Quick and Cost-Effective Estimation of Vitamin C in Multifruit Juices Using Voltammetric Methods
Abstract
1. Introduction
2. Materials and Methods
2.1. Juice Samples
2.2. Vitamin C Analysis Using a Voltammetric Method
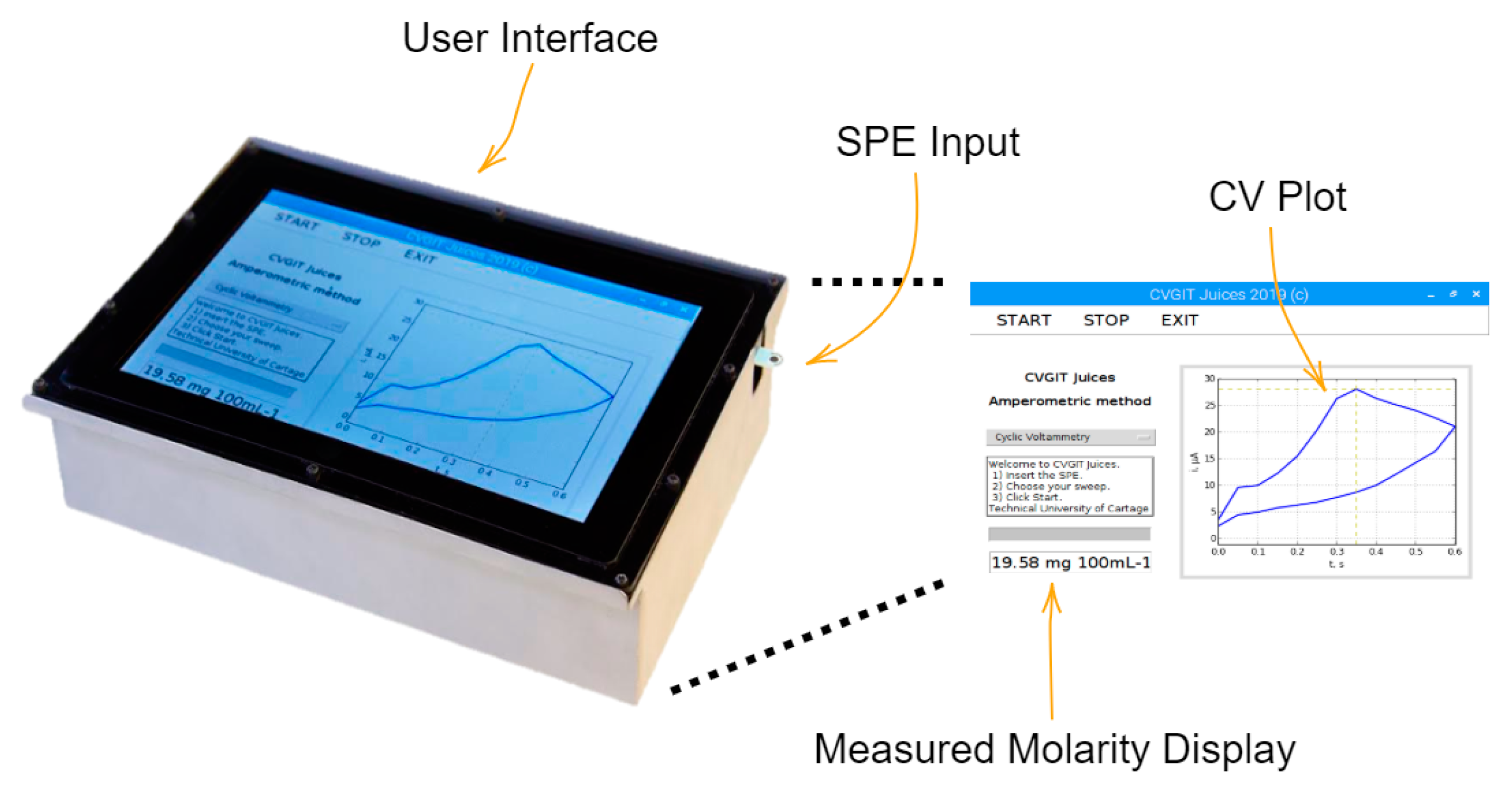
2.2.1. Field-Portable, Low-Cost Potentiostat
2.2.2. Calibration of the Proposed Device
2.2.3. Determination of the Limit of Detection (LoD)
2.3. Vitamin C Analysis Employing HPLC
2.4. Statistical Analysis
3. Results and Discussion
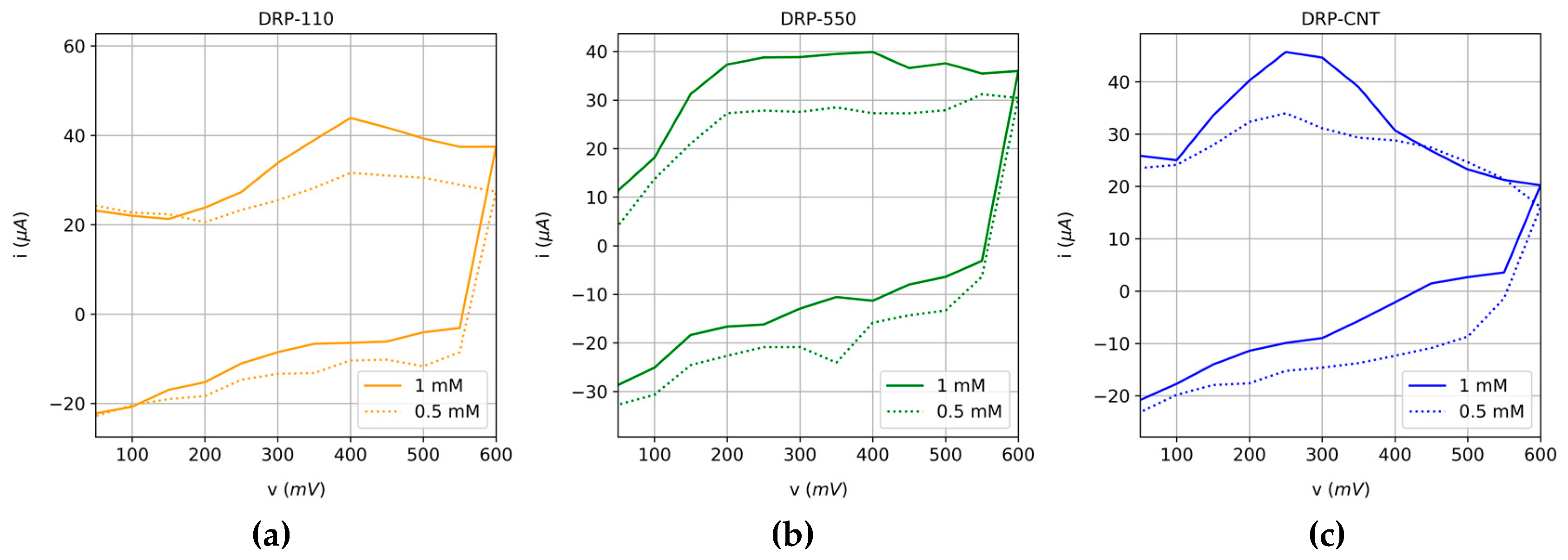
3.1. Characterization of Voltammetric Sensors
3.2. Determination of Vitamin C
3.3. Correlation between Voltammetric and HPLC Measuring Methods
3.4. Interfering Compounds
3.5. Cost per Analysis
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Carr, A.; Maggini, S. Vitamin C and immune function. Nutrients 2017, 9, 1211. [Google Scholar] [CrossRef] [PubMed]
- Davey, M.W.; Van Montagu, M.; Inzé, D.; Sanmartin, M.; Kanellis, A.; Smirnoff, N.; Benzie, I.F.F.; Strain, J.J.; Favell, D.; Fletcher, J. Plant L-ascorbic acid: Chemistry, function, metabolism, bioavailability and effects of processing. J. Sci. Food Agric. 2000, 80, 825–860. [Google Scholar] [CrossRef]
- Padayatty, S.; Katz, A.; Wang, Y.; Eck, P.; Lee, J.; Chen, S.; Corpe, C.; Dutta, A. Vitamin C as an antioxidant: Evaluation of its role in disease prevention vitamin C as an antioxidant: Evaluation of its role in. J. Am. Coll. Nutr. 2013, 37–41. [Google Scholar] [CrossRef]
- EFSA Dietary Reference Values for nutrients Summary report. EFSA Support. Publ. 2017, 14. Available online: https://www.efsa.europa.eu/en/supporting/pub/e15121 (accessed on 7 January 2020).
