Sensors for Continuous Monitoring of Surgeon’s Cognitive Workload in the Cardiac Operating Room
Abstract
1. Introduction
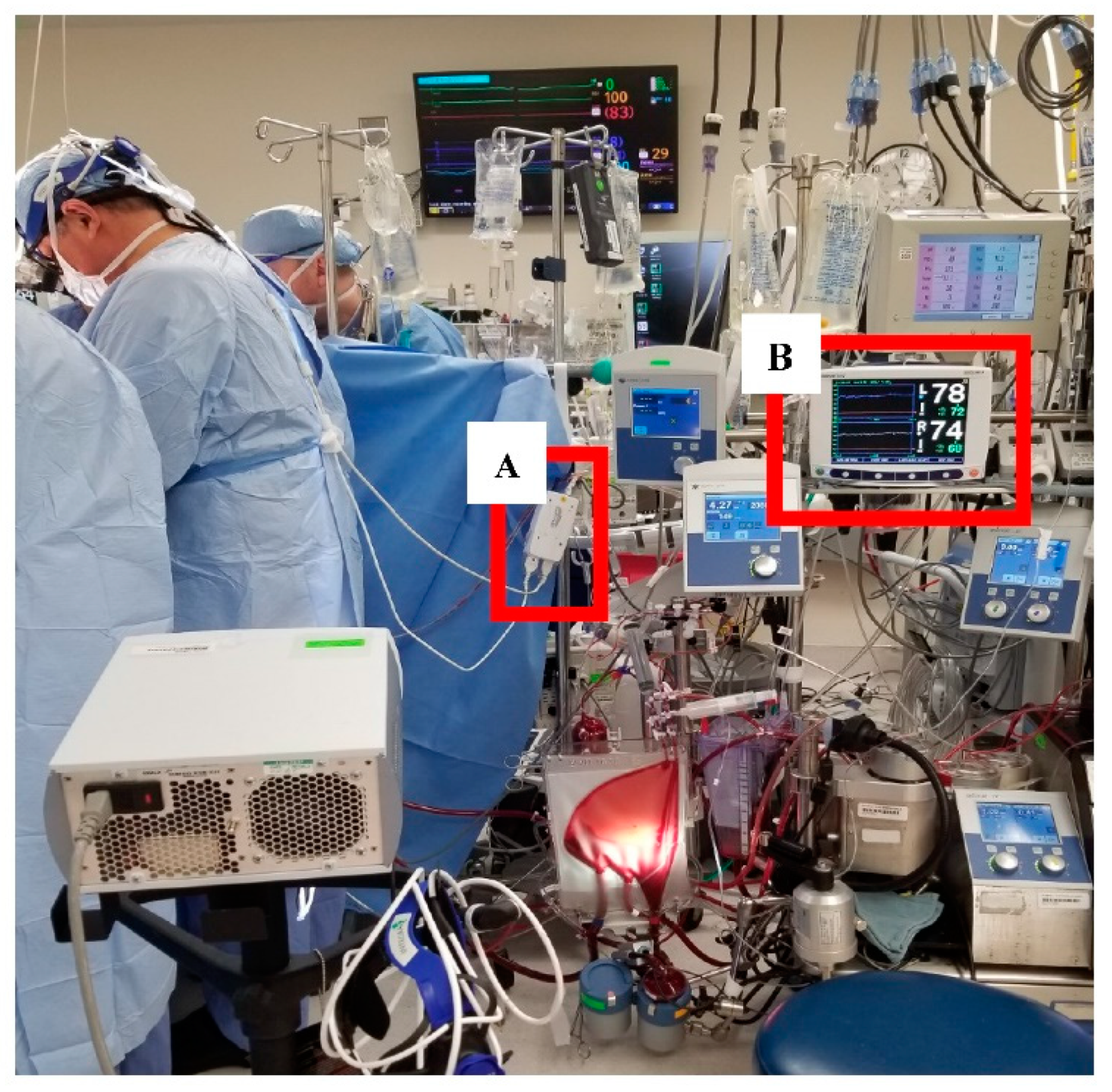
2. Materials and Methods
3. Results
3.1. Feasibility of Data Collection
3.2. Preliminary Validation
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Patel, V.L.; Kannampallil, T.G.; Shortliffe, E.H. Role of cognition in generating and mitigating clinical errors. BMJ Qual. Saf. 2015, 24, 468–474. [Google Scholar] [CrossRef] [PubMed]
- Suliburk, J.W.; Buck, Q.M.; Pirko, C.J.; Massarweh, N.N.; Barshes, N.R.; Singh, H.; Rosengart, T.K. Analysis of Human Performance Deficiencies Associated With Surgical Adverse Events. JAMA Netw. Open 2019, 2, e198067. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.D.; Yule, S.J.; Zenati, M.A. Augmented Cognition in the Operating Room. In Digital Surgery; Atallah, S., Ed.; Springer Nature: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Dias, R.D.; Ngo-Howard, M.C.; Boskovski, M.T.; Zenati, M.A.; Yule, S.J. Systematic review of measurement tools to assess surgeons’ intraoperative cognitive workload. BJS 2018, 105, 491–501. [Google Scholar] [CrossRef] [PubMed]
- Shaffer, F.; Shearman, S.; Meehan, Z.M. The Promise of Ultra-Short-Term (UST) Heart Rate Variability Measurements. Biofeedback 2016, 44, 229–233. [Google Scholar] [CrossRef]
- Maier-Hein, L.; Vedula, S.S.; Speidel, S.; Navab, N.; Kikinis, R.; Park, A.; Eisenmann, M.; Feussner, H.; Forestier, G.; Giannarou, S.; et al. Surgical data science for next-generation interventions. Nat. Biomed. Eng. 2017, 1, 691–696. [Google Scholar] [CrossRef]
- Thayer, J.F.; Hansen, A.L.; Saus-Rose, E.; Johnsen, B.H. Heart Rate Variability, Prefrontal Neural Function, and Cognitive Performance: The Neurovisceral Integration Perspective on Self-regulation, Adaptation, and Health. Ann. Behav. Med. 2009, 37, 141–153. [Google Scholar] [CrossRef]
