Biomedical Signal Acquisition Using Sensors under the Paradigm of Parallel Computing
Abstract
1. Introduction
2. Materials and Methods
2.1. Theoretical Basis
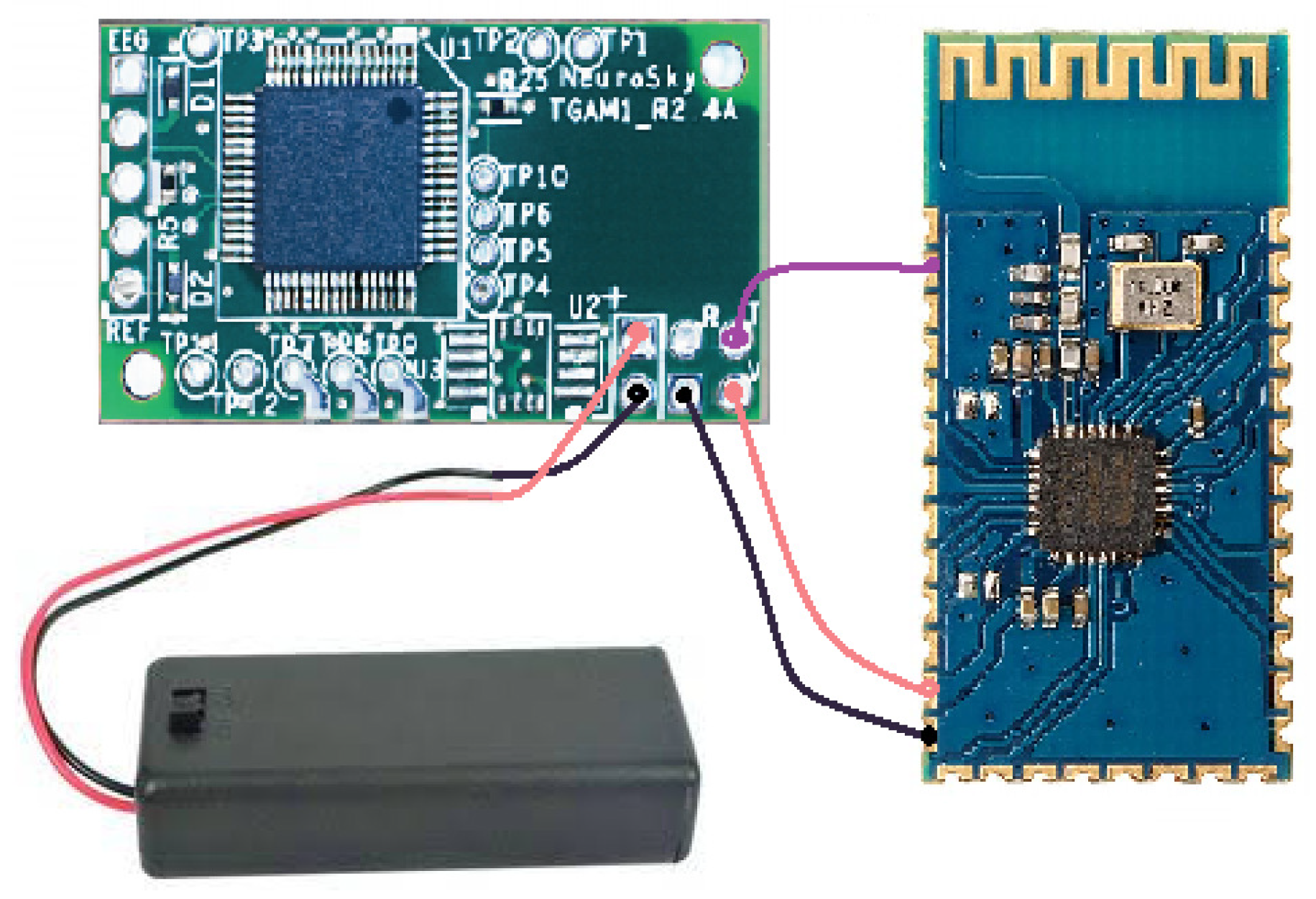
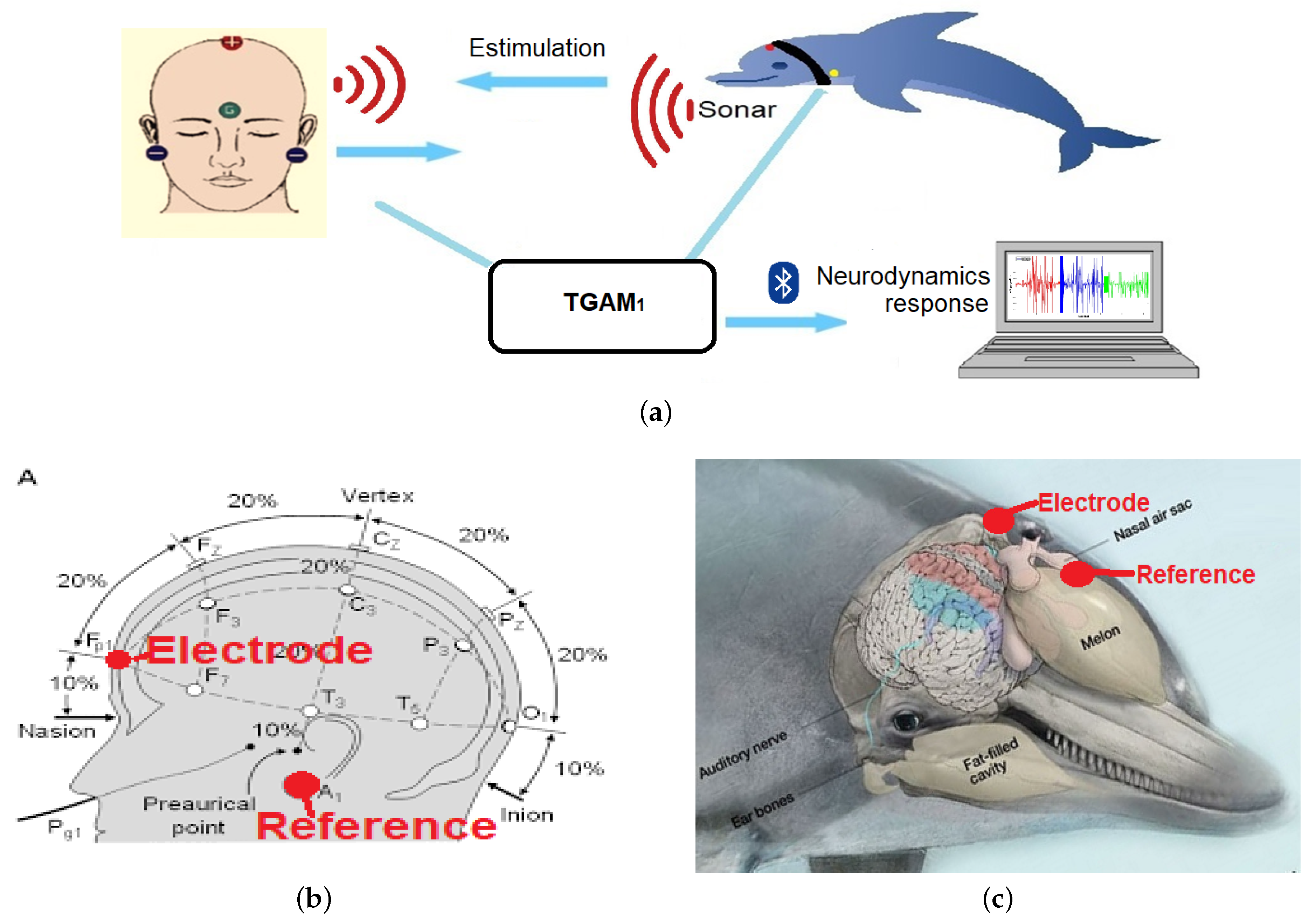
2.1.1. Electroencephalographic System: TGAM1
- (1)
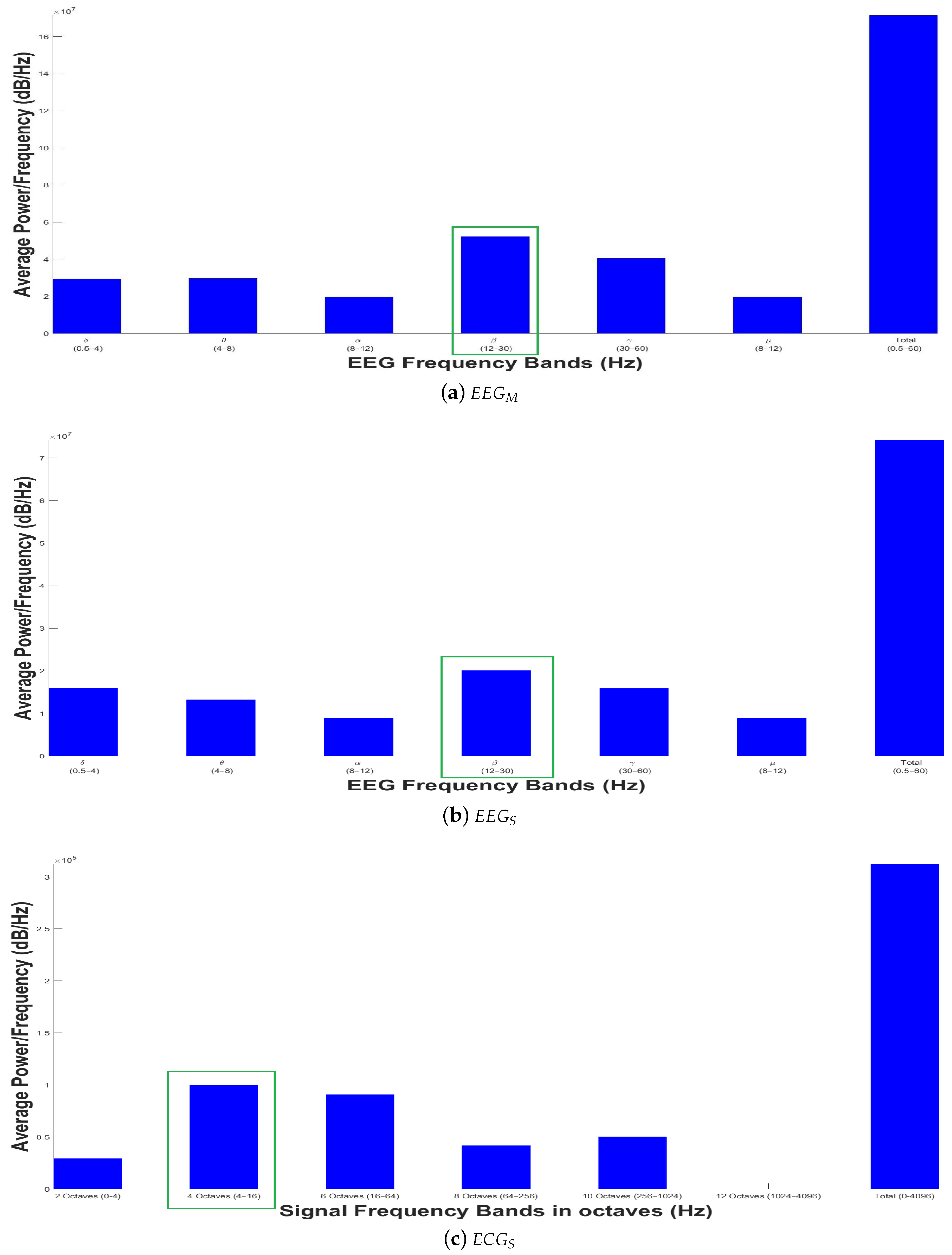
- Delta (): They oscillate from ½ to 4 Hz, and are generated when the human is in the sleep stage.
