Evaluation of High-Temperature Hydrogen Sensors Based on BaCe0.6Zr0.3Y0.1O3-α and Sr(Ce0.9Zr0.1)0.95Yb0.05O3-α Perovskites for Industrial Applications
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis and Sintering of Ceramics
2.2. Sensor Construction
2.3. Electrochemical Measurements
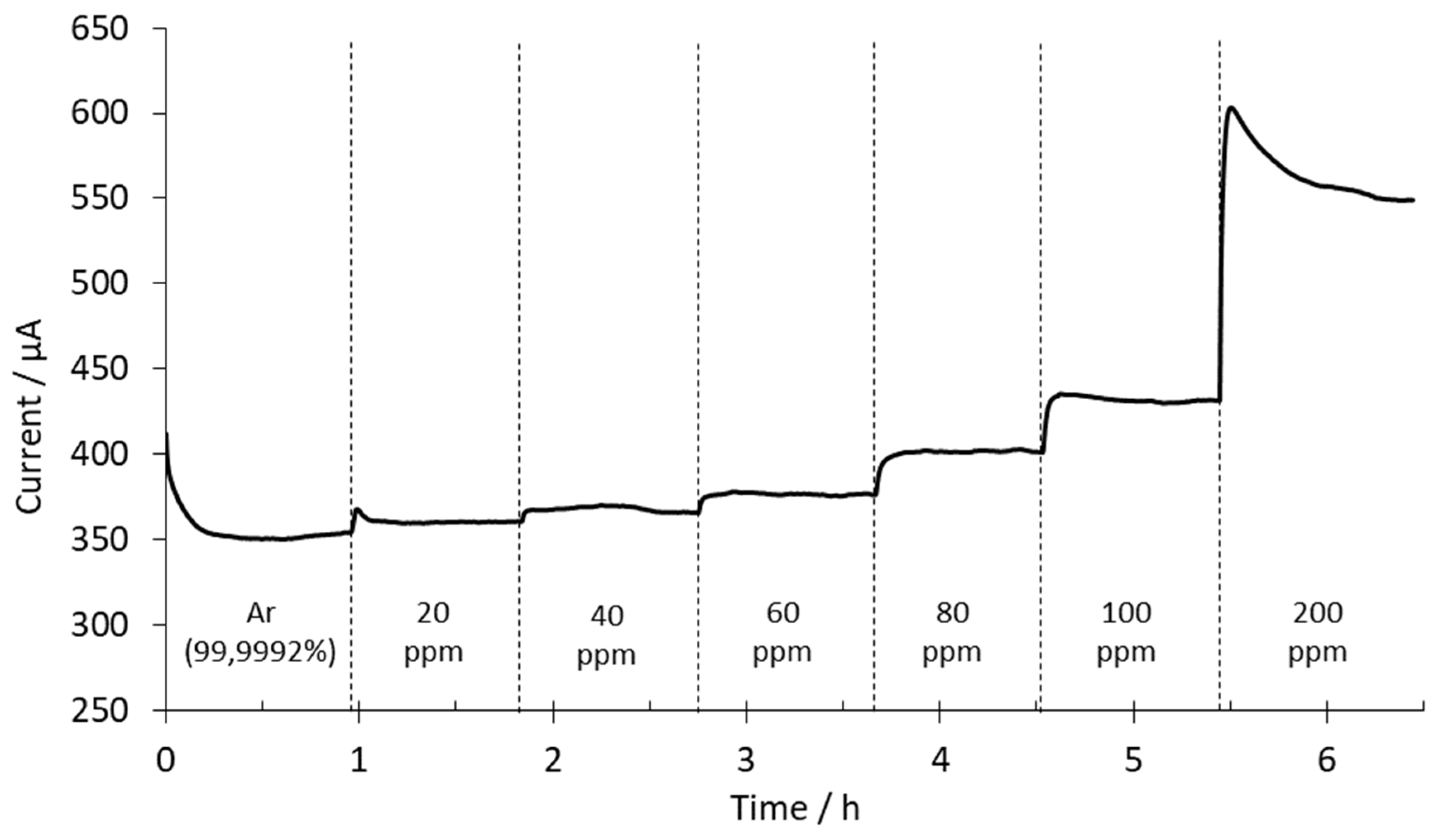
3. Results and Discussion
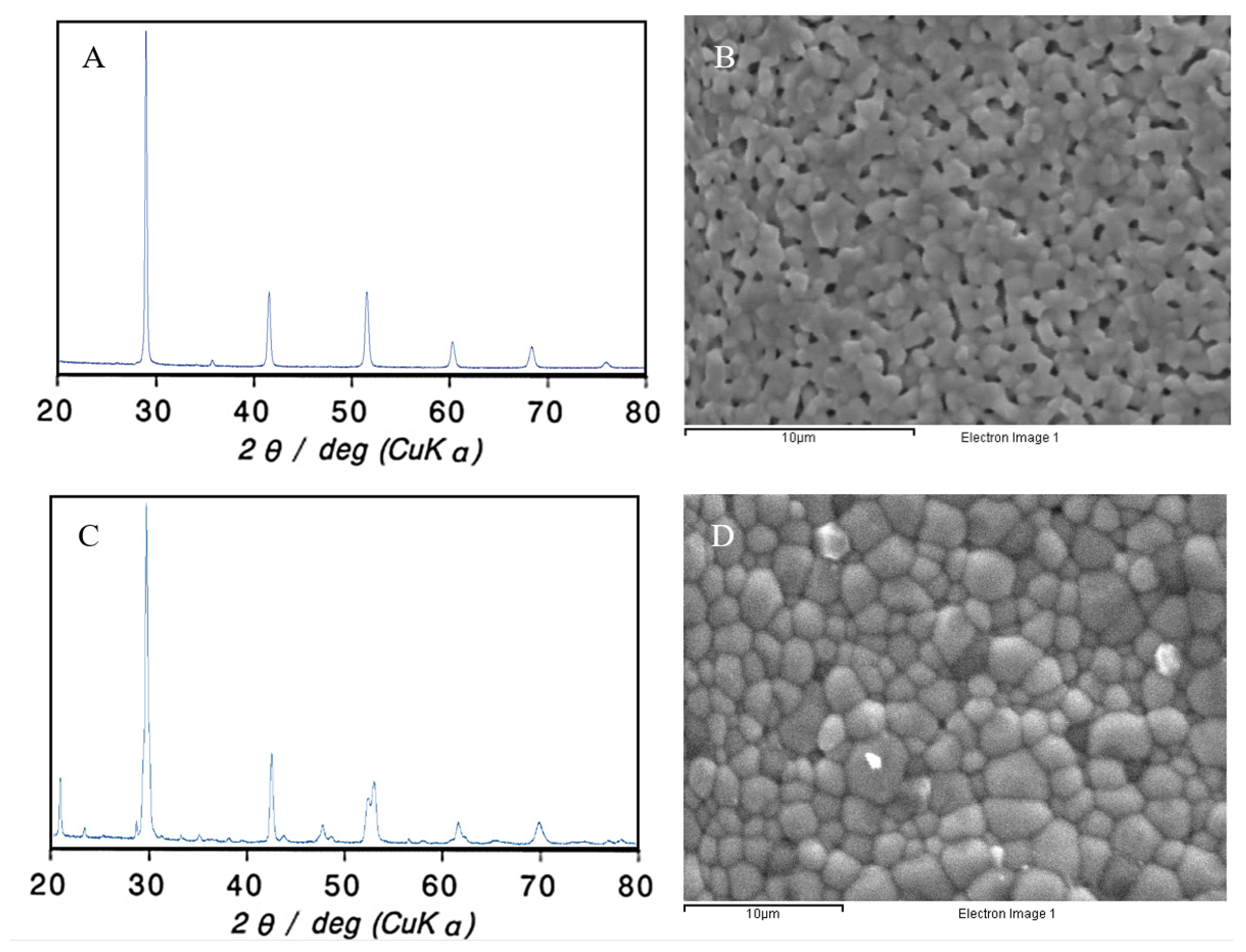
3.1. Characterization of the Proton-Conducting Electrolytes
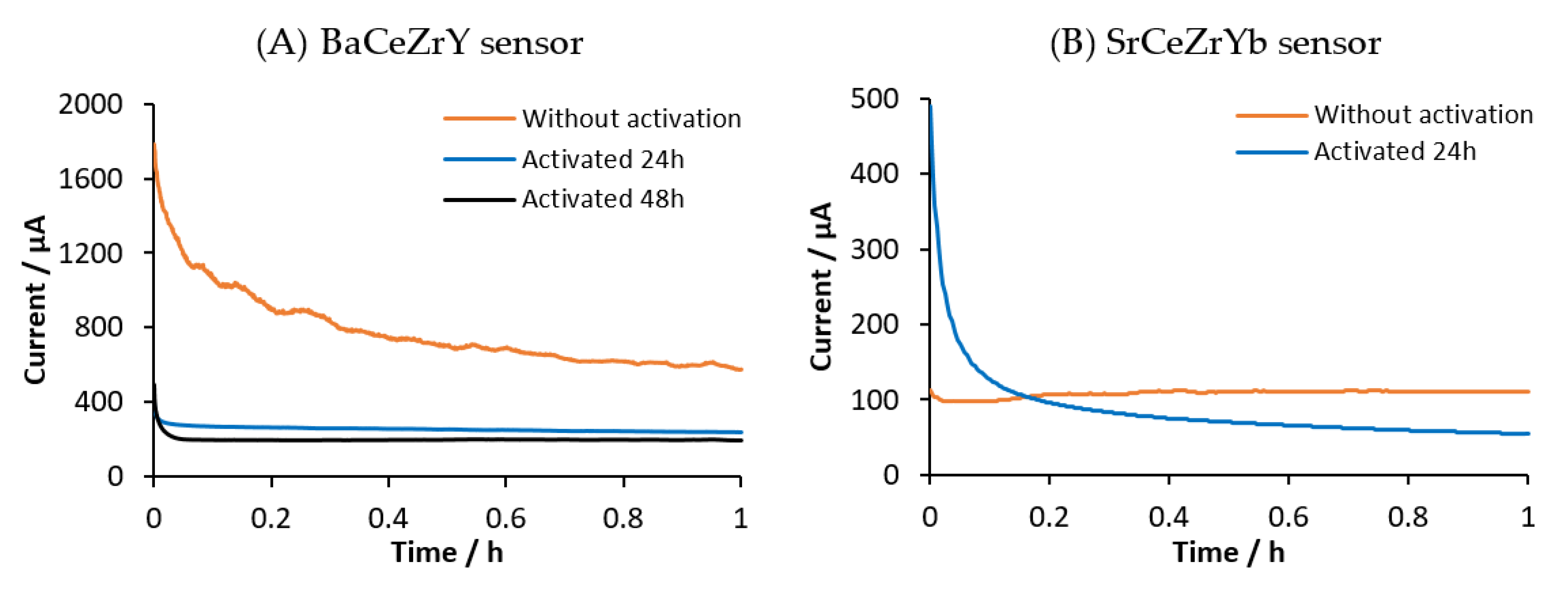
3.2. Sensor Activation
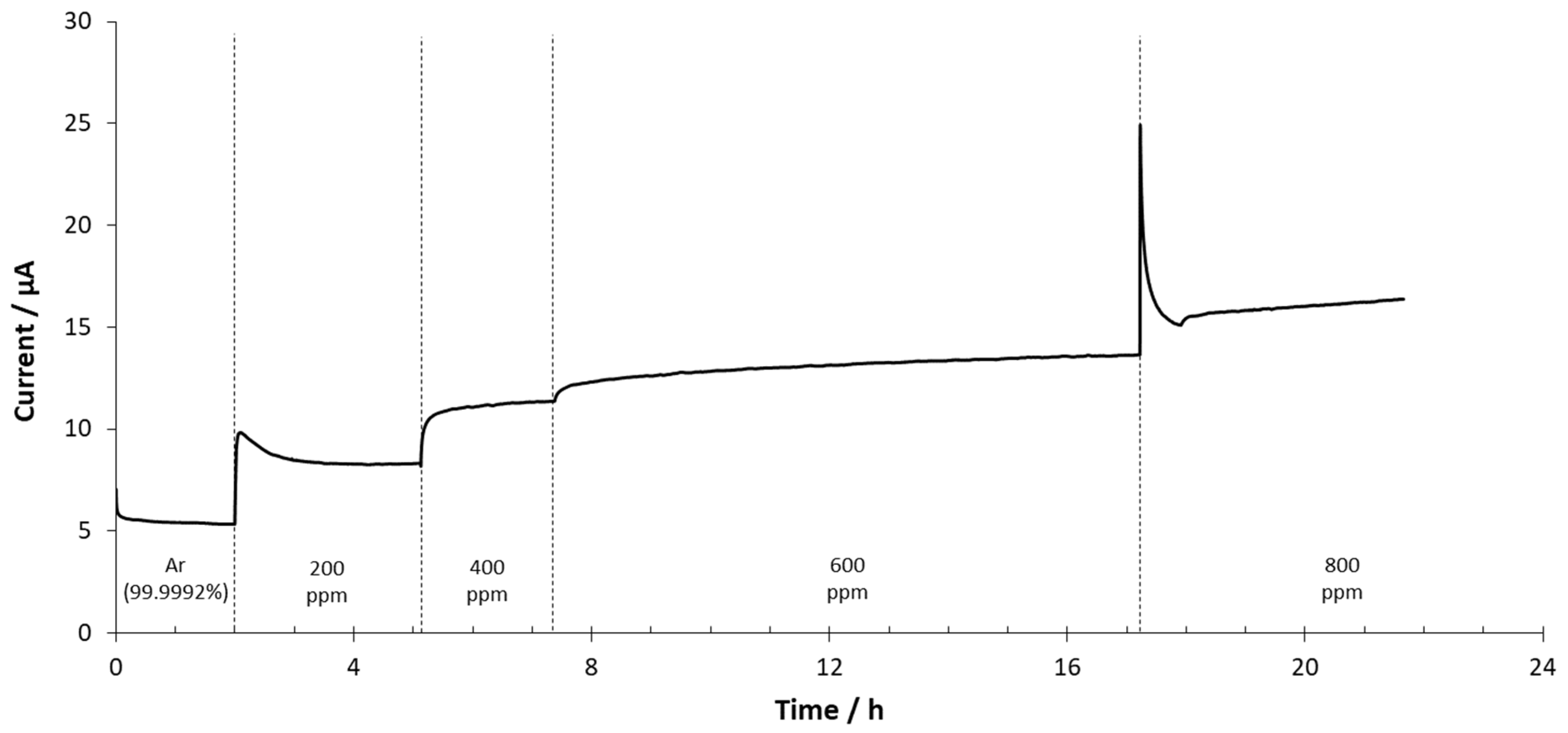
3.3. Amperometric Measurements
3.3.1. SrCeZrYb Electrolyte
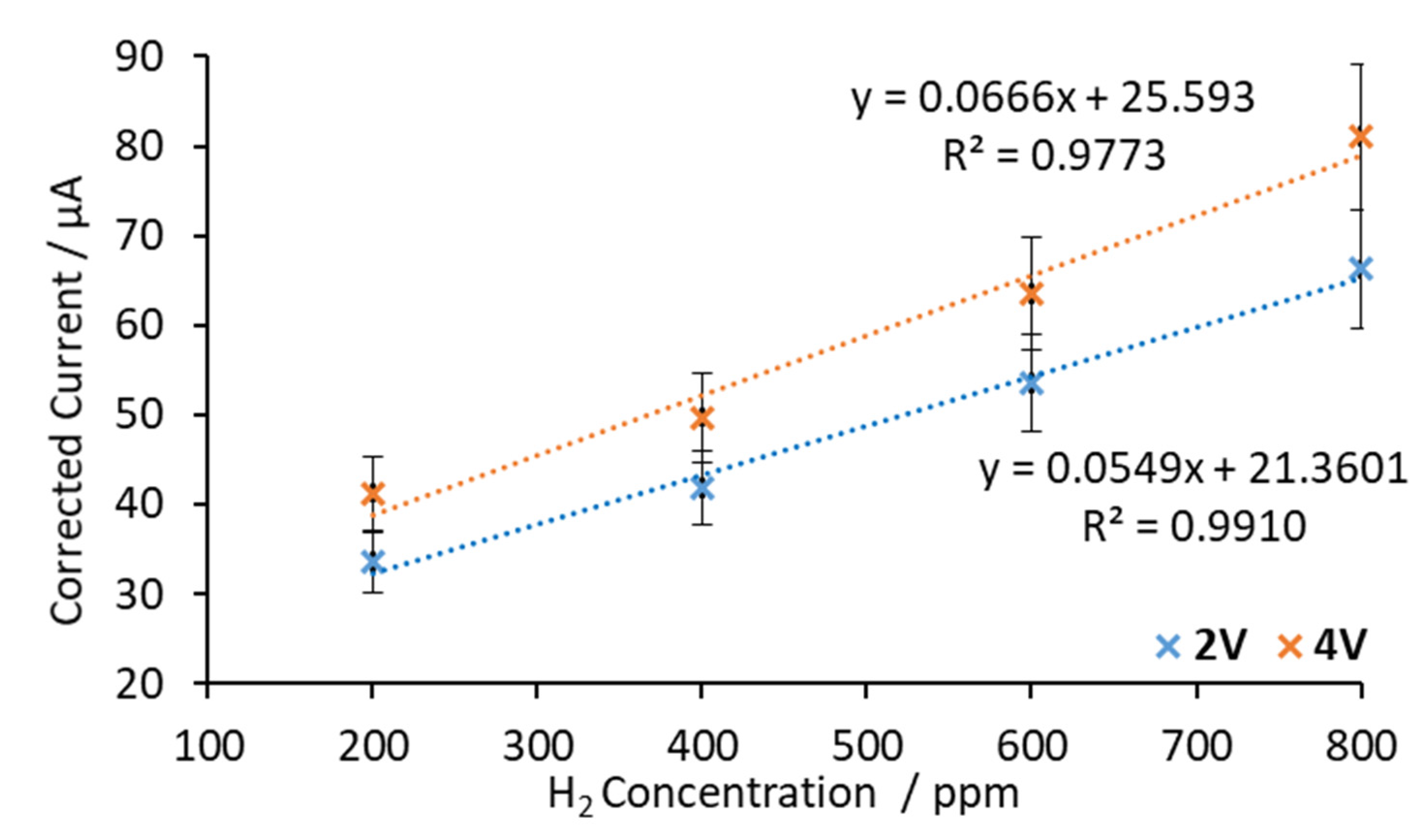
3.3.2. BaCeZrY Electrolyte
3.4. Comparison with Other Sensors Reported in the Literature
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Hoyt, W.B.; Caughey, R.H. High Temperature Metal Deterioration In Atmospheres Containing Carbon-Monoxide and Hydrogen. Corrosion 1959, 15, 21–24. [Google Scholar] [CrossRef]
- Jones, C.P.; Scott, T.B.; Petherbridge, J.R.; Glascott, J. A surface science study of the initial stages of hydrogen corrosion on uranium metal and the role played by grain microstructure. Solid State Ionics 2013, 231, 81–86. [Google Scholar] [CrossRef]
- Biezma, M. The role of hydrogen in microbiologically influenced corrosion and stress corrosion cracking. Int. J. Hydrog. Energy 2001, 26, 515–520. [Google Scholar] [CrossRef]
