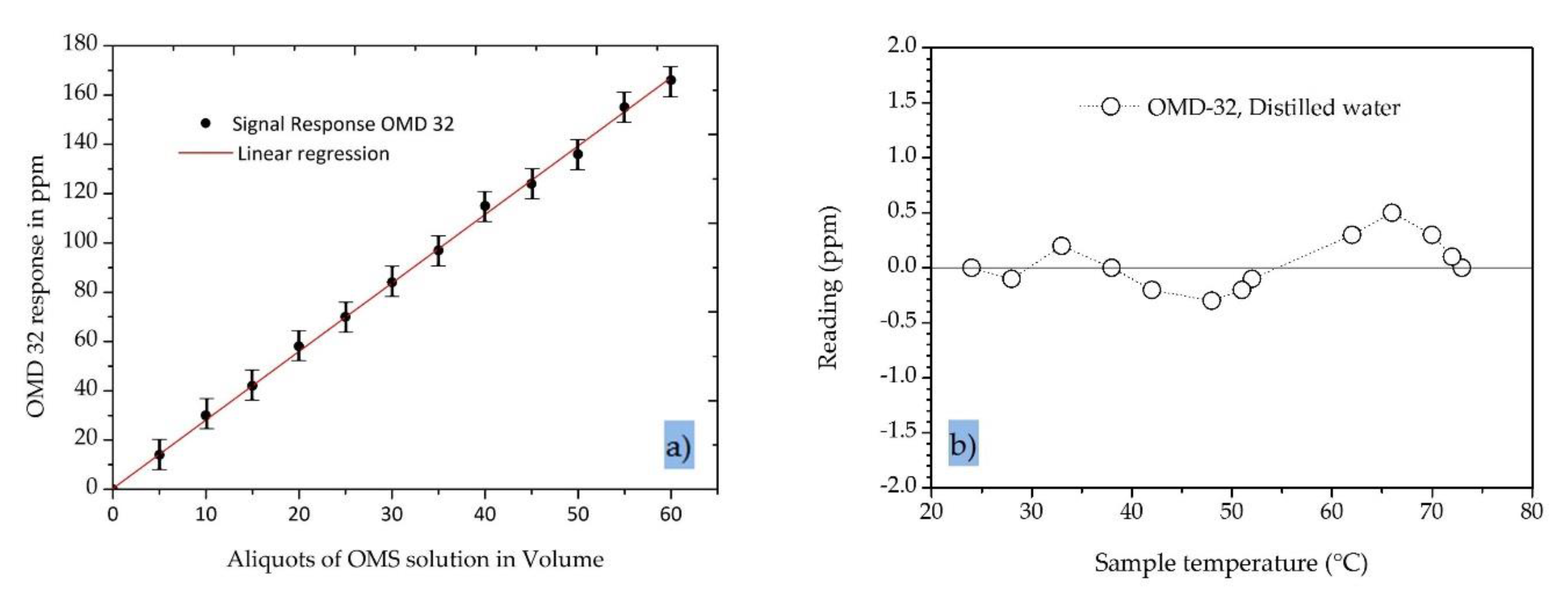
4.1. Oil-in-Water Sensor Specifications
4.2. Oily Wastewater Specifications
4.3. Ceramic Membrane Specifications
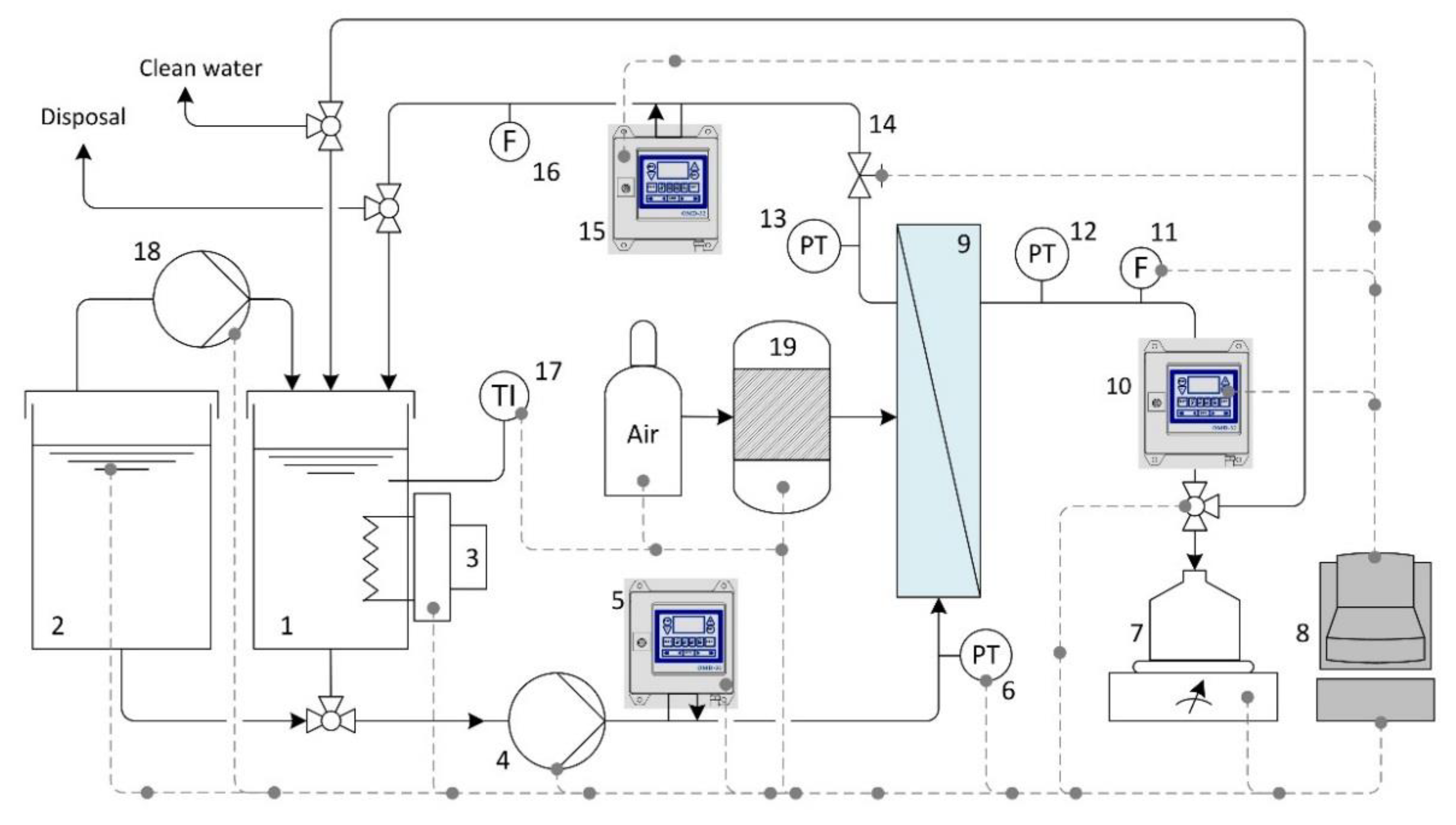
4.4. Filtration Unit Specifications
4.5. Standard Chemical Cleaning Procedures
5. Results and Discussion
5.1. Characterization of Sensor Performance—Effects of Oil Concentraion and Temperature
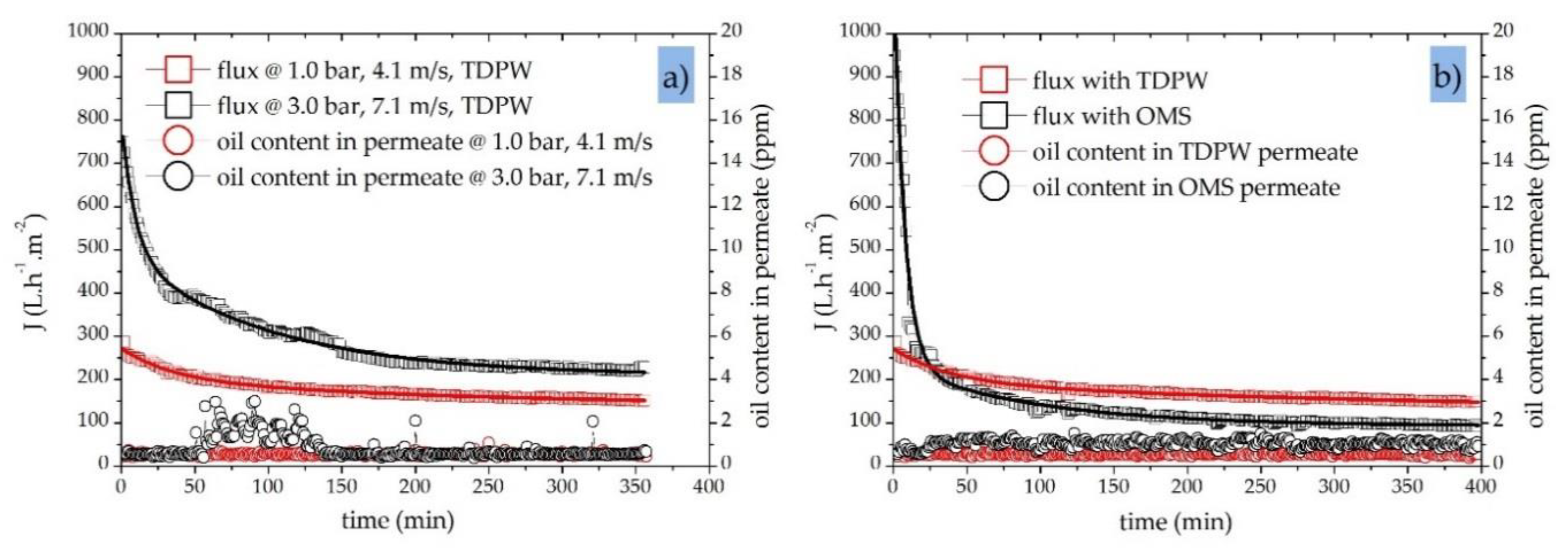
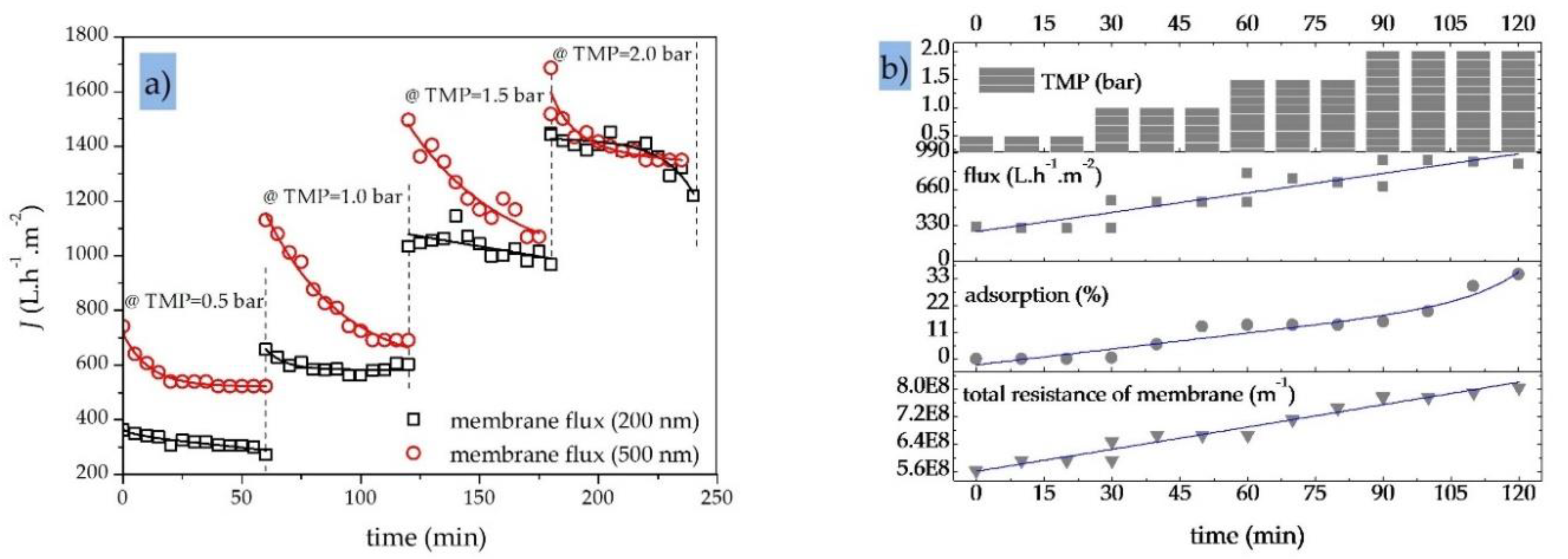
5.2. Characterization of Membrane Performance—Effects of Operating Parameters
5.3. Effect of Crossflow Velocity (CFV) and Transmembrane Pressure (TMP)
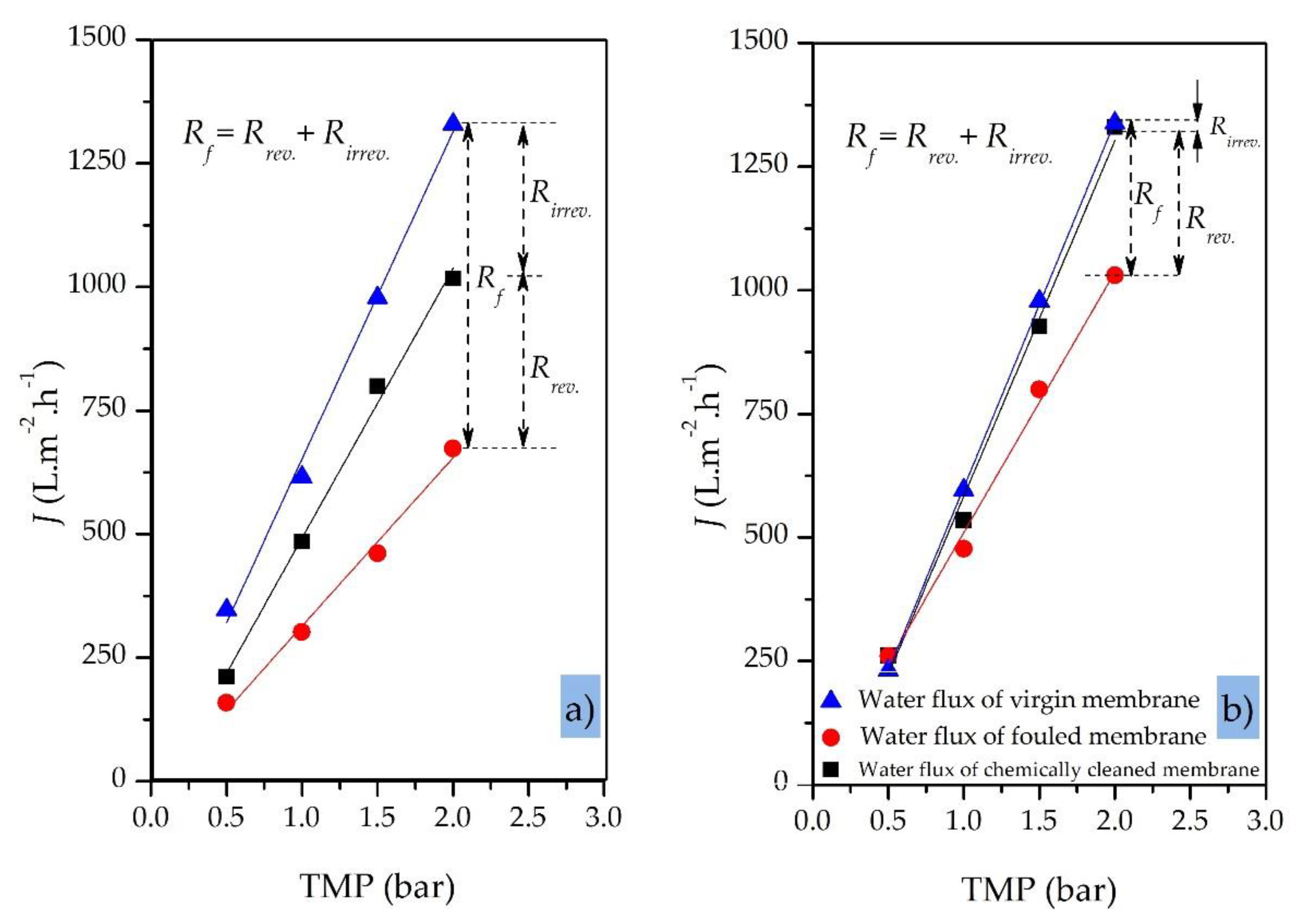
5.4. Membrane Chemical-Cleaning Procedure
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| API | American petroleum institute | OMS | oily model solution |
| Al2O3 | aluminum oxide | Ppm | parts per million |
| initial oil concentration in feed | PW | produced water | |
| initial oil concentration in feed tank | pressure on the feed side | ||
| oil concentration in feed at the time | pressure on the retentate side | ||
| Coil | oil concentration | pressure on the filtrate side | |
| oil concentration in permeate | total membrane resistance | ||
