Development of a Resonant Microwave Sensor for Sediment Density Characterization
Abstract
1. Introduction
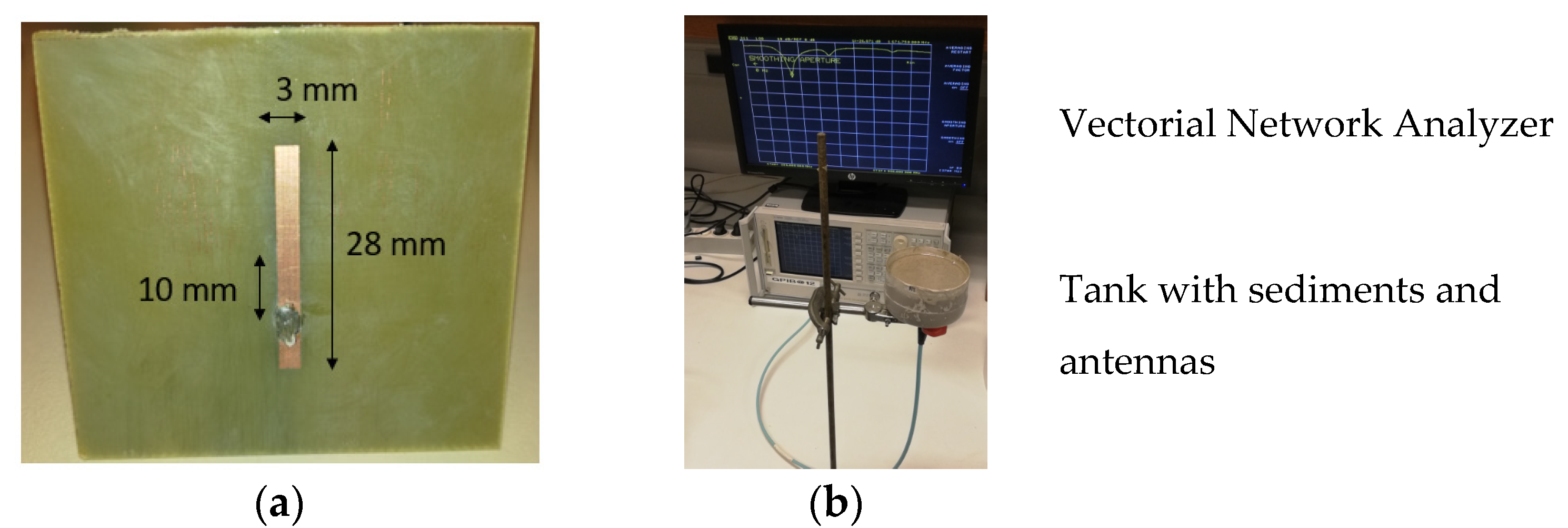
2. Materials and Methods
3. Results and Discussion
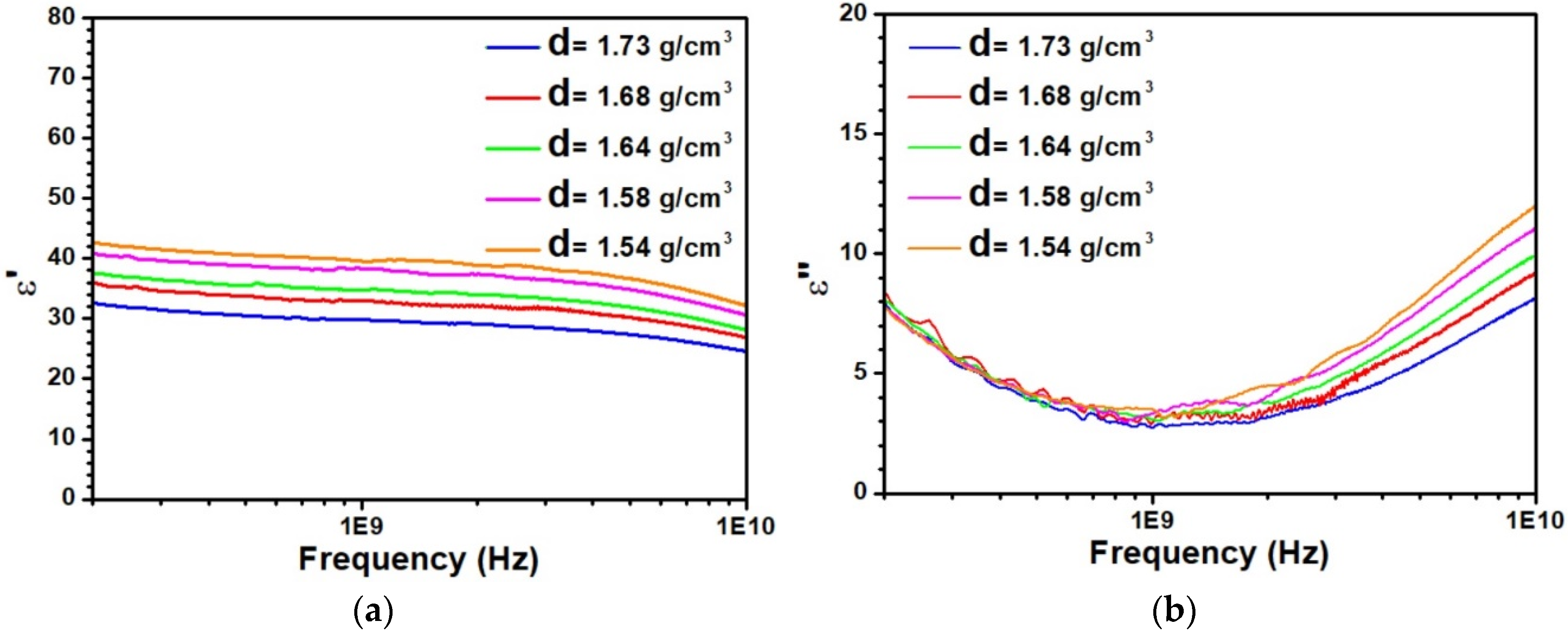
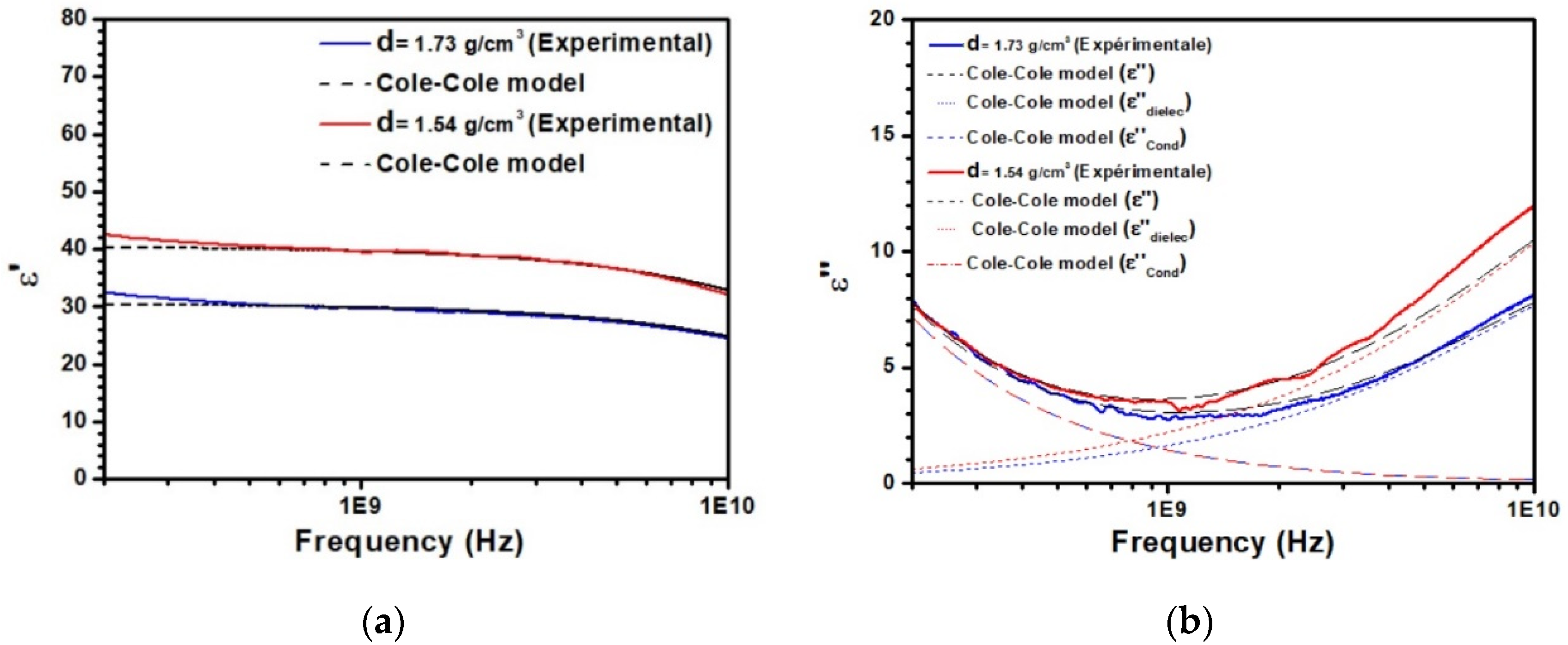
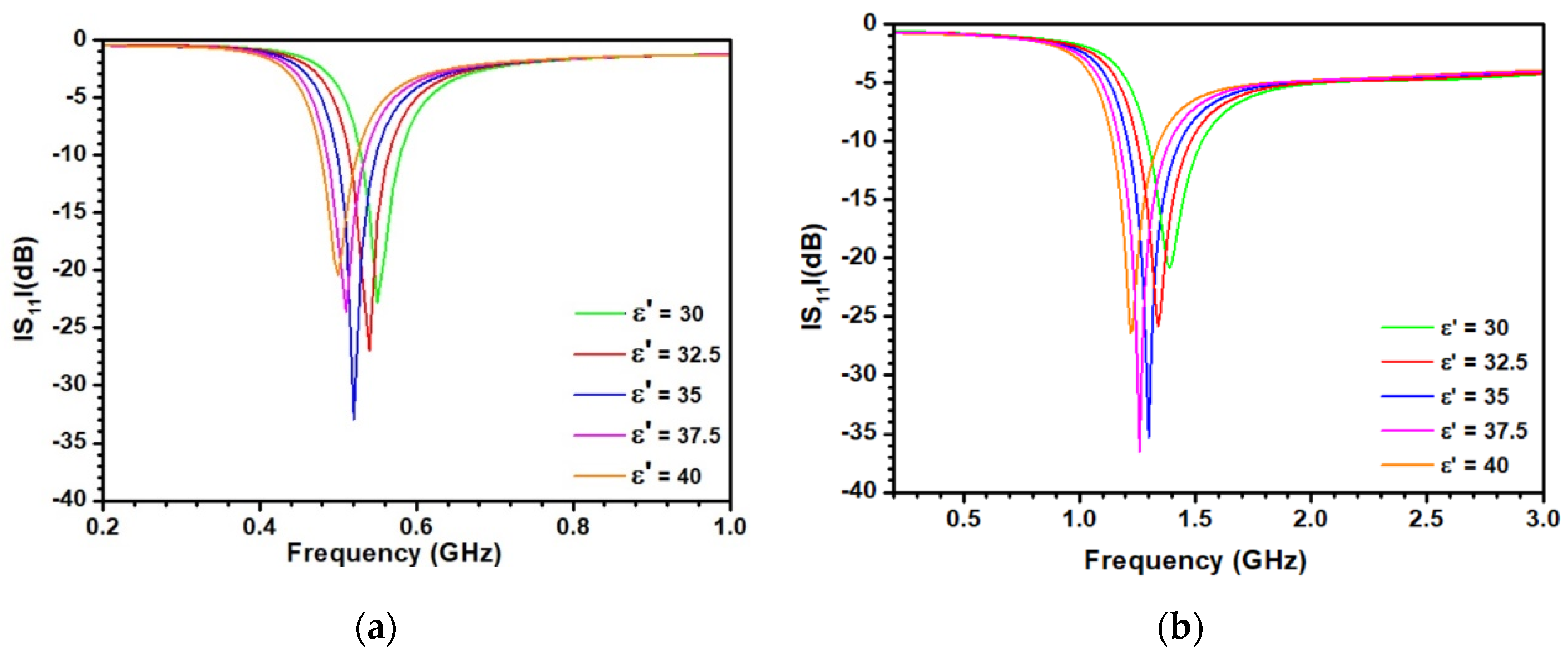
3.1. Dielectric Characterization of Kaolinite with Different Densities
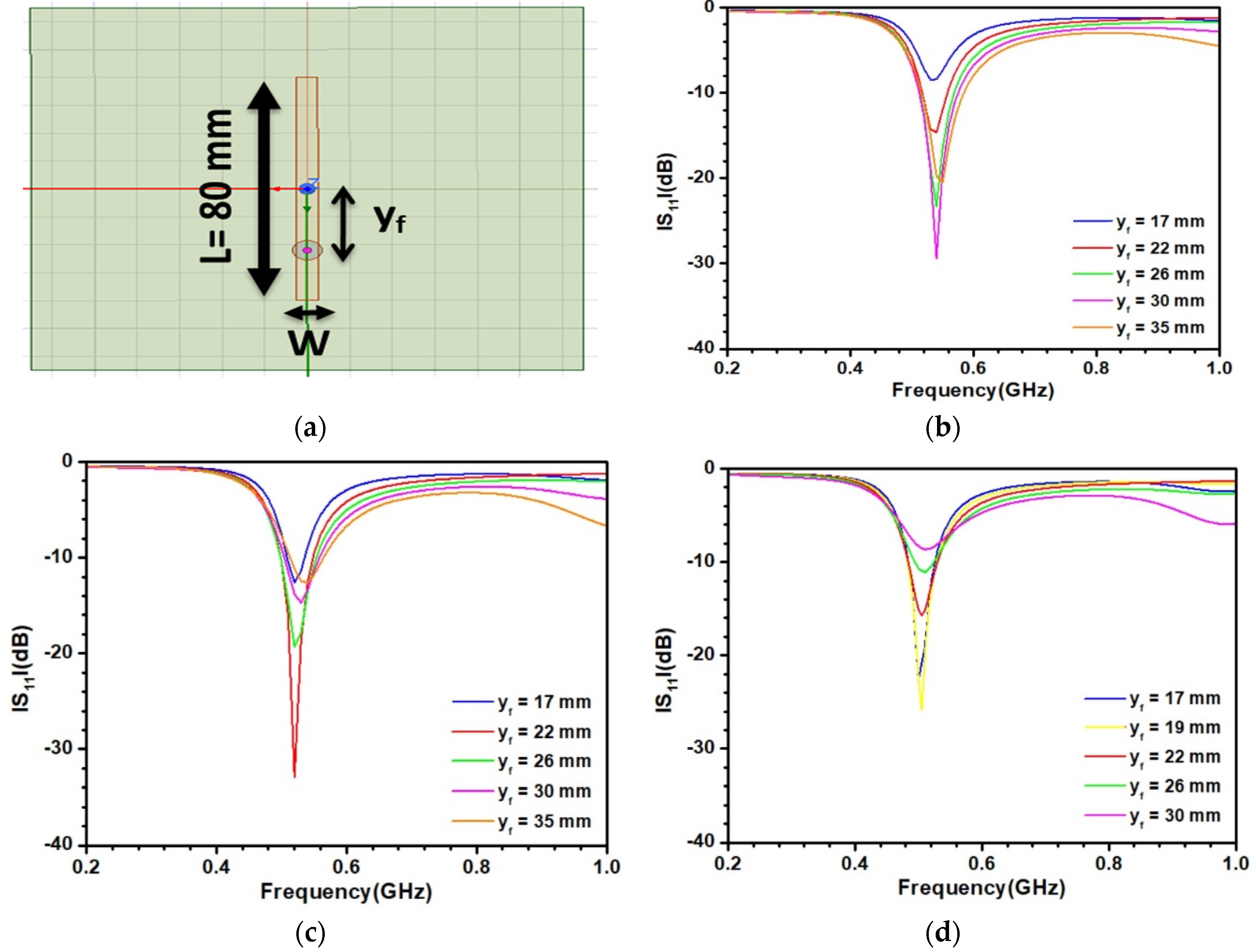
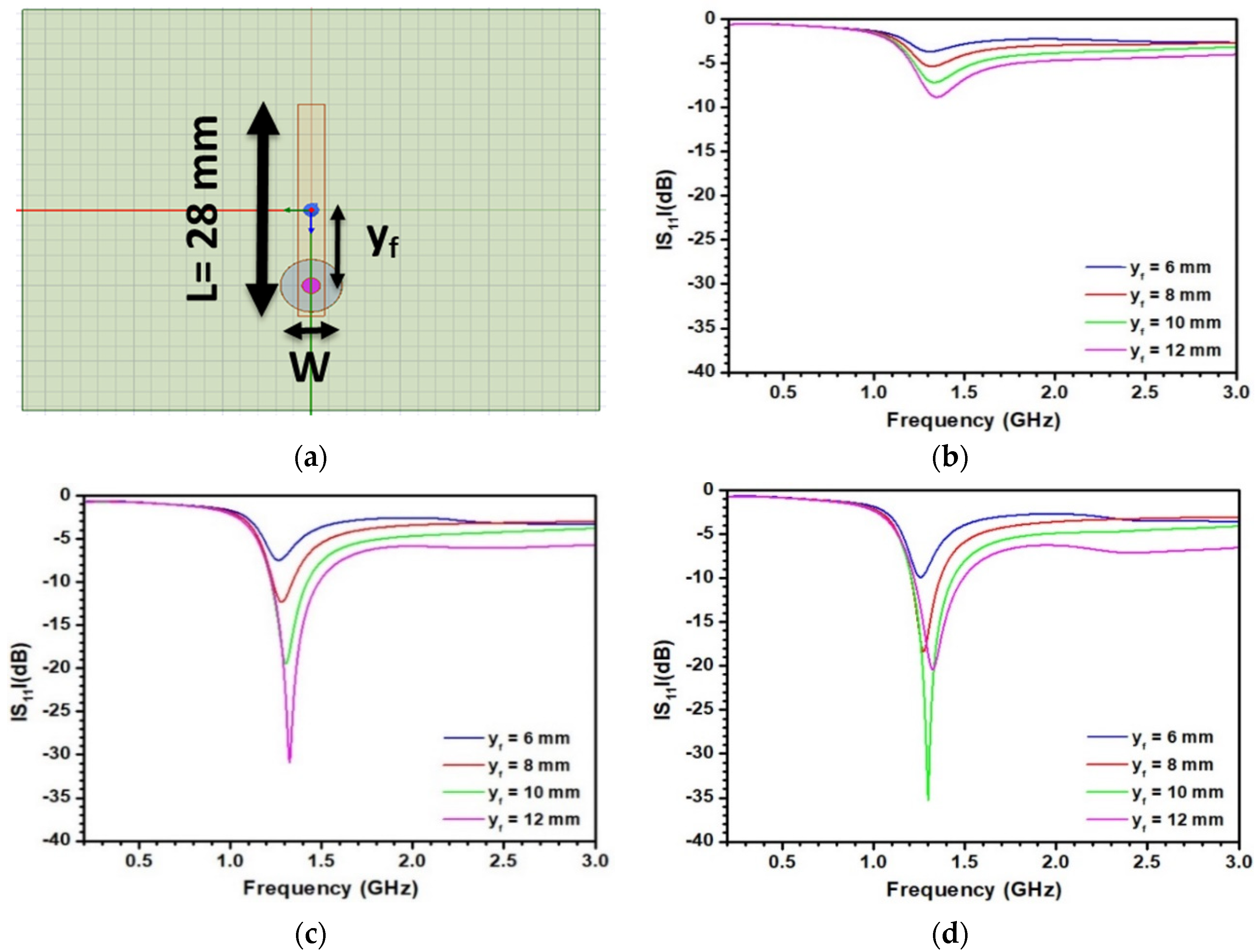
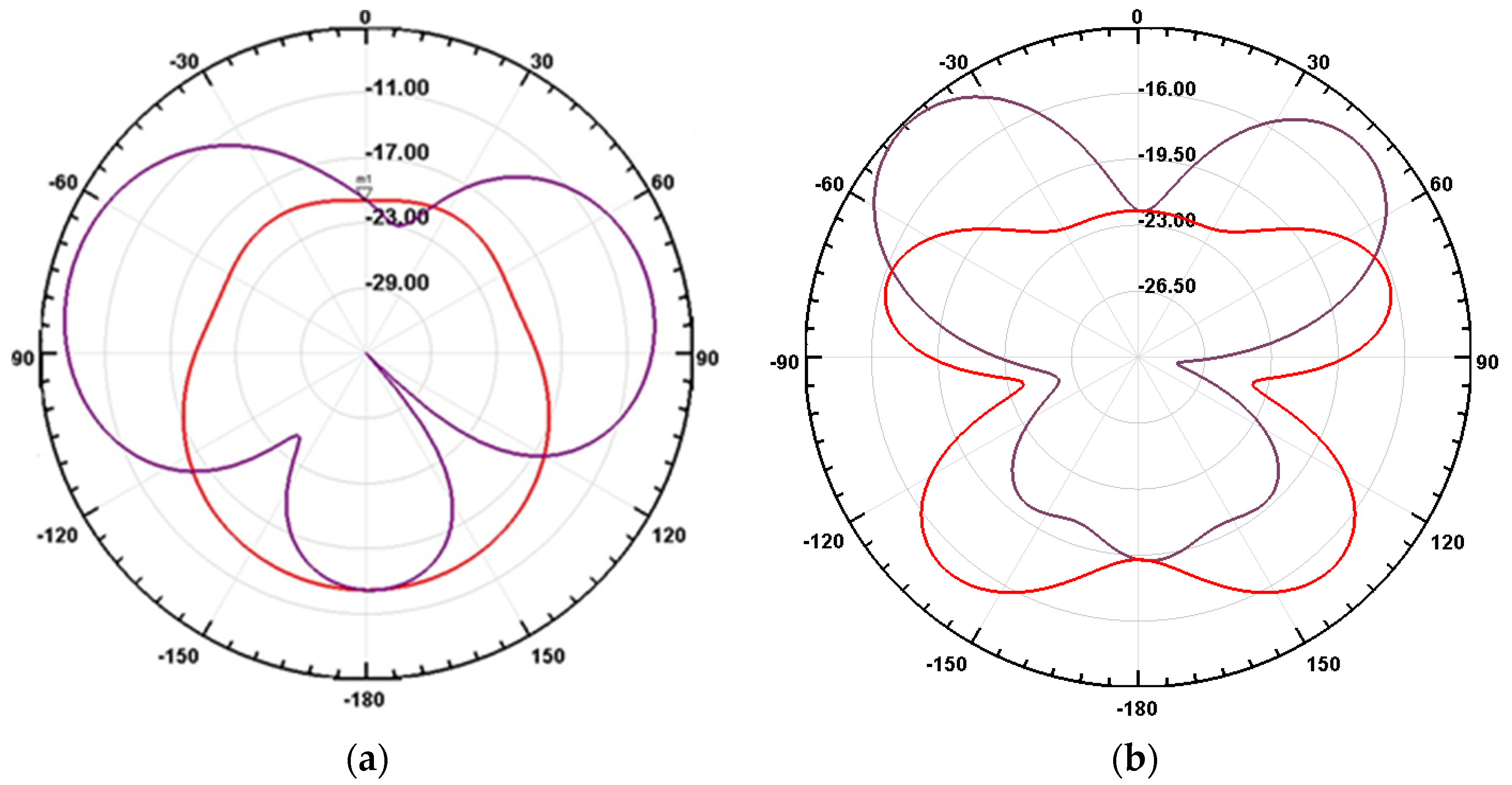
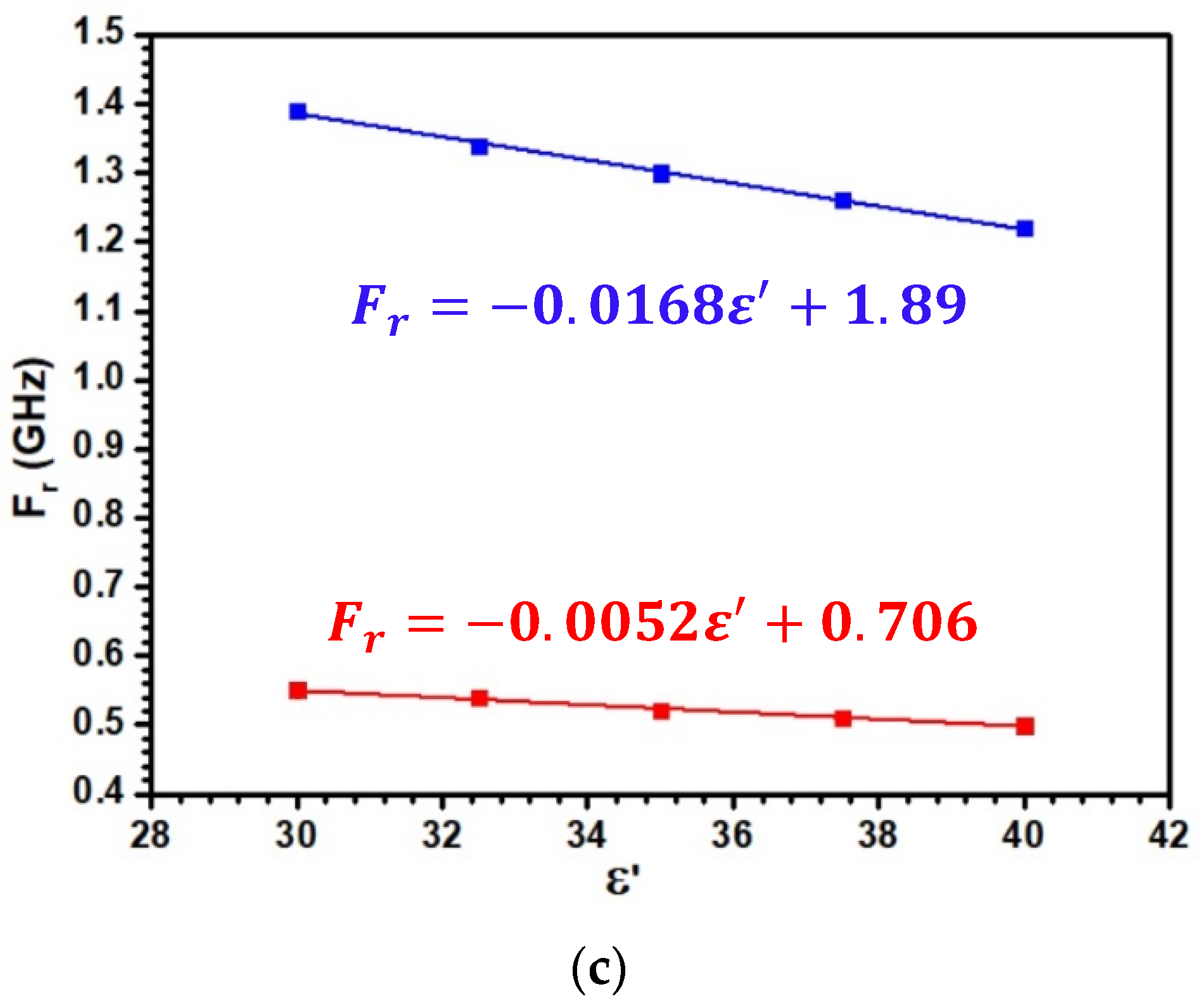