- Thayer, J.F.; Åhs, F.; Fredrikson, M.; Sollers, J.J.; Wager, T.D. A meta-analysis of heart rate variability and neuroimaging studies: Implications for heart rate variability as a marker of stress and health. Neurosci. Biobehav. Rev. 2012, 36, 747–756. [Google Scholar] [CrossRef]
- Choi, J.; Gutierrez-Osuna, R. Using Heart Rate Monitors to Detect Mental Stress. In Proceedings of the Sixth International Workshop on Wearable and Implantable Body Sensor Networks, BSN 2009, Berkeley, CA, USA, 3–5 June 2009; pp. 219–223. [Google Scholar] [CrossRef]
- Giles, D.; Draper, N.; Neil, W. Validity of the Polar V800 heart rate monitor to measure RR intervals at rest. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 116, 563–571. [Google Scholar] [CrossRef]
- Peck, E.M.; Afergan, D.; Yuksel, B.F.; Lalooses, F.; Jacob, R.J.K. Using fNIRS to Measure Mental Workload in the Real World. In Brain-Computer Interfaces; Springer Science and Business Media LLC: Berlin, Germany, 2014; pp. 117–139. [Google Scholar]
- Modi, H.N.; Singh, H.; Orihuela-Espina, F.; Athanasiou, T.; Fiorentino, F.; Yang, G.-Z.; Darzi, A.; Leff, D.R. Temporal Stress in the Operating Room. Ann. Surg. 2018, 267, 683–691. [Google Scholar] [CrossRef]
- Macnab, A.J.; Shadgan, B. Biomedical applications of wireless continuous wave near infrared spectroscopy. Biomed. Spectrosc. Imaging 2012, 1, 205–222. [Google Scholar] [CrossRef]
- Durantin, G.; Gagnon, J.-F.; Tremblay, S.; Dehais, F. Using near infrared spectroscopy and heart rate variability to detect mental overload. Behav. Brain Res. 2014, 259, 16–23. [Google Scholar] [CrossRef]
- Zenati, M.A.; Leissner, K.B.; Zorca, S.; Kennedy-Metz, L.; Yule, S.J.; Dias, R.D. First Reported Use of Team Cognitive Workload for Root Cause Analysis in Cardiac Surgery. Semin. Thorac. Cardiovasc. Surg. 2019, 31, 394–396. [Google Scholar] [CrossRef] [PubMed]
- Dias, R.D.; Zenati, M.A.; Stevens, R.; GaBany, J.M.; Yule, S.J. Physiological synchronization and entropy as measures of team cognitive load. J. Biomed. Informatics 2019, 96, 103250. [Google Scholar] [CrossRef]
