Switching from Multiplex to Multimodal Colorimetric Lateral Flow Immunosensor
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Materials
2.2. Synthesis of Gold Nanoparticles and Gold Nanostars
2.3. Labelling Immunoreagents with GNPs and GNSs
2.4. LFIA Strip Preparation
2.5. Serum Samples
2.6. The LFIA Test Procedure
3. Results and Discussion
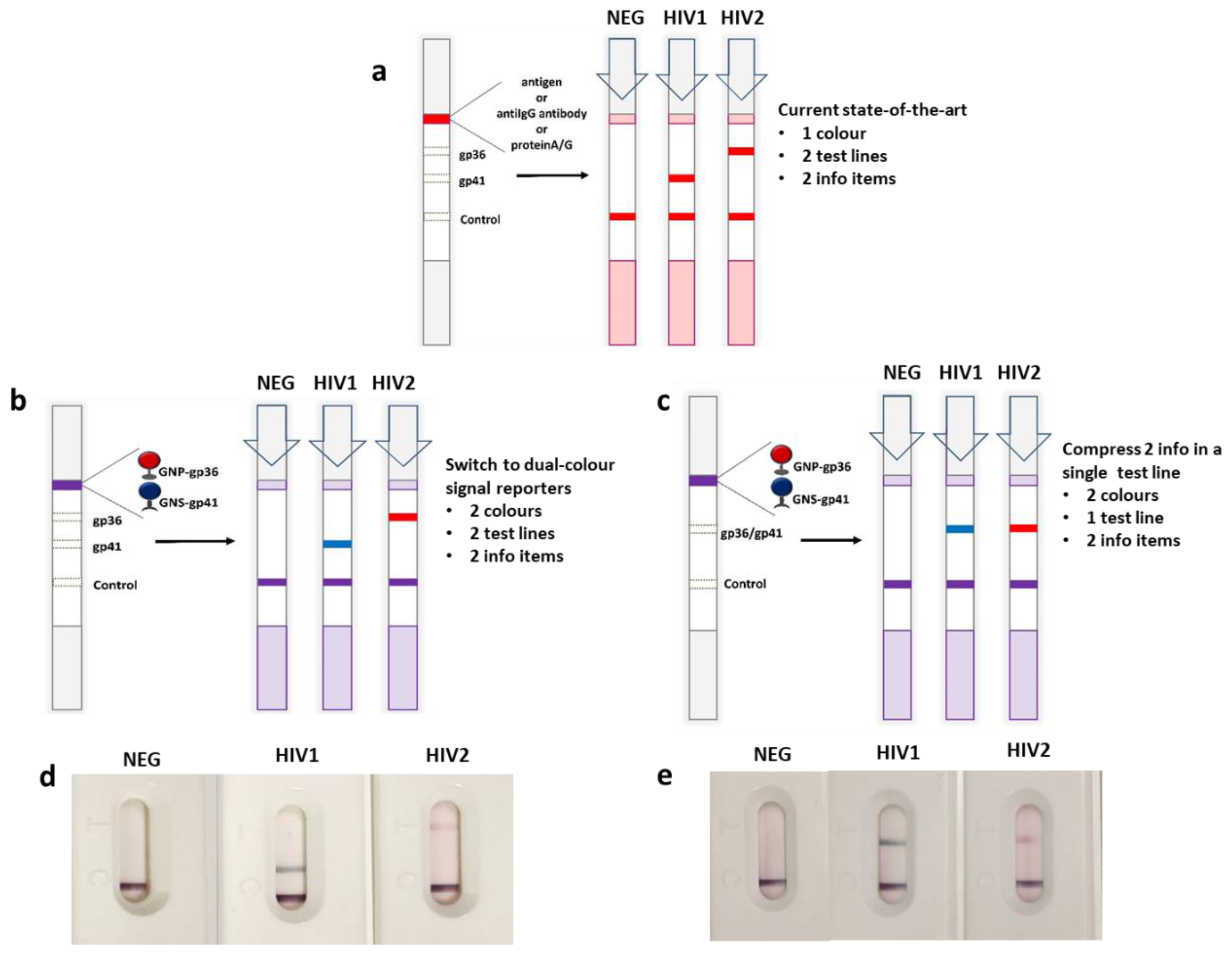
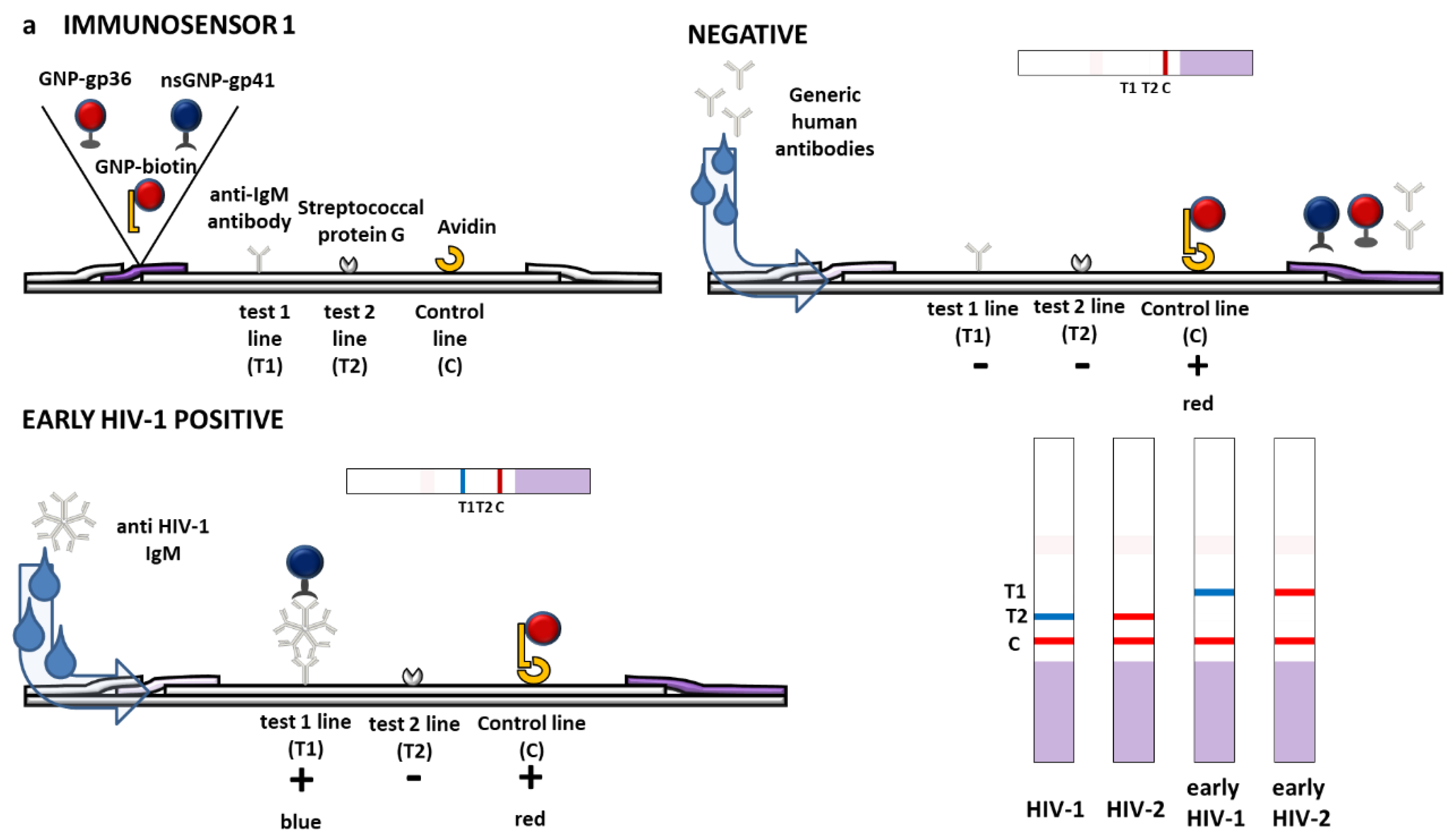
3.1. Design of Multimodal LFIA Immunosensors
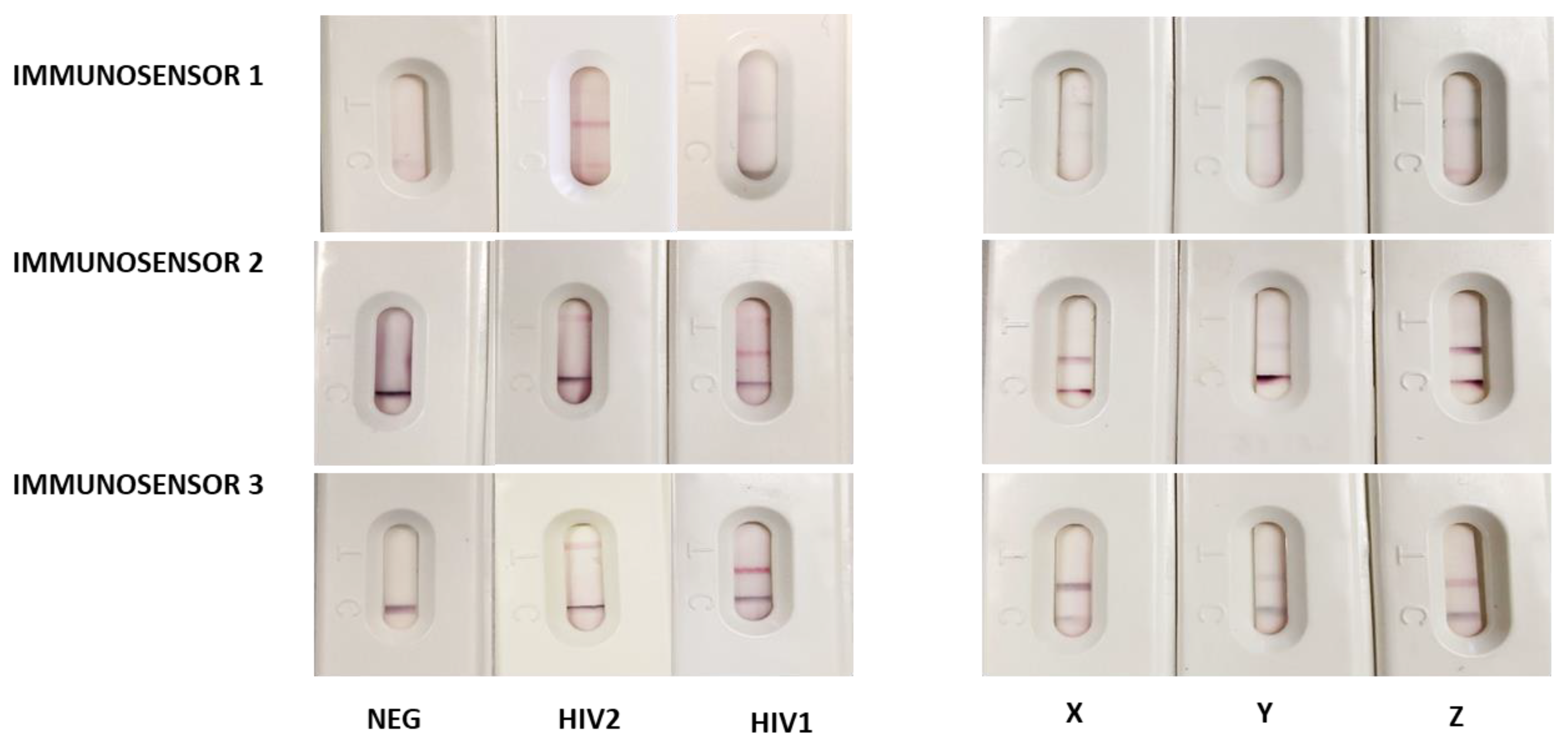
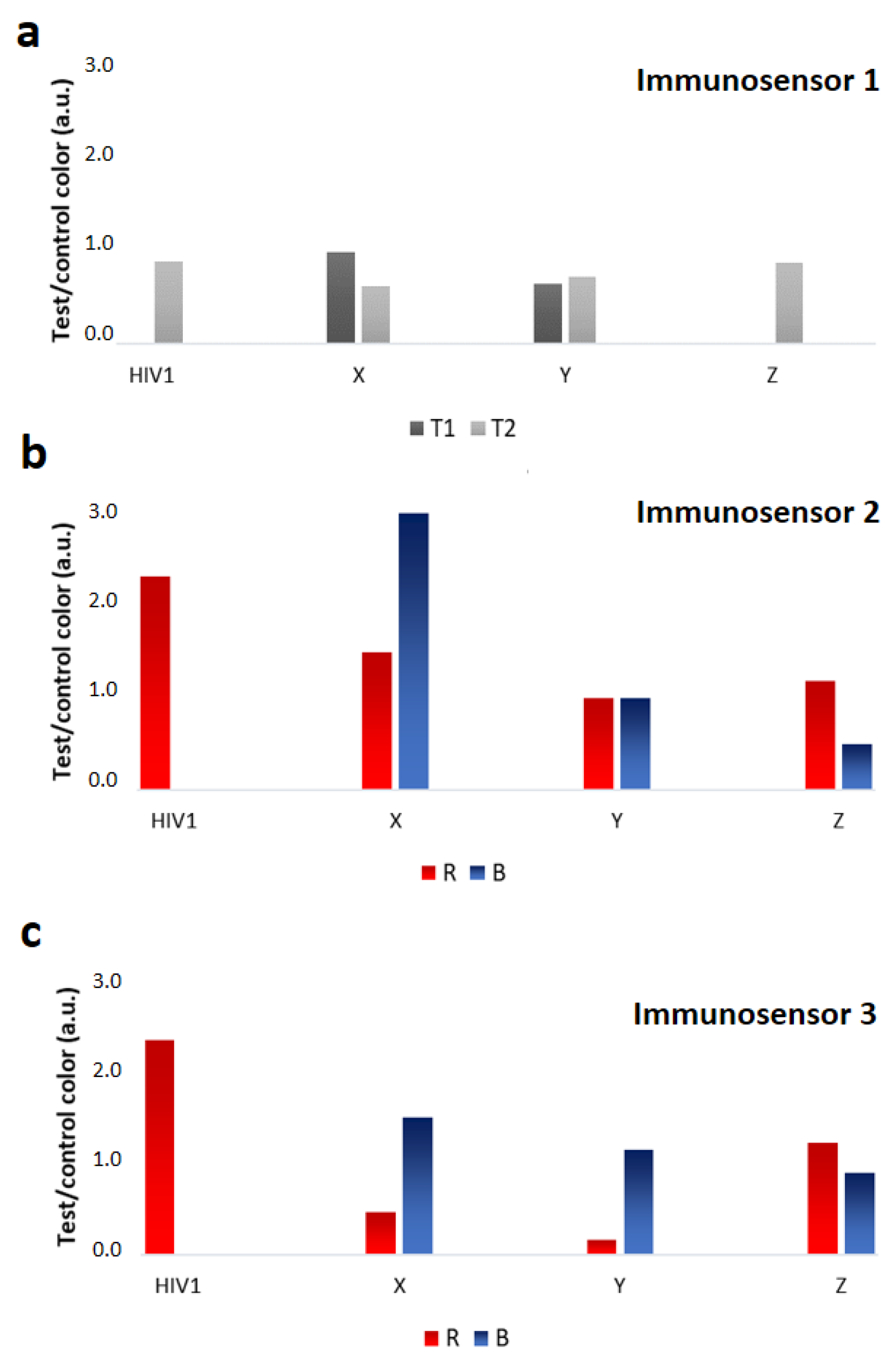
3.2. Performance of the x2LFIA Immunosensors for HIV Serotyping and Discrimination of the Infection Stage
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Wu, G.; Zaman, M.H.; Wu, G.; Zaman, M.H. Low-Cost Tools for Diagnosing and Monitoring HIV Infection in Low-Resource Settings. Bull. World Health Organ. 2012, 914–920. [Google Scholar] [CrossRef]
- WHO. Guidelines on HIV Testing Services HIV Self-Testing and Partner; World Health Organization: Geneva, Switzerland, 2016; ISBN 978-92-4-154986-8. [Google Scholar]