3.2. Optimization of the Sensitive Patch Antenna
3.3. Sensitivity of the Patch Antenna to the Density and Experimental Validation
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Kirtay, V.J. Rapid Sediment Characterization Tools; Technical Report; SSC: San Diego, CA, USA, 2008. [Google Scholar]
- Corey, J.C.; Hayes, D.W. Determination of density and water content of marine sediment in an unextruded core using fast neutron and gamma ray attenuation. Deep Sea Res. Oceanogr. Abstr. 1970, 17, 917–922. [Google Scholar] [CrossRef]
- Preiss, K. Non-destructive laboratory measurement of marine sediment density in a core barrel using gamma radiation. Deep Sea Res. Oceanogr. Abstr. 1968, 15, 401–407. [Google Scholar] [CrossRef]
- Keller, G.H. Deep-sea nuclear sediment density probe. Deep Sea Res. Oceanogr. Abstr. 1965, 12, 373–376. [Google Scholar] [CrossRef]
- Maa, J.P.Y.; Sun, K.J.; He, Q. Ultrasonic characterization of marine sediments: A preliminary study. Mar. Geol. 1997, 141, 183–192. [Google Scholar] [CrossRef]
- Bell, D.; Porter, W.; Westneat, A. Progress in the Use of Acoustics to Classify Marine Sediments. In Proceedings of the IEEE