- Kennedy-Metz, L.; Dias, R.D.; Stevens, R.H.; Yule, S.J.; Zenati, M.A. Analysis of Mirrored Psychophysiological Change of Cardiac Surgery Team Members During Open Surgery. J. Surg. Educ. 2020, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Singh, H.; Modi, H.; Yang, G.; Darzi, A.; Leff, D. Losing Your Nerve in the Operating Room—Prefrontal Attenuation is Associated with Performance Degradation under Temporal Demands. Proceedings of 10th Hamlyn Symposium on Medical Robotics, London, UK, 25–28 June 2017, 55–56. [CrossRef]
- Modi, H.M.H.; Singh, H.; Yang, G.Z.; Darzi, A.; Leff, D. Stress Resilience in Surgeons: A Neurophysiological Perspective. In Proceedings of the Hamlyn Symposium Proceedings 2018, London, UK, 24–27 June 2018; pp. 53–54. [Google Scholar] [CrossRef]
- Avrunin, G.S.; Clarke, L.A.; Conboy, H.M.; Osterweil, L.J.; Dias, R.D.; Yule, S.J.; Goldman, J.M.; Zenati, M.A. Toward improving surgical outcomes by incorporating cognitive load measurement into process-driven guidance. In Proceedings of the SEHS ‘18: Proceedings of the International Workshop on Software Engineering in Healthcare Systems, Gothenburg, Sweden, 28 May–3 June 2018; Volume 2018, pp. 2–9. [Google Scholar] [CrossRef]
- Tarvainen, M.P.; Niskanen, J.-P.; Lipponen, J.A.; Ranta-Aho, P.O.; Karjalainen, P.A. Kubios HRV—Heart rate variability analysis software. Comput. Methods Programs Biomed. 2014, 113, 210–220. [Google Scholar] [CrossRef] [PubMed]
- Feller, W. On the Kolmogorov-Smirnov Limit Theorems for Empirical Distributions. Ann. Math. Stat. 1948, 19, 177–189. [Google Scholar] [CrossRef]
- Task Force of the European Society of Cardiology and the North American Society of Pacing and Electrophysiology. Heart Rate Variability: Standards of Measurement, Physiological Interpretation and Clinical Use. Eur. Heart J. 1996, 17, 354–381. [Google Scholar] [CrossRef]