- Breeze, P. Nuclear Power. In Power Generation Technologies; Elsevier: Amsterdam, The Netherlands, 2014; pp. 353–378. [Google Scholar]
- Morse, E. Nuclear Fusion. Graduate Texts in Physics, 1st ed.; Springer International Publishing AG, Ed.; Springer International Publishing AG: Cham, Switzerland, 2018; ISBN 9783319981703. [Google Scholar]
- Mannone, F. (Ed.) Safety in Tritium Handling Technology; Eurocourses: Nuclear Science and Technology; Springer: Dordrecht, The Netherlands, 1993; Volume 1, ISBN 978-94-010-4844-6. [Google Scholar]
- Bonanos, N. Perovskite Proton Conductor. In Encyclopedia of Applied Electrochemistry; Springer: New York, NY, USA, 2014; pp. 1514–1520. [Google Scholar]
- Marrony, M. Proton-Conducting Ceramics: From Fundamentals to Applied Research, 1st ed.; Jenny Stanford Publishing: Singapore, 2015; ISBN 9789814613842. [Google Scholar]
- Benes, A.; Molinari, A.; Witte, R.; Kruk, R.; Brötz, J.; Chellali, R.; Hahn, H.; Clemens, O. Proton Conduction in Grain-Boundary-Free Oxygen-Deficient BaFeO2.5+δ Thin Films. Materials 2017, 11, 52. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez, J.A.; Fernández-García, M. (Eds.) Synthesis, Properties, and Applications of Oxide Nanomaterials; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2007; ISBN 978-0-471-72405-6. [Google Scholar]