- Crowe-White, K.; O’Neil, C.E.; Parrott, J.S.; Benson-Davies, S.; Droke, E.; Gutschall, M.; Stote, K.S.; Wolfram, T.; Ziegler, P. Impact of 100% fruit juice consumption on diet and weight status of children: An evidence-based review. Crit. Rev. Food Sci. Nutr. 2016, 56, 871–884. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.K.; Kader, A.A. Preharvest and postharvest factors influencing vitamin C content of horticultural crops. Postharvest Biol. Technol. 2000, 20, 207–220. [Google Scholar] [CrossRef]
- Cilla, A.; Alegría, A.; De Ancos, B.; Sánchez-Moreno, C.; Cano, M.P.; Plaza, L.; Clemente, G.; Lagarda, M.J.; Barberá, R. Bioaccessibility of tocopherols, carotenoids, and ascorbic acid from milk- and soy-based fruit beverages: Influence of food matrix and processing. J. Agric. Food Chem. 2012, 60, 7282–7290. [Google Scholar] [CrossRef]
- Brewer, M.S. Natural antioxidants: Sources, compounds, mechanisms of action, and potential applications. Compr. Rev. Food Sci. Food Saf. 2011, 10, 221–247. [Google Scholar] [CrossRef]
- Wu, X. Fluorimetric determination of ascorbic acid with o-phenylenediamine. Talanta 2003, 59, 95–99. [Google Scholar] [CrossRef]
- Suntornsuk, L.; Gritsanapun, W.; Nilkamhank, S.; Paochom, A. Quantitation of vitamin C content in herbal juice using direct titration. J. Pharm. Biomed. Anal. 2002, 28, 849–855. [Google Scholar] [CrossRef]
- Bassi, M.; Lubes, G.; Bianchi, F.; Agnolet, S.; Ciesa, F.; Brunner, K.; Guerra, W.; Robatscher, P.; Oberhuber, M. Ascorbic acid content in apple pulp, peel, and monovarietal cloudy juices of 64 different cultivars. Int. J. Food Prop. 2017, 20, S2626–S2634. [Google Scholar] [CrossRef]
- Zapata, S.; Dufour, J.P. Ascorbic, dehydroascorbic and isoascorbic acid simultaneous determinations by reverse phase ion interaction HPLC. J. Food Sci. 1992, 57, 506–511. [Google Scholar] [CrossRef]
- European Commission Priorities: Agriculture and Food Security. Available online: https://ec.europa.eu/jrc/en/science-area/agriculture-and-food-security (accessed on 1 November 2019).
- Pisoschi, A.M.; Pop, A.; Serban, A.I.; Fafaneata, C. Electrochemical methods for ascorbic acid determination. Electrochim. Acta 2014, 121, 443–460. [Google Scholar] [CrossRef]
- González-Sánchez, M.I.; Agrisuelas, J.; Valero, E.; Compton, R.G. Measurement of total antioxidant capacity by electrogenerated iodine at disposable screen printed electrodes. Electroanalysis 2017, 29, 1316–1323. [Google Scholar] [CrossRef]
- O’Connell, P.J.; Gormally, C.; Pravda, M.; Guilbault, G.G. Development of an amperometric L-ascorbic acid (Vitamin C) sensor based on electropolymerised aniline for pharmaceutical and food analysis. Anal. Chim. Acta 2001, 431, 239–247. [Google Scholar] [CrossRef]
- Reza Ganjali, M. Highly sensitive voltammetric sensor for determination of ascorbic acid using graphite screen printed electrode modified with ZnO/Al2O3 nanocomposite. Int. J. Electrochem. Sci. 2017, 12, 3231–3240. [Google Scholar] [CrossRef]
- Raveendran, J.; Krishnan, R.G.; Nair, B.G.; Satheesh Babu, T.G. Voltammetric determination of ascorbic acid by using a disposable screen printed electrode modified with Cu(OH)2 nanorods. Microchim. Acta 2017, 184, 3573–3579. [Google Scholar] [CrossRef]
- Aznar-Poveda, J.; Lopez-Pastor, J.A.; Garcia-Sanchez, A.J.; Garcia-Haro, J.; Otero, T.F. A cots-based portable system to conduct accurate substance concentration measurements. Sensors 2018, 18, 539. [Google Scholar] [CrossRef]
- SPCE DRP-110. Available online: http://www.dropsens.com/en/pdfs_productos/new_brochures/110-c110-c11l.pdf (accessed on 7 January 2020).
