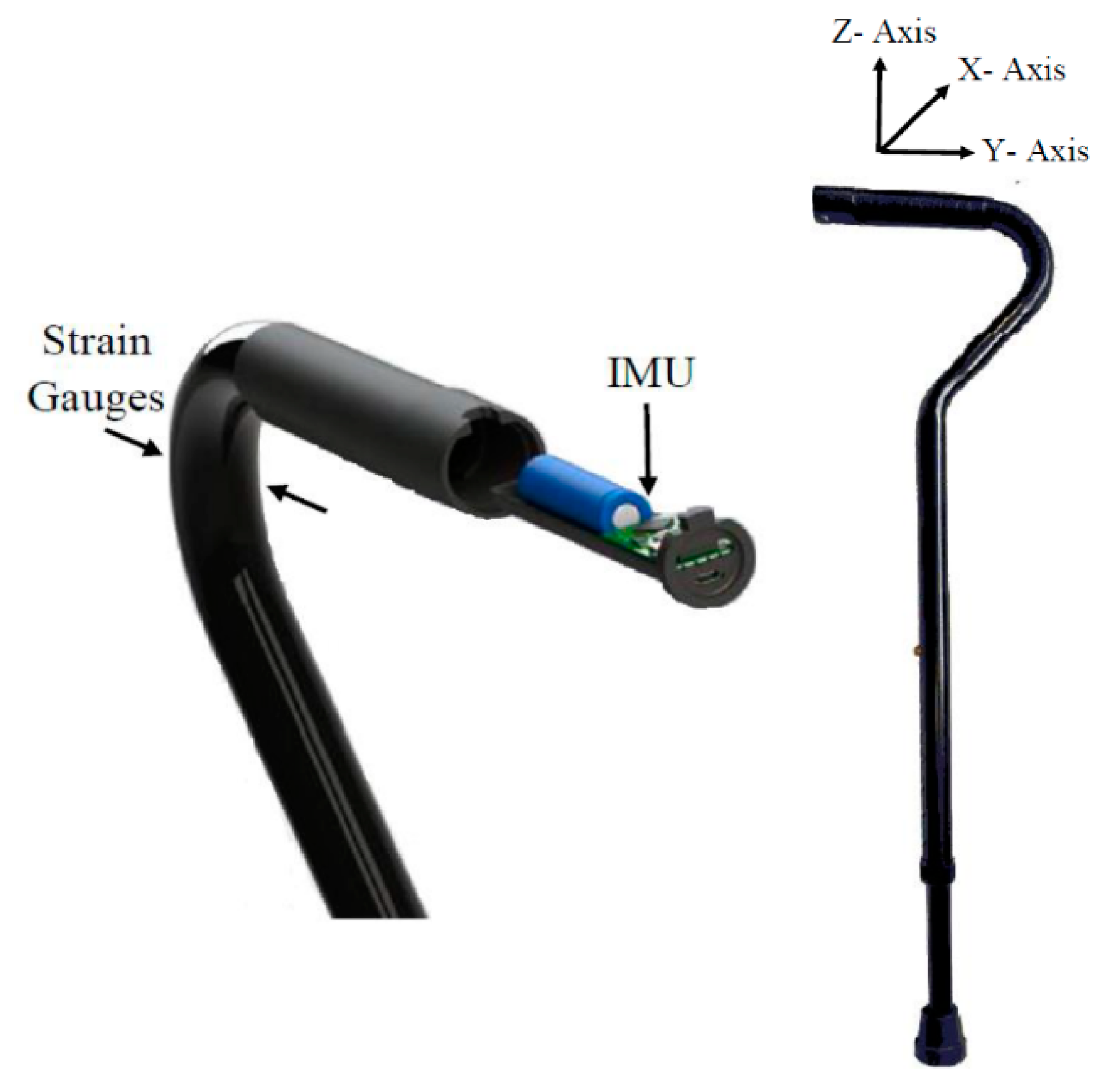
1. Introduction
Globally, healthcare systems are being stressed as a result of the aging baby-boomer demographic and the rise of chronic diseases and neurological impairments [
1,
2]. Among a variety of increased medical complications, mobility impairments due to reduced coordination, muscle strength and postural balance are a major source of concern in these populations. In order to combat this, there has been increased focused on developing innovative proactive solutions that enable self-management and early intervention to alleviate the burden on healthcare providers and caregivers [
3]. One proactive solution is to monitor people’s behaviors and mobility during everyday life as opposed to performing controlled and reactive assessments in the context of a lab or specialist clinic [
4,
5].
The gold standard for mobility and gait monitoring most often includes full-body motion capture systems, video systems, and force places that are capable of providing highly detailed and quantitative gait data [
6,
7]. These systems, however, are often prohibitively expensive for most clinical settings, require the need for additional skilled technicians, and are limited to indoor use in laboratory settings [
8,
9,
10]. Moreover, these solutions can only provide gait data for a limited number of strides thus preventing them from being a viable option for community-based, long-term monitoring.