- Snijkers, F.M.; Buekenhoudt, A.; Cooymans, J.; Luyten, J.J. Proton conductivity and phase composition in BaZr0.9Y0.1O3−δ. Scr. Mater. 2004, 50, 655–659. [Google Scholar] [CrossRef]
- Agmon, N. The Grotthuss mechanism. Chem. Phys. Lett. 1995, 244, 456–462. [Google Scholar] [CrossRef]
- Mistewicz, K. Recent Advances in Ferroelectric Nanosensors: Toward Sensitive Detection of Gas, Mechanothermal Signals, and Radiation. J. Nanomater. 2018, 2018, 1–15. [Google Scholar] [CrossRef]
- Mistewicz, K.; Nowak, M.; Paszkiewicz, R.; Guiseppi-Elie, A. SbSI Nanosensors: From Gel to Single Nanowire Devices. Nanoscale Res. Lett. 2017, 12, 97. [Google Scholar] [CrossRef]
- Shimojo, F.; Hoshino, K.; Okazaki, H. Effects of doped acceptor ions on proton diffusion in perovskite oxides: A first-principles molecular-dynamics simulation. J. Phys. Condens. Matter 1998, 10, 285–294. [Google Scholar] [CrossRef]
- Norby, T. Solid-state protonic conductors: Principles, properties, progress and prospects. Solid State Ionics 1999, 125, 1–11. [Google Scholar] [CrossRef]
- Juhera, E.; Calvet, M.; Revuelta, A.; Abellà, J.; Colominas, S. High temperature hydrogen selective solid-state electrolyte sensor fabricated by slip casting. Fusion Eng. Des. 2019, 146, 2066–2069. [Google Scholar] [CrossRef]
- Llivina, L.; Colominas, S.; Abellà, J. Evaluation of the response time of H-concentration probes for tritium sensors in lead–lithium eutectic alloy. Fusion Eng. Des. 2014, 89, 1397–1401. [Google Scholar] [CrossRef]
