1. Introduction
A vital sign is a measurable biometric signal produced by a living body that indicates life, and such signals generally pertain to variables of pulse rate, respiration, body temperature, and blood pressure [
1,
2,
3]. In order to maintain life, humans take in oxygen in the lungs, and the contraction of the heart sends oxygen to cells throughout the body by blood. Heat and blood flow pressure are generated by the movement of these organs, and biological information can be grasped from these four vital signs. Paramedics and nurses routinely measure these vital signs of patients, from which they detect valuable health-status information. Opportunities to measure vital signs in daily life are often measured after an abnormality occurs in the physical condition, such as “instructed by a doctor” or “feeling abnormal in physical condition”. Even when instructed by a doctor, vitals often measured regularly at fixed times, such as when waking up or before and after meals, and it is not common to measure continuously on a regular basis. There are many countries where the elderly population is on the rise, with Japan at the top. In recent years, manufacturers have developed wearable vital sensors that can automatically measure biological information in daily life to maintain the health of the elderly [
4,
5,
6]. The wearable sensor has the advantage that it can be attached to a living body, so it can measure vital signs anytime, anywhere. Therefore, the user can constantly measure vital signs while living a daily life just by wearing a sensor. As a result, detailed information, such as the transition of vital signs in daily life, can be measured, so that one can know more about one’s physical condition and can receive a diagnosis based on more information from a doctor.
Figure 1 shows the market size forecast for wearable devices released by the Ministry of Internal Affairs and Communications of Japan [
7]. From
Figure 1, it can be seen that the market size is expected to expand both in Japan and around the world, and that it will grow by about four times in the five years from 2015. By these projections, the wearable sensor industry is growing. With wearable sensor products, the pulse rate is mainly measured using a photoelectric pulse wave sensor. The principle of this sensor is Photoplethysmography (PPG), which measures light absorption phenomenon by hemoglobin by inserting light into the living body [
8,
9]. This sensor uses a green LED (about 550 nm) with a high absorption coefficient of hemoglobin oxide in blood vessels as a light source. The green light inserted into the living body from the light source is absorbed by the oxidized hemoglobin inside the capillaries and reflected to the outside of the living body. This reflected light is detected by a detector. The amount of oxidized hemoglobin in the capillaries changes as the heart contracts, and the signal intensity corresponding to that amount is detected. The measured signal intensity change is related to the contraction of the heart, and the heartbeat cycle can be calculated by detecting the peak intensity of the measured signal and can be calculated as the pulse rate. When the heart contracts, the amount of oxidized hemoglobin is the largest, so the measured output signal power value has the smallest. Because green light has a short penetration depth into living organisms, transmission-type sensors that use infrared light that penetrates deeper have also been developed. Such products can be used to detect early signs of stress problems and heat stroke. However, the photoelectric pulse wave sensor cannot measure respiratory rate and blood pressure, which are indicators of vital signs, at the same time.
On the other hand, as a sensor for measuring the respiratory rate, a temperature sensor using a thermistor and a pressure sensor installed on the nose are common [
10,
11,
12,
13]. Breathing is performed by up/down changes in the diaphragm and expansion/contraction changes in the lungs. Studies have measured the strain caused by breathing using FBG (Fiber Bragg grating) sensors and have calculated respiratory rates based on these. Such a method was installed in a bed/cushion [
14,
15,
16] an FBG sensor embedded in a belt-like cloth wound around the chest [
17,
18,
19]. Massaroni et al. measured the strain caused by the expansion and contraction of the lungs using an FBG sensor installed on the upper human body and calculated parameters related to respiration [
20,
21]. Thus, extant research has produced devices for measuring more real-time and complex respiratory parameters using FBG sensors. However, these studies only measure respiratory rate and cannot measure multiple vital signs at the same time. Furthermore, these studies have not well-verified which part of the body the strain by the lung and the diaphragm expansion and contraction propagates.