To address these challenges, wearable sensor technology provides greater flexibility by allowing for consistent monitoring of regular everyday activities across multiple indoor and outdoor environments. Wearable motion sensors, such as inertial measurement units (IMUs) which combine accelerometers and gyroscopes, have been widely researched for gait monitoring in elderly subjects and pathological populations [
5,
8]. A strength of these low power systems is their small size, allowing them to be placed almost anywhere on the body as a single standalone, multi-channel, or even a multi-sensor system [
9,
11,
12]. Their data provide enough detailed information to extract relevant gait characteristics including step length, number of steps, stride variability, and walking speed [
12]. However, there are some limitations that have restricted widespread adoption of these technologies among the populations that could benefit the most from their use. Firstly, there are concerns related to adoption and adherence due to the lack of technological knowledge and/or cognitive ability [
11,
12]. Secondly, there are concerns related to the obtrusiveness of these devices and opposition to behavioral change [
13].
To address these challenges while maintaining the ability to monitor in everyday environments, the assistive gait aids (ADs) that these populations often already use have been instrumented [
14,
15,
16]. Recent studies suggest that approximately 6.1 million community-dwelling American adults ambulate with the support of an AD to combat mobility impairments and mitigate instability [
17]. This has led researchers to develop instrumented ADs to help monitor and categorize gait activity [
16,
18,
19]. These works the presented mechanical design of instrumented canes equipped with IMUs and a load monitoring system intended to be used as diagnostic tools in physiotherapy clinics. The work presented in References [
18,
19] used load cells to measure axial loading information, whereas Wade et al. [
16] used force-sensing resistors incorporated into the cane handle and base to measure loading information. While these early works demonstrated the potential of this approach, limitations including cost, reliability, and lack of industrial design have prevented their successful deployment outside of training situations [
14]. In our previous works [
14,
15], the potential to perform preventative gait and mobility monitoring using affordably instrumented gait aids was demonstrated by leveraging the Internet of Things (IoT) principles to create “smart” technologies. Using this approach, accurate gait segmentation has been shown to be possible across different walking terrains [
20].
It has been shown that an instrumented wheeled walker can also be used to reflect changes in gait during rehabilitation and predict the outcomes of clinical assessments [
21]. Presented results revealed the ability of an instrumented walker to predict Tinetti assessment scores (measure of balance and gait function) on the fly. Clinician provided scores were compared to scores predicted by an instrumented walker and showed a small mean square prediction score. In our previous work, instrumented walkers have also demonstrated the ability to determine both the activity level of the individual and the type of environment in which they are walking [
15]. Incident monitoring has also been demonstrated to be possible with instrumented walking canes that are capable of detecting falls [
22,
23]. For example, Lan et al. [
23] implemented a fall detection algorithm based on subsequence matching by recognizing three stages of a fall pattern. Four types of falls (i.e., forward, backward, side, and free fall) were simulated using healthy participants. The presented results indicated the ability of the algorithm to detect the majority of the falls while achieving very low false-positive rates for non-falling conditions. While these works performed well in fall detection, they provide evidence only for situations where someone has already fallen as opposed to the preventative monitoring of near-falls or altered gait patterns. To detect subtle changes in gait, Wade et al. [
16] presented an alternative instrumented cane design that could identify when an individual was walking on stairs, walking with their eyes closed, as well as walking while looking to one’s side. The results from seven adult volunteers showed overall accuracy of 95.8% in classifying these activities using force and inertial information collected from the cane’s sensors. Recently, the relationship between user and cane movement was investigated to provide clinicians and caregivers insightful information about cane-assisted walking [
24]. Gyroscope data from shank-mounted and cane IMUs were collected for two participants undergoing post-stroke rehabilitation during a continuous standardized sequence of ambulation activities. Presented results revealed that shank-mounted and cane IMUs can capture relevant information related to a user’s gait. In our previous works, we have demonstrated the potential of a multi-sensor cane to detect changes in gait due to the fact of pain-related simulated gait perturbation [
14]. To truly enable preventative monitoring and intervention, however, these devices must all be able to function robustly during an individual’s everyday life activities. This requires the ability to detect changes in gait caused due to the presence of pain, injury, changes in walking terrains, or visual distractions.
In this work, we examined the potential of a multi-sensor cane to act, not only as an activity monitor, but also as a tool to relay information about the nature of the user’s gait. To this aim, we sought to determine the capabilities of an instrumented cane in detecting a variety of gait-related changes as validated by a shank-mounted IMU. Specifically, the desired outcomes included the ability to identify simulated changes in gait, walking terrain, simulated vision perturbations, and incorrect cane length. Improvements in these factors may help validate the instrumented multi-sensor cane as an important deployable tool for preventative monitoring of vulnerable individuals as they perform their everyday activities and routines.
3. Results
Four separate experiments were conducted to determine the ability of the multi-sensor cane to determine changes in gait caused by simulated gait abnormalities, changes in walking terrain, simulated vision perturbations, and incorrect cane length. The results of the four experiments are presented here to validate the ability of the multi-sensor cane to distinguish the various walking conditions from normal walking (control case).
3.1. Gait Abnormalities
Features were extracted from the cane’s sensor data and subsequently compared statistically. A Bonferroni comparison tested for differences among features extracted from the different gait conditions. These results, listed in
Table 3, demonstrate that the multiple features that yielded significantly different (
p < 0.05) means were plantarflexed and dorsiflexed gait with respect to the healthy control case. Moreover, maximum pitch velocity during both the swing and stance phases; as well as stride length denoted a significant difference for the dorsiflexion with respect to plantarflexion condition.
