Optimization and Analytical Behavior of Electrochemical Sensors Based on the Modification of Indium Tin Oxide (ITO) Using PANI/MWCNTs/AuNPs for Mercury Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals and Reagents
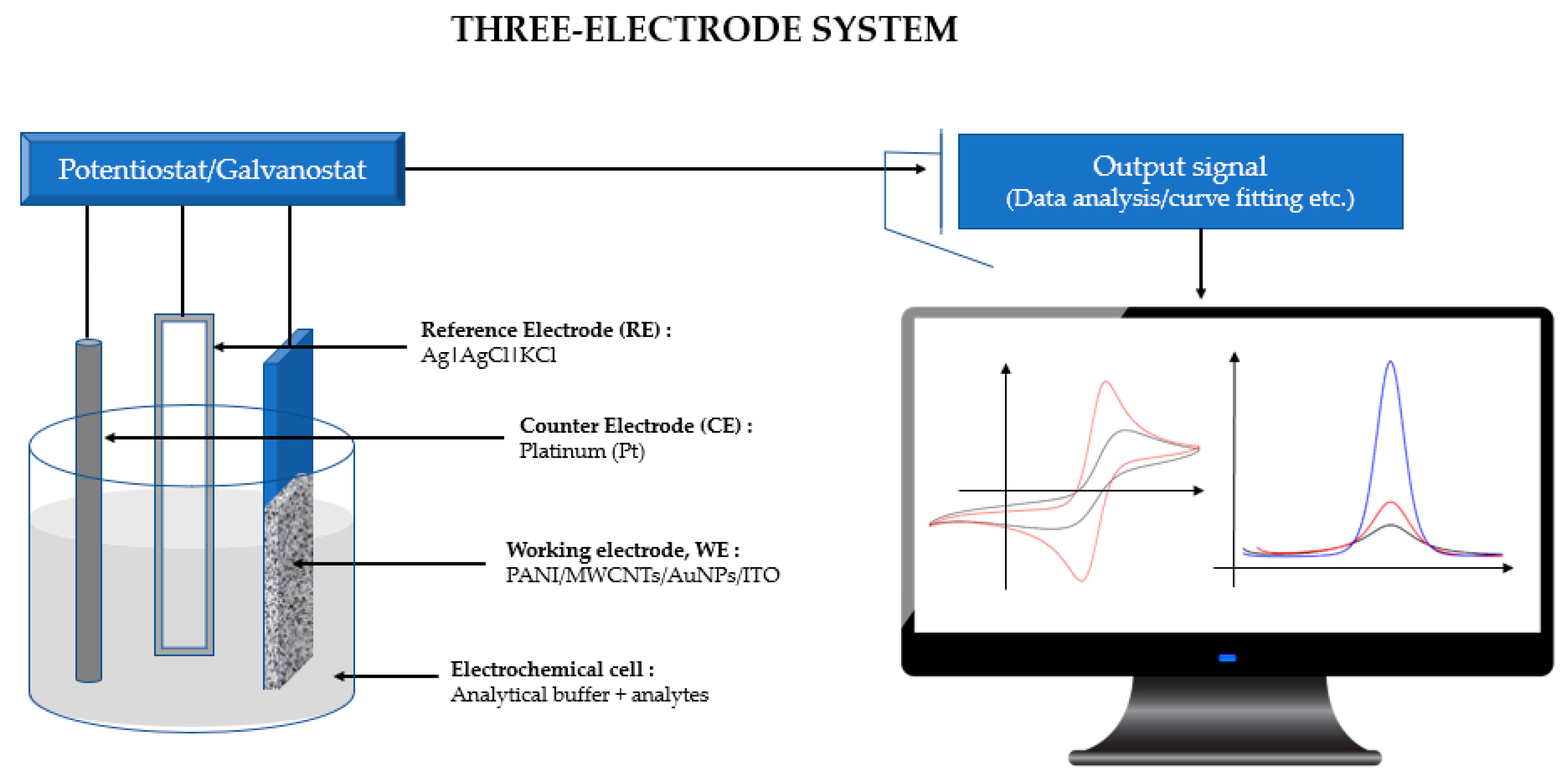
2.2. Instrumentations