- Koczula, K.M.; Gallotta, A. Lateral Flow Assays. Essays Biochem. 2016, 60, 111–120. [Google Scholar] [CrossRef] [PubMed]
- Sajid, M.; Daud, M. Designs, Formats and Applications of Lateral Flow Assay: A Literature Review. J. Saudi Chem. Soc. 2015, 19, 689–705. [Google Scholar] [CrossRef]
- Bartosh, A.V.; Sotnikov, D.V.; Hendrickson, O.D.; Zherdev, A.V.; Dzantiev, B.B. Design of Multiplex Lateral Flow Tests: A Case Study for Simultaneous Detection of Three Antibiotics. Biosensors 2020, 10, 17. [Google Scholar] [CrossRef] [PubMed]
- Anfossi, L.; Di Nardo, F.; Cavalera, S.; Giovannoli, C.; Baggiani, C. Multiplex Lateral Flow Immunoassay: An Overview of Strategies towards High-Throughput Point-of-Need Testing. Biosensors 2018, 9, 2. [Google Scholar] [CrossRef]
- Dincer, C.; Bruch, R.; Kling, A.; Dittrich, P.S.; Urban, G.A. Multiplexed Point-of-Care Testing—XPOCT. In Trends in Biotechnology; Elsevier Ltd.: Amsterdam, The Netherlands, 2017; pp. 728–742. [Google Scholar] [CrossRef]