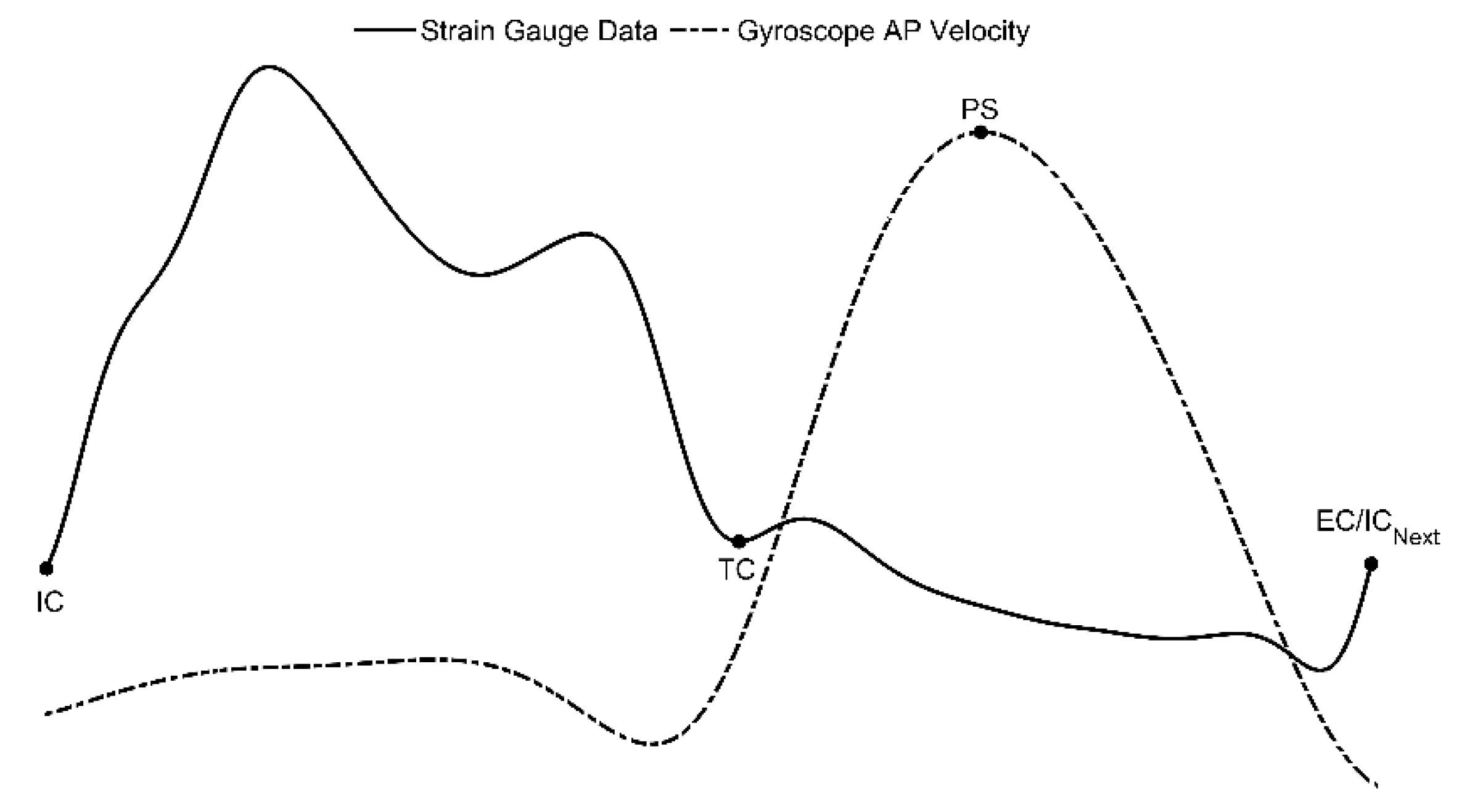
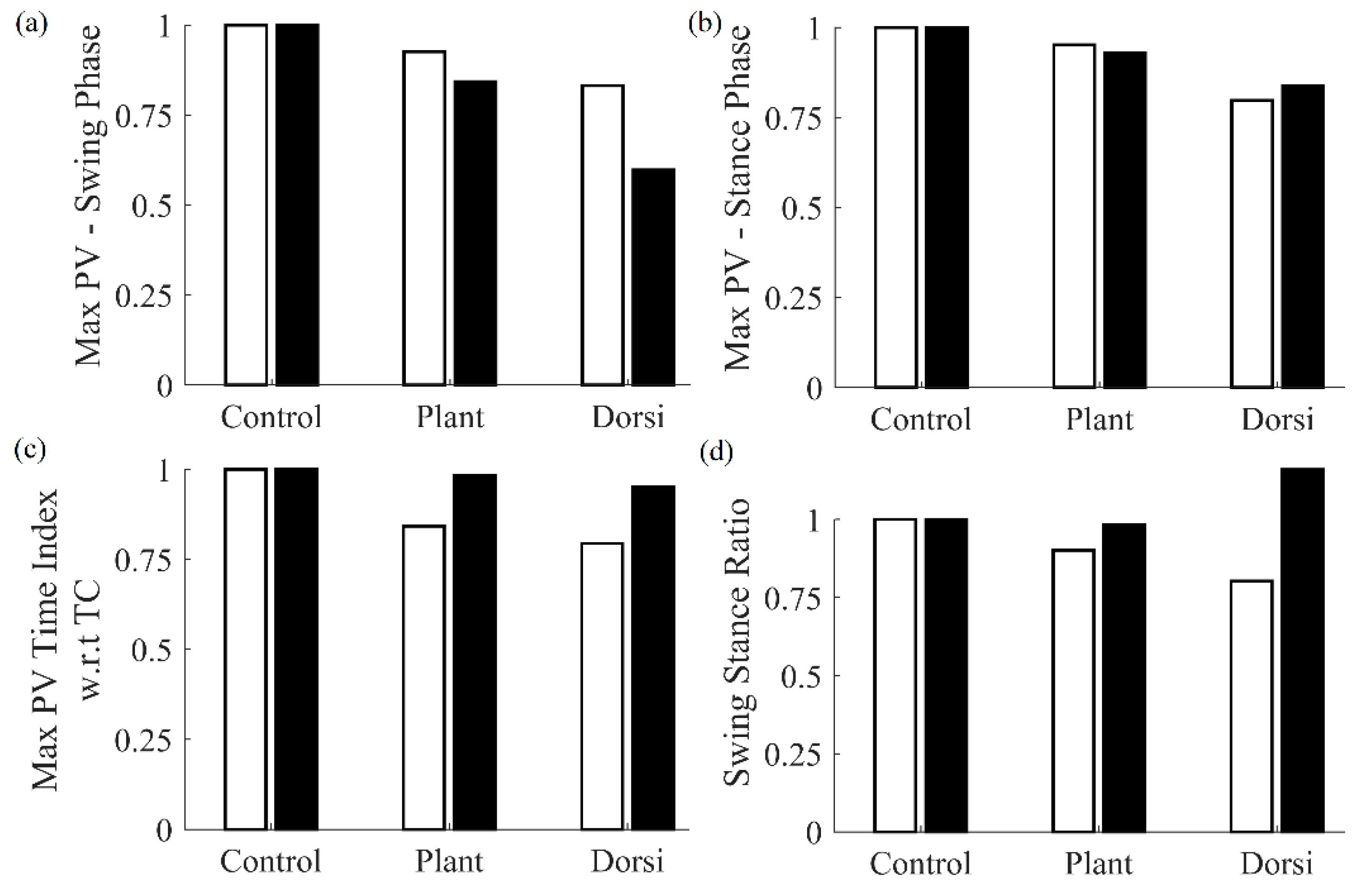
A comparison of features obtained from the cane and the shank-mounted IMU are shown in
Figure 4. All features from the cane and shank-mounted IMU follow the same trend of decreasing in value with respect to the control condition. The exception was found to be the “swing stance ratio” feature (
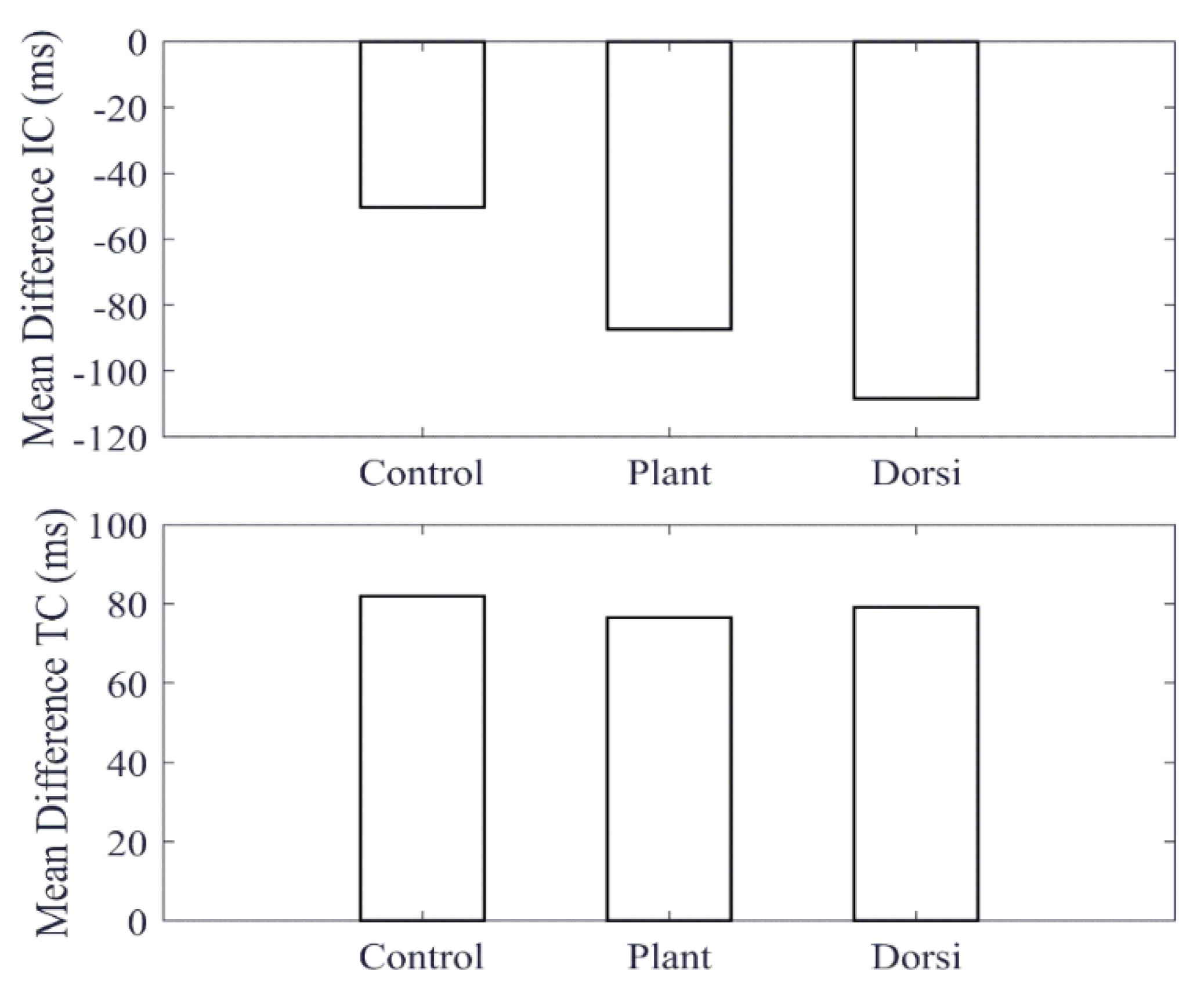
Figure 4d). By inspection, the cane data indicate that this was smaller for dorsiflexion with respect to the control case, whereas the