- SPPE DRP-550. Available online: http://www.dropsens.com/en/pdfs_productos/new_brochures/550_c550.pdf (accessed on 7 January 2020).
- SPCE DRP-110CNT. Available online: http://www.dropsens.com/en/pdfs_productos/new_brochures/110cnt-x1110cnt.pdf (accessed on 7 January 2020).
- Serafín, V.; Martínez-García, G.; Aznar-Poveda, J.; Lopez-Pastor, J.A.; Garcia-Sanchez, A.J.; Garcia-Haro, J.; Campuzano, S.; Yáñez-Sedeño, P.; Pingarrón, J.M. Determination of progesterone in Saliva using an electrochemical immunosensor and a cots-based portable potentiostat. Anal. Chim. Acta 2019, 1049, 65–73. [Google Scholar] [CrossRef]
- Serafín, V.; Arévalo, B.; Martínez-García, G.; Aznar-Poveda, J.; Lopez-Pastor, J.A.; Beltrán-Sánchez, J.F.; Garcia-Sanchez, A.J.; Garcia-Haro, J.; Campuzano, S.; Yáñez-Sedeño, P.; et al. Enhanced determination of fertility hormones in saliva at disposable immunosensing platforms using a custom designed field-portable dual potentiostat. Sensors Actuators B Chem. 2019, 299, 126934. [Google Scholar] [CrossRef]
- Saadati, N.; Abdullah, M.P.; Zakaria, Z.; Sany, S.B.T.; Rezayi, M.; Hassonizadeh, H. Limit of detection and limit of quantification development procedures for organochlorine pesticides analysis in water and sediment matrices. Chem. Cent. J. 2013, 7, 1. [Google Scholar] [CrossRef] [PubMed]
- Luna, M.C.; Martínez-Sánchez, A.; Selma, M.V.; Tudela, J.A.; Baixauli, C.; Gil, M.I. Influence of nutrient solutions in an open-field soilless system on the quality characteristics and shelf life of fresh-cut red and green lettuces (Lactuca sativa L.) in different seasons. J. Sci. Food Agric. 2013, 93, 415–421. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Fang, H.Y.; Chen, W.C.; Lin, H.M.; Chang, C.A. Electrochemical study on screen-printed carbon electrodes with modification by iron nanoparticles in Fe(CN)6 4−/3− redox system. Anal. Bioanal. Chem. 2005, 383, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Lavagnini, I.; Antiochia, R.; Magno, F. An extended method for the practical evaluation of the standard rate constant from cyclic voltammetric data. Electroanalysis 2004, 16, 505–506. [Google Scholar] [CrossRef]
- Nicholson, R.S. Theory and application of cyclic voltammetry for measurement of electrode reaction kinetics. Anal. Chem. 1965, 37, 1351–1355. [Google Scholar] [CrossRef]
- Randviir, E.P. A cross examination of electron transfer rate constants for carbon screen-printed electrodes using electrochemical impedance spectroscopy and cyclic voltammetry. Electrochim. Acta 2018, 286, 179–186. [Google Scholar] [CrossRef]
- Jadav, J.K.; Umrania, V.V.; Rathod, K.J.; Golakiya, B.A. Development of silver/carbon screen-printed electrode for rapid determination of vitamin C from fruit juices. LWT-Food Sci. Technol. 2018, 88, 152–158. [Google Scholar] [CrossRef]
- Pournaghi-Azar, M.H.; Dastangoo, H.; Fadakar, R. Differentiation of detection of ascorbic acid and dehydroascorbic acid using hydrodynamic amperometry and anodic stripping voltammetry on modified aluminum electrodes. Electroanalysis 2010, 22, 229–235. [Google Scholar] [CrossRef]
- Komorsky-Lovrić, Š.; Novak, I. Abrasive stripping square-wave voltammetry of blackberry, raspberry, strawberry, pomegranate, and sweet and blue potatoes. J. Food Sci. 2011, 76, 916–920. [Google Scholar] [CrossRef]
- Barberis, A.; Spissu, Y.; Fadda, A.; Azara, E.; Bazzu, G.; Marceddu, S.; Angioni, A.; Sanna, D.; Schirra, M.; Serra, P.A. Simultaneous amperometric detection of ascorbic acid and antioxidant capacity in orange, blueberry and kiwi juice, by a telemetric system coupled with a fullerene- or nanotubes-modified ascorbate subtractive biosensor. Biosens. Bioelectron. 2014, 67, 214–223. [Google Scholar] [CrossRef]
- Skrovankova, S.; Sumczynski, D.; Mlcek, J.; Jurikova, T.; Sochor, J. Bioactive compounds and antioxidant activity in different types of berries. Int. J. Mol. Sci. 2015, 16, 24673–24706. [Google Scholar] [CrossRef] [PubMed]
- Zanini, V.P.; De Mishima, B.L.; Labbé, P.; Solís, V. An L-lactate amperometric enzyme electrode based on L-lactate oxidase immobilized in a laponite gel on a glassy carbon electrode. application to dairy products and red wine. Electroanalysis 2010, 22, 946–954. [Google Scholar] [CrossRef]
- Tomás-Barberán, F.A. High-value co-products from plant foods: Nutraceuticals, micronutrients and functional ingredients. In Handbook of Waste Management and Co-Product Recovery in Food Processing; Woodhead Publishing: Cambridge, UK, 2007; Volume 1, pp. 448–469. [Google Scholar]
- U.S. Department of Agriculture FoodData Central. Available online: https://fdc.nal.usda.gov/ (accessed on 1 November 2019).
