Potential of Hyperspectral and Thermal Proximal Sensing for Estimating Growth Performance and Yield of Soybean Exposed to Different Drip Irrigation Regimes Under Arid Conditions
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Site and Conditions
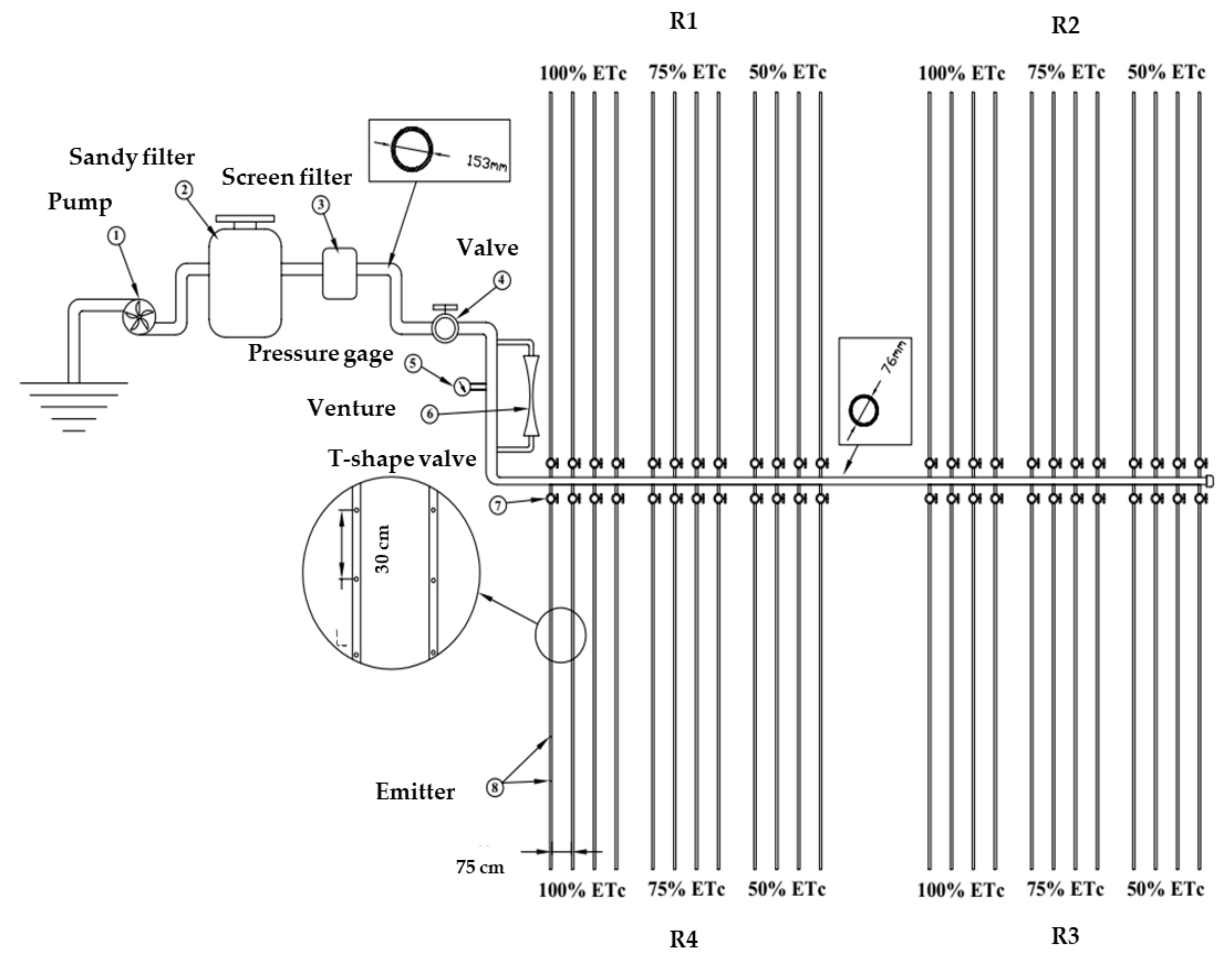
2.2. Experimental Design and Agronomic Practices
2.3. Irrigation Treatments
2.4. Measurements
2.4.1. Thermal Measurements
2.4.2. Spectral Reflectance Measurements
2.4.3. Plant Trait Measurements
2.5. Selection of Published and Newly Constructed Spectral Reflectance Indices
2.6. Statistical Analysis
3. Results and Discussion
3.1. Response of Growth Performance and Yield to Irrigation Regimes at Different Growth Stages
3.2. Thermal Canopy Temperature-Based Criteria and Performance in Assessment of Vegetative Growth Traits and Seed Yield
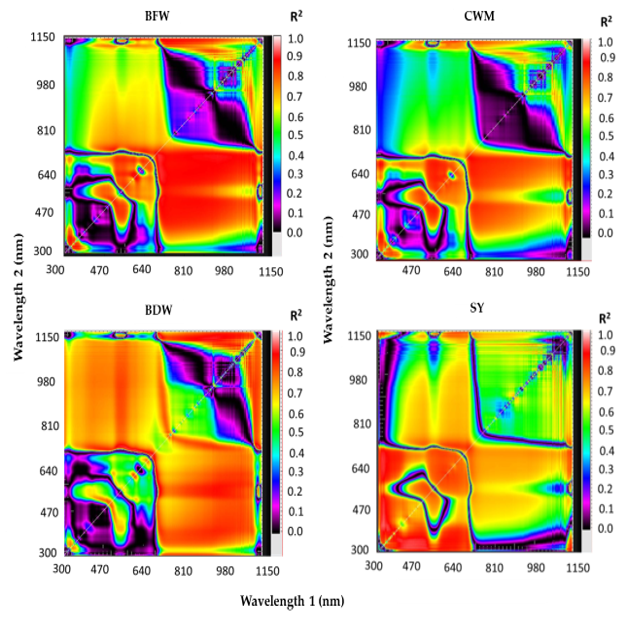
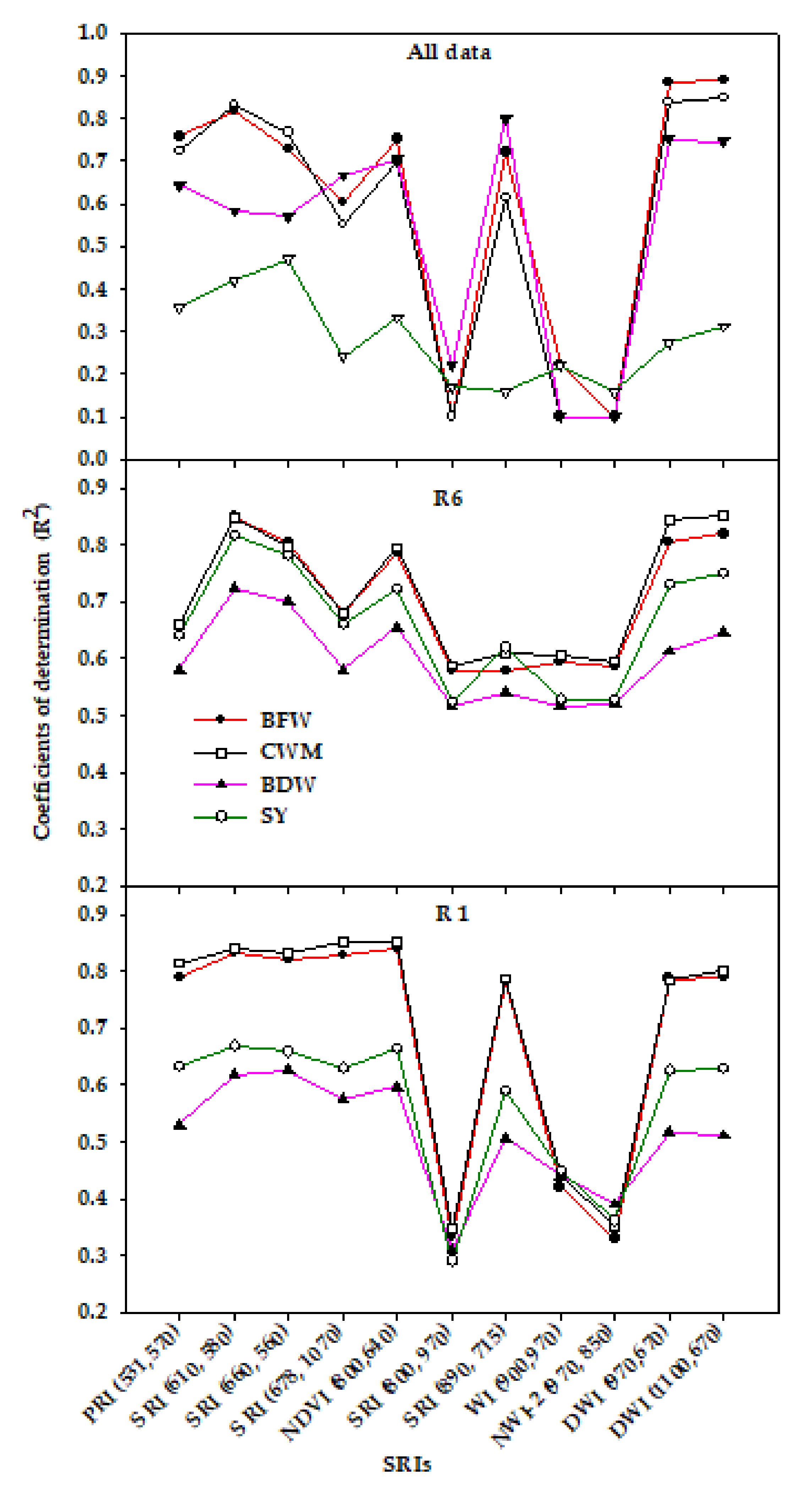
3.3. Canopy Spectral Reflectance and Performance for Assessment of Vegetative Growth Traits and Seed Yield
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- FAOSTAT. Food and Agriculture Organization of the United Nations Statistics Database. 2020. Available online: http://www.fao.org/faostat/en/#data/QC (accessed on 21 June 2020).
- Hirich, A.; Fatnassi, H.; Ragab, R.; Choukr-Allah, R. Prediction of climate change impact on corn grown in the south Morocco using the saltmed model. Irrig. Drain. 2016, 65, 9–18. [Google Scholar] [CrossRef]
- Lei, Y.; Zhang, H.; Chen, F.; Zhang, L. How rural land use management facilitates drought risk adaptation in a changing climate—A case study in arid northern China. Sci. Total Environ. 2016, 550, 192–199. [Google Scholar] [CrossRef] [PubMed]
- El-Hendawy, S.E.; Alotaibi, M.; Al-Suhaibani, N.; Al-Gaadi, K.; Hassan, W.; Dewir, Y.H.; Emam, M.A.E.-G.; Elsayed, S.; Schmidhalter, U. Comparative performance of spectral reflectance indices and multivariate modeling for assessing agronomic parameters in advanced spring wheat lines under two contrasting irrigation regimes. Front. Plant Sci. 2019, 10, 1537. [Google Scholar] [CrossRef] [PubMed]
- Fereres, F.; Soriano, M.A. Deficit irrigation for reducing agricultural water use. J. Exp. Bot. 2007, 58, 147–159. [Google Scholar] [CrossRef] [PubMed]
- El-Hendawy, S.; Hassan, W.; Al-Suhaibani, N.; Schmidhalter, U. Spectral assessment of drought tolerance indices and grain yield in advanced spring wheat lines grown under full and limited water irrigation. Agric. Water Manag. 2017, 182, 1–12. [Google Scholar] [CrossRef]
- Wijewardana, C.; Henry, W.B.; Reddy, K.R. Evaluation of drought tolerant maize germplasm to induced drought stress. Miss. Acad. Sci. 2017, 62, 316–329. [Google Scholar]
- Wijewardana, C.; Alsajri, F.A.; Irby, J.T.; Krutz, L.J.; Golden, B.; Henry, W.B.; Gao, W.; Reddy, K.R. Physiological assessment of water deficit in soybean using midday leaf water potential and spectral features. J. Plant Interact. 2019, 14, 533–543. [Google Scholar] [CrossRef]
- Farooq, M.; Wahid, A.; Ito, O.; Lee, D.J.; Siddique, K.H.M. Advances in drought resistance of rice. Crit. Rev. Plant Sci. 2009, 28, 199–217. [Google Scholar] [CrossRef]
- Pathan, S.M.; Lee, J.D.; Sleper, D.A.; Fritschi, F.B.; Sharp, R.E.; Carter, T.E.; Nelson, R.L.; King, C.A.; Schapaugh, W.T.; Ellersieck, M.R.; et al. Two soybean plant introductions display slow leaf wilting and reduced yield loss under drought. J. Agron. Crop Sci. 2014, 200, 231–236. [Google Scholar] [CrossRef]
- Popović, V.; Miladinović, J.; Vidić, M.; Vučković, S.; Dolijanović, Ž.; Ikanović, J.; Živanovic, L.; Kolarić, L. Drought Limiting factors in soybean production. The effect of irrigation on yield of soybean [Glycine max (L.) Merr.]. J. Inst. PKB Agroekonomik Belgrade 2015, 21, 11–21. [Google Scholar] [CrossRef]
- Jumrani, K.; Bhatia, V.S.; Pandey, G.P. Impact of elevated temperatures on specific leaf weight, stomatal density, photosynthesis and chlorophyll fluorescence in soybean. Photosynth. Res. 2017, 131, 333–350. [Google Scholar] [CrossRef] [PubMed]