- Borland, H.; Llivina, L.; Colominas, S.; Abellà, J. Proton conducting ceramics for potentiometric hydrogen sensors for molten metals. Fusion Eng. Des. 2013, 88, 2431–2435. [Google Scholar] [CrossRef]
- Juhera, E.; Colominas, S.; Abellà, J. Amperometric hydrogen sensors for application in fusion reactors. Fusion Eng. Des. 2017, 124, 901–904. [Google Scholar] [CrossRef]
- Juhera, E.; Colominas, S.; Abellà, J. Operating modes of electrochemical H-concentration probes for tritium sensors. Fusion Eng. Des. 2015, 98–99, 1710–1714. [Google Scholar] [CrossRef]
- Ricote, S.; Caboche, G.; Heintz, O. Synthesis and proton incorporation in BaCe0.9−xZrxY0.1O3−δ. J. Appl. Electrochem. 2009, 39, 553–557. [Google Scholar] [CrossRef]
- Hung, I.M.; Chiang, Y.J.; Wang, Y.H.; Jang, J.S.C.; Lee, S.W. Electrical properties and hydrogen flux performance of Sr(Ce0.6Zr0.4)1−xYxO3−Δ ceramic proton conductors. Int. J. Hydrogen Energy 2017, 42, 22149–22158. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, Z.; Han, J.; Wu, J.; Huang, S.; Zhu, X. Synthesis and characterization of proton conducting Sr(Ce1−xZrx)0.95Yb0.05O3−δ by the citrate method. J. Alloys Compd. 2007, 440, 270–275. [Google Scholar] [CrossRef]