- (2)
- Theta (): They oscillate between 4 and 8 Hz, and appear during meditation, tension or frustration.
- (3)
- Alpha (): They are found from 8 to 12 Hz, and are related to a mental state of relaxation.
- (4)
- Beta (): They range from 12 to 30 Hz, and are present during concentration state or when a mathematical problem is being solved.
- (5)
- Gamma (): They usually oscillate around 40 Hz, and are related to perception.
2.1.2. Electrocardiographic System: AD8232
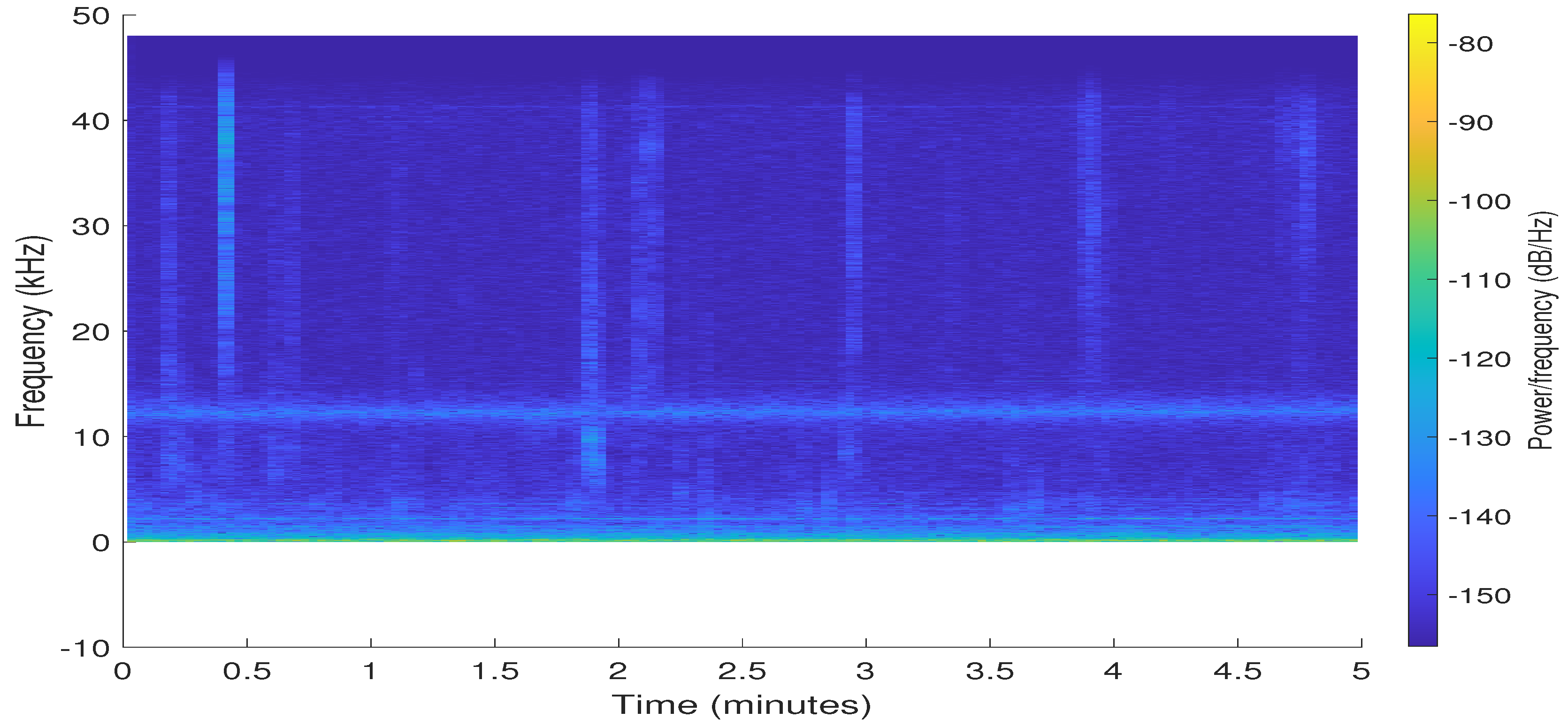
2.1.3. Bioacoustic Wave Acquisition System: Bruel & Kjær 8103
2.1.4. Image Acquisition System: Logitech C920
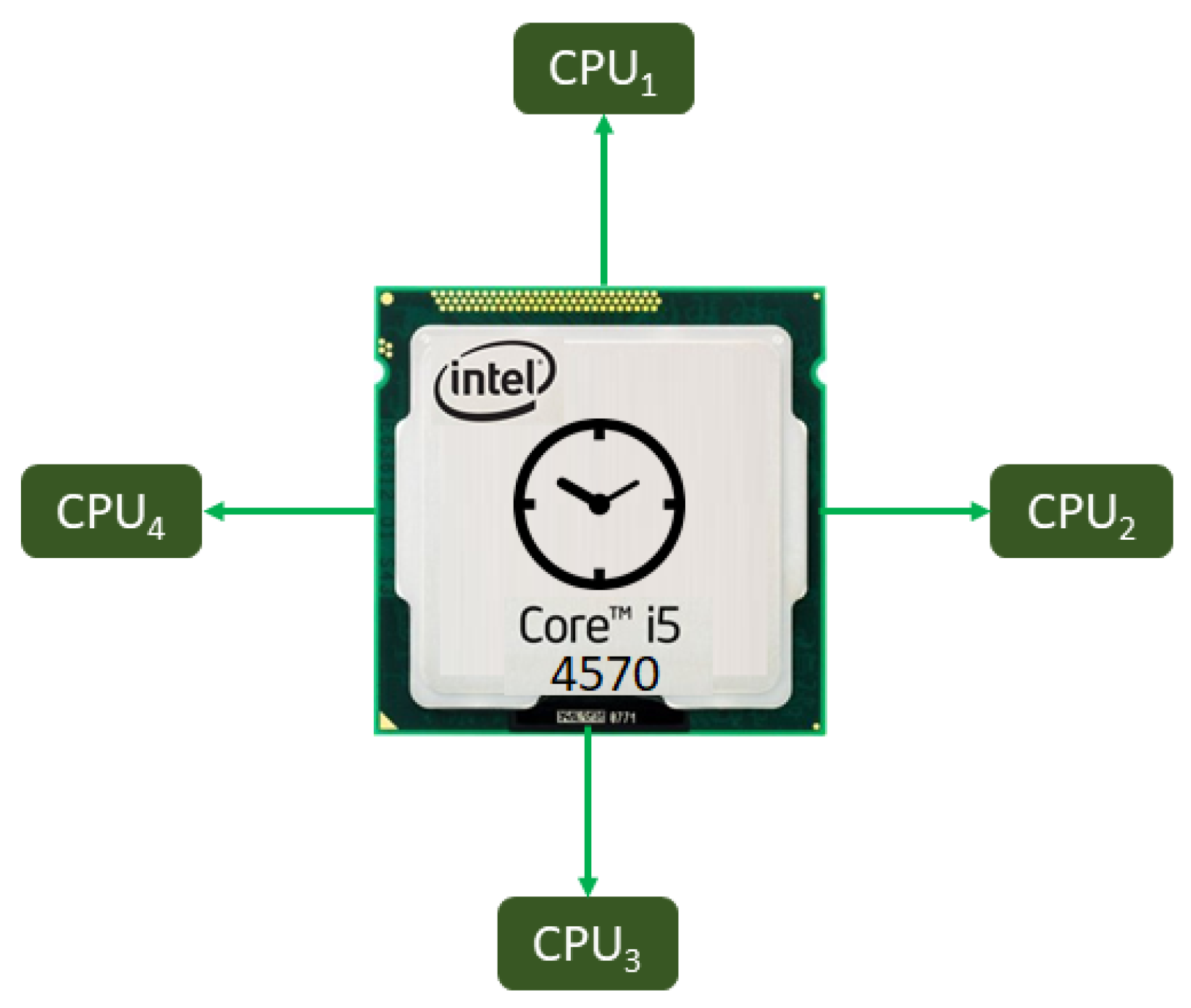
2.1.5. Parallel-Computing System: MatLab R2019b
- Applications with repetitive code segments and loops. Each iteration is evaluated separately in a parallel loop with the only restriction that these repetitions must be independent of each other.