| CFV | cross flow velocity | membrane fouling resistance | |
| CMOS | complementary metal oxide semiconductor | membrane resistance | |
| COD | chemical oxygen demand | oil rejection efficiency | |
| FRE | flux recovery efficiency | SiC | silicon carbide |
| water flux of fouled membrane | SiO2 | silicon oxide | |
| pure water flux of fresh membrane | TDS | total dissolved solids | |
| pure water flux after cleaning | TDPW | tank dewatering produced water | |
| kDa | Kilodalton | TiO2 | titanium oxide |
| membrane permeability | TOC | total organic carbon | |
| MF | Microfiltration | TMP | transmembrane pressure |
| MWCO | molecular weight cut-off | TSS | total suspended solids |
| µ | dynamic viscosity | UF | ultrafiltration |
| NF | Nanofiltraion | initial volume of feed at the time | |
| initial volume of feed at the time |
References
- Wan Ikhsan, S.N. A review of oilfield wastewater treatment using membrane filtration over conventional technology. MJAS 2017, 21. [Google Scholar] [CrossRef]
- Fakhru’l-Razi, A.; Pendashteh, A.; Abdullah, L.C.; Biak, D.R.A.; Madaeni, S.S.; Abidin, Z.Z. Review of technologies for oil and gas produced water treatment. J. Hazard. Mater. 2009, 170, 530–551. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghouti, M.A.; Al-Kaabi, M.A.; Ashfaq, M.Y.; Da’na, D.A. Produced water characteristics, treatment and reuse: A review. J. Water Process Eng. 2019, 28, 222–239. [Google Scholar] [CrossRef]
- Zheng, J.; Chen, B.; Thanyamanta, W.; Hawboldt, K.; Zhang, B.; Liu, B. Offshore produced water management: A review of current practice and challenges in harsh/Arctic environments. Mar. Pollut. Bull. 2016, 104, 7–19. [Google Scholar] [CrossRef]
- Siagian, U.W.R.; Widodo, S.; Khoiruddin; Wardani, A.K.; Wenten, I.G.; Kumoro, A.C.; Hadiyanto; Roces, S.A.; Yung, L.; Rong, X.; et al. Oilfield Produced Water Reuse and Reinjection with Membrane. MATEC Web Conf. 2018, 156, 8005. [Google Scholar] [CrossRef]
- Coca-Prados, J.; Gutiérrez-Cervelló, G. (Eds.) Water Purification and Management; Springer Science+Business Media B.V: Dordrecht, The Netherlands, 2011; ISBN 978-90-481-9775-0. [Google Scholar]
- Padaki, M.; Surya Murali, R.; Abdullah, M.S.; Misdan, N.; Moslehyani, A.; Kassim, M.A.; Hilal, N.; Ismail, A.F. Membrane technology enhancement in oil–water separation. A review. Desalination 2015, 357, 197–207. [Google Scholar] [CrossRef]