To interpret measured biological information in detail, it is useful to measure and assess multiple vital signs simultaneously [
22,
23,
24]. Thus, more complex wearable sensors are highly sought for measuring pulse rate, respiratory rate, and blood pressure at all times. Our major research proposal is to develop a wearable multi-vital-sign sensor. For this purpose, an optical fiber Bragg grating (FBG) sensor is used. In our previous work, an FBG sensor was installed at the pulsation point of the wrist strain signal so that pulsation could be measured. From the measured signals (e.g., pulse rate [
25], respiratory rate [
26], blood pressure [
27,
28], and blood glucose value [
29]), the biological parameters of the circulatory system can be assessed in real time. The pulmonary rate was calculated by measuring respiratory sinus arrhythmia, which reflects changes in pulse rate caused by changes in the autonomic nervous system. To calculate the respiratory rate using this method, the pulsation strain must be measured in a stationary state for approximately 30 s. Unfortunately, this particular capability lacks real-time calculation. To calculate the respiratory rate, the strain caused by respiration emerging from the living body must be directly measured in real time.
We propose a method using two FBG sensors to develop a sensor that simultaneously measures pulse rate, blood pressure, and respiratory rate. The important point of this method is the installation part of each FBG sensor. One FBG sensor is installed at the pulsation point on the wrist or elbow to calculate pulse rate and blood pressure. The other FBG sensor is used to measure respiratory rate. This FBG sensor should be installed in a part where respiratory strain by movement of the lungs and diaphragm can be detected. For that purpose, it is necessary to confirm to what part of the living body these strains propagate. The part where these strains can be detected most is the optimum installation part for the FBG sensor. Furthermore, the effect of respiratory strain on the pulsation points of the elbows and wrists has not been well-clarified. At the pulsation point where the strain propagated from the heart is detected, the strain due to respiration becomes noise. On the other hand, when only respiratory strain is detected, cardiac strain becomes noise. Therefore, it is necessary to confirm whether the strain due to respiration propagates to the pulsation point in the living body. In this study, we verify the extent to which the strain from a living body caused by respiration propagates. There are five measurement areas for this purpose: abdomen, chest, shoulder, elbow, and wrist. We verify that the strain signal caused by respiration can be detected from signals measured at each location. Additionally, a measurement method for simultaneously calculating multiple vital signs is considered.
2. Materials and Methods
2.1. FBG Sensor Measurement Principle and Measurement System
The FBG sensor is a PANDA-type polarization-maintaining single-mode optical fiber (SM15-PS-U25D, Fujikura Co., Ltd., Tokyo, Japan.) made of silica glass. In the sensor part, a diffraction grating, having an organic refractive index change, is installed at the core part of the optical fiber. When the FBG sensor was fabricated, the optical fiber protection films—at the location at which the FBG sensor will be engraved by using the phase mask—were removed. After the FBG sensor was engraved with a phase mask, this part was recoated with UV curable resin (UV acrylate), which is the same material as the resin stripped from the optical fiber. The FBG sensor was used in its fiber optic shape. When near-infrared light reaches this sensor, only a specific wavelength is reflected according to Equation (1):
where
λBragg is the Bragg wavelength,
neff is the refractive index of the optical fiber core, and Λ is the diffraction grating spacing. This reflected wavelength is called the “Bragg wavelength,” and it is detected by the FBG sensor system. When strain is applied to the sensor, the optical fiber is stretched. Therefore, the diffraction grating spacing (i.e., the FBG sensor) also becomes longer. According to Equation (1), the larger the grating spacing, Λ, the larger the Bragg wavelength,
λBragg. Thus, a wavelength longer than the base Bragg wavelength in the undistorted state is detected. The measured signal from the FBG sensor shows the shift length from the base Bragg wavelength on the vertical axis. Therefore, on the vertical axis, “0” indicates that no strain is applied, “+side” indicates that the optical fiber is stretched because of strain, and “−side” indicates that the optical fiber is contracted. It also shows that the larger the absolute value on the vertical axis, the larger the stretch. During inspiration, the abdomen and chest expand due to the movement of the diaphragm and lungs. When FBG sensors are installed in those areas, the optical fiber is stretched, which increases the spacing between the diffraction gratings. The signal of the FBG sensor is measured on the “+side” in the vertical axis direction. On the other hand, during exhalation, the abdomen and chest contract. Since the FBG sensors installed in those areas also shrink, the spacing between the diffraction gratings becomes smaller. Therefore, the signal of the FBG sensor is measured on the “−side” in the vertical axis direction. In this way, the expansion and contraction of the living body by respiration can be detected by the change in the waveform of the measurement signal due to the expansion and contraction of the FBG sensor.