- Zhang, J.; Wen, Z.; Huang, S.; Wu, J.; Han, J.; Xu, X. High-temperature proton conductor Sr(Ce0.6Zr0.4)0.9Y0.1O3−δ: Preparation, sintering and electrical properties. Ceram. Int. 2008, 34, 1273–1278. [Google Scholar] [CrossRef]
- Katahira, K.; Kohchi, Y.; Shimura, T.; Iwahara, H. Protonic conduction in Zr-substituted BaCeO3. Solid State Ionics 2000, 138, 91–98. [Google Scholar] [CrossRef]
- Lim, D.-K.; Park, C.-J.C.-N.; Choi, M.-B.; Park, C.-J.C.-N.; Song, S.-J. Partial conductivities of mixed conducting BaCe0.65Zr0.2Y0.15O3-δ. Int. J. Hydrog. Energy 2010, 35, 10624–10629. [Google Scholar] [CrossRef]
- The Complete Book On Glass And Ceramics Technology, 2nd ed.; NIIR Board of Consultants & Engineers, Ed.; National Institute of Industrial Research: New Delhi, India, 2005; ISBN 978-8178330334. [Google Scholar]
- Xia, C.; Mi, Y.; Wang, B.; Lin, B.; Chen, G.; Zhu, B. Shaping triple-conducting semiconductor BaCo0.4Fe0.4Zr0.1Y0.1O3-δ into an electrolyte for low-temperature solid oxide fuel cells. Nat. Commun. 2019, 10, 1707. [Google Scholar] [CrossRef]
- Matzke, T.; Cappadonia, M. Proton conductive perovskite solid solutions with enhanced mechanical stability. Solid State Ionics 1996, 86–88, 659–663. [Google Scholar] [CrossRef]
- Pionke, M.; Mono, T.; Schweika, W.; Schober, H.; Springer, T. Proton diffusion in Ba[Ca1+x)/3Nb2−x]O3−x/2 studied by quasielastic neutron scattering. Phys. B Condens. Matter 1997, 234–236, 95–96. [Google Scholar] [CrossRef]