- Programs with a series of tasks that do not depend on each other. A parallel loop can also be implemented.
- Evaluation of the same code on different sets of data at the same time. To do this, a set of workers is used for working at the same time with the same code, but with different data.
- Information is too large to be stored in the computer or server memory. Therefore, it must be distributed among different workers in such a way that each one works with a part of the data.
- Performance improvement if the code runs in parallel or on a GPU.
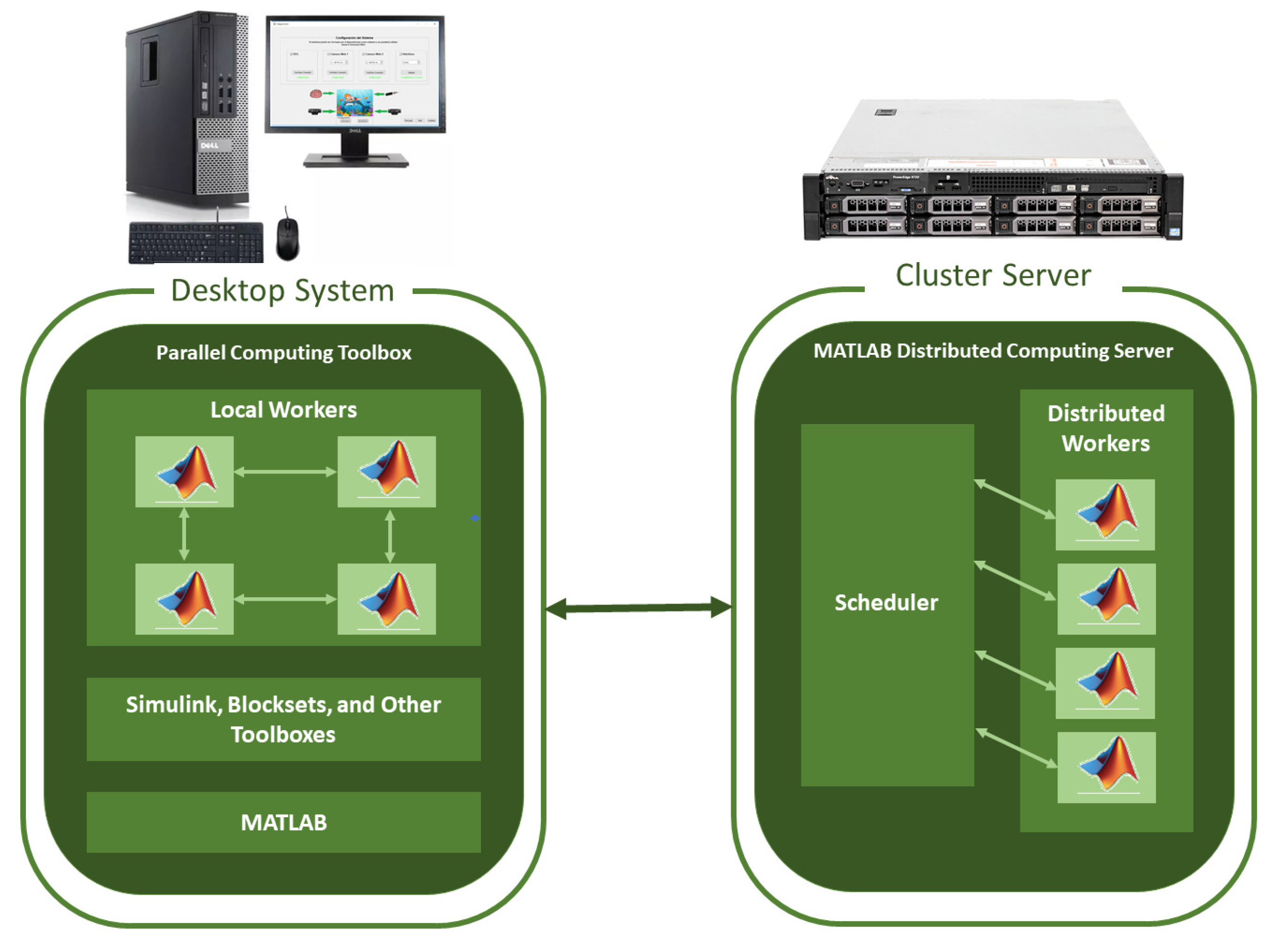
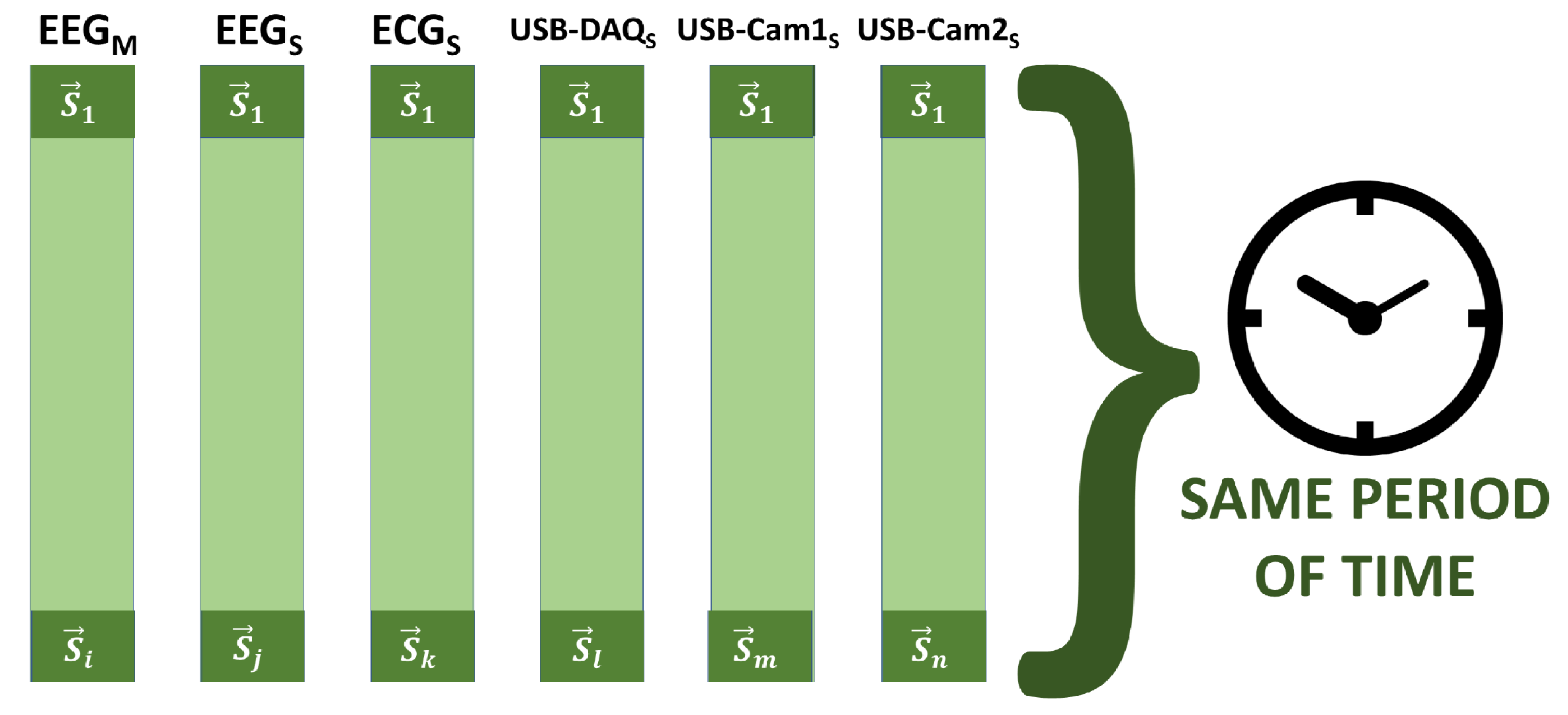
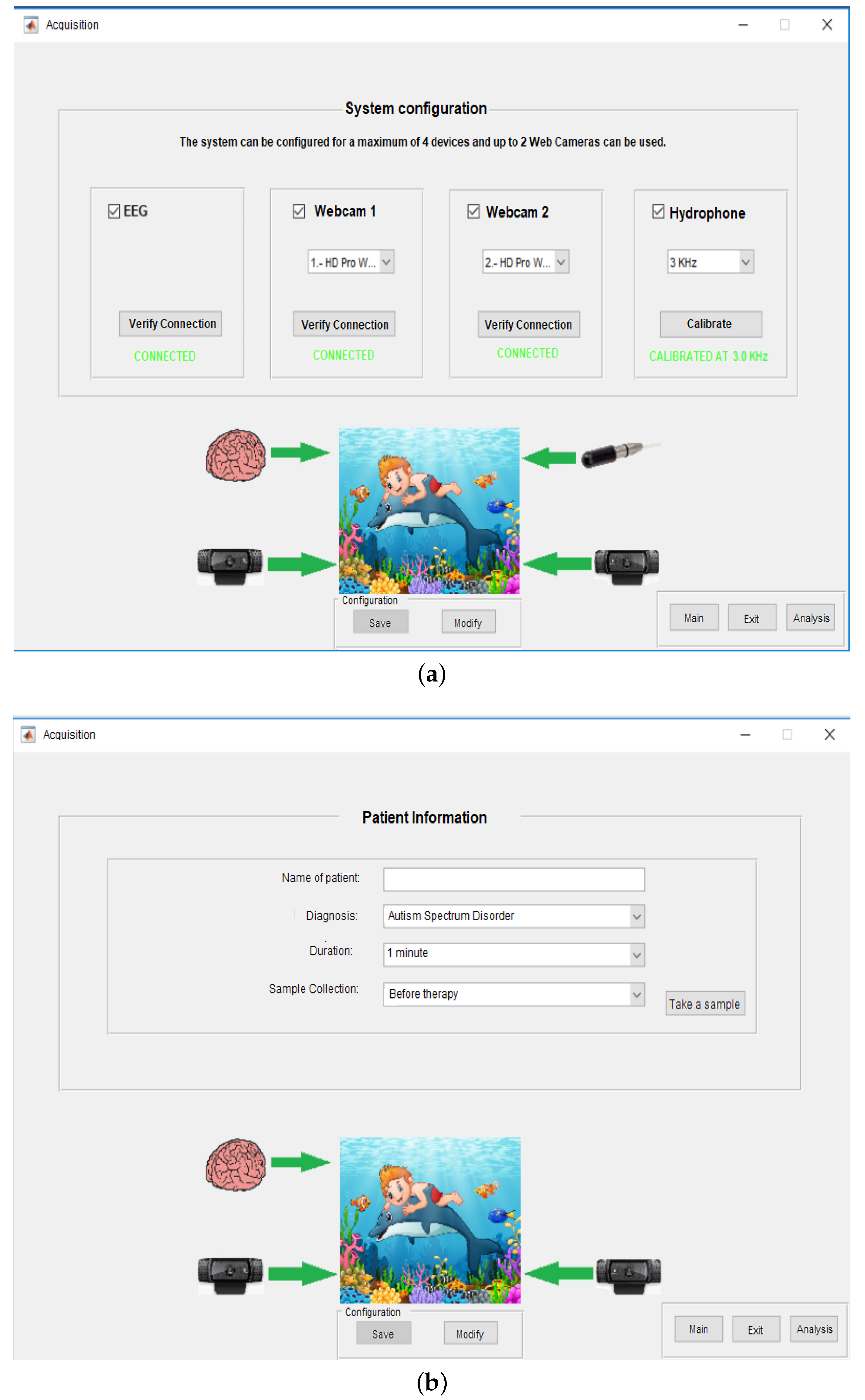
2.2. System for Monitoring Biomedical Signals Using Parallel Computing