- Li, J.; Macdonald, J. Multiplexed Lateral Flow Biosensors: Technological Advances for Radically Improving Point-of-Care Diagnoses. In Biosensors and Bioelectronics; Elsevier Ltd.: Amsterdam, The Netherlands, 2016; pp. 177–192. [Google Scholar] [CrossRef]
- Yang, M.; Goolia, M.; Xu, W.; Bittner, H.; Clavijo, A. Development of a Quick and Simple Detection Methodology for Foot-and-Mouth Disease Virus Serotypes O, A and Asia 1 Using a Generic Rapid Assay Device. Virol. J. 2013, 10, 125. [Google Scholar] [CrossRef]
- Assure HIV Test. Available online: https://www.fda.gov/media/139792/download (accessed on 1 October 2020).
- Li, Z.; Yi, Y.; Luo, X.; Xiong, N.; Liu, Y.; Li, S.; Sun, R.; Wang, Y.; Hu, B.; Chen, W.; et al. Development and Clinical Application of a Rapid IgM-IgG Combined Antibody Test for SARS-CoV-2 Infection Diagnosis. J. Med. Virol. 2020, 92, 1518–1524. [Google Scholar] [CrossRef]
- HCV/HIV/HBsAg Rapid Test (One Strip)-Hangzhou Biotest Biotech Co., LTD. Available online: http://en.biotests.com.cn/product/278147348 (accessed on 1 October 2020).
- Daskalakis, D. HIV Diagnostic Testing: Evolving Technology and Testing Strategies. Top. Antivir. Med. 2011, 19, 18–22. [Google Scholar]