shank-mounted IMU data indicate an increase. This can be explained due to the difference in the IC and TC events determined by the MSMF and GPD segmentation algorithms as shown in
Figure 5. A negative difference in the IC suggests that the GPD algorithm determined IC events later in time, whereas a positive difference in TC means that GPD determined the TC event to occurs earlier.
3.2. Walking Terrains
Table 4 lists the results of a Bonferroni comparison of the differences in features for uphill and downhill walking with respect to level walking. The differences in upstairs and downstairs walking with respect to level walking are presented in
Table 5.
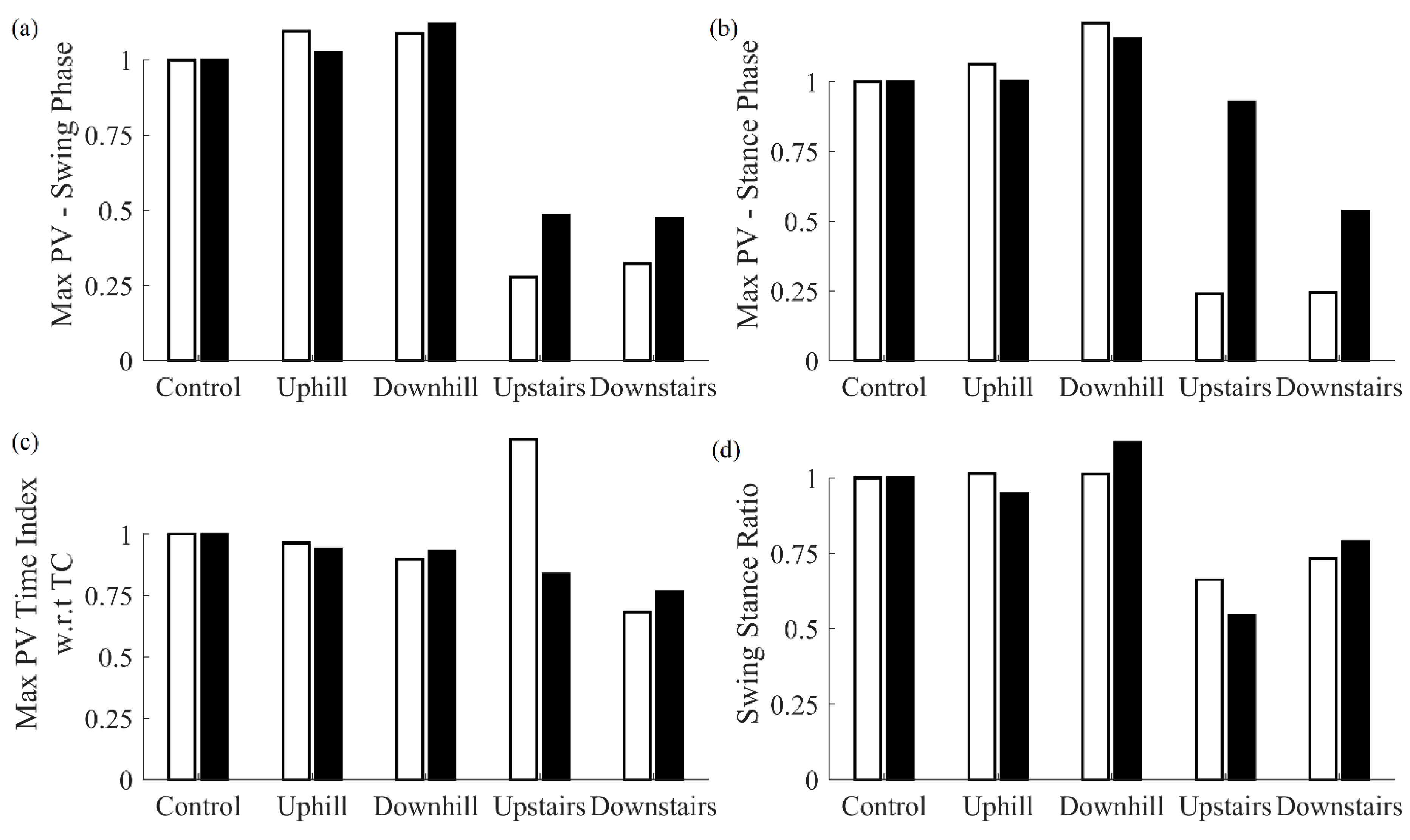
When walking on uphill or downhill terrains, pitch velocity during the swing and stance phases was higher with respect to the control case, and no significant difference was observed in the swing stance ratio. By contrast, while walking up and down stairs, pitch velocity during the swing and stance phases was lower with respect to the control and a significant decrease in swing stance ratio was observed.