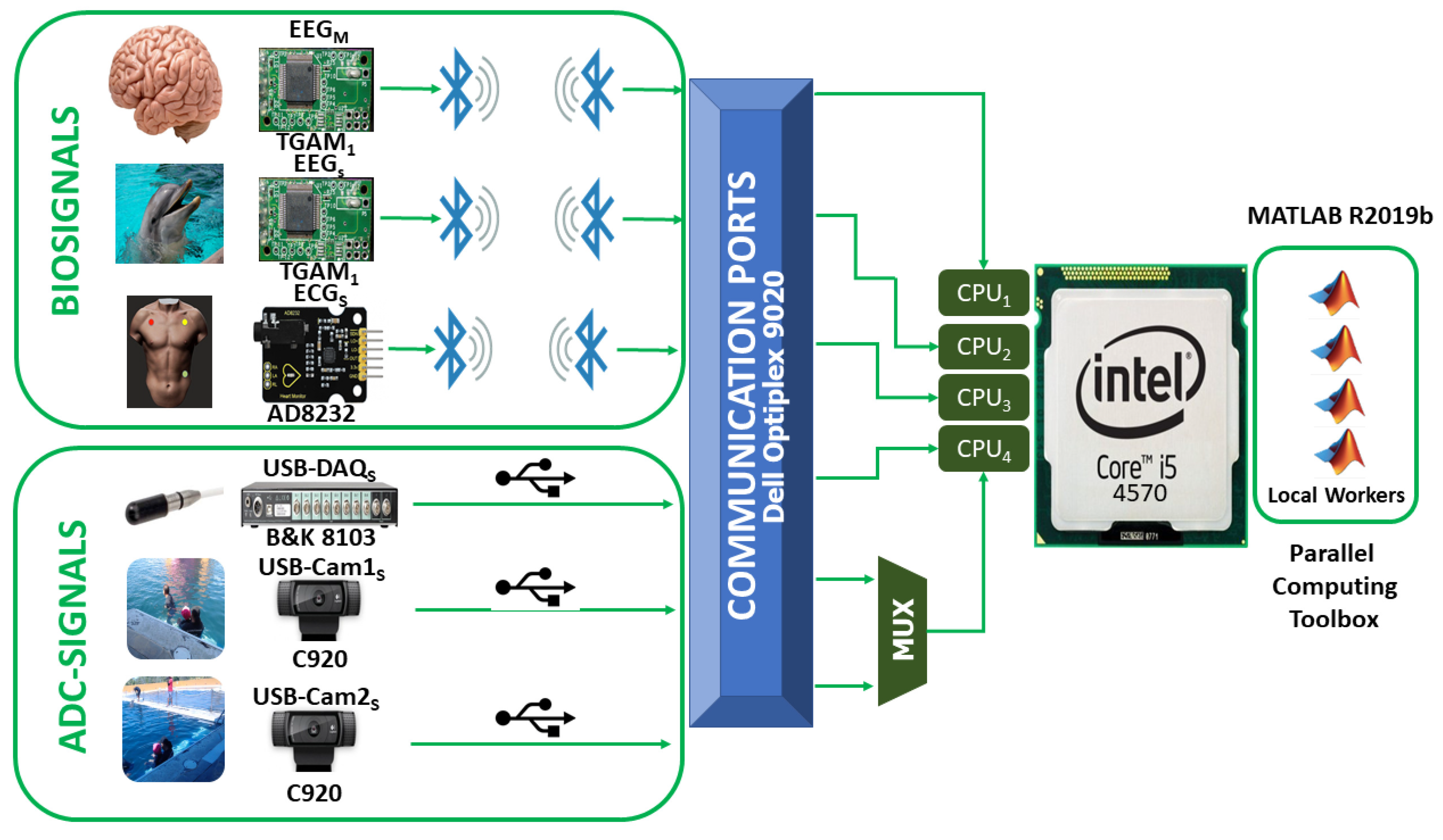
2.2.1. Architecture
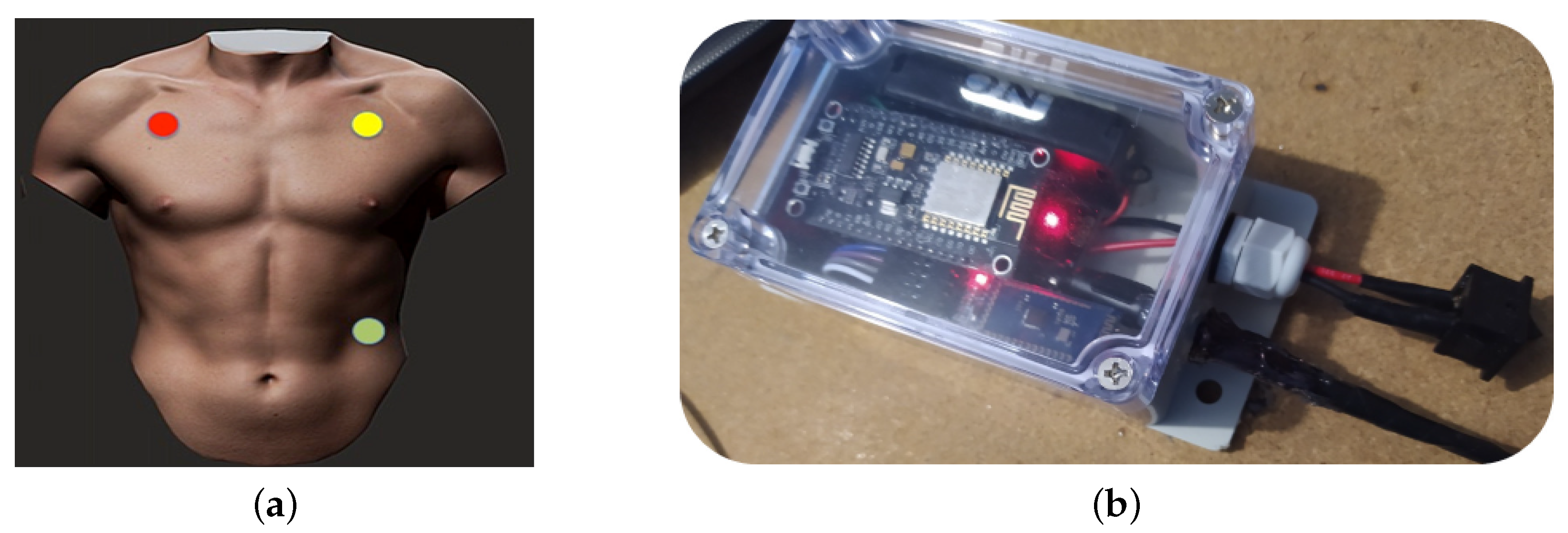
- EEG electrode attached to a patient with neurodevelopmental problems.
- EEG electrode attached to a bottlenose dolphin (tursiops truncatus).
- ECG electrode attached to a patient with neurodevelopmental problems.
- USB-DAQ Bruel & Kjær Type 3760 with an 8103-hydrophone (introduced into a tank with salt water).
- Logitech C920 USB Webcam for a front view of the DAT.
- Logitech C920 USB Webcam for a side view of the DAT.
2.2.2. Design of Biomedical Signal Interfaces
- Salinity: 18 to 36 parts per thousand.
- Hydrogen Potential (pH): between 6 and 8 units.
- Temperature: from 5 to C.
- Pressure: 1 Atmosphere (ATM).