- Boyle, M.D.P. Bacterial Immunoglobulin-Binding Proteins, 2nd ed.; Elsevier: Oxford, UK, 1998; pp. 323–327. [Google Scholar] [CrossRef]
- Wang, W.; Su, X.; Ouyang, H.; Wang, L.; Fu, Z. A Novel Immunochromatographic Assay Based on a Time-Resolved Chemiluminescence Strategy for the Multiplexed Detection of Ractopamine and Clenbuterol. Anal. Chim. Acta 2016, 917, 79–84. [Google Scholar] [CrossRef]
- Wang, C.; Hou, F.; Ma, Y. Simultaneous Quantitative Detection of Multiple Tumor Markers with a Rapid and Sensitive Multicolor Quantum Dots Based Immunochromatographic Test Strip. Biosens. Bioelectron. 2015, 68, 156–162. [Google Scholar] [CrossRef]
- Yen, C.-W.; de Puig, H.; Tam, J.O.; Gómez-Márquez, J.; Bosch, I.; Hamad-Schifferli, K.; Gehrke, L. Multicolored Silver Nanoparticles for Multiplexed Disease Diagnostics: Distinguishing Dengue, Yellow Fever, and Ebola Viruses. Lab Chip 2015, 15, 1638–1641. [Google Scholar] [CrossRef] [PubMed]