This system comprises an FBG interrogator in which the light-source and detection units are packaged and an optical fiber in which the FBG sensor is installed. The outline of the FBG interrogator is shown in
Figure 2. Our edge-filter module is used with the FBG interrogator [
30]. This edge filter has polarization dependent loss. This polarization-dependent loss had a large effect on the measured signal waveform; therefore, this loss had to be reduced. The light source SLD emits polarized light. Bragg wavelength light had to be reached at the edge filter while maintaining this polarized light, so PANDA-type polarization-maintaining single-mode optical fiber was used. In this FBG interrogator, when the light of the Bragg wavelength reached the edge filter, it was divided into reflected light and transmitted light. The amount of divided reflected light and transmitted light was detected by each photodiode. The detected light amount was calculated by (R − T)/(R + T) from the reflected light amount R and the transmitted light amount T. There was nothing in the strain (base Bragg wavelength); the amount of reflected light and transmitted light were each divided by 50%, and the output from the FBG interrogator was 0. When the FBG sensor was extended, the Bragg wavelength became longer, the amount of reflected light from the edge filter increased, and the amount of transmitted light decreased, so the output became “+”. Conversely, when the FBG sensor contracted, the Bragg wavelength became shorter, and the output of the FBG interrogator became “−”.
Two FBG sensors, with different base Bragg wavelengths, were installed in one optical fiber. The near-infrared light emitted from the light source was inserted into the core of the optical fiber and reached each sensor. The FBG sensor-1 reflected near-infrared light in the 1543 nm band, and the FBG sensor-2 reflected its Bragg wavelength in the 1561 nm band. Each near-infrared light signal was detected, and the displacement length of each Bragg wavelength from the time of calibration was calculated. The calibration was performed using the FBG sensor installed on the desk and without the strain. The sampling rate of the FBG sensor was 1 kHz. The measured signal was processed by a 0.03 to 0.5 Hz bandpass filter (BPF) to remove noise. Therefore, the BPF-processed signal (vertical axis: output value from the BPF-processed FBG sensor, horizontal axis: measurement time) was used as the signal measured by the FBG sensor.
2.2. Simultaneous Measurement Experiment during Breathing Using FBG Sensor and Respiratory Temperature Sensor
The relationship between the FBG sensor measurement signal and the respiration time was also verified. As shown in
Figure 3, a medical mask was attached to the subject’s oral cavity and an FBG sensor was installed on the abdomen. A thermistor type temperature sensor (Sato Keiryoki MFG. Co., Ltd., Tokyo, Japan. SK-L200TH2α) was installed in the airflow path of the medical mask. This temperature sensor measured the outside air temperature during inspiration and the exhaled air temperature from inside the body during expiration. Because each temperature was different, the temperatures measured during inspiration and expiration differed. Therefore, because the temperature changes periodically during inspiration and expiration, one cycle of the measurement signal was calculated as one respiration time. The sampling frequency of this temperature sensor was 1 Hz, and the temperature resolution was 0.1 °C. Each sensor was measured simultaneously when the subject breathed. The time synchronization of both sensors was performed by the following method. First, each sensor was installed to the subject, and the measurement start button of each sensor was clicked at the same time. The subject stopped breathing for 5 s from the start of measurement, and then started breathing. The fluctuation point of the signal measured after 5 s was treated as the measurement start point (Measurement time is 0), and the measurement signals at each sensor were synchronized in time. The time for one respiration was 6 s (inspiration: 3 s, expiration: 3 s). The measurement time was approximately 60 s and approximately 10 respirations were measured in one measurement; four measurements were taken. Because the signal measured by the FBG sensor also changed periodically, the time of each cycle was calculated. The time calculated by each sensor was compared and the respiration time from the signal measured by the FBG sensor was verified.
The FBG sensor was very sensitive to temperature, so it was necessary to pay attention to the temperature crossing sensitivity. The following measurement environment adjustment and calibration measurements were performed in advance in all experiments. The room temperature and humidity were unified at 20 degrees and 65% RH. Immediately after the FBG sensor was installed to the living body, the Bragg wavelength was shifted due to the temperature difference. However, it was confirmed that the baseline fluctuation of the measurement signal did not appear as a calibration measurement 30 min after the FBG sensor was installed to the living body. The temperature of the living body did not change rapidly during the subsequent signal measurement. By these countermeasures, the measurement signal had almost no effect on the temperature cross-sensitivity.