International Conference on Engineering in the Ocean Environment, Seattle, WA, USA, 25–28 September 1973; pp. 354–360. [Google Scholar]
- Ridd, P.; Day, G.; Thoma, S.; Harradence, J.; Fox, D.; Bunt, J.; Renagi, O.; Jago, C. Measurement of Sediment Deposition Rates using an Optical Backscatter Sensor. Estuar. Coast. Shelf Sci. 2001, 2, 155–163. [Google Scholar] [CrossRef]
- Oh, M.; Kim, Y.; Park, J. Factors affecting the complex permittivity spectrum of soil at a low frequency range of 1 kHz–10 MHz. Environ. Geol. 2006, 51, 821–833. [Google Scholar] [CrossRef]
- Datsios, Z.G.; Mikropoulos, P.N. Characterization of the frequency dependence of the electrical properties of sandy soil with variable grain size and water content. IEEE Trans. Dielectr. Electr. Insul. 2019, 26, 904–912. [Google Scholar] [CrossRef]
- Josh, M.; Clennell, B. Broadband electrical properties of clays and shales: Comparative investigations of remolded and preserved samples. Geophysics 2015, 80, 129–143. [Google Scholar] [CrossRef]
- Stevens Water Monitoring Systems Inc. Available online: https://www.stevenswater.com/products/hydraprobe (accessed on 1 January 2020).