- Münch, W. A molecular dynamics study of the high proton conducting phase of CsHSO4. Solid State Ionics 1995, 77, 10–14. [Google Scholar] [CrossRef]
- Kreuer, K.D. Proton-Conducting Oxides. Annu. Rev. Mater. Res. 2003, 33, 333–359. [Google Scholar] [CrossRef]
- Kreuer, K. H/D isotope effect of proton conductivity and proton conduction mechanism in oxides. Solid State Ionics 1995, 77, 157–162. [Google Scholar] [CrossRef]
- Keeble, D.J.; Wicklein, S.; Dittmann, R.; Ravelli, L.; Mackie, R.A.; Egger, W. Identification of A-and B-site cation vacancy defects in perovskite oxide thin films. Phys. Rev. Lett. 2010, 105, 226102. [Google Scholar] [CrossRef]
- Barison, S.; Battagliarin, M.; Cavallin, T.; Doubova, L.; Fabrizio, M.; Mortalò, C.; Boldrini, S.; Malavasi, L.; Gerbasi, R. High conductivity and chemical stability of BaCe1−x−yZrxYyO3−δ proton conductors prepared by a sol–gel method. J. Mater. Chem. 2008, 18, 5120. [Google Scholar] [CrossRef]
- Singhal, S.C.; Kendall, K. High-Temperature Solid Oxide Fuel Cells: Fundamentals, Design and Applications, 1st ed.; Singhal, S.C., Kendall, K., Eds.; Elsevier: Amsterdam, The Netherlands, 2003; ISBN 9781856173872. [Google Scholar]
- Kalyakin, A.; Lyagaeva, J.; Medvedev, D.; Volkov, A.; Demin, A.; Tsiakaras, P. Characterization of proton-conducting electrolyte based on La0.9Sr0.1YO3-δ and its application in a hydrogen amperometric sensor. Sens. Actuators B Chem. 2016, 225, 446–452. [Google Scholar] [CrossRef]