- Jumrani, K.; Bhatia, V.S. Impact of combined stress of high temperature and water deficit on growth and seed yield of soybean. Physiol. Mol. Biol. Plants 2018, 24, 37–50. [Google Scholar] [CrossRef] [PubMed]
- Zhou, M.; Li, D.; Li, Z.; Hu, Q.; Yang, C.; Zhu, L.; Luo, H. Constitutive expression of a miR319 Gene alters plant development and enhances salt and drought tolerance in transgenic creeping Bentgrass. Plant Physiol. 2013, 161, 1375–1391. [Google Scholar] [CrossRef] [PubMed]
- Kumar, N.; Poddar, A.; Shankar, V.; Ojha, C.S.P.; Adeloye, A.J. Crop water stress index for scheduling irrigation of indian mustard (Brassica juncea) based on water use efficiency considerations. J. Agron. Crop Sci. 2019, 206, 148–159. [Google Scholar] [CrossRef]
- Nadeem, M.; Li, J.; Yahya, M.; Sher, A.; Ma, C.; Wang, X.; Qiu, L. Research Progress and Perspective on Drought Stress in Legumes: A Review. Int. J. Mol. Sci. 2019, 20, 2541. [Google Scholar] [CrossRef]
- Gutierrez, M.; Reynolds, M.P.; Raun, W.R.; Stone, M.L.; Klatt, A.R. Spectral water indices for assessing yield in elite bread wheat genotypes in well irrigated, water stressed, and high temperature conditions. Crop Sci. 2010, 50, 197–214. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, C.; Guo, N.; Li, Y.; Jian, S.; Yu, K. Determining the canopy water stress for spring wheat using canopy hyperspectral reflectance data in loess plateau semiarid regions. Spectrosc. Lett. 2015, 48, 492–498. [Google Scholar] [CrossRef]
- Elsayed, S.; Rischbeck, P.; Schmidhalter, U. Comparing the performance of active and passive reflectance sensors to assess the normalized relative canopy temperature and grain yield of drought-stressed barley cultivars. Field Crops Res. 2015, 177, 148–160. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Al-Suhaibani, N.; Elsayed, S.; Hassan, W.M.; Dewir, Y.H.; Refay, Y.; Abdella, K.A. Potential of the existing and novel spectral reflectance indices for estimating the leaf water status and grain yield of spring wheat exposed to different irrigation rates. Agric. Water Manag. 2019, 217, 356–373. [Google Scholar] [CrossRef]
- Zhang, F.; Zhou, G. Estimation of vegetation water content using hyperspectral vegetation indices: A comparison of crop water indicators in response to water stress treatments for summer maize. BMC Ecol. 2019, 19, 18. [Google Scholar] [CrossRef]
- Medrano, H.; Escalona, J.M.; Bota, J.; Gulías, J.; Flexas, J. Regulation of photosynthesis of C3 plants in response to progressive drought: Stomatal conductance as a reference parameter. Ann. Bot. 2002, 89, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Hura, T.; Hura, K.; Grzesiak, M.; Rzepka, A. Effect of long-term drought stress on leaf gas exchange and fluorescence parameters in C3 and C4 plants. Acta Physiol. Plant. 2007, 29, 103–113. [Google Scholar] [CrossRef]
- Jones, H.G.; Serraj, R.; Loveys, B.R.; Xiong, L.; Wheaton, A.; Price, A.H. Thermal infrared imaging of crop canopies for the remote diagnosis and quantification of plant responses to water stress in the field. Funct. Plant Biol. 2009, 36, 978–989. [Google Scholar] [CrossRef] [PubMed]
- Lapidot, O.; Ignat, T.; Rud, R.; Rog, I.; Alchanati, V.; Klein, T. Use of thermal imaging to detect evaporative cooling in coniferous and broadleaved tree species of the Mediterranean maquis. Agric. For. Meteorol. 2019, 271, 285–294. [Google Scholar] [CrossRef]
- Mak, M.; Babla, M.; Xu, S.; Carrigan, A.O.; Liu, X.; Gong, Y.; Holford, P.; Chen, Z. Leaf mesophyll K+, H+ and Ca2+ fluxes are involved in drought-induced decrease in photosynthesis and stomatal closure in soybean. Environ. Exp. Bot. 2014, 98, 1–12. [Google Scholar] [CrossRef]
- Mutava, R.N.; Jebakumar, S.; Prince, K.; Hasan, N.; Song, L.; Valliyodan, B.; Chen, W.; Nguyen, H.T. Understanding abiotic stress tolerance mechanisms in soybean: A comparative evaluation of soybean response to drought and flooding stress. Plant Physiol. Biochem. 2014, 86, 109–120. [Google Scholar] [CrossRef]
- Atti, S.; Bonnell, R.; Smith, D.; Prasher, S. Response of an indeterminate soybean {Glycine Max (L.) Merr} to chronic water deficit during reproductive development under greenhouse conditions. Can. Water Resour. J. 2004, 29, 209–222. [Google Scholar] [CrossRef]