- Kurisu, F.; Ramanathan, A.; Kazmi, A.A.; Kumar, M. (Eds.) Trends in Asian Water Environmental Science and Technology; Capital Publishing Company: New Delhi, India, 2017; ISBN 978-3-319-39259-2. [Google Scholar]
- Tanudjaja, H.J.; Hejase, C.A.; Tarabara, V.V.; Fane, A.G.; Chew, J.W. Membrane-based separation for oily wastewater: A practical perspective. Water Res. 2019, 156, 347–365. [Google Scholar] [CrossRef]
- Elshorbagy, W.; Chowdhury, R. (Eds.) Water Treatment; InTech: London, UK, 2013; ISBN 978-953-51-0928-0. [Google Scholar]
- Jiménez, S.; Micó, M.M.; Arnaldos, M.; Medina, F.; Contreras, S. State of the art of produced water treatment. Chemosphere 2018, 192, 186–208. [Google Scholar] [CrossRef]
- Igunnu, E.T.; Chen, G.Z. Produced water treatment technologies. Int. J. Low-Carbon Tech. 2014, 9, 157–177. [Google Scholar] [CrossRef] [Green Version]
- Jepsen, K.; Bram, M.; Pedersen, S.; Yang, Z. Membrane Fouling for Produced Water Treatment: A Review Study From a Process Control Perspective. Water 2018, 10, 847. [Google Scholar] [CrossRef] [Green Version]
- Clark, C.E.; Veil, J.A. Produced Water Volumes and Management Practices in the United States. Available online: https://doi.org/10.2172/1007397 (accessed on 19 February 2020).