- Di Nardo, F.; Alladio, E.; Baggiani, C.; Cavalera, S.; Giovannoli, C.; Spano, G.; Anfossi, L. Color-Encoded Lateral Flow Immunoassay for the Simultaneous Detection of Aflatoxin B1 and Type-B Fumonisins in a Single Test Line. Talanta 2019, 192, 288–294. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Tian, J.; Zhao, Y.; Zhao, S. Ag/Au Nanoparticles Coated Graphene Electrochemical Sensor for Ultrasensitive Analysis of Carcinoembryonic Antigen in Clinical Immunoassay. Sens. Actuators B Chem. 2015, 206, 570–576. [Google Scholar] [CrossRef]
- Di Nardo, F.; Baggiani, C.; Giovannoli, C.; Spano, G.; Anfossi, L. Multicolor Immunochromatographic Strip Test Based on Gold Nanoparticles for the Determination of Aflatoxin B1 and Fumonisins. Microchim. Acta 2017, 184, 1295–1304. [Google Scholar] [CrossRef]
- Kim, J.; Cao, X.E.; Finkelstein, J.L.; Cárdenas, W.B.; Erickson, D.; Mehta, S. A Two-Color Multiplexed Lateral Flow Immunoassay System to Differentially Detect Human Malaria Species on a Single Test Line. Malar. J. 2019, 18, 313. [Google Scholar] [CrossRef]
- Available online: https://www.unaids.org/sites/default/files/media_asset/2019-UNAIDS-data.p16 (accessed on 1 October 2020).