- Kalyakin, A.; Fadeyev, G.; Demin, A.; Gorbova, E.; Brouzgou, A.; Volkov, A.; Tsiakaras, P. Application of Solid oxide proton-conducting electrolytes for amperometric analysis of hydrogen in H2+N2+H2O gas mixtures. Electrochim. Acta 2014, 141, 120–125. [Google Scholar] [CrossRef]
- Farabolini, W.; Ciampichetti, A.; Dabbene, F.; Fütterer, M.A.; Giancarli, L.; Laffont, G.; Puma, A.L.; Raboin, S.; Poitevin, Y.; Ricapito, I.; et al. Tritium control modelling for a helium cooled lithium–lead blanket of a fusion power reactor. Fusion Eng. Des. 2006, 81, 753–762. [Google Scholar] [CrossRef]





| Operational Conditions | Sensitivity/μA·ppm−1 | Range/ppm | Response Time/min |
|---|---|---|---|
| 500 °C—2 V | 0.0134 | 200–800 | 15 |
| 600 °C—2 V | 0.0549 | 200–800 | 8 |
| Operational Conditions | Sensitivity /μA·ppm−1 | Range/ppm | Response Time/min |
|---|---|---|---|
| 500 °C—2 V | 0.6086 | 60–200 | 6 |
| 600 °C—2 V | 1.4044 | 40–200 | 5 |
| 500 °C—4 V | 1.2272 | 60–200 | 4 |
| 600 °C—4 V | 2.5052 | 40–200 | 4 |
| Reference | Electrolyte | Operational Conditions | Sensitivity/μA·ppm−1 | Range/ppm |
|---|---|---|---|---|
| [38] | La0.9Sr0.1YO3-α | 550 °C—2 V | 0.0077 | 1000–33,000 |
| [39] | La0.95Sr0.05YO3-α | 800 °C—0.75 V | 0.2085 | 5000–40,000 |