- Kim, J.; Babajanyan, A.; Hovsepyan, A.; Lee, K.; Friedman, B. Microwave dielectric resonator biosensor for aqueous glucose solution. Rev. Sci. Instrum. 2008, 79, 086107. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Withayachumnankul, W.; Al-Sarawi, S.; Abbott, D. High-sensitivity metamaterial-inspired sensor for microfluidic dielectric characterization. IEEE Sens. J. 2014, 14, 1345–1351. [Google Scholar] [CrossRef]
- Vélez, P.; Su, L.; Grenier, K.; Mata-Contreras, J.; Dubuc, D.; Martín, F. Microwave microfluidic sensor based on a microstrip splitter/combiner configuration and split ring resonators (SRRs) for dielectric characterization of liquids. IEEE Sens. J. 2017, 17, 6589–6598. [Google Scholar] [CrossRef]
- Sharafadinzadeh, N.; Abdolrazzaghi, M.; Daneshmand, M. Highly sensitive microwave split ring resonator sensor using gap extension for glucose sensing. IEEE MTT-S Int. Microw. Symp. Dig. Pavia Italy Sep. 2017, 1–3. [Google Scholar] [CrossRef]
- Vélez, P.; Grenier, K.; Mata-Contreras, J.; Dubuc, D.; Martín, F. Highly-sensitive microwave sensors based on open complementary split ring resonators (OCSRRs) for dielectric characterization andsolute concentration measurement in liquids. IEEE Access 2018, 6, 48324–48338. [Google Scholar] [CrossRef]
- Hotte, D.; Siragusa, R.; Duroc, Y.; Tedjini, S. A Humidity Sensor Based on V-Band Slotted Waveguide Antenna Array, 4 October 2017, Article Number 8058639. In Proceedings of the 2017 IEEE MTT-S International Microwave Symposium, IMS 2017, Honololu, HI, USA, 4–9 June 2017; pp. 602–604. [Google Scholar]
- Austin, J.; Gupta, A.; McDonnell, R.; Reklaitis, G.V.; Harris, M.T. A novel microwave sensor to determine particulate blend composition on-line. Anal. Chim. Acta 2014, 819, 82–93. [Google Scholar] [CrossRef] [PubMed]
- Bernou, C.; Rebiere, D.; Pistré, J. Microwave sensors: A new sensing principle. Application to humidity detection. Sens. Actuators B 2000, 68, 88–93. [Google Scholar] [CrossRef]
- Kim, Y.H.; Jang, K.; Yoon, Y.J.; Kim, Y.J. A novel relative humidity sensor based on microwave resonators and a customized polymeric film. Sens. Actuators B 2006, 117, 315–322. [Google Scholar] [CrossRef]
- Amin, E.M.; Karmakar, N.; Winther-Jensen, B. Polyvinyl-alcohol (PVA)-based RF humidity sensor in microwave frequency. Prog. Electromagn. Res. B 2013, 54, 149–166. [Google Scholar] [CrossRef]
- Toba, T.; Kitagawa, A. Wireless Moisture Sensor Using a Microstrip Antenna. J. Sens. 2011, 827969. [Google Scholar] [CrossRef]