For all terrains, maximum strain increased with respect to the control case. In addition, stride length slightly decreased for uphill and downhill walking, whereas a significant decrease in stride length was observed for upstairs and downstairs walking. Lastly, no significant difference in mean difference in IC as determined by the MSMF and GPD approaches was observed for downhill walking, whereas a significant difference was observed for uphill walking. For upstairs and downstairs walking, a significant difference in IC determined by these approaches was observed with respect to control case. For some features, significant differences were observed in downhill walking with respect to uphill walking. When comparing downstairs walking with respect to upstairs walking, significant differences were observed for maximum pitch velocity during swing phase, stride length, and difference in IC determined by the two approaches.
Figure 6 shows a comparison of features obtained for various walking terrains for the cane and shank-mounted IMU. All features for both follow the same trend with respect to control case except for Max PV Time Index with respect to TC.
3.3. Impaired Vision
Next, we investigated the ability of the cane to detect any changes in gait due to the fact of impaired vision. The results presented in
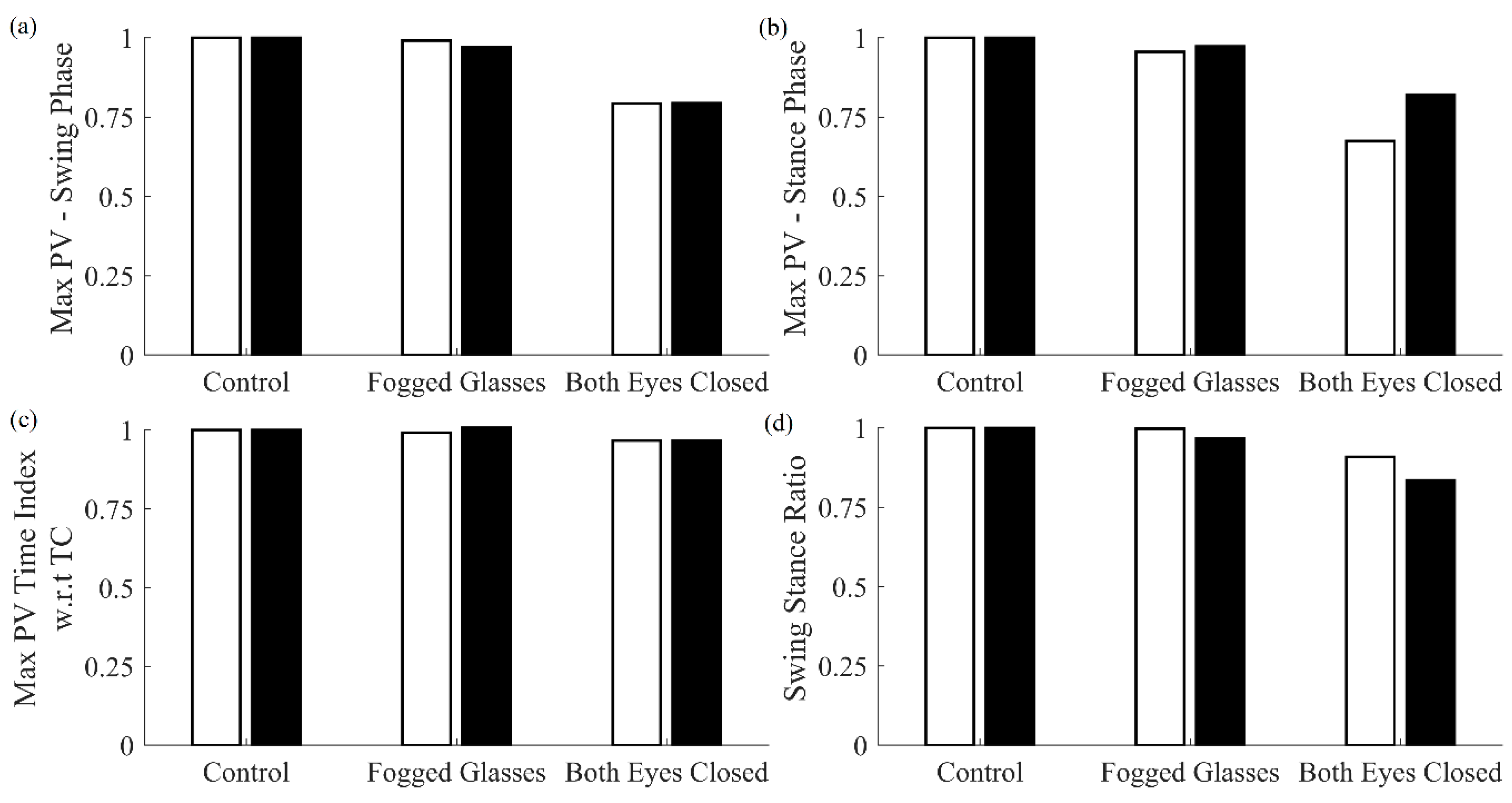
Table 6 show that swing stance ratio for the impaired vision conditions saw significant differences with respect to the control case. Maximum strain increased significantly for impaired vision conditions with respect to the control case. Moreover, the mean difference in IC, as determined by the MSMF and GPD approaches, increased significantly for impaired vision conditions with respect to the control case.