2.3. Measurement Experiment of Respiratory Strain Using FBG Sensor
In our experiment, the FBG sensor waveforms during breathing and apnea were verified. We expected that the strain of the living body would differ greatly between breathing and apnea. As shown in
Figure 4a, the subject’s measurement posture was sitting. This helped prevent strain caused by body movement. As shown in
Figure 4b, the FBG sensor was installed on the abdomen. The subject repeated breathing for 30 s, and they then stopped breathing for 30 s. The time for one respiration was 6 s (inspiration: 3 s, expiration: 3 s), breathing was performed five times in 30 s. In these 60 s, the FBG sensor measured the strain from the living body.
The measured signal waveform was analyzed as shown in
Figure 5.
Figure 5 shows an example of the signal waveform measured during 5 rounds of breathing. Five top peaks of the measured signal waveform were detected (red plots in
Figure 5), and the average value was calculated as the “average top peak value”. The breathing time for 4 breaths was calculated from the measurement time interval of the top peak (red-dotted arrows in
Figure 5). Similarly, five bottom peaks of the measurement signal were detected (green plots in
Figure 5), and the average value was calculated as the average bottom peak value. The time interval of the bottom peak was calculated (green-dashed arrows in
Figure 5) as well as the top peak. The sum of the absolute values on the vertical axis from the top peak to the bottom peak detected was detected as the longest amplitude of one breath (black arrow in
Figure 5). The longest amplitude during breathing was calculated from the average value of the five longest amplitudes. The reaction strain due to respiration was detected between 30 and 40 s after the end of respiration, the signal after 40 s was used as the signal measured in the apnea state. Of the signals after 40 s, the maximum output value on the vertical axis was detected as “Max in Apnea” and the minimum output value was detected as “Min. In Apnea” (sky blue arrow in
Figure 5). The total of these absolute values was calculated as the longest amplitude in apnea. Furthermore, as the rate of change of the longest amplitude during respiration and apnea, the ratio between the longest amplitude value during respiration and the longest amplitude during apnea was calculated. The number of experiments was 5. In that experiment, the vertical direction of the FBG sensor signal due to respiration was verified.
2.4. Measurement Experiment of Propagation Range of Respiratory Strain to each Living Body Part
In this experiment, two FBG sensors were installed on the living body. The subjects were three men in their twenties, measured in a sitting position. The subjects breathed for 30 s using a 6 s cycle. Five parts were selected for measuring the range of respiratory strain propagation: abdomen, chest, shoulders, elbows, and wrists. The abdomen and chest are the trunk of the living body and are the places where the movement of the diaphragm and lungs due to breathing can be detected. The elbow and wrist are the parts where the pulsating points of the arteries are located, and the strain due to the contraction of the heart can be detected. If respiratory strain propagates to these parts, it may be possible to measure cardiovascular and respiratory information at the same time by installing a single FBG sensor. The shoulder is the part that corresponds to the end of the trunk. The elbows and wrists are located beyond the shoulder or elbow joints when viewed from the trunk side of the abdomen and chest. If respiratory strain is measured at the shoulders only and not at the elbows or wrists, the result is that respiratory strain cannot propagate beyond the joints. For the above reasons, 5 measurement parts were selected.
As shown in
Figure 6a, FBG sensor-1 (
λBragg = 1543 nm) was installed on the subject’s abdomen, and FBG sensor-2 (
λBragg = 1561 nm) was installed on the chest. In this state, two FBG sensors were measured simultaneously. The signal measured by this FBG sensor-1 was used as a reference value for respiratory strain. Next, the FBG sensor-2 was installed on the shoulder and measured at the same time as FBG sensor-1 on the abdomen (
Figure 6b). Then, the FBG sensor-2 was installed on the elbow or wrist (
Figure 6c or
Figure 6d), and the same measurement was performed; each part was measured 5 times.