- Hoseini, N.; Olokede, S.; Daneshmand, M. A novel miniaturized asymmetric CPW split ring resonator with extended field distribution pattern for sensing applications. Sens. Actuators A Phys. 2020. [Google Scholar] [CrossRef]
- Khalifeh, R.; Gallée, F.; Lescop, B.; Talbot, P.; Rioual, S. Application of Fully Passive Wireless Sensors to the Monitoring of Reinforced Concrete Structure Degradation. In Proceedings of the 9th European Workshop on Structural Health Monitoring (EWSHM 2018), Manchester, UK, 10–13 July 2018. [Google Scholar]
- Lázaro, A.; Villarino, R.; Costa, F.; Genovesi, S.; Gentile, A.; Buoncristiani, L.; Girbau, D. Chipless Dielectric Constant Sensor for Structural Health Testing. IEEE Sens. J. 2018, 18, 5576–5585. [Google Scholar] [CrossRef]
- Khalifeh, R.; Lescop, B.; Gallée, F.; Rioual, S. Development of a Radio Frequency resonator for monitoring water diffusion in organic coatings. Sens. Actuators A Phys. 2016, 247, 30–36. [Google Scholar] [CrossRef]
- Li, Z.; Jin, Z.; Shao, S.; Zhao, T.; Wang, P. Influence of Moisture Content on Electromagnetic Response of Concrete Studied Using a Homemade Apparatus. Sensors 2019, 19, 4637. [Google Scholar] [CrossRef] [PubMed]
- Meunier, J.; Sultan, N.; Jegou, P.; Harmegnies, F. The Penfeld Seabed Penetrometer. In Proceedings of the Europe Oceans 2005, Brest, France, 20–23 June 2005; Volume 2, pp. 1309–1314. [Google Scholar]
- Tenzer, R.; Gladkikh, V. Assessment of Density Variations of Marine Sediments with Ocean and Sediment Depths. Sci. World J. 2014, 823296. [Google Scholar] [CrossRef] [PubMed]
- Dong, X.; Wang, Y.H. The Effects of the pH-Influenced Structure on the Dielectric Properties of Kaolinite–Water Mixtures. Soil Sci. Soc. Am. J. 2008, 72, 1532–1541. [Google Scholar] [CrossRef]
- Lukichev, A.A. Relaxation function for the non-Debye relaxation spectra description. Chem. Phys. 2014, 428, 29–33. [Google Scholar] [CrossRef]
- Cole, K.S.; Cole, R.H. Dispersion and Absorption in Dielectrics I. Alternating Current Characteristics. J. Chem. Phys. 1941, 9, 341–351. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Scott, J.; Ghorbani, K. Ultrahigh-Sensitivity Microwave Sensor for Microfluidic Complex Permittivity Measurement. IEEE Trans. Microw. Theory Tech. 2019, 67, 4269–4277. [Google Scholar] [CrossRef]
- Ebrahimi, A.; Scott, J.; Ghorbani, K. Differential Sensors Using Microstrip Lines Loaded With Two Split–Ring Resonators. IEEE Sens. J. 2018, 18, 5786–5793. [Google Scholar] [CrossRef]