- Arora, S.; Tierney, T.; Sevdalis, N.; Aggarwal, R.; Nestel, D.; Woloshynowych, M.; Darzi, A.; Kneebone, R. The Imperial Stress Assessment Tool (ISAT): A Feasible, Reliable and Valid Approach to Measuring Stress in the Operating Room. World J. Surg. 2010, 34, 1756–1763. [Google Scholar] [CrossRef]
- Fishburn, F.A.; Norr, M.E.; Medvedev, A.V.; Vaidya, C.J. Sensitivity of fNIRS to cognitive state and load. Front. Hum. Neurosci. 2014, 8, 76. [Google Scholar] [CrossRef] [PubMed]
- Ayaz, H.; Shewokis, P.A.; Bunce, S.C.; Izzetoglu, K.; Willems, B.; Onaral, B. Optical brain monitoring for operator training and mental workload assessment. NeuroImage 2012, 59, 36–47. [Google Scholar] [CrossRef]
- Kennedy-Metz, L.; Parker, S.H. Biofeedback as a stress management tool: A systematic review. Cogn. Technol. Work. 2018, 21, 161–190. [Google Scholar] [CrossRef]
- Afergan, D.; Hincks, S.W.; Shibata, T.; Jacob, R.J.K. Phylter: A System for Modulating Notifications in Wearables Using Physiological Sensing. In Proceedings of the 9th International Conference, AC 2015, Held as Part of HCI International 2015, Los Angeles, CA, USA, 2–7 August 2015; Volume 9183, pp. 167–177. [Google Scholar] [CrossRef]
- Bailey, B.P.; Konstan, J.A. On the need for attention-aware systems: Measuring effects of interruption on task performance, error rate, and affective state. Comput. Hum. Behav. 2006, 22, 685–708. [Google Scholar] [CrossRef]
- Dias, R.D.; Conboy, H.M.; GaBany, J.M.; Clarke, L.A.; Osterweil, L.J.; Arney, D.; Goldman, J.M.; Riccardi, G.; Avrunin, G.S.; Yule, S.J.; et al. Intelligent Interruption Management System to Enhance Safety and Performance in Complex Surgical and Robotic Procedures. In Lecture Notes in Computer Science; Springer Science and Business Media LLC: Berlin, Germany, 2018; Volume 11041, pp. 62–68. [Google Scholar]