2.2.3. ADC-SIGNALS Configuration

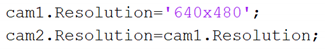
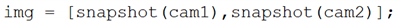
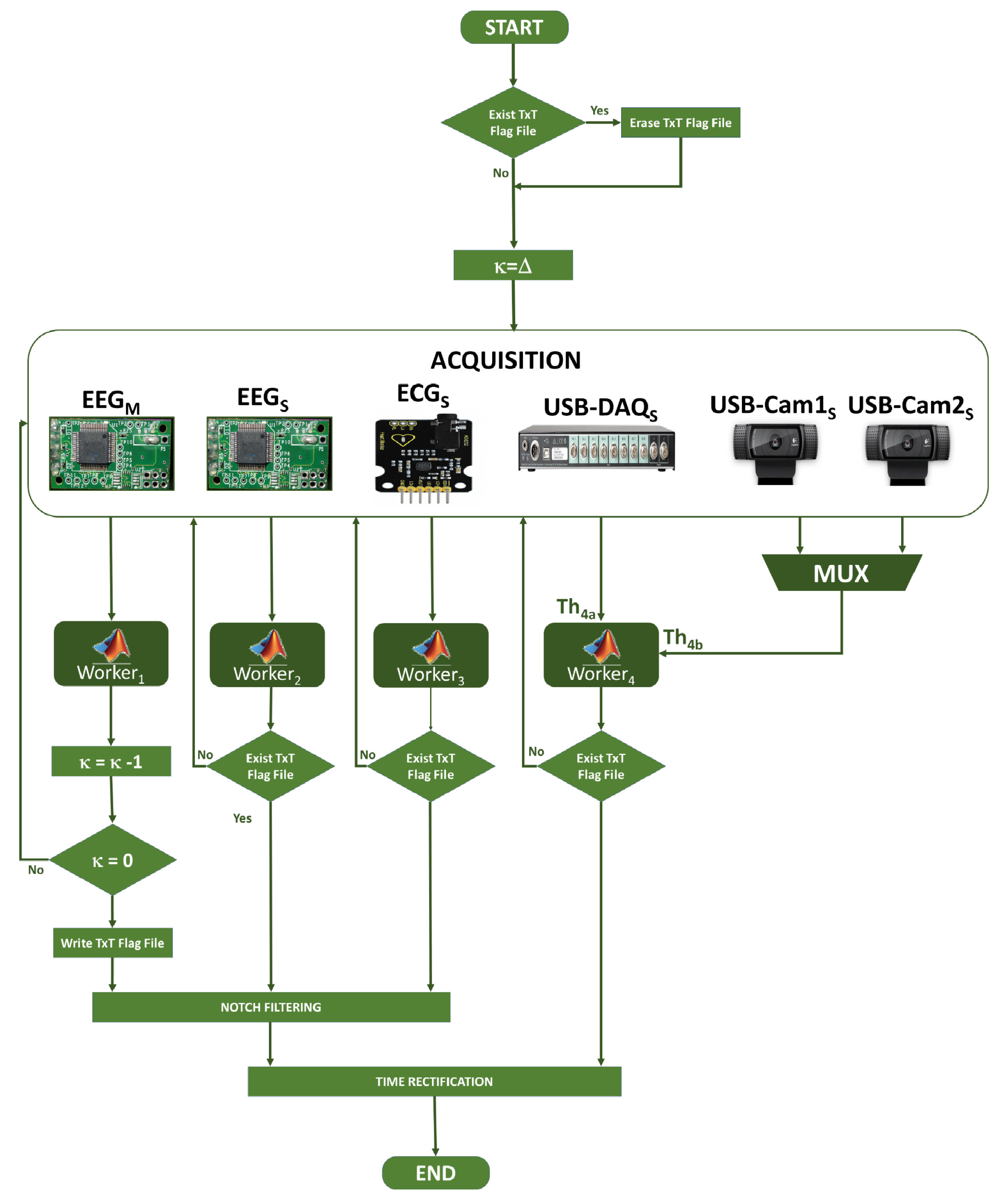
2.2.4. General Algorithm




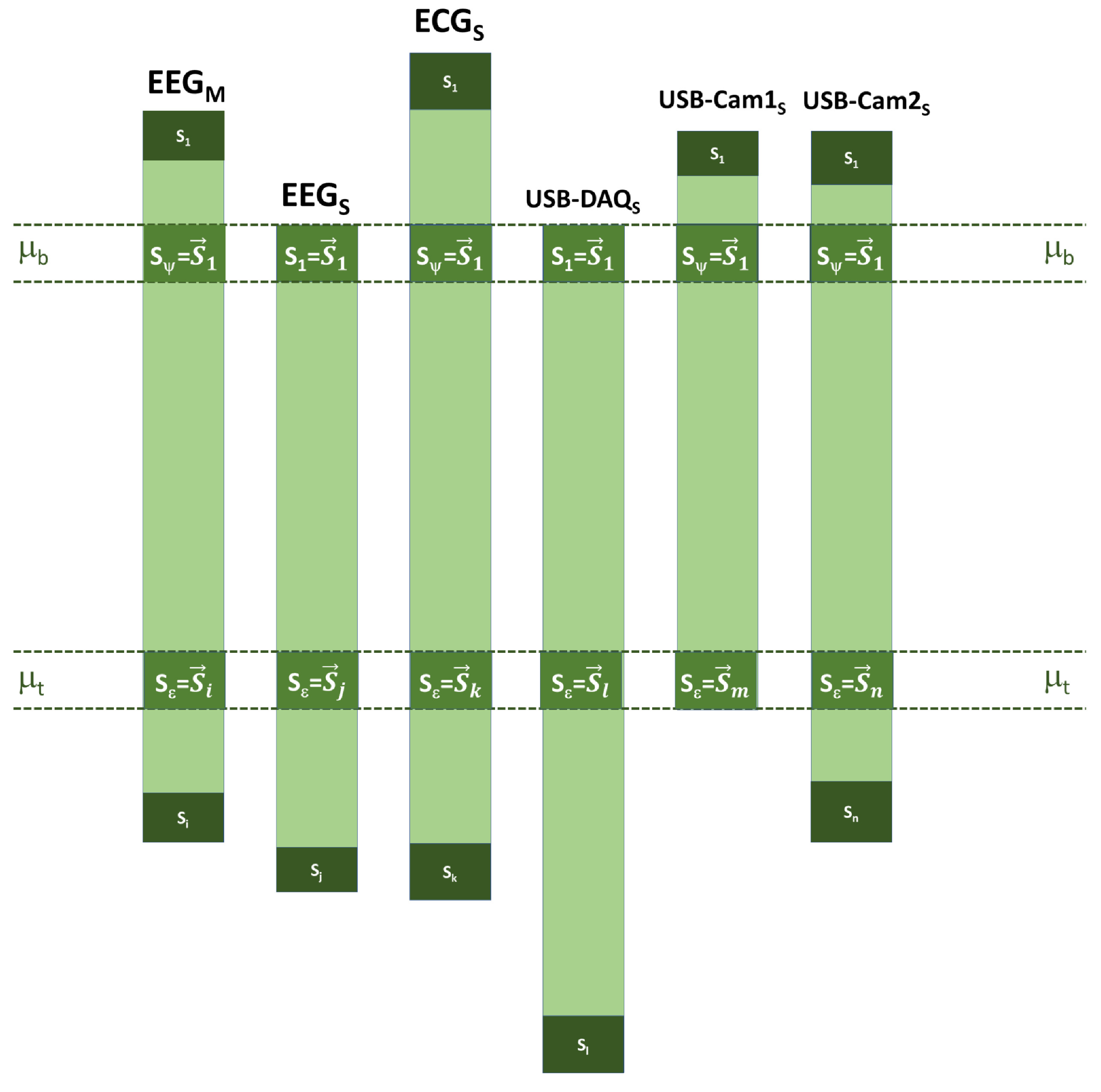
2.2.5. Synchronization
3. Results
3.1. Integrated Development Environment
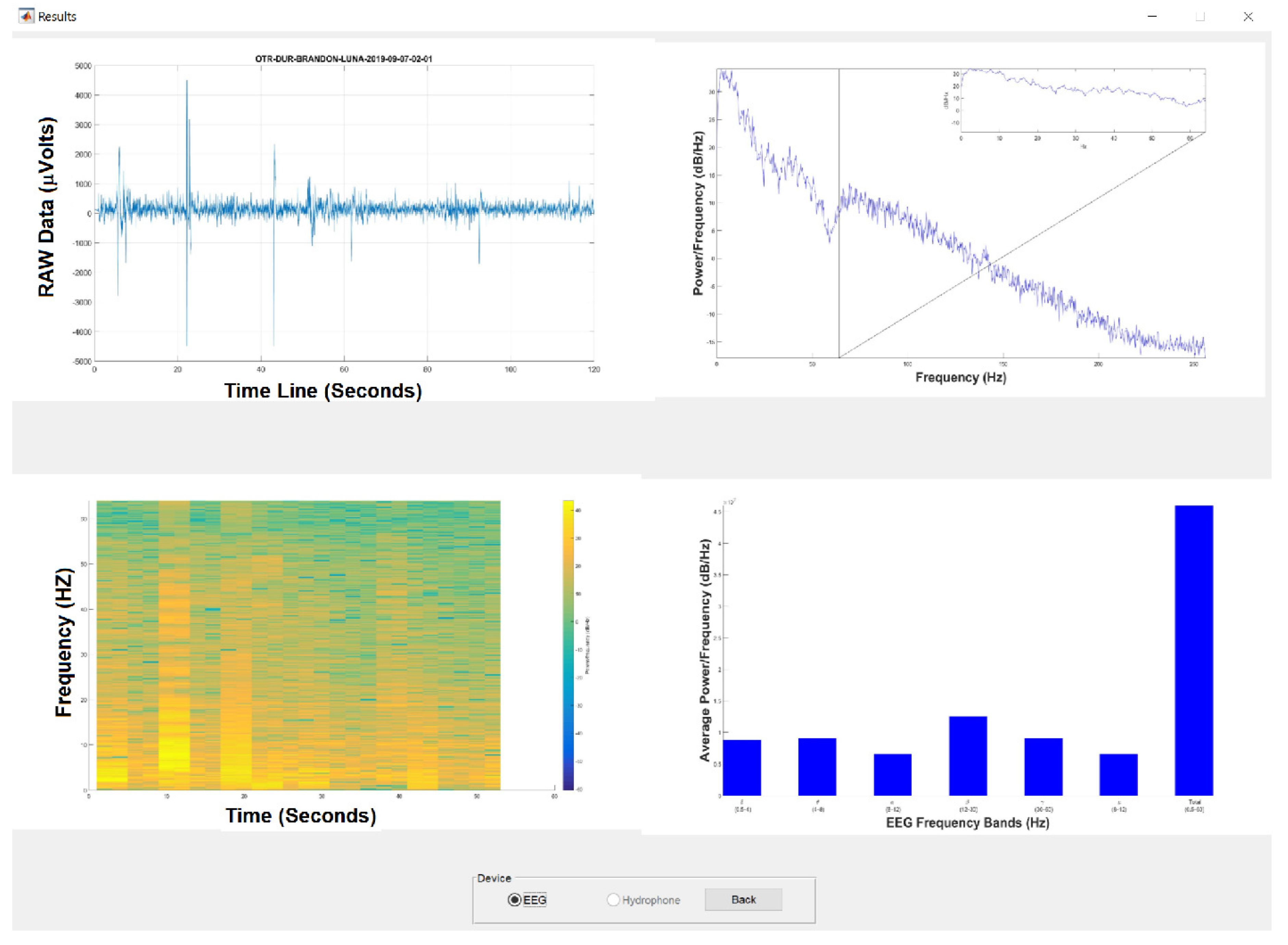
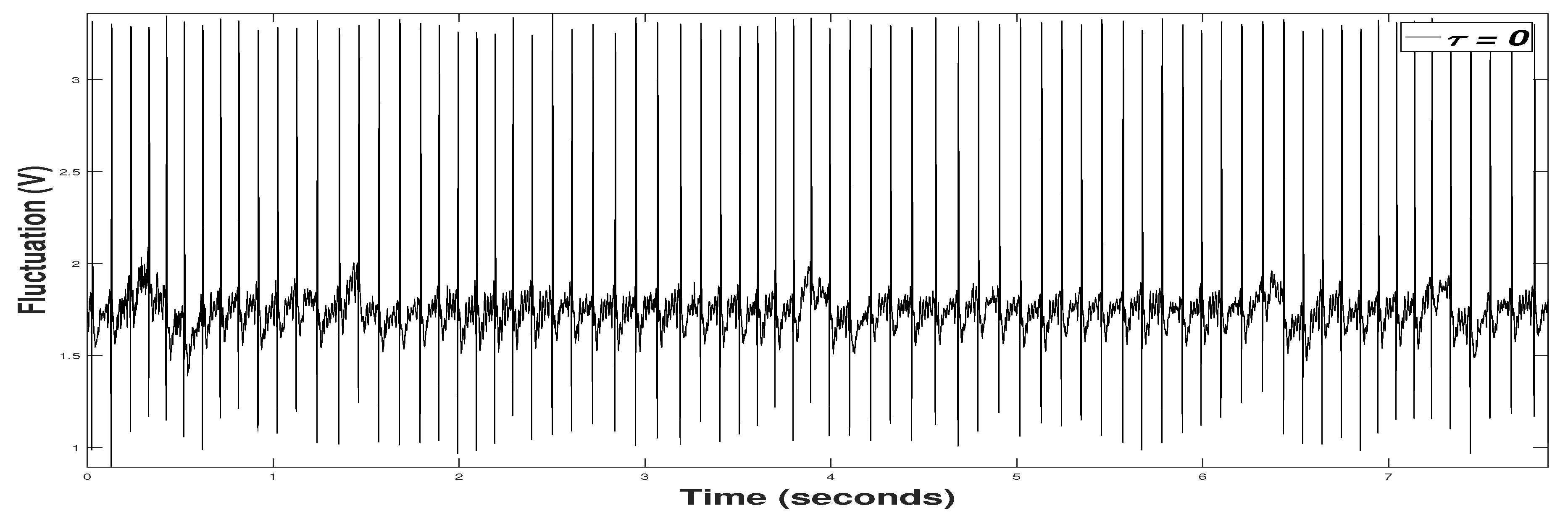
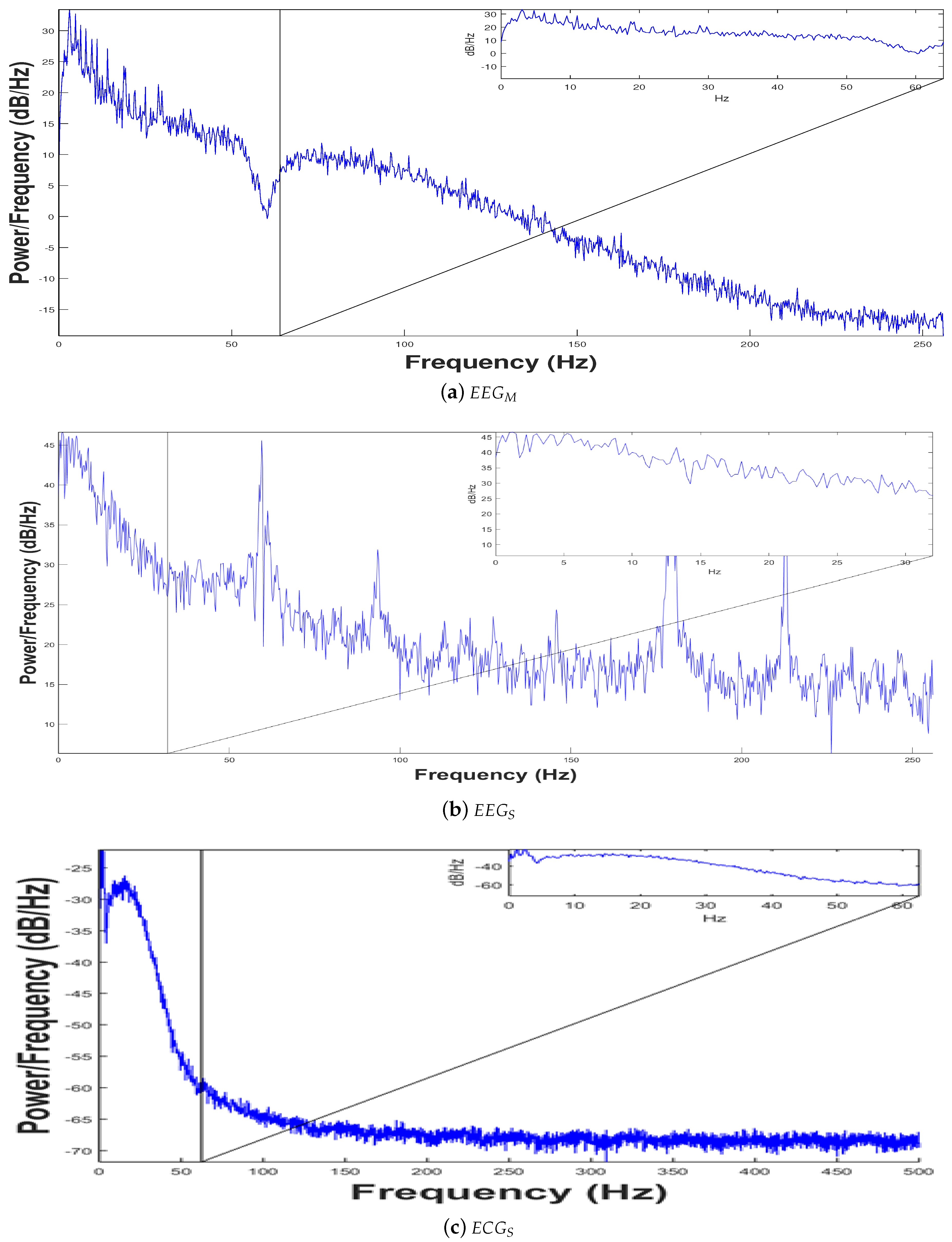
3.2. Signal Preprocessing
3.3. Experimental Results
- Values equal to 200 indicate no connection, so the data recorded by the sensor is noise.
- Values < 51 point out that the recorded data are consistent.
- Values equal to 0 mean an optimal connection.
- baudrate is the speed that is transmitting, 115,200 baud.
- bytesize is the size of data, 8 bits.
- parity is the parity of an error checking way, used in serial communication.
- stopbits are the stop bits to signal the end of communication.