- Sands, A. Diagnostics for HIV Diagnosis. 2015. Available online: https://apps.who.int/iris/bitstream/handle/10665/180221/WHO_HIV_2015.26_eng.pdf (accessed on 16 November 2020).
- Granade, T.C.; Workman, S.; Wells, S.K.; Holder, A.N.; Owen, S.M.; Pau, C.-P. Rapid Detection and Differentiation of Antibodies to HIV-1 and HIV-2 Using Multivalent Antigens and Magnetic Immunochromatography Testing. Clin. Vaccine Immunol. 2010, 17, 1034–1039. [Google Scholar] [CrossRef]
- Meda, N.; Gautier-Charpentier, L.; Soudré, R.B.; Dahourou, H.; Ouedraogo-Traoré, R.; Ouangré, A.; Bambara, A.; Kpozehouen, A.; Sanou, H.; Valéa, D.; et al. Serological Diagnosis of Human Immunodeficiency Virus in Burkina Faso: Reliable, Practical Strategies Using Less Expensive Commercial Test Kits. Bull. World Health Organ. 1999, 77, 731–739. [Google Scholar]
- Bottiger, B.; Karlsson, A.; Andreasson, P.; Naucleir, A.; Costa, C.M.; Norrby, E.; Biberfeld, G. Envelope Cross-Reactivity between Human Immunodeficiency Virus Types 1 and 2 Detected by Different Serological Methods: Correlation between Cross-Neutralization and Reactivity against the Main Neutralizing Site. J. Virol. 1990, 64, 3492–3499. [Google Scholar] [CrossRef]
- Song, S.; Liu, N.; Zhao, Z.; Njumbe Ediage, E.; Wu, S.; Sun, C.; De Saeger, S.; Wu, A. Multiplex Lateral Flow Immunoassay for Mycotoxin Determination. Anal. Chem. 2014, 86, 4995–5001. [Google Scholar] [CrossRef]
- Peng, J.; Wang, Y.; Liu, L.; Kuang, H.; Li, A.; Xu, C. Multiplex Lateral Flow Immunoassay for Five Antibiotics Detection Based on Gold Nanoparticle Aggregations. RSC Adv. 2016, 6, 7798–7805. [Google Scholar] [CrossRef]
- Zhang, M.-Z.; Wang, M.-Z.; Chen, Z.-L.; Fang, J.-H.; Fang, M.-M.; Liu, J.; Yu, X.-P. Development of a Colloidal Gold-Based Lateral-Flow Immunoassay for the Rapid Simultaneous Detection of Clenbuterol and Ractopamine in Swine Urine. Anal. Bioanal. Chem. 2009, 395, 2591. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Liu, Y.; Wang, M.; Chen, Y.; Jiang, W. A Multiplex Immunochromatographic Test Using Gold Nanoparticles for the Rapid and Simultaneous Detection of Four Nitrofuran Metabolites in Fish Samples. Anal. Bioanal. Chem. 2018, 410, 223–233. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Yang, J.; Yang, S.; Sang, Q.; Teng, M.; Li, Q.; Deng, R.; Feng, L.; Hu, X.; Zhang, G. Development of an Immunochromatographic Lateral Flow Strip for the Simultaneous Detection of Aminoglycoside Residues in Milk. RSC Adv. 2018, 8, 9580–9586. [Google Scholar] [CrossRef]
- Liu, B.; Gong, H.; Wang, Y.; Zhang, X.; Li, P.; Qiu, Y.; Wang, L.; Hua, X.; Guo, Y.; Wang, M.; et al. A Gold Immunochromatographic Assay for Simultaneous Detection of Parathion and Triazophos in Agricultural Products. Anal. Methods 2018, 10, 422–428. [Google Scholar] [CrossRef]
- Taranova, N.A.; Berlina, A.N.; Zherdev, A.V.; Dzantiev, B.B. ‘Traffic Light’ Immunochromatographic Test Based on Multicolor Quantum Dots for the Simultaneous Detection of Several Antibiotics in Milk. Biosens. Bioelectron. 2015, 63, 255–261. [Google Scholar] [CrossRef]