- Han, J.H.; Choi, H.N.; Park, S.; Chung, T.D.; Lee, W.Y. Mesoporous platinum electrodes for amperometric determination of sugars with anion exchange chromatography. Anal. Sci. 2010, 26, 995–1000. [Google Scholar] [CrossRef][Green Version]
- Wu, X.L.; Zhou, H.B.; Wang, S.J.; Ye, B.X. Determination of magnesium and calcium in biological samples by potentiometric stripping analysis. J. Chinese Chem. Soc. 2010, 57, 647–652. [Google Scholar] [CrossRef]
- Lima, A.S.; Bocchi, N.; Gomes, H.M.; Teixeira, M.F.S. An electrochemical sensor based on nanostructured hollandite-type manganese oxide for detection of potassium ions. Sensors 2009, 9, 6613–6625. [Google Scholar] [CrossRef] [PubMed]
- Kadara, R.O.; Haggett, B.G.D.; Birch, B.J. Disposable sensor for measurement of vitamin B2 in nutritional premix, cereal, and milk powder. J. Agric. Food Chem. 2006, 54, 4921–4924. [Google Scholar] [CrossRef]
- Brunetti, B.; Desimoni, E. Voltammetric determination of vitamin B6 in food samples and dietary supplements. J. Food Compos. Anal. 2014, 33, 155–160. [Google Scholar] [CrossRef]
- Lovander, M.D.; Lyon, J.D.; Parr, D.L.; Wang, J.; Parke, B.; Leddy, J. Review-electrochemical properties of 13 vitamins: A critical review and assessment. J. Electrochem. Soc. 2018, 165, G18–G49. [Google Scholar] [CrossRef]
- Thompson, G. Electrochemical Detection of Antioxidants Senior Honors. Ph.D. Thesis, University of New Hampshire, Durham, NH, USA, 2016. [Google Scholar]
- Pisoschi, A.M.; Pop, A.; Negulescu, G.P.; Pisoschi, A. Determination of ascorbic acid content of some fruit juices and wine by voltammetry performed at Pt and carbon paste electrodes. Molecules 2011, 16, 1349–1365. [Google Scholar] [CrossRef]




| Multifruit juice I | Multifruit juice II | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Calibration | H2SO4 0.5 M | H2SO4 0.5 M pH 3.5 | Calibration | H2SO4 0.5 M | H2SO4 0.5 M pH 3.5 | ||||
| SPE | Pearson Coeff. (r) | RMSE | Pearson Coeff. (r) | RMSE | SPE | Pearson Coeff. (r) | RMSE | Pearson Coeff. (r) | RMSE |
| DRP-110 | 0.8806 | 3.5790 | 0.8806 | 2.3323 | DRP-110 | 0.6826 | 6.2140 | 0.6826 | 6.506 |
| DRP-550 | 0.9801 | 1.6442 | 0.9801 | 2.0016 | DRP-550 | 0.8457 | 5.8858 | 0.8457 | 4.2524 |
| DRP-110CNT | 0.9216 | 3.4315 | 0.9216 | 2.2786 | DRP-110CNT | −0.0431 | 5.0622 | −0.0431 | 5.8018 |
| Portion (100 mL) | Orange juice (I) | Grape juice (I) | Mango nectar (I) | Carrot juice (I) | Apple juice (I) | Pomeg-ranate juice (II) | Guavas juice (II) | Blueberries juice (II) | Root-beet juice (II) | Black-berries juice (II) | Max (I, II) | Oxidation Peak (V) | TIE (µA) | Refs. |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Sugars (g) | 9.17 | 14.2 | 12.45 | 3.91 | 10.97 | 1.61 | 13 | 7.92 | 6.64 | 7.7 | 14.2 | * | * | Glucose, fructose, and linked flavonoids tested in lab. Additionally, see [39] |