Top peaks and bottom peaks were detected from the signal waveforms measured at each part, as with
Figure 5. From these values, the average top peak value (Ave. Top peak), average bottom peak value (Ave. Bottom peak) and Longest amplitude value (Longest) were calculated. The ratio was calculated from the longest amplitude value of the abdomen measured at the same time as the longest amplitude value calculated for the chest, shoulders, elbows, and wrists (Longest amplitude value in abdomen/Longest amplitude in each part). When this ratio is smaller, it shows that the strain measured at each part is larger against the strain measured at the abdomen. When the ratio of the longest amplitude value to the abdomen measured at each part was smaller than the ratio of the longest amplitude value during breathing and apnea in
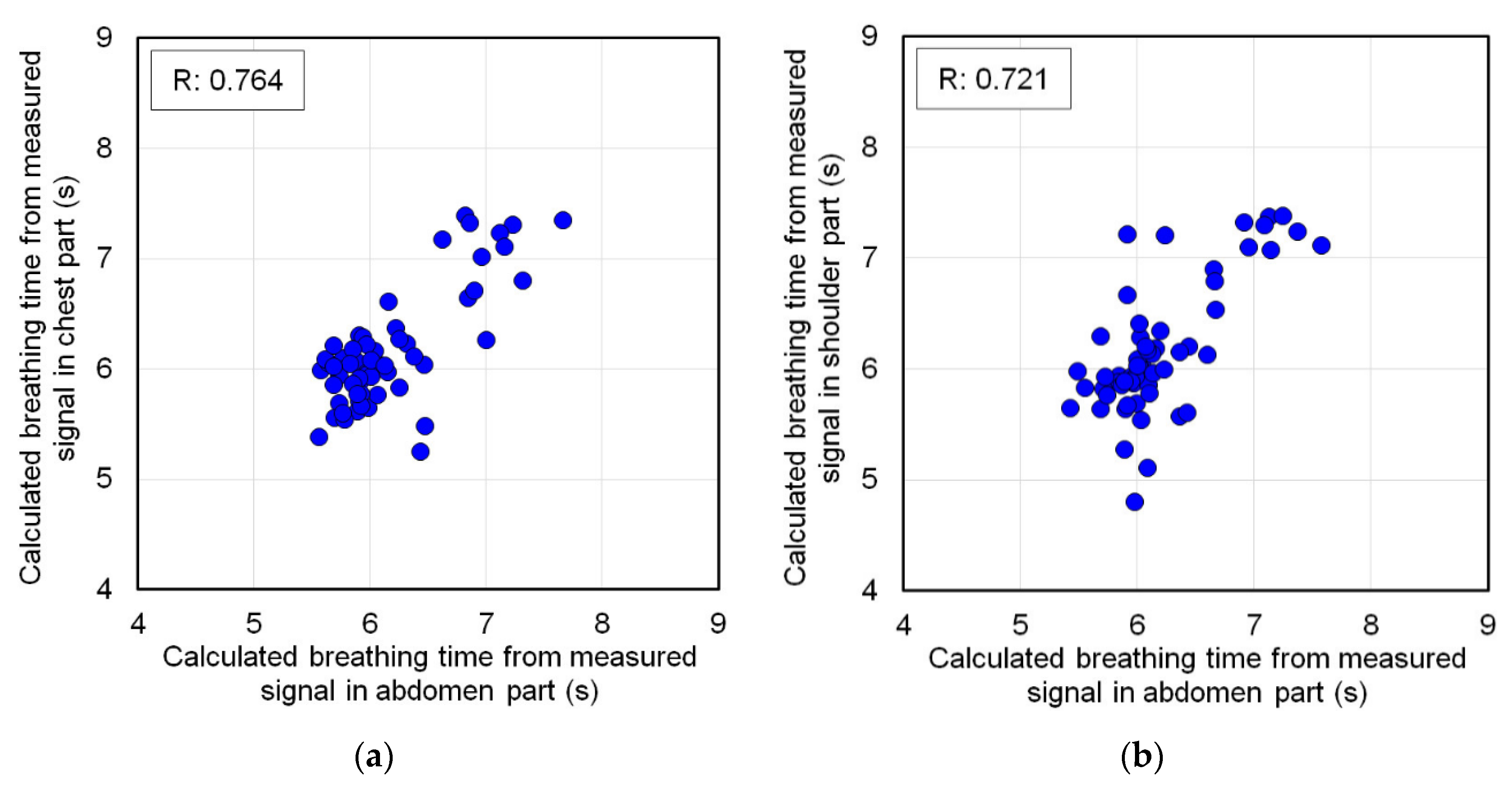
Section 2.2, it was judged that respiratory strain could be measured. In this case, the time interval of the top peak of the signal measured at each part was detected, and this was defined as each respiratory time. The time interval of the top peak was also detected from the signal waveform of the abdomen measured at the same time. The measurement accuracy of the respiratory time calculation was verified from the correlation scatter plot of the respiratory time by each partial signal against the respiratory time by the abdomen signal.