- Ha, H.K.; Maa, J.P.Y.; Holland, C.W. Acoustic density measurements of consolidating cohesive sediment beds by means of a non-intrusive “Micro-Chirp” acoustic system. Geo Mar. Lett. 2010, 30, 585–593. [Google Scholar] [CrossRef]
- Peyman, A.; Gabriel, C.; Grant, E.H. Complex Permittivity of Sodium Chloride Solutions at Microwave Frequencies. Bioelectromagnetics 2007, 28, 264–274. [Google Scholar] [CrossRef]
- Chahadih, A.; Cresson, P.Y.; Hamouda, Z.; Gu, S.; Mismer, C.; Lasri, T. Microwave/microfluidic sensor fabricated on a flexible kapton substrate for complex permittivity characterization of liquids. Sens. Actuators A Phys. 2015, 229, 128–135. [Google Scholar] [CrossRef]
- Abbasi, Z.; Daneshmand, M. Contactless pH Measurement Based on High Resolution Enhanced Q Microwave Resonator. In Proceedings of the 2018 IEEE/MTT-S International Microwave Symposium –IMS, Philadelphia, PA, USA, 10–15 June 2018. [Google Scholar]
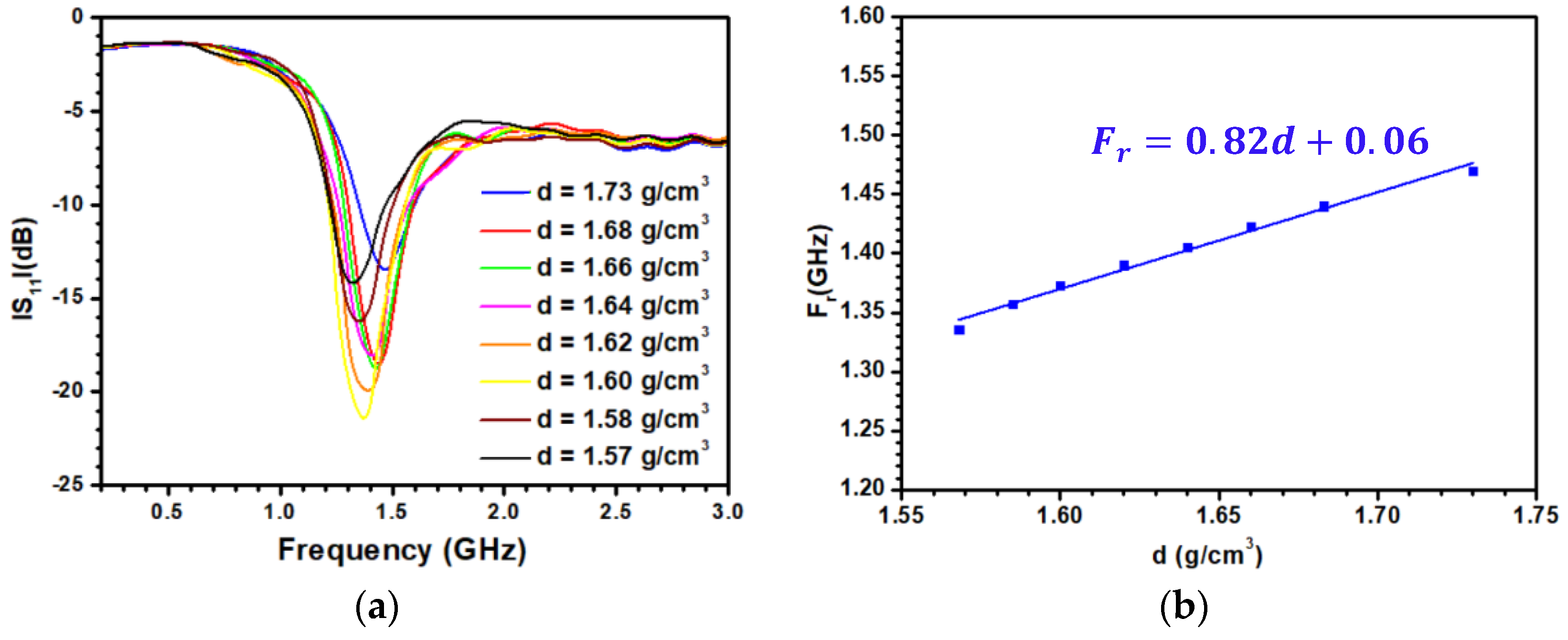
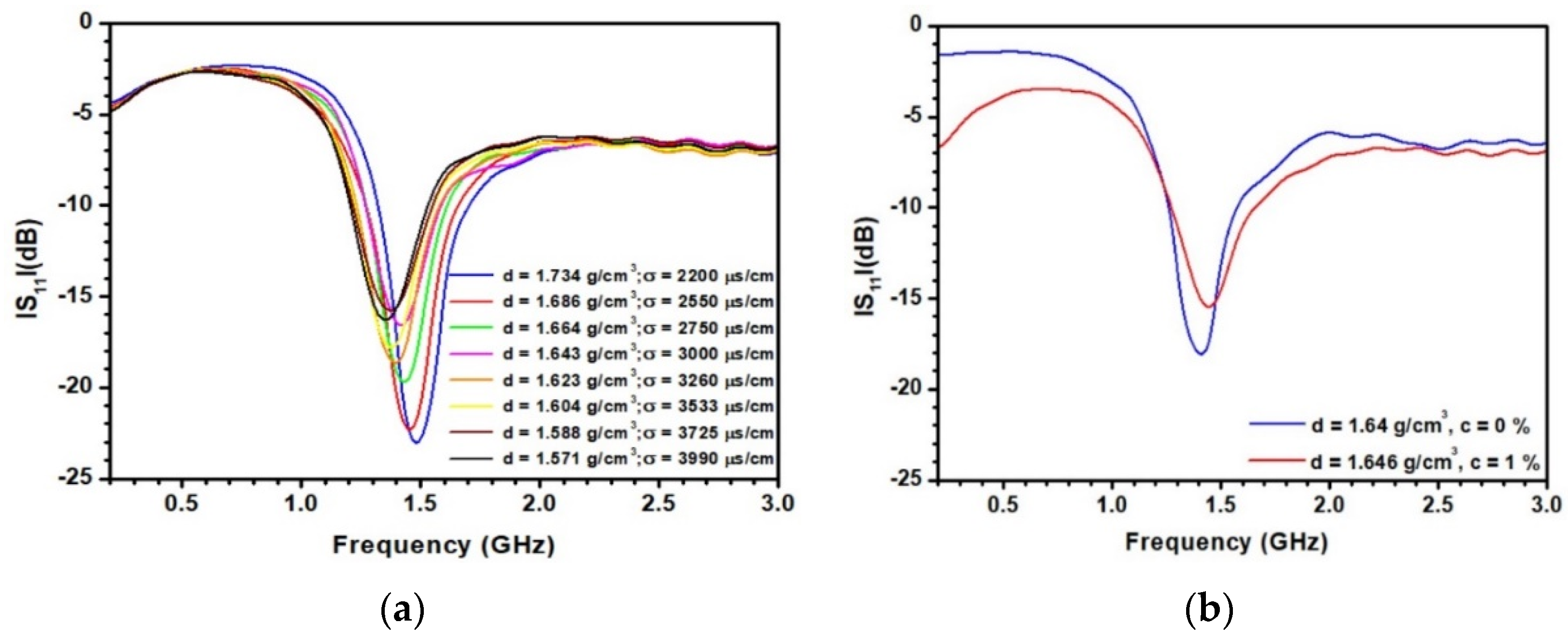
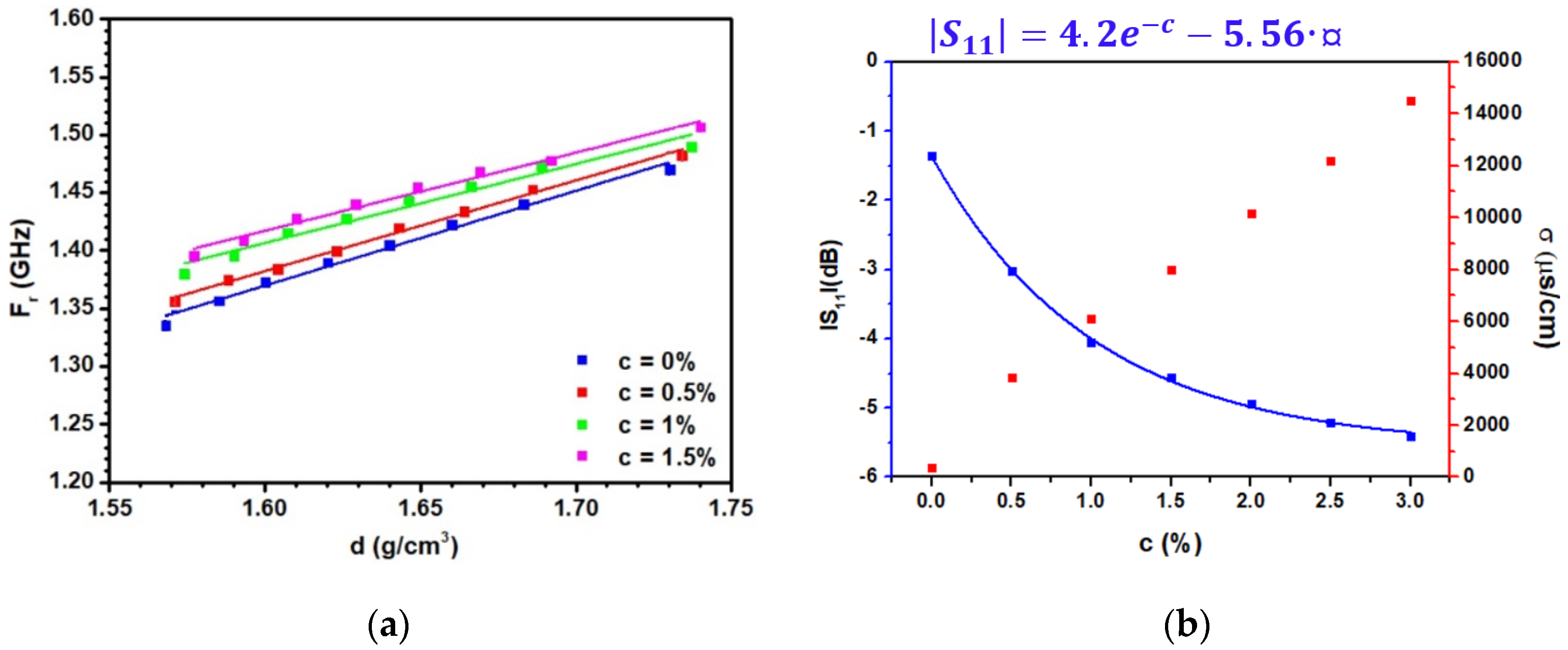
| d(g/cm3) | (s) | |||||
|---|---|---|---|---|---|---|
| 1.73 | 30.6 | 2 | 0.2 | 5 × 10−12 | 800 | 450 |
| 1.68 | 34 | 2 | 0.2 | 5 × 10−12 | 800 | 530 |
| 1.64 | 35.6 | 2 | 0.2 | 5 × 10−12 | 800 | 500 |
| 1.58 | 38.8 | 2 | 0.2 | 5 × 10−12 | 800 | 510 |
| 1.54 | 40.6 | 2 | 0.2 | 5 × 10−12 | 800 | 480 |
| C (%) | (c) | (c) |
|---|---|---|
| 0 | 0.06 | 0.82 |
| 0.5 | 0.13 | 0.783 |
| 1 | 0.316 | 0.682 |
| 1.5 | 0.338 | 0.674 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mansour, R.; Rioual, S.; Lescop, B.; Talbot, P.; Abboud, M.; Farah, W.; Tanné, G. Development of a Resonant Microwave Sensor for Sediment Density Characterization. Sensors 2020, 20, 1058. https://doi.org/10.3390/s20041058
Mansour R, Rioual S, Lescop B, Talbot P, Abboud M, Farah W, Tanné G. Development of a Resonant Microwave Sensor for Sediment Density Characterization. Sensors. 2020; 20(4):1058. https://doi.org/10.3390/s20041058
Chicago/Turabian StyleMansour, R., S. Rioual, B. Lescop, P. Talbot, M. Abboud, W. Farah, and G. Tanné. 2020. "Development of a Resonant Microwave Sensor for Sediment Density Characterization" Sensors 20, no. 4: 1058. https://doi.org/10.3390/s20041058
APA StyleMansour, R., Rioual, S., Lescop, B., Talbot, P., Abboud, M., Farah, W., & Tanné, G. (2020). Development of a Resonant Microwave Sensor for Sediment Density Characterization. Sensors, 20(4), 1058. https://doi.org/10.3390/s20041058