| Calcium (mg) | 6 | 11 | 17 | 24 | 0 | 11 | 0 | 8 | 17 | 14 | 24 | * | * | [40] |
| Magnesium (mg) 1 | 10 | 10 | 3 | 14 | 0 | 7 | 0 | 0 | 0 | 21 | 14 | * | * | [40] |
| Potassium (mg) | 188 | 104 | 24 | 292 | 80 | 214 | 0 | 75 | 219 | 135 | 292 | * | * | [41] |
| Vitamin C 2 (mg) | 30 | 25 | 15.2 | 8.5 | 30.4 | 0.1 | 24 | 4.2 | 3.8 | 11.3 | 30.4 | +0.4 | - | [14,44,46] |
| Thiamin (mg) B1 | 0 | 0.017 | 0.003 | 0.092 | 0 | 0.015 | 0 | 0 | 0 | 0.012 | 0.092 | +0.6 | 0.31 | [44] |
| Riboflavin (mg) B2 | 0.028 | 0.015 | 0.003 | 0.055 | 0 | 0.05 | 0 | 0 | 0 | 0.018 | 0.055 | * | * | [42,44] |
| Vitamin B6 (mg) | 0.05 | 0.036 | 0.015 | 0.217 | 0 | 0.04 | 0 | 0 | 0 | 0.021 | 0.217 | * | * | [43,44] |
| Vitamin A (mg) | 0 | 0 | 35 | 956 | 0 | 0 | 100 | 0 | 0 | 6 | 956 | * | * | [44] |
| Vitamin E (mg) | 0 | 0 | 0.21 | 1.16 | 0 | 0.38 | 0 | 0 | 0 | 0.9 | 1.16 | * | * | [44] |
| Vitamin K (mg) | 0 | 0.4 | 0.8 | 15.5 | 0 | 10.4 | 0 | 0 | 0 | 15.2 | 15.5 | * | * | [44] |
| Carotene, beta (mg) | 0 | 5 | 402 | 9303 | 0 | 0 | 0 | 0 | 0 | 74 | 9303 | * | * | [45] |
| Lutein (mg) | 0 | 57 | 0 | 333 | 0 | 0 | 0 | 0 | 0 | 68 | 333 | * | * | [45] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
López-Pastor, J.-A.; Martínez-Sánchez, A.; Aznar-Poveda, J.; García-Sánchez, A.-J.; García-Haro, J.; Aguayo, E. Quick and Cost-Effective Estimation of Vitamin C in Multifruit Juices Using Voltammetric Methods. Sensors 2020, 20, 676. https://doi.org/10.3390/s20030676
López-Pastor J-A, Martínez-Sánchez A, Aznar-Poveda J, García-Sánchez A-J, García-Haro J, Aguayo E. Quick and Cost-Effective Estimation of Vitamin C in Multifruit Juices Using Voltammetric Methods. Sensors. 2020; 20(3):676. https://doi.org/10.3390/s20030676
Chicago/Turabian StyleLópez-Pastor, Jose-Antonio, Ascensión Martínez-Sánchez, Juan Aznar-Poveda, Antonio-Javier García-Sánchez, Joan García-Haro, and Encarnación Aguayo. 2020. "Quick and Cost-Effective Estimation of Vitamin C in Multifruit Juices Using Voltammetric Methods" Sensors 20, no. 3: 676. https://doi.org/10.3390/s20030676
APA StyleLópez-Pastor, J.-A., Martínez-Sánchez, A., Aznar-Poveda, J., García-Sánchez, A.-J., García-Haro, J., & Aguayo, E. (2020). Quick and Cost-Effective Estimation of Vitamin C in Multifruit Juices Using Voltammetric Methods. Sensors, 20(3), 676. https://doi.org/10.3390/s20030676