Maximum pitch velocity during both the swing and stance phase, as well as stride length, decreased significantly for both eyes closed conditions. No significant differences in these features were observed for the fogged glasses case with respect to the control case.
Results for the comparison of features for both cane and shank-mounted IMU are presented in
Figure 7. As can be seen, the results obtained using shank-mounted IMUs follow similar trends as those obtained using the cane.
3.4. Incorrect Cane Length
Lastly, the cane’s ability to detect changes in gait due to the incorrect configuration of the cane length was investigated (
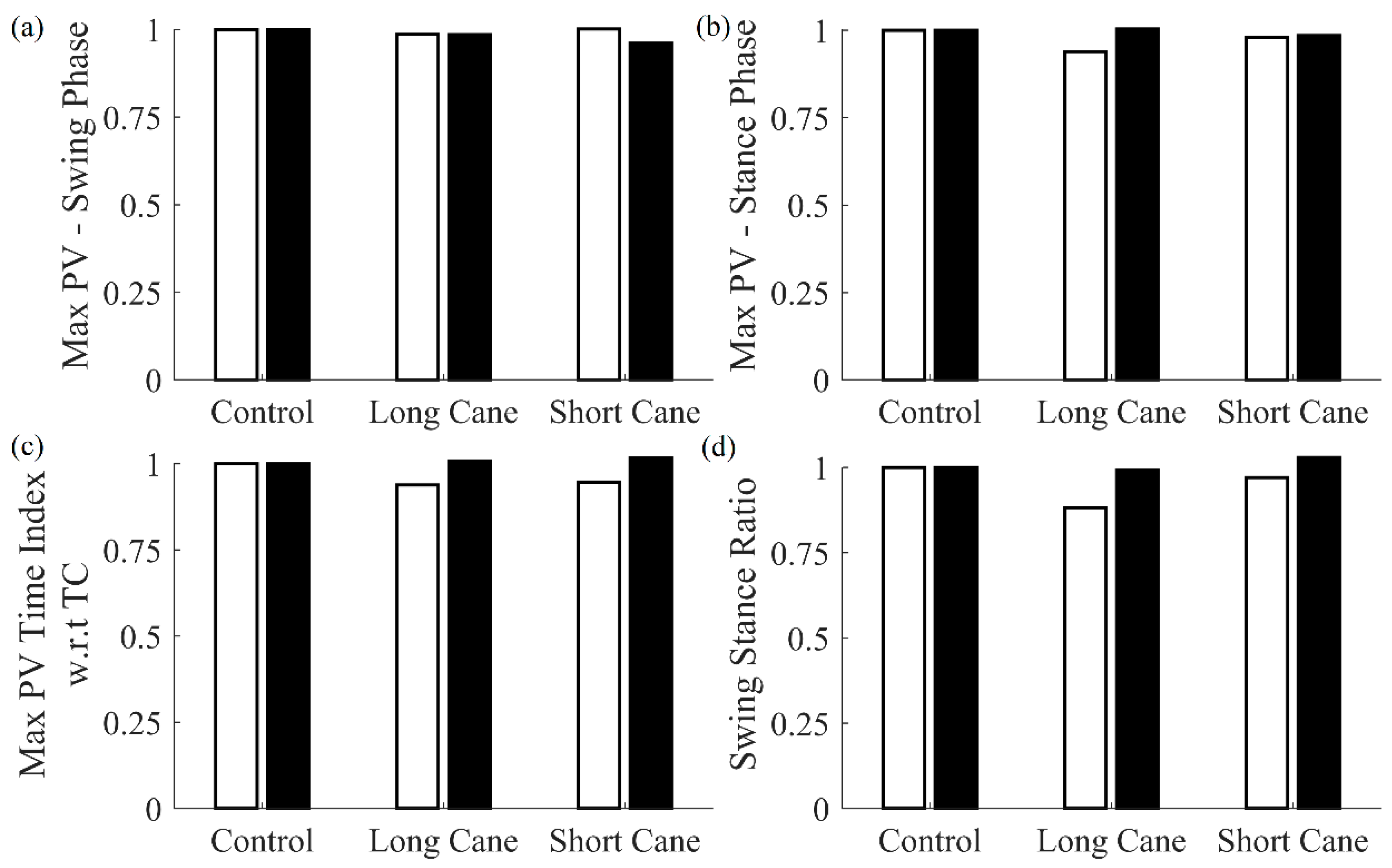
Table 7). Users were found to rely more on the cane for incorrect cane lengths. Intuitively, stride length increased while walking with a longer cane, whereas the opposite trend was observed for a shorter cane. An increased mean difference in IC, as determined by MSMF with respect to GPD, indicates a change in walking pattern due to the incorrect cane length. No significant difference was observed in the other features for the shorter cane case. For the longer cane condition, significant difference was observed in the swing stance ratio.