- Gebreslase, G.A. Review on Membranes for the Filtration of Aqueous Based Solution: Oil in Water Emulsion. J. Membr. Sci. Technol. 2018, 8. [Google Scholar] [CrossRef]
- Hosseini, S.S.; Fakharian Torbati, S.; Alaei Shahmirzadi, M.A.; Tavangar, T. Fabrication, characterization, and performance evaluation of polyethersulfone/TiO2 nanocomposite ultrafiltration membranes for produced water treatment. Polym. Adv. Technol. 2018, 29, 2619–2631. [Google Scholar] [CrossRef]
- Dabir, S.; Deng, W.; Sahimi, M.; Tsotsis, T. Fabrication of silicon carbide membranes on highly permeable supports. J. Membr. Sci. 2017, 537, 239–247. [Google Scholar] [CrossRef]
- Tran-Duc, T.; Phan-Thien, N.; Wang, J. A theoretical study of permeability enhancement for ultrafiltration ceramic membranes with conical pores and slippage. Phys. Fluids 2019, 31, 22003. [Google Scholar] [CrossRef]
- Lin, F.; Zhang, S.; Ma, G.; Qiu, L.; Sun, H.; Weng, C.-H.; Weerasinghe, R. Application of Ceramic Membrane in Water and Wastewater Treatment. E3S Web Conf. 2018, 53, 4032. [Google Scholar] [CrossRef] [Green Version]
- Gitis, V.; Rothenberg, G. Ceramic Membranes. New Opportunities and Practical Applications; Wiley-VCH: Weinheim, Germany, 2016; ISBN 9783527334933. [Google Scholar]
- He, Z.; Lyu, Z.; Gu, Q.; Zhang, L.; Wang, J. Ceramic-based Membranes for Water and Wastewater Treatment. Colloids Surf. A 2019. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Busse, N.; Kerker, S.; Schmitz, O.; Hilpert, M.; Czermak, P. Treatment of the Bleaching Effluent from Sulfite Pulp Production by Ceramic Membrane Filtration. Membranes 2015, 6, 7. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, M.; Kerker, S.; Schmitz, O.; Schmidt, A.A.; Czermak, P. Evaluation of the fouling potential of ceramic membrane configurations designed for the treatment of oilfield produced water. Sep. Sci. Technol. 2017, 53, 349–363. [Google Scholar] [CrossRef]
- Ćwirko, K.; Kalbarczyk-Jedynak, A. Performance and Selectivity of Ceramic Membranes in the Ultrafiltration of Model Emulsion in Saline. Manage. Syst. Prod. Eng. 2017, 25, 88–92. [Google Scholar] [CrossRef] [Green Version]
- Magalhães, H.L.F.; de Lima, A.G.B.; de Farias Neto, S.R.; Alves, H.G.; de Souza, J.S. Produced water treatment by ceramic membrane: A numerical investigation by computational fluid dynamics. Adv. Mech. Eng. 2017, 9. [Google Scholar] [CrossRef] [Green Version]
- Zsirai, T.; Al-Jaml, A.K.; Qiblawey, H.; Al-Marri, M.; Ahmed, A.; Bach, S.; Watson, S.; Judd, S. Ceramic membrane filtration of produced water: Impact of membrane module. Sep. Purif. Technol. 2016, 165, 214–221. [Google Scholar] [CrossRef] [Green Version]
- Fraga, M.C.; Sanches, S.; Crespo, J.G.; Pereira, V.J. Assessment of a New Silicon Carbide Tubular Honeycomb Membrane for Treatment of Olive Mill Wastewaters. Membranes 2017, 7, 12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Wit, P.; Kappert, E.J.; Lohaus, T.; Wessling, M.; Nijmeijer, A.; Benes, N.E. Highly permeable and mechanically robust silicon carbide hollow fiber membranes. J. Membr. Sci. 2015, 475, 480–487. [Google Scholar] [CrossRef] [Green Version]
- Moritz, T.; Benfer, S.; Árki, P.; Tomandl, G. Influence of the surface charge on the permeate flux in the dead-end filtration with ceramic membranes. Sep. Purif. Technol. 2001, 25, 501–508. [Google Scholar] [CrossRef]