- Jerbi, T.; Wuyts, N.; Cane, M.A.; Faux, P.F.; Draye, X. High resolution imaging of maize (Zea mays) leaf temperature in the field: The key role of the regions of interest. Funct. Plant Biol. 2015, 42, 858–864. [Google Scholar] [CrossRef]
- Elsayed, S.; Elhoweity, M.; Ibrahim, H.H.; Dewir, Y.H.; Migdadic, H.M.; Schmidhalter, U. Thermal imaging and passive reflectance sensing to estimate the water status and grain yield of wheat under different irrigation regimes. Agric. Water Manag. 2017, 189, 98–110. [Google Scholar] [CrossRef]
- Dejonge, K.C.; Taghvaeian, S.; Trout, T.J.; Comas, L.H. Comparison of canopy temperature-based water stress indices for maize. Agric. Water Manag. 2015, 156, 51–62. [Google Scholar] [CrossRef]
- Masseroni, D.; Addin, J.; Tyrrell, R.; Mareels, I.; Gandolfi, C.; Facchi, A. Towards a smart automated surface irrigation management in rice-growing areas in Italy. Agric. Eng. 2017, 48, 42–48. [Google Scholar] [CrossRef]
- Pou, A.; Diago, M.P.; Medrano, H.; Baluja, J.; Tardaguila, J. Validation of thermal indices for water status identification in grapevine. Agric. Water Manag. 2014, 134, 60–72. [Google Scholar] [CrossRef]
- Yu, M.H.; Ding, G.D.; Gao, G.L.; Zhao, Y.Y.; Sai, L.Y.K. Using plant temperature to evaluate plant response of stomatal conductance to soil moisture deficit. Forest 2015, 6, 3748–3762. [Google Scholar] [CrossRef]
- Asemanrafat, M.; Honar, T. Effect of water stress and plant density on canopy temperature, yield components and protein concentration of red beans (Phaseolus vulgaris L. cvAkhtar). Int. J. Plant Prod. 2017, 11, 241–258. [Google Scholar]
- Lobos, G.A.; Matus, I.; Rodriguez, A.; Romero-Bravo, S.; Araus, J.L.; Pozo, A.D. Wheat genotypic variability in grain yield and carbon isotope discrimination under Mediterranean conditions assessed by spectral reflectance. J. Integr. Plant Biol. 2014, 56, 470–479. [Google Scholar] [CrossRef]
- Rapaport, T.; Hochberg, U.; Cochavi, A.; Karnieli, A.; Rachmilevitch, S. The potential of the spectral ‘water balance index’ (WABI) for crop irrigation scheduling. New Phytol. 2017, 216, 741–757. [Google Scholar] [CrossRef] [PubMed]
- Becker, E.; Schmidhalter, U. Evaluation of yield and drought using active and passive spectral sensing systems at the reproductive stage in wheat. Front. Plant Sci. 2017, 8, 379. [Google Scholar] [CrossRef]
- Silva-Perez, V.; Molero, G.; Serbin, S.P.; Condon, A.G.; Reynolds, M.P.; Furbank, R.T.; Evans, J.R. Hyperspectral reflectance as a tool to measure biochemical and physiological traits in wheat. J. Exp. Bot. 2018, 69, 483–496. [Google Scholar] [CrossRef]
- Lobos, G.A.; Romero, S.; Escobar, A.; Estrada, F.; Caligari, P.D.S.; Poblete-Echeverría, C.; Ahumada, L.; Zuñiga, M. Fast and non-destructive prediction of gas exchange in olive orchards (Olea europaea L.) under different soil water conditions. Acta Hortic. 2014, 1057, 329–334. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Al-Suhaibani, N.; Alotaibi, M.; Hassan, W.; Elsayed, S.; Tahir, M.U.; Ahmed Ibrahim Mohamed, A.I.; Schmidhalter, U. Estimating growth and photosynthetic properties of wheat grown in simulated saline field conditions using hyperspectral reflectance sensing and multivariate analysis. Sci. Rep. 2019, 9, 1647. [Google Scholar] [CrossRef]
- Seelig, H.D.; Hoehn, A.; Stodieck, L.S.; Klaus, D.M.; Adams, W.W.; Emery, W.J. Relations of remote sensing leaf water indices to leaf water thickness in cowpea, bean, and sugar beet plants. Remote Sens. Environ. 2008, 112, 445–455. [Google Scholar] [CrossRef]
- Bayat, B.; van der Tol, C.; Verhoef, W. Remote sensing of grass response to drought stress using spectroscopic techniques and canopy reflectance model inversion. Remote Sens. 2016, 8, 557. [Google Scholar] [CrossRef]
- Peñuelas, J.; Filella, I.; Biel, C.; Serrano, L.; Savé, R. The reflectance at the 950–970 nm region as an indicator of plant water status. Int. J. Remote Sens. 1993, 14, 1887–1905. [Google Scholar] [CrossRef]