Results of the comparison between the features extracted by the cane and shank-mounted IMU follow similar trends (see
Figure 8).
4. Discussion
Proactive monitoring and intervention may help to alleviate the growing demands on global healthcare systems. This will require new and innovative solutions that can capture relevant information about patients without intruding on their quality of life. Instrumenting existing assistive devices that a person already uses may be an effective method of capturing changes in gait caused due to the fact of pain, walking terrains, altered vision, etc. in a minimally invasive way. This is important as changes in gait, particularly in response to pain originating from chronic disease, are known to be associated with reduced mobility, disability, and quality of life. Decreases in the level of activity or avoiding different walking terrains, such as stairs, can also be indicative of a person’s health. Similarly, changes in vision caused due to the fact of aging may also be an indicator of health. Improper configuration of assistive device settings can result in bad posture, potentially exacerbating problems and further impacting quality of life. Recognizing negative trends and differences in activity levels in daily life may provide vital information that could allow proactive monitoring of an individual’s well-being. Early interventions from healthcare professionals, especially for aging populations, may help reduce incidents and enable aging in place.
The design of the tested multi-sensor cane provides a low-cost method for detecting deviations from healthy walking patterns without requiring clinical intervention or assessment. In this study, the cane consistently demonstrated the ability to detect changes in gait due to the presence of perturbed gait. The mean differences observed between healthy controls and perturbed gait conditions indicate the device can identify changes from a baseline state. Reduced pitch velocity during stance and swing phases indicate that users tended to swing their cane more slowly for perturbed gait conditions. Smaller values of maximum pitch velocity index with respect to the TC event indicate that users reached their maximum velocity earlier for perturbed gait conditions, possibly suggesting apprehension towards the end of the stride. Similarly, small values of swing stance ratio indicate that users tended to spend more time in stance phase for perturbed gait conditions. This is likely due to the lower stability and confidence. Higher values of maximum strain also indicate that the users relied more on the cane while walking under perturbed gait conditions. Moreover, the negative mean difference in maximum strain index with respect to the IC event for the stance phase indicates that users reached their peak load later in stance phase for perturbed gait conditions. It can also be seen that stride length significantly decreased for perturbed gait conditions. Lastly, the mean difference in IC event, determined by taking the difference between the IC events determined by the MSMF and GPD approaches, increased for perturbed gait. Together, this collection of metrics may be used to build indicators of changes in gait due to the presence of pain or other factors.
Changes in gait due to the fact of walking terrains were also detectable by the multi-sensor cane. Specifically, the swing and stance phases demonstrated higher values of pitch velocity for uphill and downhill terrains with respect to the control case. This finding suggests that the users were swinging their canes faster while on these terrains. By contrast, while walking on stairs, a reverse trend in pitch velocity during swing and stance phase was observed for both upstairs and downstairs terrains. This indicates that users tended to swing their cane slower while encountering stairs. In addition, smaller values of swing stance ratio for stair-walking indicates that users tended to spend more time in stance phase. As observed during the perturbed gait conditions, users again tended to rely more on their canes for different walking terrains. The mean difference in IC events determined by the MSMF and GPD algorithms increased for all walking terrains except for downhill walking, indicating that changes in the walking pattern occurred due to the different terrains.
Changes in gait due to the fact of reduced vision were also investigated with results indicating the ability of the multi-sensor cane to detect some changes from normal vision. Intuitively, the “both eyes closed” case was notably different. A lack of vision decreased the pitch velocity during swing and stance phases indicating that people walked slower when their vision was impaired. There was some evidence that users relied more on their cane when their vision was impaired but not occluded; however, no significant changes were observed. Stride length decreased significantly for the “both eyes closed” case indicating decreased walking confidence due to the loss of vision. Lastly, mean difference in IC event determined by MSMF with respect to GPD increased, indicating a change in walking pattern because of a change in vision. The ability to detect gait changes during impaired or occluded vision could be used to indicate unsafe walking conditions such as during the night without proper lighting.
Finally, the results suggested that the multi-sensor cane is capable of identifying differences between cane lengths. Particularly, users tended to rely more on cane due to the improper cane lengths, and stride length increased and decreased in response to cane length with respect to the control case. The mean difference in IC, as determined by the MSMF and GPD approaches, increased for improper cane lengths, indicating a change in the walking pattern. Given these results, future work could seek to develop an automated cane length adjustment protocol that optimizes gait.
Overall, the results presented here validate the ability of the multi-sensor cane to detect changes in gait due to the presence of simulated pain, walking terrains, perturbed vision, and improper setting of the cane. These results were validated using a shank-mounted IMU which provided results similar to those obtained by the cane. This is an important finding because, although the load through the cane is distributed through the upper body, it is still able to identify changes in gait caused due to the lower extremity. This strengthens the case for instrumented ADs as a deployable tool that could provide valuable information and feedback to their users, healthcare providers, and loved ones.