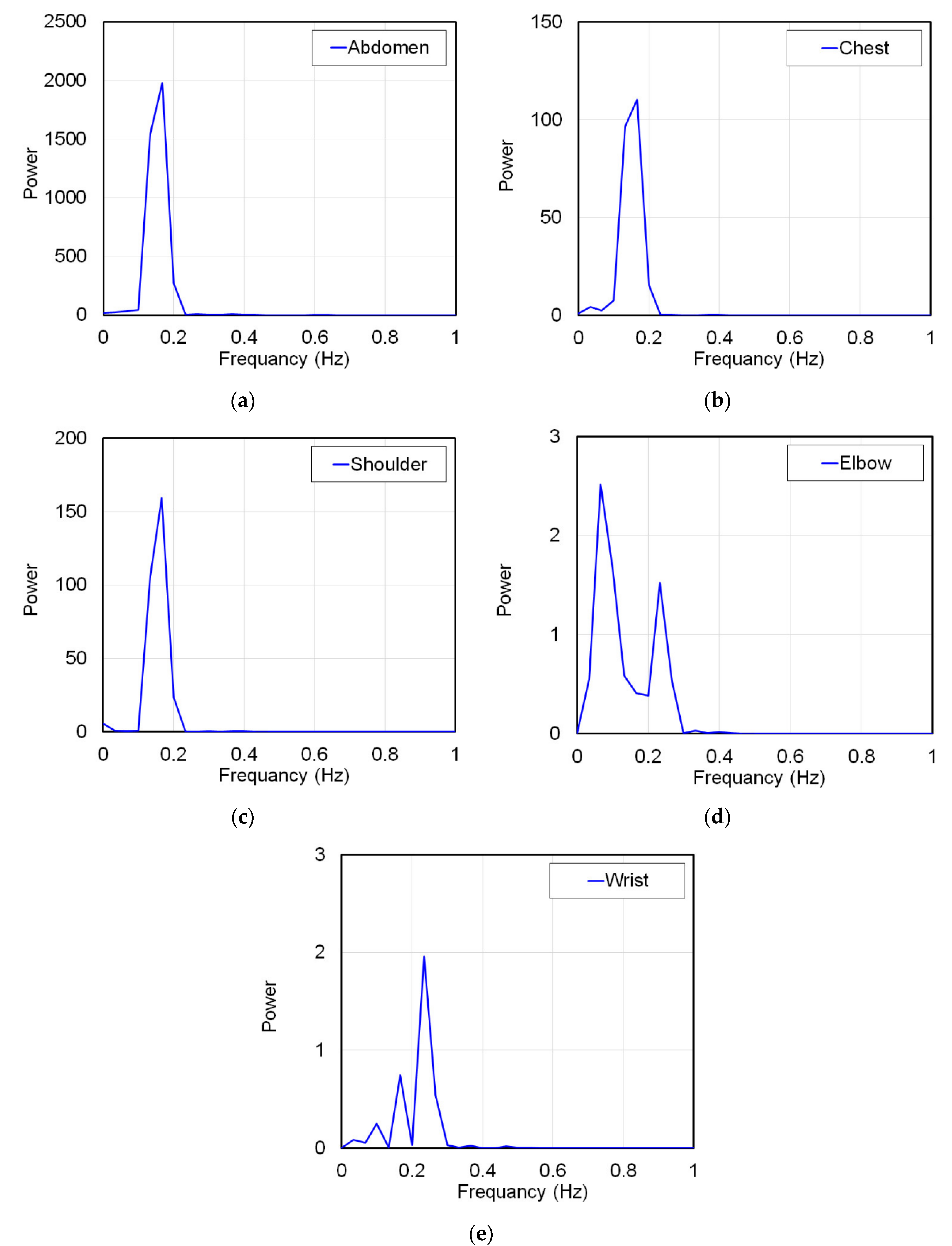
On the other hand, if the ratio of the longest amplitude value to the abdomen measured at each part was larger than the ratio of the longest amplitude value during breathing and apnea, respiratory strain was not measured. In this case, this ratio indicates that only strains smaller than the amplitude value of strains unrelated to respiration measured during apnea are measured. Therefore, as a matter of course, the strain due to respiration cannot be measured by the FBG sensor. To prove this, the signal waveforms measured at each part were frequency-analyzed by Fourier transform. The subject was breathing in a 6 s cycle, and the breathing frequency was 0.167 Hz. Other frequencies were not respiratory strain. Therefore, if the frequency analysis shows a frequency other than 0.167 Hz, it means that the strain due to respiration could not be measured. From the above all results, the propagation range of strain due to respiration to each living body part was verified.