- Zenati, M.A.; Kennedy-Metz, L.; Dias, R.D. Cognitive Engineering to Improve Patient Safety and Outcomes in Cardiothoracic Surgery. Semin. Thorac. Cardiovasc. Surg. 2020, 32, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Cilhoroz, B.; Giles, D.; Zaleski, A.; Taylor, B.; Fernhall, B.; Pescatello, L. Validation of the Polar V800 heart rate monitor and comparison of artifact correction methods among adults with hypertension. PLoS ONE 2020, 15, e0240220. [Google Scholar] [CrossRef]
- Bizzego, A.; Battisti, A.; Gabrieli, G.; Esposito, G.; Furlanello, C. pyphysio: A physiological signal processing library for data science approaches in physiology. SoftwareX 2019, 10, 100287. [Google Scholar] [CrossRef]
| Bypass Phase | Sub-Phase | Pearson’s r | N | p-Value | Notable Events |
|---|---|---|---|---|---|
| 1. Pre-bypass | 0.47 | 58 | <0.001 | ||
| 1a. Sternotomy | 0.58 | 17 | 0.014 | Resident errors requiring verbal corrections | |
| 1b. Heparinization | 0.04 | 17 | 0.869 | ||
| 1c. Cannulation | −0.53 | 9 | 0.142 | Resident errors requiring attending to take over | |
| 1d. Other | 0.24 | 15 | 0.387 | ||
| 2. On Bypass | 0.31 | 87 | 0.003 | ||
| 2a. Initiate Bypass | 0.68 | 4 | 0.318 | ||
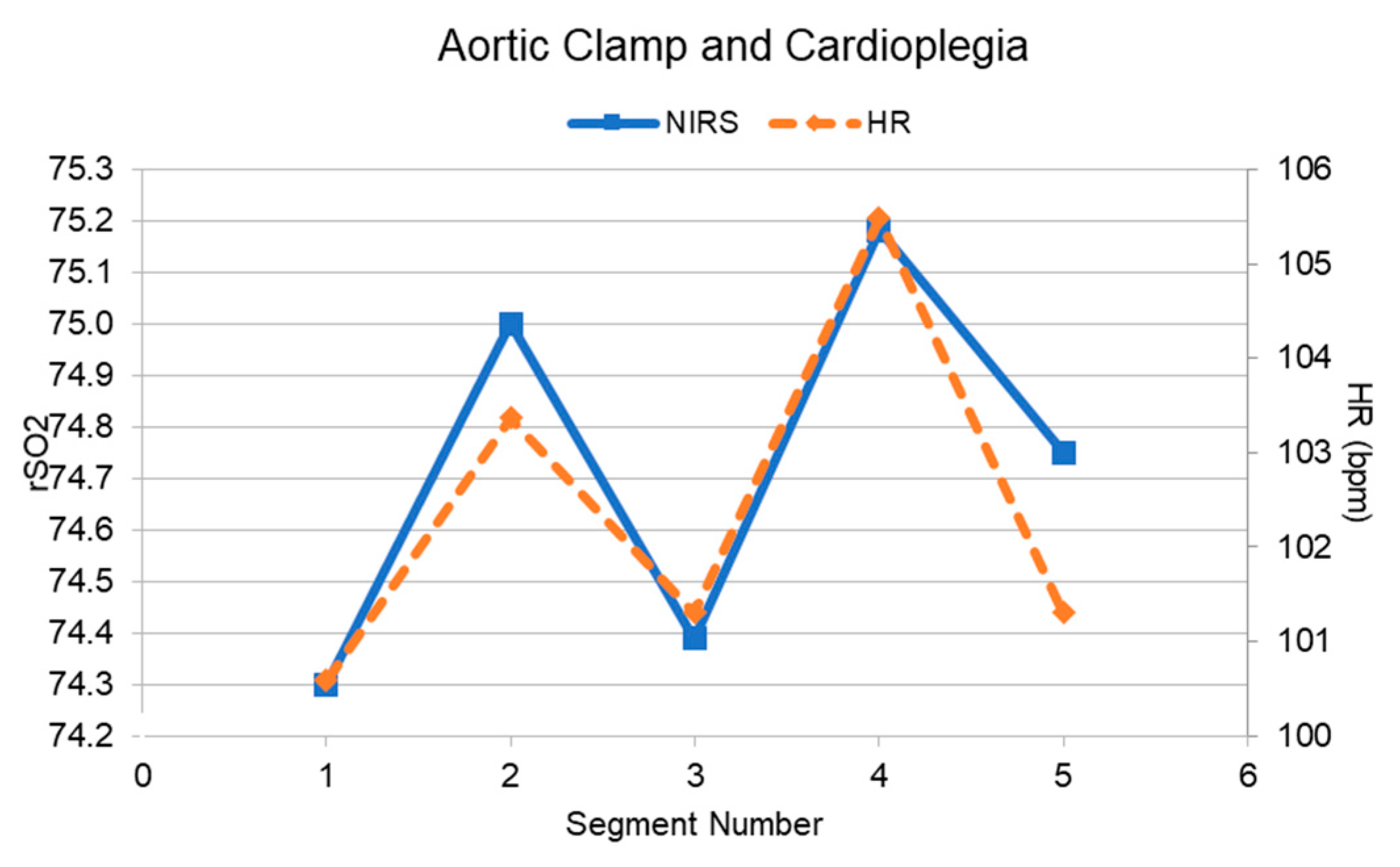
| 2b. Aortic Clamp and Cardioplegia | 0.91 | 5 | 0.031 | Temporal pressure (observed) | |
| 2c. Aortotomy | −0.19 | 66 | 0.118 | Patient anatomy difficulty, irrespective of resident performance | |
| 2d. Other | −0.49 | 12 | 0.106 | ||
| 3. Post-bypass | −0.14 | 32 | 0.432 | ||
| 3a. Separate from Bypass | −0.12 | 23 | 0.581 | ||
| 3b. Other | 0.21 | 9 | 0.589 | ||
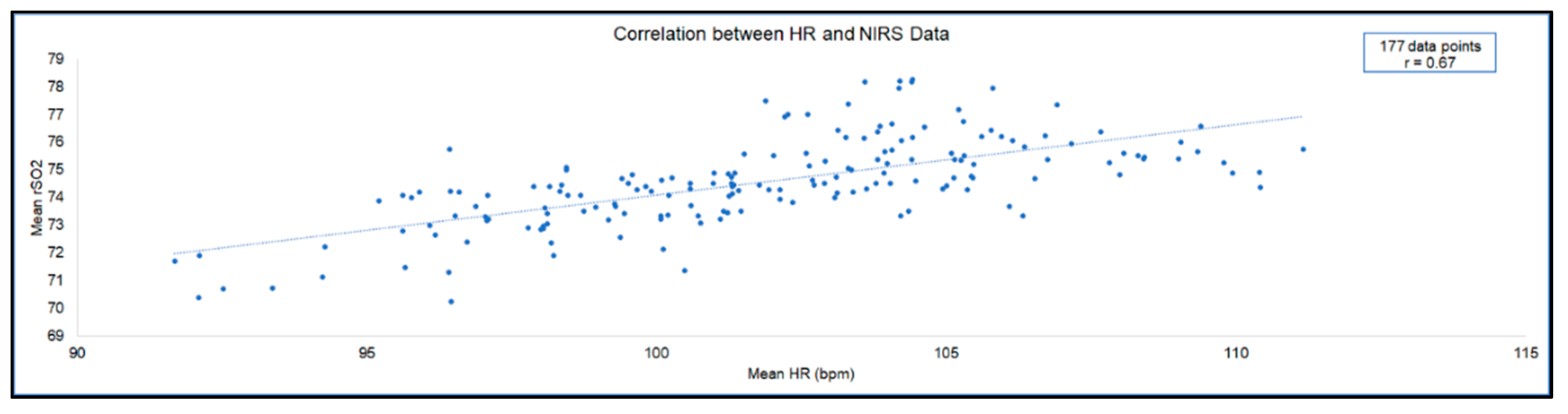
| Complete case | 0.67 | 177 | <0.001 |
| Sub-Phase | Minimum Difference | Maximum Difference |
|---|---|---|
| 1a. Sternotomy | 19.97 | 29.14 |
| 1b. Heparinization | 21.34 | 25.17 |
| 1c. Cannulation | 23.38 | 28.52 |
| 2a. Initiate Bypass | 23.20 | 27.18 |
| 2b. Aortic Clamp and Cardioplegia | 26.29 | 30.30 |
| 2c. Aortotomy | 24.39 | 35.50 |
| 3a. Separate from Bypass | 24.72 | 36.06 |