- Winterhalter, L.; Mistele, B.; Jampatong, S.; Schmidhalter, U. High throughput phenotyping of canopy water mass and canopy temperature in well-watered and drought stressed tropical maize hybrids in the vegetative stage. Eur. J. Agron. 2011, 35, 22–32. [Google Scholar] [CrossRef]
- Christenson, B.S.; Schapaugh, W.T.; Nan, A.J.; Price, K.P.; Prasad, V.; Fritz, A.K. Predicting soybean relative maturity and seed yield using canopy reflectance. Crop Sci. 2016, 56, 625–643. [Google Scholar] [CrossRef]
- Garriga, M.; Romero-Bravo, S.; Estrada, F.; Escobar, A.; Matus, I.A.; del Pozo, A.; Astudillo, C.A.; Lobos, G.A. Assessing wheat traits by spectral reflectance: Do we really need to focus on predicted trait-values or directly identify the elite genotypes group? Front. Plant Sci. 2017, 8, 280. [Google Scholar] [CrossRef] [PubMed]
- El-Hendawy, S.E.; Al-Suhaibani, N.; Elsayed, S.; Refay, Y.; Alotaibi, M.; Dewir, Y.H.; Hassan, W.M.; Schmidhalter, U. Combining biophysical parameters, spectral indices and multivariate hyperspectral models for estimating yield and water productivity of spring wheat across different agronomic practices. PLoS ONE 2019, 14, e0212294. [Google Scholar]
- Allen, R.G.; Pereira, L.S.; Raes, D.; Smith, M. Crop Evapotranspiration Guidelines for Computing Crop Water Requirements; (Irrigation and Drainage Paper 56); FAO of the United Nations: Rome, Italy, 1998. [Google Scholar]
- Gonzalez-Dugo, M.P.; Moran, M.S.; Mateos, L.; Bryant, R. Canopy temperature variability as an indicator of crop water stress severity. Irrig. Sci. 2006, 24, 233. [Google Scholar] [CrossRef]
- Jones, H.G. Application of thermal imaging and infrared sensing in plant physiology and eco-physiology. In Advances in Botanical Research; Academic Press: Cambridge, MA, USA, 2004; pp. 107–163. [Google Scholar]
- Jackson, R.D. Canopy temperature and crop water stress. In Advances in Irrigation; Academic Press, Inc.: Cambridge, MA, USA, 1981. [Google Scholar]
- Zarco-Tejada, P.J.; Gonzalez-Dugo, V.; Berni, J.A.J. Fluorescence, temperature and narrow-band indices acquired from a UAV platform for water stress detection using a micro-hyperspectral imager and a thermal camera. Remote Sens. Environ. 2011, 117, 322–337. [Google Scholar] [CrossRef]
- Yi, Q.; Bao, A.; Wang, Q.; Zhao, J. Estimation of leaf water content in cotton by means of hyperspectral indices. Comput. Electron. Agric. 2013, 90, 144–151. [Google Scholar] [CrossRef]
- Kim, Y.; Glenn, D.M.; Park, J.; Ngugi, H.K.; Lehman, B.L. Hyperspectral image analysis for plant stress detection. In Proceedings of the American Society of Agricultural and Biological Engineers, Annual International Meeting, Pittsburgh, PA, USA, 20–23 June 2010. Paper No. 1009114. [Google Scholar]
- Köksal, E.S. Hyperspectral reflectance data processing through cluster and principal component analysis for estimating irrigation and yield related indicators. Agric. Water Manag. 2011, 98, 1317–1328. [Google Scholar] [CrossRef]
- Clevers, J.G.P.W.; Kooistra, L.; Schaepman, M.E. Using spectral information from the NIR water absorption features for the retrieval of canopy water content. Int. J. Appl. Earth Obs. Geoinf. 2008, 10, 338–397. [Google Scholar] [CrossRef]
- Desclaux, D.; Huynh, T.T.; Roumet, P. Identification of soybean plant characteristics that indicate the timing of drought stress. Crop Sci. 2000, 40, 716–722. [Google Scholar] [CrossRef]
- Ku, Y.S.; Au-Yeung, W.K.; Yung, Y.L.; Li, M.W.; Wen, C.Q.; Liu, X.; Lam, H.M. Drought stress and tolerance in soybean. In A Comprehensive Survey of International Soybean Research—Genetics, Physiology, Agronomy and Nitrogen Relationships; Board, J.E., Ed.; InTech: New York, NY, USA, 2013; pp. 209–237. [Google Scholar]
- Khan, M.S.A.; Karim, M.A.; Haque, M.M. Genotypic differences in growth and ions accumulation in soybean under NaCl salinity and water stress conditions. Bangladesh Agron. J. 2014, 18, 267–288. [Google Scholar] [CrossRef][Green Version]
- Board, J.E.; Kahlon, C.S. Soybean yield formation: What controls it and how it can be improved. Soybean Physiol. Biochem. 2011. [Google Scholar] [CrossRef][Green Version]