- Cho, K.; Seo, K.W.; Shin, S.G.; Lee, S.; Park, C. Process stability and comparative rDNA/rRNA community analyses in an anaerobic membrane bioreactor with silicon carbide ceramic membrane applications. Sci. Total Environ. 2019, 666, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Costa, J.A.; Farias, N.C.; Queirós, Y.G.C.; Mansur, C.R.E. Determination of oil-in-water using nanoemulsions as solvents and UV visible and total organic carbon detection methods. Talanta 2013, 107, 304–311. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Neff, J. (Eds.) Produced Water. Environmental Risks and Advances in Mitigation Technologies; Springer Science+Business Media LLC: New York, NY, USA, 2011; ISBN 978-1-4614-0045-5. [Google Scholar]
- He, L.M.; Kear-Padilla, L.L.; Lieberman, S.H.; Andrews, J.M. Rapid in situ determination of total oil concentration in water using ultraviolet fluorescence and light scattering coupled with artificial neural networks. Anal. Chim. Acta 2003, 478, 245–258. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Ashaghi, K.S.; Engel, L.; Willershausen, D.; Mund, P.; Bolduan, P.; Czermak, P. Characterization and application of different ceramic membranes for the oil-field produced water treatment. Desalination 2009, 245, 533–540. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Schmitz, O.; Kerker, S.; Liebermann, F.; Czermak, P. Dynamic cross-flow filtration of oilfield produced water by rotating ceramic filter discs. Desalin. Water Treat. 2013, 51, 1762–1768. [Google Scholar] [CrossRef] [Green Version]
- Ebrahimi, M.; Kerker, S.; Daume, S.; Geile, M.; Ehlen, F.; Unger, I.; Schütz, S.; Czermak, P. Innovative ceramic hollow fiber membranes for recycling/reuse of oilfield produced water. Desalin. Water Treat. 2014, 55, 3554–3567. [Google Scholar] [CrossRef]
- Ebrahimi, M.; Willershausen, D.; Ashaghi, K.S.; Engel, L.; Placido, L.; Mund, P.; Bolduan, P.; Czermak, P. Investigations on the use of different ceramic membranes for efficient oil-field produced water treatment. Desalination 2010, 250, 991–996. [Google Scholar] [CrossRef] [Green Version]
- Chicea, D.; Rei, S.M. Using Dynamic Light Scattering for Monitoring the Size of the Suspended Particles in Wastewater. Transylvanian Rev. Systematical Ecol. Res. 2019, 21, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Goddeeris, C.; Cuppo, F.; Reynaers, H.; Bouwman, W.G.; van den Mooter, G. Light scattering measurements on microemulsions: Estimation of droplet sizes. Int. J. Pharm. 2006, 312, 187–195. [Google Scholar] [CrossRef] [PubMed]
- Khan, M.F.; Singh, M.K.; Sen, S. Measuring Size, Size Distribution, and Polydispersity of Water-in-Oil Microemulsion Droplets using Fluorescence Correlation Spectroscopy: Comparison to Dynamic Light Scattering. J. Phys. Chem. B 2016, 120, 1008–1020. [Google Scholar] [CrossRef]
- Bohren, C.F.; Huffman, D.R. Absorption and Scattering of Light by Small Particles; Wiley-VCH: Weinheim, Germany, 2008; ISBN 9780471293408. [Google Scholar]
- Mie, G. Beiträge zur Optik trüber Medien, speziell kolloidaler Metallösungen. Ann. Phys. 1908, 330, 377–445. [Google Scholar] [CrossRef]
- Mishchenko, M.I. Light scattering by randomly oriented axially symmetric particles. J. Opt. Soc. Am. A 1991, 8, 871. [Google Scholar] [CrossRef]
- Mishchenko, M.I.; Mackowski, D.W. Electromagnetic scattering by randomly oriented bispheres: Comparison of theory and experiment and benchmark calculations. J. Quant. Spectrosc. Radiat. Transfer 1996, 55, 683–694. [Google Scholar] [CrossRef]
- Mishchenko, M.I.; Travis, L.D.; Macke, A. Scattering of light by polydisperse, randomly oriented, finite circular cylinders. Appl. Opt. 1996, 35, 4927–4940. [Google Scholar] [CrossRef]
- Maguire, C.M.; Rösslein, M.; Wick, P.; Prina-Mello, A. Characterisation of particles in solution - a perspective on light scattering and comparative technologies. Sci. Technol. Adv. Mater. 2018, 19, 732–745. [Google Scholar] [CrossRef]