| Bypass Phase | Sub-Phase | Pearson’s r | N | p-Value | Notable Events |
|---|---|---|---|---|---|
| 1. Pre-bypass | 0.18 | 58 | 0.185 | ||
| 1a. Sternotomy | −0.17 | 17 | 0.497 | Resident errors requiring verbal corrections | |
| 1b. Heparinization | 0.25 | 17 | 0.324 | ||
| 1c. Cannulation | −0.02 | 9 | 0.968 | Resident errors requiring attending to take over | |
| 1d. Other | 0.01 | 15 | 0.960 | ||
| 2. On Bypass | −0.06 | 87 | 0.582 | ||
| 2a. Initiate Bypass | −0.26 | 4 | 0.740 | ||
| 2b. Aortic Clamp and Cardioplegia | −0.99 | 5 | <0.001 | Temporal pressure (observed) | |
| 2c. Aortotomy | −0.10 | 66 | 0.428 | Patient anatomy difficulty, irrespective of resident performance | |
| 2d. Other | −0.31 | 12 | 0.333 | ||
| 3. Post-bypass | −0.18 | 32 | 0.330 | ||
| 3a. Separate from Bypass | −0.26 | 23 | 0.230 | ||
| 3b. Other | 0.06 | 9 | 0.882 | ||
| Complete case | −0.11 | 177 | 0.151 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kennedy-Metz, L.R.; Dias, R.D.; Srey, R.; Rance, G.C.; Furlanello, C.; Zenati, M.A. Sensors for Continuous Monitoring of Surgeon’s Cognitive Workload in the Cardiac Operating Room. Sensors 2020, 20, 6616. https://doi.org/10.3390/s20226616
Kennedy-Metz LR, Dias RD, Srey R, Rance GC, Furlanello C, Zenati MA. Sensors for Continuous Monitoring of Surgeon’s Cognitive Workload in the Cardiac Operating Room. Sensors. 2020; 20(22):6616. https://doi.org/10.3390/s20226616
Chicago/Turabian StyleKennedy-Metz, Lauren R., Roger D. Dias, Rithy Srey, Geoffrey C. Rance, Cesare Furlanello, and Marco A. Zenati. 2020. "Sensors for Continuous Monitoring of Surgeon’s Cognitive Workload in the Cardiac Operating Room" Sensors 20, no. 22: 6616. https://doi.org/10.3390/s20226616
APA StyleKennedy-Metz, L. R., Dias, R. D., Srey, R., Rance, G. C., Furlanello, C., & Zenati, M. A. (2020). Sensors for Continuous Monitoring of Surgeon’s Cognitive Workload in the Cardiac Operating Room. Sensors, 20(22), 6616. https://doi.org/10.3390/s20226616