- Qin, Y.-J.; Sha, R.; Feng, Y.-C.; Huang, Y.-C.; Yan-Chun Huang, C. Comparison of Double Antigen Sandwich and Indirect Enzyme-Linked Immunosorbent Assay for the Diagnosis of Hepatitis C Virus Antibodies. J. Clin. Lab. Anal. 2020. [Google Scholar] [CrossRef]
- Xiang, T.; Jiang, Z.; Zheng, J.; Lo, C.; Tsou, H.; Ren, G.; Zhang, J.; Huang, A.; Lai, G. A Novel Double Antibody Sandwich-Lateral Flow Immunoassay for the Rapid and Simple Detection of Hepatitis C Virus. Int. J. Mol. Med. 2012, 30, 1041–1047. [Google Scholar] [CrossRef]
- Stamatatos, L.; Morris, L.; Burton, D.R.; Mascola, J.R. Neutralizing Antibodies Generated during Natural HIV-1 Infection: Good News for an HIV-1 Vaccine? Nat. Med. 2009, 15, 866–870. [Google Scholar] [CrossRef]
- Tomaras, G.D.; Haynes, B.F. HIV-1-Specific Antibody Responses during Acute and Chronic HIV-1 Infection. J. AIDS HIV Res. 2009, 4, 373–379. [Google Scholar] [CrossRef]
- Prince, H.E.; Yeh, C.; Lapé-Nixon, M. Utility of IgM/IgG Ratio and IgG Avidity for Distinguishing Primary and Secondary Dengue Virus Infections Using Sera Collected More than 30 Days after Disease Onset. Clin. Vaccine Immunol. 2011, 18, 1951–1956. [Google Scholar] [CrossRef]
- Lugito, C.N.P.H.; Kurniawan, A. Immunoglobulin G (IgG) to IgM ratio in secondary adult dengue infection using samples from early days of symptoms onset. BMC Infect Dis. 2015, 15, 276. [Google Scholar] [CrossRef]
- Zhang, D.; Huang, L.; Liu, B.; Ni, H.; Sun, L.; Su, E.; Chen, H.; Gu, Z.; Zhao, X. Quantitative and Ultrasensitive Detection of Multiplex Cardiac Biomarkers in Lateral Flow Assay with Core-Shell SERS Nanotags. Biosens. Bioelectron. 2018, 106, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Calderón-Santiago, M.; Priego-Capote, F.; Turck, N.; Robin, X.; Jurado-Gámez, B.; Sanchez, J.C.; Luque de Castro, M.D. Human Sweat Metabolomics for Lung Cancer Screening. Anal. Bioanal. Chem. 2015, 407, 5381–5392. [Google Scholar] [CrossRef] [PubMed]
- Bansal, T.; Pandey, A.; Deepa, D.; Asthana, A.K. C-Reactive Protein (CRP) and Its Association with Periodontal Disease: A Brief Review. J. Clin. Diagn. Res. 2014, 8, 21–24. [Google Scholar] [CrossRef]
- Tsai, T.-T.; Huang, T.-H.; Ho, N.Y.-J.; Chen, Y.-P.; Chen, C.-A.; Chen, C.-F. Development of a Multiplex and Sensitive Lateral Flow Immunoassay for the Diagnosis of Periprosthetic Joint Infection. Sci. Rep. 2019, 9, 15679. [Google Scholar] [CrossRef] [PubMed]
- Turkevich, J.; Stevenson, P.C.; Hillier, J. A Study of the Nucleation and Growth Processes in the Synthesis of Colloidal Gold. Discuss. Faraday Soc. 1951, 11, 55–75. [Google Scholar] [CrossRef]
- European Commission EU2009/886/EC: Commission Decision of 27 November 2009 amending Decision 2002/364/EC on common technical specifications for in vitro diagnostic medical devices. Off. J. Eur. Union 2009, L318, 25–40.