| This work | Sr(Ce0.9Zr0.1)0.95Yb0.05O3-α | 500 °C—2 V | 0.0134 | 200–800 |
| 600 °C—2 V | 0.0549 | 200–800 | ||
| This work | BaCe0.6Zr0.3Y0.1O3-α | 500 °C—2 V | 0.6086 | 60–200 |
| 600 °C—4 V | 2.5052 | 40–200 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hinojo, A.; Soriano, I.; Abellà, J.; Colominas, S. Evaluation of High-Temperature Hydrogen Sensors Based on BaCe0.6Zr0.3Y0.1O3-α and Sr(Ce0.9Zr0.1)0.95Yb0.05O3-α Perovskites for Industrial Applications. Sensors 2020, 20, 7258. https://doi.org/10.3390/s20247258
Hinojo A, Soriano I, Abellà J, Colominas S. Evaluation of High-Temperature Hydrogen Sensors Based on BaCe0.6Zr0.3Y0.1O3-α and Sr(Ce0.9Zr0.1)0.95Yb0.05O3-α Perovskites for Industrial Applications. Sensors. 2020; 20(24):7258. https://doi.org/10.3390/s20247258
Chicago/Turabian StyleHinojo, Antonio, Iván Soriano, Jordi Abellà, and Sergi Colominas. 2020. "Evaluation of High-Temperature Hydrogen Sensors Based on BaCe0.6Zr0.3Y0.1O3-α and Sr(Ce0.9Zr0.1)0.95Yb0.05O3-α Perovskites for Industrial Applications" Sensors 20, no. 24: 7258. https://doi.org/10.3390/s20247258
APA StyleHinojo, A., Soriano, I., Abellà, J., & Colominas, S. (2020). Evaluation of High-Temperature Hydrogen Sensors Based on BaCe0.6Zr0.3Y0.1O3-α and Sr(Ce0.9Zr0.1)0.95Yb0.05O3-α Perovskites for Industrial Applications. Sensors, 20(24), 7258. https://doi.org/10.3390/s20247258