- Frederick, J.R.; Camp, C.R.; Bauer, P.J. Drought-stress effects on branch and main stem seed yield and yield components of determinate soybean. Crop Sci. 2001, 41, 759–763. [Google Scholar] [CrossRef]
- Liu, G.; Guo, A.; Ren, S.; An, S.; Zhao, H. Compensatory effects of re-watering on summer maize threatened by water stress at seedling period. Chin. J. Ecol. 2004, 23, 24–29. [Google Scholar]
- Makbul, S.; Guler, N.S.; Durmus, N.; Guven, S. Changes in anatomical and physiological parameters of soybean under drought stress. Turk. J. Bot. 2011, 35, 369–377. [Google Scholar]
- Chowdhury, J.; Karim, M.; Khaliq, Q.; Solaiman, A.; Ahmed, J. Screening of soybean (Glycine max L.) genotypes under water stress condition. Bangladesh J. Agric. Res. 2016, 41, 441–450. [Google Scholar] [CrossRef][Green Version]
- José, A.; Rodrigues, J.; Filho, M.; Rodrigues, C.; Sales, G.; Célia, R.; Pires, D.M.; Machado, E.C. Source-sink relationships in two soybean cultivars with indeterminate growth under water deficit. Bragantia 2018, 77, 23–35. [Google Scholar]
- Wei, Y.; Jin, J.; Jiang, S.; Ning, S.; Liu, L. Quantitative response of soybean development and yield to drought stress during different growth stages in the Huaibei Plain, China. Agronomy 2018, 8, 97. [Google Scholar] [CrossRef]
- Kashiwagi, J.; Krishnamurthy, L.; Upadhyaya, H.D.; Gaur, P.M. Rapid screening technique for canopy temperature status and its relevance to drought tolerance improvement in chickpea. J. SAT Agric. Res. 2008, 6, 105–114. [Google Scholar]
- Poirier-Pocovi, M.; Bailey, N.B. Sensitivity analysis of four crop water stress indices to ambient environmental conditions and stomatal conductance. Sci. Hortic. 2020, 259, 108825. [Google Scholar] [CrossRef]
- Colaizzi, P.D.; O’Shaughnessy, S.; Evett, S.; Howell, T.A. Using plant canopy temperature to improve irrigated crop management. In Proceedings of the 24th Annual Central Plains Irrigation Conference, Colby, KS, USA, 21–22 February 2012. [Google Scholar]
- Prashar, A.; Yildiz, J.; McNicol, J.W.; Bryan, G.J.; Jones, H.G. Infra-red thermography for high throughput field phenotyping in Solanum tuberosum. PLoS ONE 2013, 8, e65816. [Google Scholar] [CrossRef]
- Taghvaeian, C.S.; Chávez, J.S.; Hansen, N.C. Infrared thermometry to estimate crop water stress index and water use of irrigated maize in Northeastern. Remote Sens. 2012, 4, 3619–3637. [Google Scholar] [CrossRef]
- Han, M.; Zhang, H.; DeJonge, K.C.; Comas, L.H.; Gleason, S. Comparison of three crop water stress index models with sap flow measurements in maize. Agric. Water Manag. 2018, 203, 366–375. [Google Scholar] [CrossRef]
- Irmak, S.; Haman, D.Z.; Bastug, R. Determination of crop water stress index for irrigation timing and yield estimation of corn. Agron. J. 2002, 92, 1221–1227. [Google Scholar] [CrossRef]
- Steele, D.D.; Stegman, E.C.; Gregor, B.L. Field comparison of irrigation scheduling methods for corn. Trans. ASAE 1994, 37, 1197–1203. [Google Scholar] [CrossRef]
- O’Shaughnessy, S.A.; Evett, S.R.; Colaizzi, P.D.; Howell, T.A. Using radiation thermography and thermometry to evaluate crop water stress in soybean and cotton. Agric. Water Manag. 2011, 98, 1523–1535. [Google Scholar] [CrossRef]
- Kumar, M.; Govindasamy, V.; Rane, J.; Singh, A.K.; Choudhary, R.L.; Raina, S.K.; George, P.; Aher, L.K.; Singh, N.P. Canopy temperature depression (CTD) and canopy greenness associated with variation in seed yield of soybean genotypes grown in semi-arid environment. S. Afr. J. Bot. 2017, 113, 230–238. [Google Scholar] [CrossRef]
- Balota, M.; Payne, W.A.; Evett, S.R.; Lazar, M.D. Canopy temperature depression sampling to assess grain yield and genotypic differentiation in winter wheat. Crop Sci. 2007, 47, 1518–1529. [Google Scholar] [CrossRef]
- El-Hendawy, S.E.; Al-Suhaibani, N.; Dewir, Y.H.; El-Sayed, S.; Alotaibi, M.; Hassan, W.M.; Refay, Y.; Tahir, M.U. Ability of modified spectral reflectance indices for estimating growth and photosynthetic efficiency of wheat under saline field conditions. Agronomy 2019, 9, 35. [Google Scholar] [CrossRef]