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Vashisht, M.; Bhatia, M. Role of Mathematics in Image Processing. In Proceedings of the 2019 International Conference on Machine Learning, Big Data, Cloud and Parallel Computing (COMITCon), Faridabad, India, 14–16 February 2019; pp. 538–543. [Google Scholar]
- Pereira, M.; Eduardo de Morais, C.; Matsumoto, L. IDM—A New Parallel Methodology to Calculate the Determinant of Matrices of the Order n, with Computational Complexity O(n). IEEE Lat. Am. Trans. 2012, 10, 1357–1363. [Google Scholar] [CrossRef]
- Cruz, M.A.; Ortigoza, R.S.; Sánchez, C.M.; Guzmán, V.M.H.; Gutiérrez, J.S.; Lozada, J.C.H. Parallel Computing as a Tool for Tuning the Gains of Automatic Control Laws. IEEE Lat. Am. Trans. 2017, 15, 1189–1196. [Google Scholar] [CrossRef]
- Qu, H.; Zhang, J.; Lin, Z.; Chen, H. Parallel Acceleration of SAM Algorithm and Performance Analysis. IEEE J. Sel. Top. Appl. Earth Obs. Remote Sens. 2013, 6, 1172–1178. [Google Scholar] [CrossRef]
- Choy, R.; Edelman, A. Parallel MATLAB: Doing it Right. Proc. IEEE 2005, 93, 331–341. [Google Scholar] [CrossRef]
- The MathWorks Inc. Parallel Computing Toolbox; The MathWorks Inc.: Natick, MA, USA, 2020. [Google Scholar]
- Yao, Y.; Chang, J.; Xia, K. A Case of Parallel EEG Data Processing upon a Beowulf Cluster. In Proceedings of the 2009 15th International Conference on Parallel and Distributed Systems, Shenzhen, China, 8–11 December 2009; pp. 799–803. [Google Scholar]
- Gow, D.W., Jr.; Keller, C.J.; Eskandar, E.; Meng, N.; Cash, S.S. Parallel versus serial processing dependencies in the perisylvian speech network: A Granger analysis of intracranial EEG data. Brain Lang. 2009, 110, 43–48. [Google Scholar] [CrossRef]
- Wang, L.; Chen, D.; Ranjan, R.; Khan, S.U.; KolOdziej, J.; Wang, J. Parallel Processing of Massive EEG Data with MapReduce. In Proceedings of the 2012 IEEE 18th International Conference on Parallel and Distributed Systems, Singapore, 17–19 December 2012; pp. 164–171. [Google Scholar]
- Zhang, D.; Yao, L. Cascade and Parallel Convolutional Recurrent Neural Networks on EEG-Based Intention Recognition for Brain Computer Interface; Association for the Advancement of Artificial Intelligence: New Orleans, LA, USA, 2017. [Google Scholar]
- Maksimenko, V.A.; Grubov, V.V.; Kirsanov, D.V. Use of parallel computing for analyzing big data in EEG studies of ambiguous perception. In Dynamics and Fluctuations in Biomedical Photonics XV; Tuchin, V.V., Larin, K.V., Leahy, M.J., Wang, R.K., Eds.; International Society for Optics and Photonics, SPIE: Bellingham, WA, USA, 2018; Volume 10493, pp. 159–166. [Google Scholar] [CrossRef]
- Lau-Zhu, A.; Lau, M.P.; McLoughlin, G. Mobile EEG in research on neurodevelopmental disorders: Opportunities and challenges. Dev. Cogn. Neurosci. 2019, 36, 100635. [Google Scholar] [CrossRef] [PubMed]
- Alsuradi, H.; Park, W.; Eid, M. EEG-Based Neurohaptics Research: A Literature Review. IEEE Access 2020, 8, 49313–49328. [Google Scholar] [CrossRef]
- Qi, S.; Yishan, W. Detection and Analysis of ECG Based on Parallel Computing Technology. In Proceedings of the 2009 International Forum on Computer Science-Technology and Applications, Chongqing, China, 25–27 December 2009; Volume 1, pp. 106–109. [Google Scholar]
- Zhang, M.; Li, R.; Lu, Z.; Zou, C. An ECG Parallel Scheduling Algorithm for the Distributed Systems. In Proceedings of the 2006 Seventh International Conference on Parallel and Distributed Computing, Applications and Technologies (PDCAT’06), Taipei, Taiwan, 4–7 December 2006; pp. 484–487. [Google Scholar]
- Munoz, H.; Dascalu, S.; Wu, R.; Barford, L.; Harris, F. Image Processing Using Multiple GPUs on Webcam Image Streams. In Proceedings of the 16th International Conference on Information Technology-New Generations (ITNG 2019), Las Vegas, NV, USA, 1–3 April 2019; Springe: Cham, Switzerland, 2019; pp. 325–331. [Google Scholar] [CrossRef]
- Lee, S.; Yang, C. A Real Time Object Recognition and Counting System for Smart Industrial Camera Sensor. IEEE Sens. J. 2017, 17, 2516–2523. [Google Scholar] [CrossRef]
- Hendricks, B.; Keen, E.M.; Wray, J.L.; Alidina, H.M.; McWhinnie, L.; Meuter, H.; Picard, C.R.; Gulliver, T.A. Automated Monitoring and Analysis of Marine Mammal Vocalizations in Coastal Habitats. In Proceedings of the 2018 OCEANS—MTS/IEEE Kobe Techno-Oceans (OTO), Kobe, Japan, 28–31 May 2018; pp. 1–10. [Google Scholar]
- Van Uffelen, L.J.; Roth, E.H.; Howe, B.M.; Oleson, E.M.; Barkley, Y. A Seaglider-Integrated Digital Monitor for Bioacoustic Sensing. IEEE J. Ocean. Eng. 2017, 42, 800–807. [Google Scholar] [CrossRef]
- Collins, T.F.; Wyglinski, A.M. Dataflow in MATLAB: Algorithm Acceleration Through Concurrency. IEEE Access 2017, 5, 2308–2318. [Google Scholar] [CrossRef]
- Keith, D.B.; Hoge, C.C.; Frank, R.M.; Malony, A.D. Parallel ICA methods for EEG neuroimaging. In Proceedings of the 20th IEEE International Parallel Distributed Processing Symposium, Rhodes Island, Greece, 25–29 April 2006; pp. 1–10. [Google Scholar]
- Li, K.G.; Shapiai, M.I.; Adam, A.; Ibrahim, Z. Feature scaling for EEG human concentration using particle swarm optimization. In Proceedings of the 2016 8th International Conference on Information Technology and Electrical Engineering (ICITEE), Yogyakarta, Indonesia, 5–6 October 2016; pp. 1–6. [Google Scholar] [CrossRef]
- Zhang, L.; Lv, Q.; Xu, Y. Single channel brain-computer interface control system based on TGAM module. In Proceedings of the 2017 10th International Congress on Image and Signal Processing, BioMedical Engineering and Informatics (CISP-BMEI), Shanghai, China, 14–16 October 2017; pp. 1–5. [Google Scholar]
- Jinchuang, Z.; Hao, Z.; Wenli, F.; Xingxing, Z. Design and implementation of multi-parameter portable biological information measurement system. In Proceedings of the 2016 Chinese Control and Decision Conference (CCDC), Yinchuan, China, 28–30 May 2016; pp. 3762–3765. [Google Scholar]
- Jimenez, C.O.S.; Mesa, H.G.A.; Rebolledo-Mendez, G.; de Freitas, S. Classification of cognitive states of attention and relaxation using supervised learning algorithms. In Proceedings of the 2011 IEEE International Games Innovation Conference (IGIC), Orange, CA, USA, 2–3 November 2011; pp. 31–34. [Google Scholar] [CrossRef]
- Prasad, A.S.; Kavanashree, N. ECG Monitoring System Using AD8232 Sensor. In Proceedings of the 2019 International Conference on Communication and Electronics Systems (ICCES), Coimbatore, India, 17–19 July 2019; pp. 976–980. [Google Scholar]
- Gifari, M.W.; Zakaria, H.; Mengko, R. Design of ECG Homecare:12-lead ECG acquisition using single channel ECG device developed on AD8232 analog front end. In Proceedings of the 2015 International Conference on Electrical Engineering and Informatics (ICEEI), Bali, Indonesia, 10–11 August 2015; pp. 371–376. [Google Scholar]
- Gong, Z.; Ding, Y. Design and Implementation of Wearable Dynamic Electrocardiograph Real-Time Monitoring Terminal. IEEE Access 2020, 8, 6575–6582. [Google Scholar] [CrossRef]
- Chowdhury, M.H.; Hossain, Q.D.; Saha, P.; Rahaman, M.M. Design, fabrication and performance evaluation of a three electrode ECG recorder. In Proceedings of the 2016 International Conference on Innovations in Science, Engineering and Technology (ICISET), Chittagong, Bangladesh, 28–29 October 2016; pp. 1–4. [Google Scholar]