- Seebacher, G.; Schmidt, A.A.; Offermann, J. Oil in Water Monitoring Using Advanced Light Scattering. In Proceedings of the ASME/USCG 2013 3rd Workshop on Marine Technology and Standards, Arlington, VA, USA, 24–25 July 2013; pp. 1–12, ISBN 978-0-7918-9940-3. [Google Scholar]
- Banda-Cruz, E.E.; Padrón-Ortega, S.I.; Gallardo-Rivas, N.V.; Rivera-Armenta, J.L.; Páramo-García, U.; Zavala, N.P.D.; Mendoza-Martínez, A.M. Crude oil UV spectroscopy and light scattering characterization. J. Pet. Sci. Technol. 2016, 34, 732–738. [Google Scholar] [CrossRef]
- Bannwarth, S.; Trieu, T.; Oberschelp, C.; Wessling, M. On-line monitoring of cake layer structure during fouling on porous membranes by in situ electrical impedance analysis. J. Membr. Sci. 2016, 503, 188–198. [Google Scholar] [CrossRef]
- Kurada, K.V.; De Tanmay, S. Modeling of cross flow hollow fiber ultrafiltration for treatment of effluent from Railway Workshop. J. Membr. Sci. 2018, 551, 223–233. [Google Scholar] [CrossRef]
- Janus, T.; Ulanicki, B. A Behavioural Membrane Fouling Model for Integrated Simulation of Membrane Bioreactors for Wastewater Treatment. Procedia Eng. 2015, 119, 1328–1337. [Google Scholar] [CrossRef] [Green Version]
- Miller, D.J.; Kasemset, S.; Paul, D.R.; Freeman, B.D. Comparison of membrane fouling at constant flux and constant transmembrane pressure conditions. J. Membr. Sci. 2014, 454, 505–515. [Google Scholar] [CrossRef]
- Alresheedi, M.T.; Basu, O.D.; Barbeau, B. Chemical cleaning of ceramic ultrafiltration membranes—Ozone versus conventional cleaning chemicals. Chemosphere 2019, 226, 668–677. [Google Scholar] [CrossRef]
- Thuvander, J.; Zarebska, A.; Hélix-Nielsen, C.; Jönsson, A.-S.J. Wood Chem. Technol. 2018, 38, 276–285. [Google Scholar] [CrossRef]
- Tian, J.-y.; Ernst, M.; Cui, F.; Jekel, M. Correlations of relevant membrane foulants with UF membrane fouling in different waters. Water Res. 2013, 47, 1218–1228. [Google Scholar] [CrossRef]
- László Kiss, Z.; Talpas, L.; Seres, Z.; Beszédes, S.; Hodúr, C.; László, Z. Treatment of model oily waste water by microfiltration. Per. Pol. Chem. Eng. 2013, 57, 21. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Hankins, N.P. Introduction to Membrane Processes for Water Treatment. In Emerging Membrane Technology for Sustainable Water Treatment; Elsevier: Amsterdam, The Netherlands, 2016; pp. 15–52. ISBN 9780444633125. [Google Scholar]
- Simonič, M. Membrane surface properties and their effects on real waste oil-in-water emulsion ultrafiltration. WSA 2019, 45. [Google Scholar] [CrossRef] [Green Version]
- Darvishzadeh, T.; Tarabara, V.V.; Priezjev, N.V. Oil droplet behavior at a pore entrance in the presence of crossflow: Implications for microfiltration of oil–water dispersions. J. Membr. Sci. 2013, 447, 442–451. [Google Scholar] [CrossRef] [Green Version]
- Abadi, S.R.H.; Sebzari, M.R.; Hemati, M.; Rekabdar, F.; Mohammadi, T. Ceramic membrane performance in microfiltration of oily wastewater. Desalination 2011, 265, 222–228. [Google Scholar] [CrossRef]
- Masoudnia, K.; Raisi, A.; Aroujalian, A.; Fathizadeh, M. A hybrid microfiltration/ultrafiltration membrane process for treatment of oily wastewater. Desalin. Water Treat. 2014, 55, 901–912. [Google Scholar] [CrossRef]
- Hankins, N.P.; Singh, R. Emerging Membrane Technology for Sustainable Water Treatment; Elsevier: Amsterdam, The Netherlands, 2016; ISBN 9780444633125. [Google Scholar]
- Yunos, K.F.M.; Mazlan, N.A.; Naim, M.N.M.; Baharuddin, A.S.; Hassan, A.R. Ultrafiltration of palm oil mill effluent: Effects of operational pressure and stirring speed on performance and membranes fouling. Environ. Eng. Res. 2019, 24, 263–270. [Google Scholar] [CrossRef] [Green Version]