- Lobos, G.A.; Escobar-Opazo, A.; Estrada, F.; Romero-Bravo, S.; Garriga, M.; del Pozo, A.; Poblete-Echeverría, C.; Gonzalez-Talice, J.; González-Martinez, L.; Caligari, P. Spectral reflectance modeling by wavelength selection: Studying the scope for blueberry physiological breeding under contrasting water supply and heat conditions. Remote Sens. 2019, 11, 329. [Google Scholar] [CrossRef]
- Yue, J.; Feng, H.; Tian, Q.; Zhou, C.A. Robust spectral angle index for remotely assessing soybean canopy chlorophyll content in different growing stages. Plant Methods 2020, 16, 104. [Google Scholar] [CrossRef] [PubMed]
- Vescovo, L.; Wohlfahrt, G.; Balzarolo, M.; Pilloni, S.; Sottocornola, M.; Rodeghiero, M.; Gianelle, D. New spectral vegetation indices based on the near-infrared shoulder wavelengths for remote detection of grassland phytomass. Int. J. Remote Sens. 2012, 33, 2178–2195. [Google Scholar] [CrossRef]
- Yao, X.; Jia, W.; Si, H.; Guo, Z.; Tian, Y.; Liu, X.; Cao, W.; Zhu, Y. Exploring novel bands and key index for evaluating leaf equivalent water thickness in wheat using hyperspectra influenced by nitrogen. PLoS ONE 2014, 9, e96352. [Google Scholar] [CrossRef]
- Weber, V.S.; Araus, J.L.; Cairns, J.E.; Sanchez, C.; Melchinger, A.E.; Orsini, E. Prediction of grain yield using reflectance spectra of canopy and leaves in maize plants grown under different water regimes. Field Crops Res. 2012, 128, 82–90. [Google Scholar] [CrossRef]
- Rischbeck, P.; Elsayed, S.; Mistele, B.; Barmeier, G.; Heil, K.; Schmidhalter, U. Data fusion of spectral, thermal and canopy height parameters for improved yield prediction of drought stressed spring barley. Eur. J. Agron. 2016, 78, 44–59. [Google Scholar] [CrossRef]
- Wang, C.; Feng, M.; Yang, W.; Ding, G.; Xiao, L.; Li, G.; Liu, T. Extraction of sensitive bands for monitoring the winter wheat (Triticum aestivum) growth status and yields based on the spectral reflectance. PLoS ONE 2017, 12, e0167679. [Google Scholar] [CrossRef][Green Version]
- Carter, G.A. Primary and secondary effects of water content on the spectral reflectance of leaves. Am. J. Bot. 1991, 78, 916–924. [Google Scholar] [CrossRef]
- Scoffoni, C.; Vuong, C.; Diep, S.; Cochard, H.; Sack, L. Leaf shrinkage with dehydration: Coordination with hydraulic vulnerability and drought tolerance. Plant Physiol. 2014, 164, 1772–1788. [Google Scholar] [CrossRef]
- Suárez, L.; Zarco-Tejada, P.J.; Sepulcre-Cantó, G.; Pérez-Priego, O.; Miller, J.R.; Jiménez-Muñoz, J.C.; Sobrino, J. Assessing canopy PRI for water stress detection with diurnal airborne imagery. Remote Sens. Environ. 2008, 112, 560–575. [Google Scholar] [CrossRef]
- Prasad, B.; Carver, B.F.; Stone, M.L.; Babar, M.A.; Raun, W.R.; Klatt, A.R. Potential use of spectral reflectance indices as a selection tool for grain yield in winter wheat under Great Plains conditions. Crop Sci. 2007, 47, 1426–1440. [Google Scholar] [CrossRef]
- El-Hendawy, S.; Al-Suhaibani, N.; Salem, A.; Ur Rehman, S.; Schmidhalter, U. Spectral reflectance indices as a rapid nondestructive phenotyping tool for estimating different morphophysiological traits of contrasting spring wheat germplasms under arid conditions. Turk. J. Agric. For. 2015, 39, 572–587. [Google Scholar] [CrossRef]
- Elazab, A.; Bort, J.; Zhou, B.; Serret, M.D.; Nieto-Taladriz, M.T.; Araus, J.L. The combined use of vegetation indices and stable isotopes to predict durum wheat grain yield under contrasting water conditions. Agric. Water Manag. 2015, 158, 196–208. [Google Scholar] [CrossRef]
- Aparicio, N.; Villegas, D.; Casadesús, J.; Araus, J.L.; Royo, C. Spectral vegetation indices and nondestructive tools for determining durum wheat yield. Agron. J. 2000, 92, 83–91. [Google Scholar] [CrossRef]
- Babar, M.A.; Reynolds, M.P.; van Ginkel, M.; Klatt, A.R.; Raun, W.R.; Stone, M.L. Spectral reflectance indices as a potential indirect selection criteria for wheat yield under irrigation. Crop Sci. 2006, 46, 578–588. [Google Scholar] [CrossRef]