- Shin, S.Y.; Lee, M.K.; Kim, S.Y.; Jang, S.Y.; Hahm, K. The Use of Multiple Antigenic Peptide (Map) In The Immunodiagnosis Of Human Immunodeficiency Virus Infection. Biochem. Mol. Biol. Int. 1997, 43, 713–721. [Google Scholar] [CrossRef]
- Fitzgerald, J.; Leonard, P.; Danaher, M.; O’Kennedy, R. Rapid Simultaneous Detection of Anti-protozoan Drugs Using a Lateral-Flow Immunoassay Format. Appl. Biochem. Biotechnol. 2015, 176, 387–398. [Google Scholar] [CrossRef]
- Zhu, M.; Jia, Y.; Peng, L.; Ma, J.; Li, X.; Shi, F. A highly sensitive dual-color lateral flow immunoassay for brucellosis using one-step synthesized latex microspheres. Anal. Methods 2019, 11, 2937–2942. [Google Scholar] [CrossRef]


| ID (#) | Serotype | Type Elapsed from Infection (Days) | Seroconversion |
|---|---|---|---|
| NEG (#10) | Negative | - | - |
| HIV1 (#8) | HIV1 | >90 | complete |
| HIV2 (#5) | HIV2 | >90 | complete |
| X (9081-03) | HIV1 | 27 | In progress |
| Y (9089-06) | HIV1 | 26 | In progress |
| Z (9019-03) | HIV1 | 38 | In progress |
| Reporter | Capture | |||||||
|---|---|---|---|---|---|---|---|---|
| Format | Adsorbed on GNP | Adsorbed on GNS | Optical Density (Ratio) b | Test Line 1 (T1) | Test Line 2 (T2) | Control Line (C) | ||
| xLFIA | A | gp36 | biotin a | gp41 | 2.5 (1 + 0.5 + 1) | gp36 | gp41 | avidin |
| B | gp36 | biotin a | gp41 | 2.5 (1 + 0.5 + 1) | gp36/gp41 | - | avidin | |
| x2LFIA | 1 | gp36 | biotin a | gp41 | 2.5 (1 + 0.5 + 1) | anti-IgM | protein G | avidin |
| 2 | protein G | anti-IgM | 3 (1.5 + 1.5) | gp36 | gp41 | protein G | ||
| 3 | gp36 | gp41 | anti-IgM | 3 (1 + 1 + 1) | gp36 | gp41 | protein G | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cavalera, S.; Di Nardo, F.; Forte, L.; Marinoni, F.; Chiarello, M.; Baggiani, C.; Anfossi, L. Switching from Multiplex to Multimodal Colorimetric Lateral Flow Immunosensor. Sensors 2020, 20, 6609. https://doi.org/10.3390/s20226609
Cavalera S, Di Nardo F, Forte L, Marinoni F, Chiarello M, Baggiani C, Anfossi L. Switching from Multiplex to Multimodal Colorimetric Lateral Flow Immunosensor. Sensors. 2020; 20(22):6609. https://doi.org/10.3390/s20226609
Chicago/Turabian StyleCavalera, Simone, Fabio Di Nardo, Luca Forte, Francesca Marinoni, Matteo Chiarello, Claudio Baggiani, and Laura Anfossi. 2020. "Switching from Multiplex to Multimodal Colorimetric Lateral Flow Immunosensor" Sensors 20, no. 22: 6609. https://doi.org/10.3390/s20226609
APA StyleCavalera, S., Di Nardo, F., Forte, L., Marinoni, F., Chiarello, M., Baggiani, C., & Anfossi, L. (2020). Switching from Multiplex to Multimodal Colorimetric Lateral Flow Immunosensor. Sensors, 20(22), 6609. https://doi.org/10.3390/s20226609