- Pérez-Gálvez, R.; Guadix, E.M.; Bergé, J.-P.; Guadix, A. Operation and cleaning of ceramic membranes for the filtration of fish press liquor. J. Membr. Sci. 2011, 384, 142–148. [Google Scholar] [CrossRef] [Green Version]

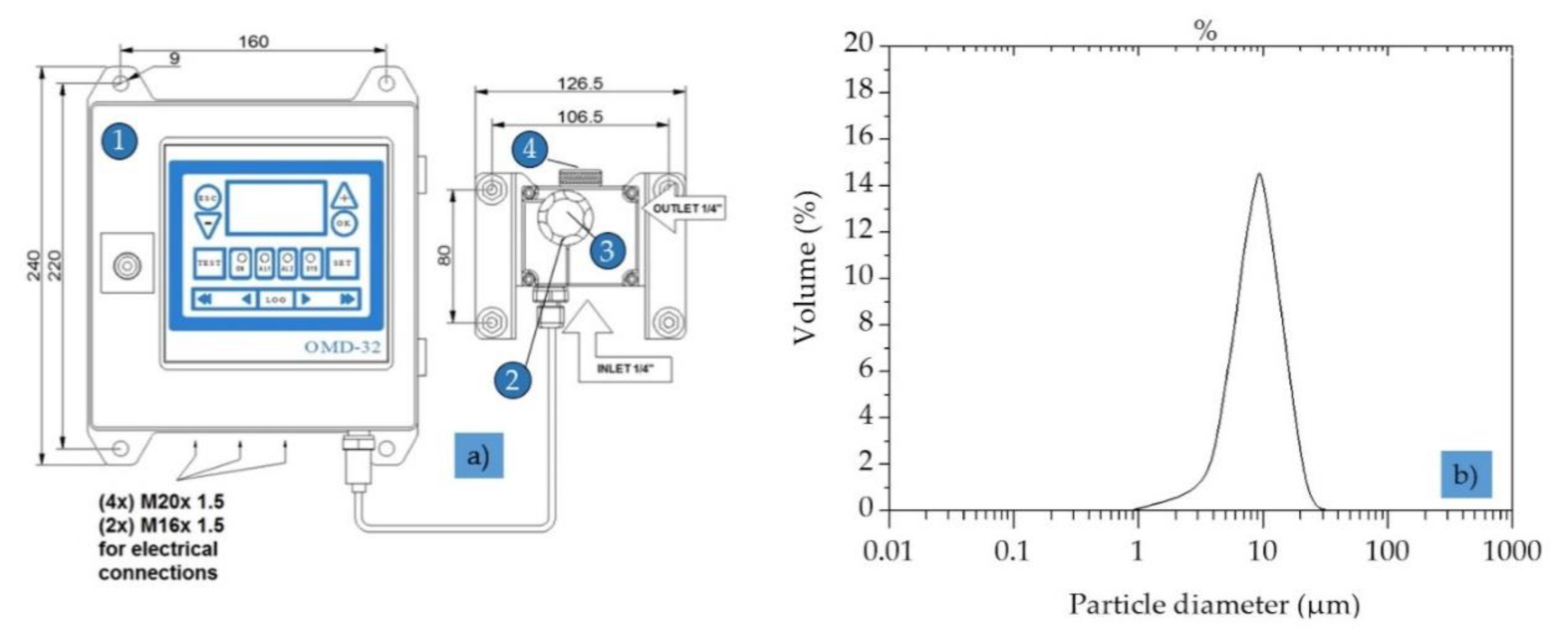

| Major Parameter | Value | Unit |
|---|---|---|
| TSS | <1000 | mg·L−1 |
| TDS | <400,000 | mg·L−1 |
| TOC | 0–1500 | mg·L−1 |
| Total organic acids | <10,000 | mg·L−1 |
| Total oil | 2–600 | mg·L−1 |
| pH | 4.3–10 | – |
| Density | 1014–1140 | kg·m−3 |
| Oil droplet size | 2.0–30 | μm |
| Range | 0–200 ppm |
| Accuracy | up to ±1.0 ppm below 10 ppm |
| Resolution | 1.0 ppm (0.1 ppm below 10 ppm) |
| Response time | <5.0 s |
| Sample water pressure | max. 10 bar |
| Sample water temperature | up to 90 °C |
| Major Parameter | OMS | TDPW | Oilfield PW |
|---|---|---|---|
| pH | 7.0–7.8 | 4.3–10 | 4.0–10 |
| TOC | 20–100 mgL−1 | 200–2000 mgL−1 | 0–1500 mgL−1 |
| Dispersed oil | 30–300 mgL−1 | 200–1000 mgL−1 | 2.0–400 mgL−1 |
| Conductivity | 160–220 µS·cm−1 | 20,000–80,000 µS·cm−1 | n.a. |
| Parameter | Description | ||
|---|---|---|---|
| Nominal pore size | 500 nm | 200 nm | 40 nm |
| pH range | 0–14 | 0–14 | 0–14 |
| Membrane length | 0.45 m | 0.45 m | 0.25 m |
| Number of channels | 37 | 37 | 31 |
| Channel diameter | 0.0034 m | 0.0034 m | 0.0030 m |
| Membrane filtration area | 0.18 m2 | 0.18 m2 | 0.09 m2 |
| Manufacturer | 1 | 1 | 2 |
| Cut-Off (nm) | Feed Source | Filtration Mode | CFV (m·s−1) | TMP (Bar) | Duration (min) | Temp. (°C) | COil Feed (ppm) | Initial Flux L·h−1·m−2) | Final Flux (L·h−1·m−2) | Flux Decline (%) | FRE (%) | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 40 | OMS | FBM | 4.1 | 1.0 | 420 | 50 | 30 | 950 | 88 | 91 | 99 | >98 |
| 40 | OMS | FBM | 4.1 | 2.0 | 420 | 50 | 30 | 878 | 162 | 82 | 95 | >98 |
| 40 | OMS | FBM | 7.1 | 3.0 | 420 | 50 | 30 | 1086 | 235 | 78 | 80 | >97 |
| 40 | TDPW | FBM | 4.1 | 1.0 | 420 | 50 | 200 | 275 | 148 | 46 | 90 | >98 |
| 40 | TDPW | FBM | 7.1 | 3.0 | 400 | 50 | 200 | 1163 | 229 | 80 | 79 | >96 |
| 200 | OMS | FBM | 1.0 | 1.0 | 240 | 40 | 100 | 777 | 600 | 23 | 99 | >98 |
| 200 | OMS | TRM | 1.0 | 1.0 | 240 | 40 | 200 | 590 | 455 | 23 | 97 | >98 |
| 200 | OMS | TRM | 1.0 | 0.5 | 240 | 40 | 200 | 573 | 506 | 12 | 98 | >96 |
| 200 | OMS | TRM | 1.0 | 0.5 | 240 | 40 | 100 | 363 | 273 | 25 | 97 | >98 |
| 500 | OMS | TRM | 1.0 | 1.0 | 240 | 40 | 200 | 2260 | 317 | 84 | 94 | >98 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ebrahimi, M.; Schmidt, A.A.; Kaplan, C.; Schmitz, O.; Czermak, P. Innovative Optical-Sensing Technology for the Online Fouling Characterization of Silicon Carbide Membranes during the Treatment of Oily Water. Sensors 2020, 20, 1161. https://doi.org/10.3390/s20041161
Ebrahimi M, Schmidt AA, Kaplan C, Schmitz O, Czermak P. Innovative Optical-Sensing Technology for the Online Fouling Characterization of Silicon Carbide Membranes during the Treatment of Oily Water. Sensors. 2020; 20(4):1161. https://doi.org/10.3390/s20041161
Chicago/Turabian StyleEbrahimi, Mehrdad, Axel A. Schmidt, Cagatay Kaplan, Oliver Schmitz, and Peter Czermak. 2020. "Innovative Optical-Sensing Technology for the Online Fouling Characterization of Silicon Carbide Membranes during the Treatment of Oily Water" Sensors 20, no. 4: 1161. https://doi.org/10.3390/s20041161
APA StyleEbrahimi, M., Schmidt, A. A., Kaplan, C., Schmitz, O., & Czermak, P. (2020). Innovative Optical-Sensing Technology for the Online Fouling Characterization of Silicon Carbide Membranes during the Treatment of Oily Water. Sensors, 20(4), 1161. https://doi.org/10.3390/s20041161