| Year | Months | Temperature (°C) | Wind Speed (m s−1) | Relative Humidity (%) | Total Solar Radiation (MJ m−2 day−1) | Net Solar Radiation (MJ m−2 day−1) | |
|---|---|---|---|---|---|---|---|
| Maximum | Minimum | ||||||
| 2016 | April | 30.4 | 16.2 | 0.74 | 53.4 | 23.4 | 12.51 |
| May | 32.1 | 17.0 | 0.81 | 47.2 | 25.6 | 14.71 | |
| June | 34.9 | 17.1 | 0.61 | 53.3 | 26.1 | 15.00 | |
| July | 35.5 | 22.0 | 0.53 | 62.6 | 22.9 | 14.31 | |
| August | 35.9 | 22.7 | 0.47 | 61.8 | 20.8 | 12.00 | |
| 2017 | April | 32.7 | 17.0 | 0.62 | 51.2 | 24.5 | 14.41 |
| May | 33.5 | 16.0 | 0.74 | 49.3 | 26.0 | 15.47 | |
| June | 35.9 | 20.8 | 0.65 | 56.4 | 27.6 | 17.14 | |
| July | 36.5 | 23.2 | 0.51 | 59.7 | 23.8 | 14.08 | |
| August | 35.7 | 23.0 | 0.42 | 63.1 | 22.3 | 13.43 | |
| Spectral Reflectance Indices | Formula | References |
|---|---|---|
| Photochemical reflectance index (PRI, (531,570)) | (R531 − R570)/(R531 + R570) | [53] |
| Simple ratio based on 610 and 550 nm (SRI(610,580)) | R610/R580 | This work |
| Simple ratio based on 660 and 560 nm (SRI(660,560)) | R660/R560 | This work |
| Simple ratio based on 678 and 1070 nm (SRI(678,1070)) | R678/R1070 | [54] |
| Normalized difference vegetation index (NDVI(800,640)) | (R800 − R640)/(R800 + R640) | [55] |
| Simple ratio based on 800 and 970 nm (SRI(800,970)) | R800/R970 | [56] |
| Simple ratio based on 890 and 715 nm (SRI(890,715)) | R890/R715 | [45] |
| Water index (WI(900,970)) | R900/R970 | [57] |
| Normalized water index 2 (NWI-2(970,850)) | (R970 − R850)/(R970 + R850) | [17] |
| Development of water index (DWI970–670) | R970/R670 | This work |
| Development of Water index (DWI1100–670) | R1100/R670 | This work |
| Irrigation Water Regimes | ||||||
|---|---|---|---|---|---|---|
| 100% ETc | 75% ETc | 50% ETc | 100% ETc | 75% ETc | 50% ETc | |
| 2016 | 2017 | |||||
| SY (Mg ha−1) | 3.18a | 2.45b | 1.63c | 3.25a | 2.57b | 1.654c |
| R1 | R6 | |||||
| BFW (Mg ha−1) | 6.11a | 5.10ab | 3.96b | 13.32a | 9.01b | 5.17c |
| BDW (Mg ha−1) | 1.29a | 1.19ab | 1.07b | 4.08a | 3.33b | 2.44c |
| CWM (Mg ha−1) | 4.83a | 3.92ab | 2.90b | 9.24a | 5.68b | 2.74c |
| CWSI | 0.18c | 0.45b | 0.62a | 0.29c | 0.61b | 0.78a |
| NRCT | 0.17c | 0.42b | 0.58a | 0.30c | 0.60b | 0.79a |
| PRI(531,570) | −0.084a | −0.100b | −0.122c | −0.040a | −0.065b | −0.091c |
| SRI(610,580) | 0.952c | 1.00b | 1.048a | 0.862c | 0.934b | 0.999a |
| SRI(660,560) | 0.731c | 0.878b | 1.055a | 0.526c | 0.712b | 0.919a |
| SRI(678,1070) | 0.254c | 0.358b | 0.501a | 0.091c | 0.167b | 0.208a |
| NDVI(800,640) | 0.800a | 0.454b | 0.351c | 0.819a | 0.665b | 0.593c |
| SRI(800,970) | 1.071a | 1.045b | 1.026c | 0.991a | 0.903b | 0.843c |
| SRI(890,715) | 1.726a | 1.444b | 1.325c | 2.556a | 2.047b | 2.051b |
| WI(900,970) | 1.071a | 1.050b | 1.029c | 1.094a | 1.028b | 0.985c |
| NWI-2(970,850) | −0.039c | −0.026b | −0.016a | −0.022c | 0.018b | 0.046a |
| DWI(970,670) | 4.408a | 2.908b | 2.150c | 13.794a | 6.905b | 5.401b |
| DWI(1100,670) | 3.230a | 2.379b | 1.658c | 7.959a | 4.354b | 3.552b |
| Treatments | BFW | BDW | CWM | SY | |||||
|---|---|---|---|---|---|---|---|---|---|
| CWSI | NRCT | CWSI | NRCT | CWSI | NRCT | CWSI | NRCT | ||
| Growth stages | R1 | 0.89 L* | 0.90 L* | 0.67 L* | 0.75 L* | 0.88 L* | 0.87 L* | 0.82 L* | 0.82 L* |
| R6 | 0.94 L* | 0.90 L* | 0.88 L* | 0.90 L* | 0.93 L* | 0.83 L* | 0.90 L* | 0.84 L* | |
| Irrigation water regimes | 100%ETc | 0.63 L* | 0.79 L* | 0.65 L* | 0.71 L* | 0.62 Q* | 0.82 L* | 0.002 L | 0.20 Q |
| 75% ETc | 0.72 L* | 0.78 L* | 0.72 L* | 0.77 L* | 0.84 Q* | 0.74 L* | 0.003 L | 0.14 Q | |
| 50% ETc | 0.78 L* | 0.78 L* | 0.72 L* | 0.69 L* | 0.54 Q* | 0.61 Q* | 0.16 Q | 0.21 Q | |
| R1 | 100%ETc | 0.39 L* | 0.30 Q* | 0.05 Q | 0.14 Q | 0.50 L* | 0.52 Q* | 0.01 L | 0.35 Q* |
| 75%ETc | 0.02 L | 0.36 Q* | 0.20 Q | 0.02 Q | 0.03 L | 0.27 Q* | 0.02 L | 0.53 Q* | |
| 50%ETc | 0.55 L* | 0.38 L* | 0.42 Q* | 0.90 Q* | 0.13 Q | 0.35 Q* | 0.17 Q | 0.80 Q* | |
| R6 | 100%ETc | 0.003 L | 0.85 Q* | 0.01 L | 0.19 Q | 0.001 L | 0.83 Q* | 0.48 Q* | 0.42 Q* |
| 75% ETc | 0.12 Q | 0.30 Q* | 0.20 Q | 0.09 L | 0.21 L | 0.43 Q* | 0.43 Q* | 0.41 Q* | |