- Cichoń, A.; Borucki, S. Diagnostics of technical condition on load tap changers by acoustic emission method using different types of measuring transducers. In Proceedings of the 2012 International Conference on High Voltage Engineering and Application, Shanghai, China, 17–20 September 2012; pp. 621–624. [Google Scholar]
- Kuzlu, M.; Dinçer, H.; Öztürk, S. Desing of an underwater communication receiver pre-amplifier [Desing read Design]. In Proceedings of the 2010 IEEE 18th Signal Processing and Communications Applications Conference, Diyarbakir, Turkey, 22–24 April 2010; pp. 37–40. [Google Scholar]
- Feng, Y.; Zhang, H.; Wang, J.; Xu, Y.; Chen, J.; Yang, D.; Zhang, Y.; Chen, C.; Zhang, G.; Wang, J.; et al. Design of a Nonvacuum-Cooling Compact CCD Camera for Scientific Detection. IEEE Trans. Nucl. Sci. 2019, 66, 2286–2292. [Google Scholar] [CrossRef]
- Kublanov, V.; Purtov, K.; Kontorovich, M. Video-based vital sign monitoring system of patients in intensive care unit. In Proceedings of the 2017 International Multi-Conference on Engineering, Computer and Information Sciences (SIBIRCON), Novosibirsk, Russia, 18–24 September 2017; pp. 556–560. [Google Scholar]
- Afrisal, H.; Faris, M.; Utomo P., G.; Grezelda, L.; Soesanti, I.; Andri, F.M. Portable smart sorting and grading machine for fruits using computer vision. In Proceedings of the 2013 International Conference on Computer, Control, Informatics and Its Applications (IC3INA), Jakarta, Indonesia, 19–21 November 2013; pp. 71–75. [Google Scholar]
- Cresson, R.; Hautreux, G. A Generic Framework for the Development of Geospatial Processing Pipelines on Clusters. IEEE Geosci. Remote Sens. Lett. 2016, 13, 1706–1710. [Google Scholar] [CrossRef]
- Villaverde, A.F.; Becker, K.; Banga, J.R. PREMER: A Tool to Infer Biological Networks. IEEE/ACM Trans. Comput. Biol. Bioinform. 2018, 15, 1193–1202. [Google Scholar] [CrossRef] [PubMed]
- Jian, B.; Chen, C.; Lin, C.; Yau, H. Optimization Method of IR Thermography Facial Image Registration. IEEE Access 2019, 7, 93501–93510. [Google Scholar] [CrossRef]
- Moreno Escobar, J.J.; Morales Matamoros, O.; Tejeida Padilla, R.; Lina Reyes, I.; Chanona Hernández, L.; Ramírez Gutiérrez, A.G. Brain-Inspired Healthcare Smart System Based on Perception-Action Cycle. Appl. Sci. 2020, 10, 3532. [Google Scholar] [CrossRef]
- Morales Matamoros, O.; Moreno Escobar, J.J.; Tejeida Padilla, R.; Lina Reyes, I. Neurodynamics of Patients during a Dolphin-Assisted Therapy by Means of a Fractal Intraneural Analysis. Brain Sci. 2020, 10, 403. [Google Scholar] [CrossRef] [PubMed]
- Liggins, M.E.; Hall, D.L.; Llinas, J. (Eds.) Handbook of Multisensor Data Fusion: Theory and Practice, 2nd ed.; The Electrical Engineering and Applied Signal Processing Series; CRC Press: Boca Raton, FL, USA, 2009. [Google Scholar]
- Blasch, E.; Kadar, I.; Salerno, J.; Kokar, M.M.; Das, S.; Powell, G.M.; Corkill, D.D.; Ruspini, E.H. Issues and challenges of knowledge representation and reasoning methods in situation assessment (Level 2 Fusion). In Signal Processing, Sensor Fusion, and Target Recognition XV; International Society for Optics and Photonics: Bellingham, WA, USA, 2006; p. 623510. [Google Scholar] [CrossRef]
- Bosch Roig, I. Algoritmos de detección distribuida en sistemas monosensor. Riunet 2008. [Google Scholar] [CrossRef]
- Sevier, S.; Tekeoglu, A. Analyzing the Security of Bluetooth Low Energy. In Proceedings of the 2019 International Conference on Electronics, Information, and Communication (ICEIC), Auckland, New Zealand, 22–25 January 2019; pp. 1–5. [Google Scholar]
- Castro, P.; Huber, M. Marine Biology; McGraw-Hill: New York, NY, USA, 2016. [Google Scholar]
- Mandelbrot, B. Multifractals and 1/f Noise: Wild Self-Affinity in Physics; Springer: Berlin, Germany, 1999. [Google Scholar]
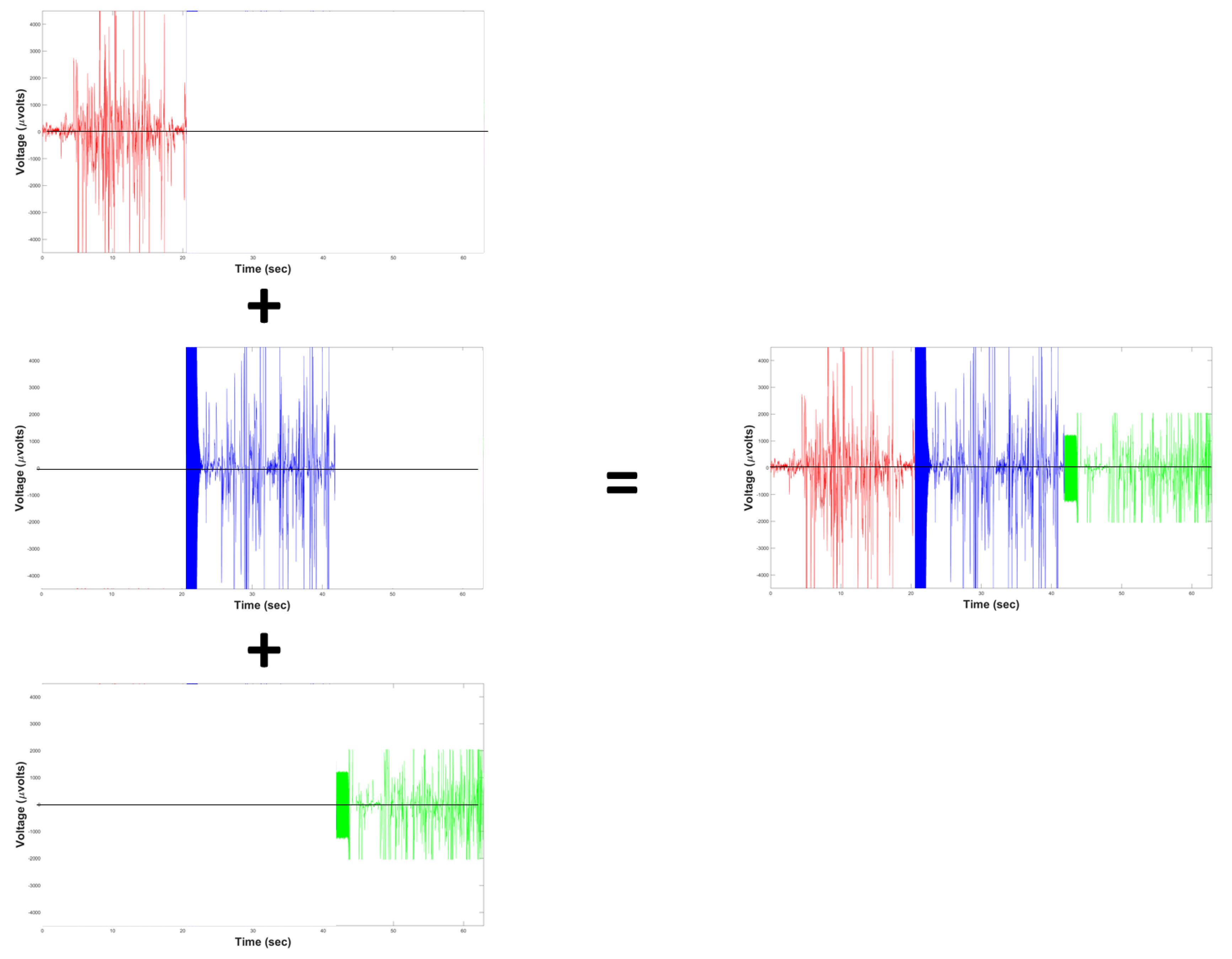













| Feature | [9] | [10] | [14] | [15] | [16] | [17] | [18] | [19] | Proposal |
|---|---|---|---|---|---|---|---|---|---|
| Article of | Analysis | Analysis | Analysis | Design | Design | Analysis and Design | Analysis | Analysis and Design | Analysis and Design |
| Signal Acquisition | No | Yes | No | No | Yes | Yes | Yes | Yes | Yes |
| Signal Processing | Yes | Yes | Yes | No | No | Yes | Yes | Yes | Yes |
| Sensors | EEG | EEG | ECG | ECG | Camera | Camera | Bioacoustic, GPS | Bioacoustic, GPS | EEG, ECG, Webcam, Bioacoustic |
| Number of channels | 6 | 14 | 2 | 1 | 1 | 1 | 4 | 3 | 6 |
| Epoch Synchronization | No | No | No | No | No | No | No | No | Yes |
| Analysis Tools | Hilbert-Huang spectral entropy | Confusion matrix | Magnitud Response | Demonstration | Speedup Factor | None | Confusion matrix | PowerSpectrum Density | PowerSpectrum Density and Fractal Geometry |
| Diagnosis | No | No | Yes | No | No | No | No | Yes | No |
| Experimentation | Indoors | Indoors | Indoors | Indoors | Outdoors | Outdoors | Outdoors | Outdoors | Indoors and Outdoors |
| Parallel Computing | Local | Local | Distributed | Distributed | Local | Local | Distributed | Local | Local |
| Parallel-Computing Usage | Processing | Acquisition | Processing | Processing | Acquisition | Acquisition and Processing | Acquisition | Acquisition and Processing | Acquisition and Processing |
| Artificial Intelligence Tool | None | Neural Networks | None | None | None | SURF key points | Signal Classification | None | None |
| Programming language | Python | C/C++ | Matlab | None | C/C++ | C/C++ | Python | Matlab | Matlab |
| CPU Cores | 1 | 1 | 3 | 2 | 2 | 2 | 2 | 2 | 4 |
| Self-Affine Analysis | Power Spectrum Density | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| Patient | BEFORE | During DAT | AFTER | BEFORE | During DAT | AFTER | |||
| H | CrossOver | H | CrossOver | H | CrossOver | ||||
| 1 | 0.4405 | 119 | 0.4572 | 99 | 0.4235 | 136 | 1.74 | 8.30 | 1.41 |
| 2 | 0.1978 | 33 | 0.2178 | 127 | 0.2151 | 185 | 1.53 | 7.32 | 2.35 |
| Average | 0.3192 | 76 | 0.3375 | 113 | 0.3193 | 161 | 1.64 | 7.81 | 1.88 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moreno Escobar, J.J.; Morales Matamoros, O.; Tejeida Padilla, R.; Chanona Hernández, L.; Posadas Durán, J.P.F.; Pérez Martínez, A.K.; Lina Reyes, I.; Quintana Espinosa, H. Biomedical Signal Acquisition Using Sensors under the Paradigm of Parallel Computing. Sensors 2020, 20, 6991. https://doi.org/10.3390/s20236991
Moreno Escobar JJ, Morales Matamoros O, Tejeida Padilla R, Chanona Hernández L, Posadas Durán JPF, Pérez Martínez AK, Lina Reyes I, Quintana Espinosa H. Biomedical Signal Acquisition Using Sensors under the Paradigm of Parallel Computing. Sensors. 2020; 20(23):6991. https://doi.org/10.3390/s20236991
Chicago/Turabian StyleMoreno Escobar, Jesús Jaime, Oswaldo Morales Matamoros, Ricardo Tejeida Padilla, Liliana Chanona Hernández, Juan Pablo Francisco Posadas Durán, Ana Karen Pérez Martínez, Ixchel Lina Reyes, and Hugo Quintana Espinosa. 2020. "Biomedical Signal Acquisition Using Sensors under the Paradigm of Parallel Computing" Sensors 20, no. 23: 6991. https://doi.org/10.3390/s20236991
APA StyleMoreno Escobar, J. J., Morales Matamoros, O., Tejeida Padilla, R., Chanona Hernández, L., Posadas Durán, J. P. F., Pérez Martínez, A. K., Lina Reyes, I., & Quintana Espinosa, H. (2020). Biomedical Signal Acquisition Using Sensors under the Paradigm of Parallel Computing. Sensors, 20(23), 6991. https://doi.org/10.3390/s20236991