4. Conclusions
In this study, the strain on a living body caused by respiration was measured using an FBG sensor, and the measured signal waveform was verified. Additionally, respiratory strain was measured in the abdomen, chest, shoulder, elbow, and wrist, and the propagation range of this strain was verified. As a result, it was shown that the waveforms measured during breathing and apnea were significantly different. The respiratory cycle measured by the respiratory sensor and the peak interval time of the FBG sensor measurement signal waveform almost matched. Therefore, it was confirmed that the FBG sensor could accurately measure the respiratory strain. This strain signal was measured in the abdomen, chest, and shoulder, and the amplitude of the strain signal decreased with distance from the abdomen. This indicates that the respiratory strain propagated to the shoulder. Thus, the respiratory strain could be measured at any part of the trunk. On the other hand, respiratory strain was not measured in the elbows or wrists. Therefore, it was confirmed that the respiratory strain did not propagate to the peripheral parts of the body. From these results, we showed that respiratory strain propagates throughout the trunk, but not in the peripheral part.
Research on a sensor that can measure pulse rate and respiratory rate at the same time has been reported by Leal-Junior et al. [
31]. We aim to develop a sensor system that can measure blood pressure and blood glucose level at the same time in addition to the simultaneous measurement of these two parameters. For that purpose, it is important that the strain due to breathing and the strain due to pulsation do not affect each other. In the signal that measures the pulsation strain, the signal due to respiratory strain is noise. Therefore, it affects the signal waveform to be analyzed, and the measurement accuracy for calculating blood pressure and blood glucose level decreases. In this paper, it was shown that respiratory strain did not propagate to the wrist, which measures pulsation strain. This is very important in our sensor system development. The developed FBG interrogator has two channels, it is possible to install two FBG sensors on the living body as shown in
Figure 6d. The FBG sensor-1 is installed on the chest or abdomen as in this paper to measure respiratory strain. The respiratory rate can be calculated from this measurement signal. The FBG sensor-2 is installed at the pulsation point of the wrist and measures the pulsation strain. The pulse rate can be calculated from the average peak interval time of this measurement signal. A calibration curve has already been constructed focusing on the lengths of the top and bottom peaks (vertical axis direction) of the measurement signal, and blood pressure can be calculated from this calibration curve. The blood glucose level is also calculated from the calibration curve constructed by focusing on the measurement signal waveform. Pulse rate, blood pressure and blood glucose level can be calculated from one and the same signal, only the calculation method is different. As described above, the strain due to respiration and pulsation is measured by the two channel FBG interrogator, and multiple vital sign parameters, such as respiratory rate, pulse rate, blood pressure and blood glucose level can be calculated simultaneously from these signals.
Currently, many vital sign sensors are sold, but surprisingly few sensors can measure pulse rate and respiratory rate simultaneously. Electronic blood pressure monitors can measure pulse rate at the same time, but few can measure respiratory rate. However, in clinical settings, multiple sensors are required to simultaneously measure pulse rate, blood pressure, and respiratory rate. FBG sensors have the potential to overcome these problems. A wearable FBG interrogator was developed and can now be used as a wearable multi-vital sign sensor [
30]. We expect this report to be an important step towards realizing the development of these sensors.