| 50% ETc | 0.05 L | 0.32 Q* | 0.49 L* | 0.96 L* | 0.52 Q* | 0.58 Q* | 0.80 L* | 0.61 L* | |
| SRIs | R1 | R6 | ||||||
|---|---|---|---|---|---|---|---|---|
| BFW | CWM | BDW | SY | BFW | CWM | BDW | SY | |
| 100% ETc | ||||||||
| PRI(531,570) | 0 | 0.05 | 0.23 | 0.05 | 0.10 | 0.21 | 0.46 | 0.19 |
| SRI(610,580) | 0.03 | 0.13 | 0.13 | 0.12 | 0.49 | 0.65 | 0.26 | 0.34 |
| SRI(660,560) | 0.03 | 0.12 | 0.12 | 0.10 | 0.48 | 0.64 | 0.25 | 0.35 |
| SRI(678,1070) | 0.03 | 0.18 | 0.30 | 0.11 | 0.67 | 0.72 | 0.04 | 0.34 |
| NDVI(800,640) | 0 | 0.10 | 0.32 | 0.05 | 0.59 | 0.70 | 0.12 | 0.39 |
| SRI(800,970) | 0.17 | 0.49 | 0.27 | 0.14 | 0.45 | 0.56 | 0.15 | 0.45 |
| SRI(890,715) | 0 | 0.09 | 0.42 | 0.03 | 0.54 | 0.48 | 0.02 | 0.35 |
| WI(900,970) | 0.14 | 0.41 | 0.25 | 0.14 | 0.44 | 0.54 | 0.14 | 0.47 |
| NWI-2(970,850) | 0.16 | 0.46 | 0.27 | 0.12 | 0.46 | 0.56 | 0.14 | 0.46 |
| DWI(970,670) | 0.05 | 0.22 | 0.27 | 0.08 | 0.54 | 0.68 | 0.20 | 0.40 |
| DWI(1100,670) | 0.06 | 0.25 | 0.25 | 0.09 | 0.60 | 0.66 | 0.05 | 0.53 |
| 75% ETc | ||||||||
| PRI(531,570) | 0 | 0 | 0 | 0.70 | 0.14 | 0.14 | 0.02 | 0.05 |
| SRI(610,580) | 0 | 0 | 0.01 | 0.48 | 0.25 | 0.30 | 0 | 0.01 |
| SRI(660,560) | 0.01 | 0 | 0.00 | 0.50 | 0.28 | 0.27 | 0.03 | 0 |
| SRI(678,1070) | 0.01 | 0 | 0.08 | 0.62 | 0.35 | 0.29 | 0.07 | 0.17 |
| NDVI(800,640) | 0 | 0.03 | 0.08 | 0.71 | 0.18 | 0.22 | 0 | 0 |
| SRI(800,970) | 0.02 | 0.01 | 0 | 0.46 | 0.01 | 0.02 | 0 | 0.16 |
| SRI(890,715) | 0.03 | 0.08 | 0.12 | 0.71 | 0.15 | 0.13 | 0.02 | 0.05 |
| WI(900,970) | 0 | 0 | 0 | 0.63 | 0.01 | 0.01 | 0 | 0.20 |
| NWI-2(970,850) | 0 | 0 | 0 | 0.54 | 0.02 | 0.02 | 0 | 0.17 |
| DWI(970,670) | 0.01 | 0 | 0.07 | 0.59 | 0.38 | 0.36 | 0.05 | 0.10 |
| DWI(1100,670) | 0.03 | 0 | 0.06 | 0.54 | 0.35 | 0.30 | 0.06 | 0.19 |
| 50% ETc | ||||||||
| PRI(531,570) | 0.12 | 0.47 | 0.15 | 0.35 | 0.11 | 0.03 | 0 | 0 |
| SRI(610,580) | 0.28 | 0.31 | 0.27 | 0.37 | 0.08 | 0.03 | 0.01 | 0 |
| SRI(660,560) | 0.22 | 0.33 | 0.20 | 0.35 | 0.06 | 0.02 | 0.01 | 0 |
| SRI(678,1070) | 0.28 | 0.39 | 0.20 | 0.31 | 0.13 | 0.03 | 0 | 0 |
| NDVI(800,640) | 0.29 | 0.42 | 0.26 | 0.39 | 0.11 | 0.03 | 0 | 0 |
| SRI(800,970) | 0.05 | 0.38 | 0.08 | 0.36 | 0.13 | 0 | 0.23 | 0.02 |
| SRI(890,715) | 0.33 | 0.44 | 0.29 | 0.40 | 0.17 | 0.04 | 0 | 0.00 |
| WI(900,970) | 0.09 | 0.44 | 0.12 | 0.32 | 0.03 | 0 | 0.15 | 0.01 |
| NWI-2(970,850) | 0.07 | 0.41 | 0.11 | 0.36 | 0.07 | 0 | 0.20 | 0.02 |
| DWI(970,670) | 0.34 | 0.35 | 0.27 | 0.35 | 0.10 | 0.03 | 0 | 0.00 |
| DWI(1100,670) | 0.19 | 0.49 | 0.09 | 0.47 | 0.11 | 0.03 | 0 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Elmetwalli, A.H.; El-Hendawy, S.; Al-Suhaibani, N.; Alotaibi, M.; Tahir, M.U.; Mubushar, M.; Hassan, W.M.; Elsayed, S. Potential of Hyperspectral and Thermal Proximal Sensing for Estimating Growth Performance and Yield of Soybean Exposed to Different Drip Irrigation Regimes Under Arid Conditions. Sensors 2020, 20, 6569. https://doi.org/10.3390/s20226569
Elmetwalli AH, El-Hendawy S, Al-Suhaibani N, Alotaibi M, Tahir MU, Mubushar M, Hassan WM, Elsayed S. Potential of Hyperspectral and Thermal Proximal Sensing for Estimating Growth Performance and Yield of Soybean Exposed to Different Drip Irrigation Regimes Under Arid Conditions. Sensors. 2020; 20(22):6569. https://doi.org/10.3390/s20226569
Chicago/Turabian StyleElmetwalli, Adel H., Salah El-Hendawy, Nasser Al-Suhaibani, Majed Alotaibi, Muhammad Usman Tahir, Muhammad Mubushar, Wael M. Hassan, and Salah Elsayed. 2020. "Potential of Hyperspectral and Thermal Proximal Sensing for Estimating Growth Performance and Yield of Soybean Exposed to Different Drip Irrigation Regimes Under Arid Conditions" Sensors 20, no. 22: 6569. https://doi.org/10.3390/s20226569
APA StyleElmetwalli, A. H., El-Hendawy, S., Al-Suhaibani, N., Alotaibi, M., Tahir, M. U., Mubushar, M., Hassan, W. M., & Elsayed, S. (2020). Potential of Hyperspectral and Thermal Proximal Sensing for Estimating Growth Performance and Yield of Soybean Exposed to Different Drip Irrigation Regimes Under Arid Conditions. Sensors, 20(22), 6569. https://doi.org/10.3390/s20226569