2.3. Electrode Preparation and Modification
2.4. Electrode Characterizations
2.5. Optimization of Analyte and Operating Conditions
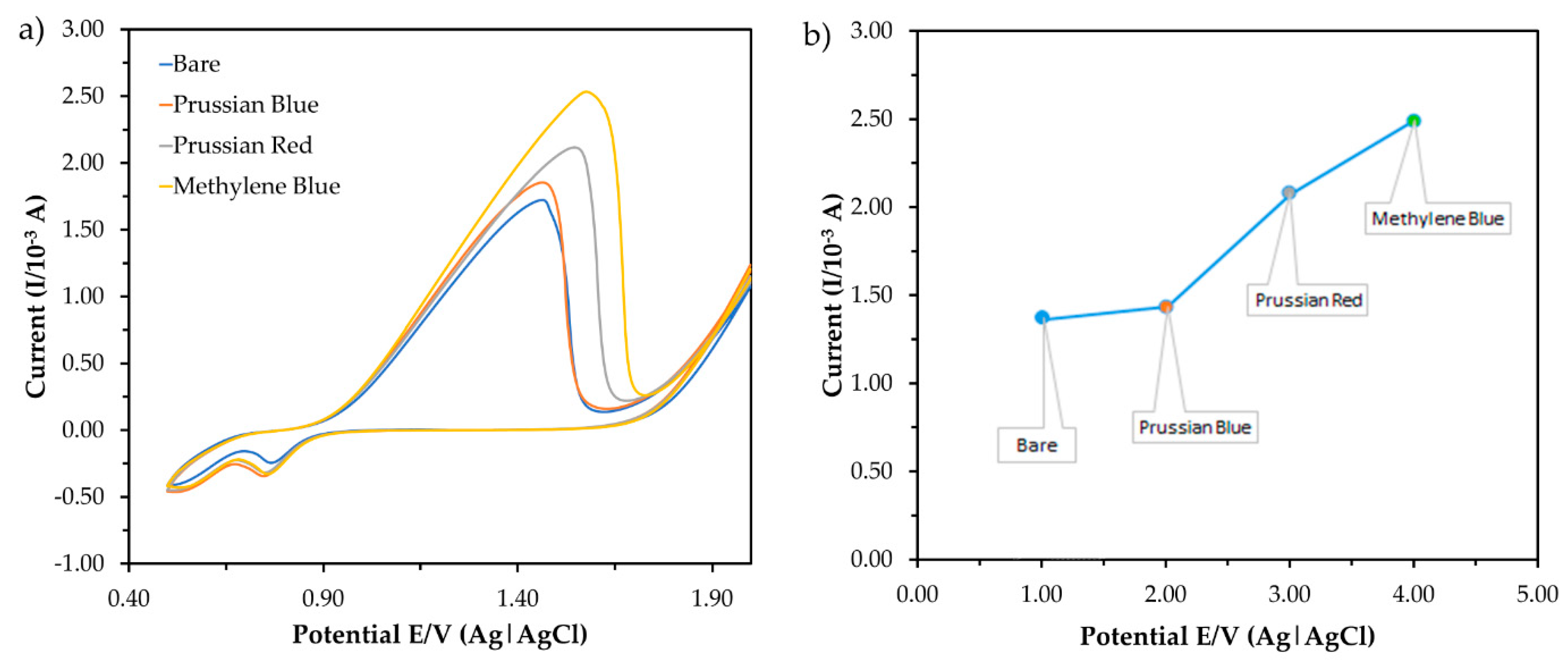
2.5.1. Types of Redox Indicator
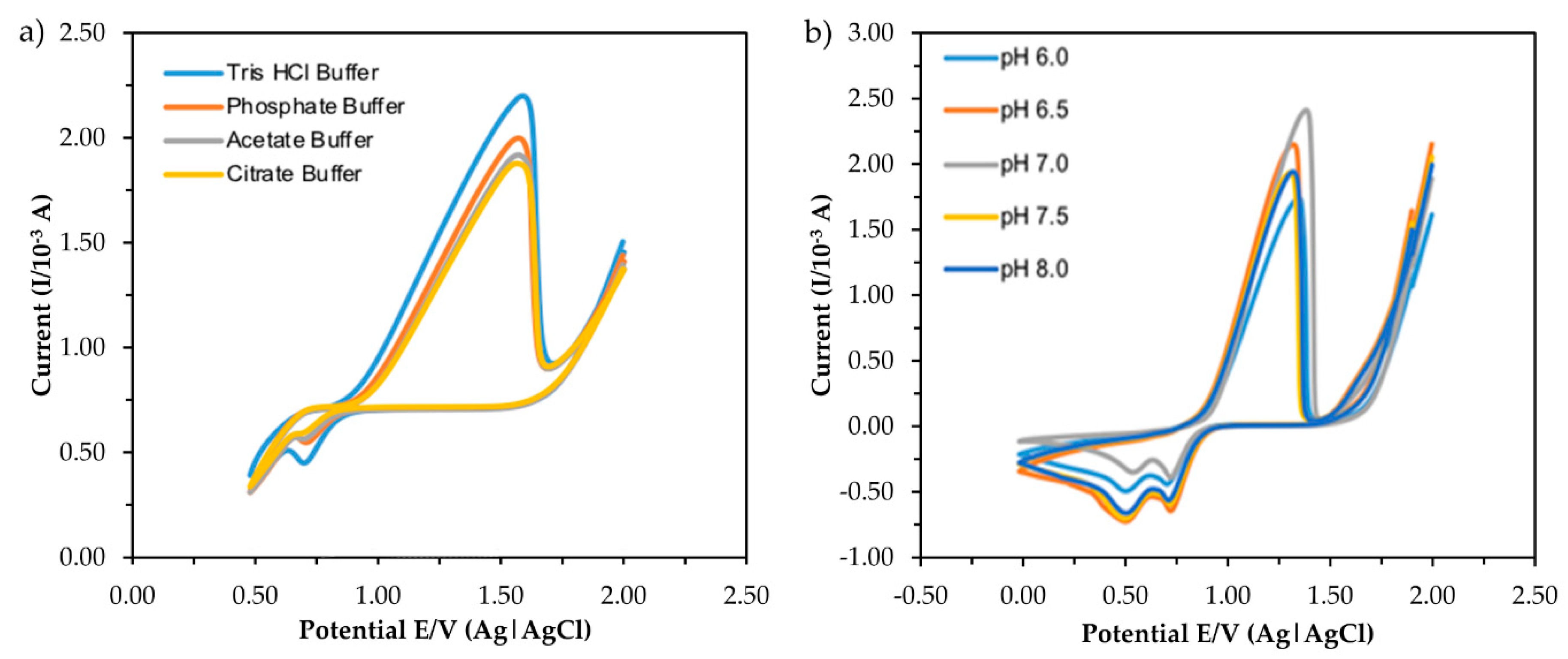
2.5.2. Types of Analytical Buffer
2.5.3. The pH of the Analytical Buffer
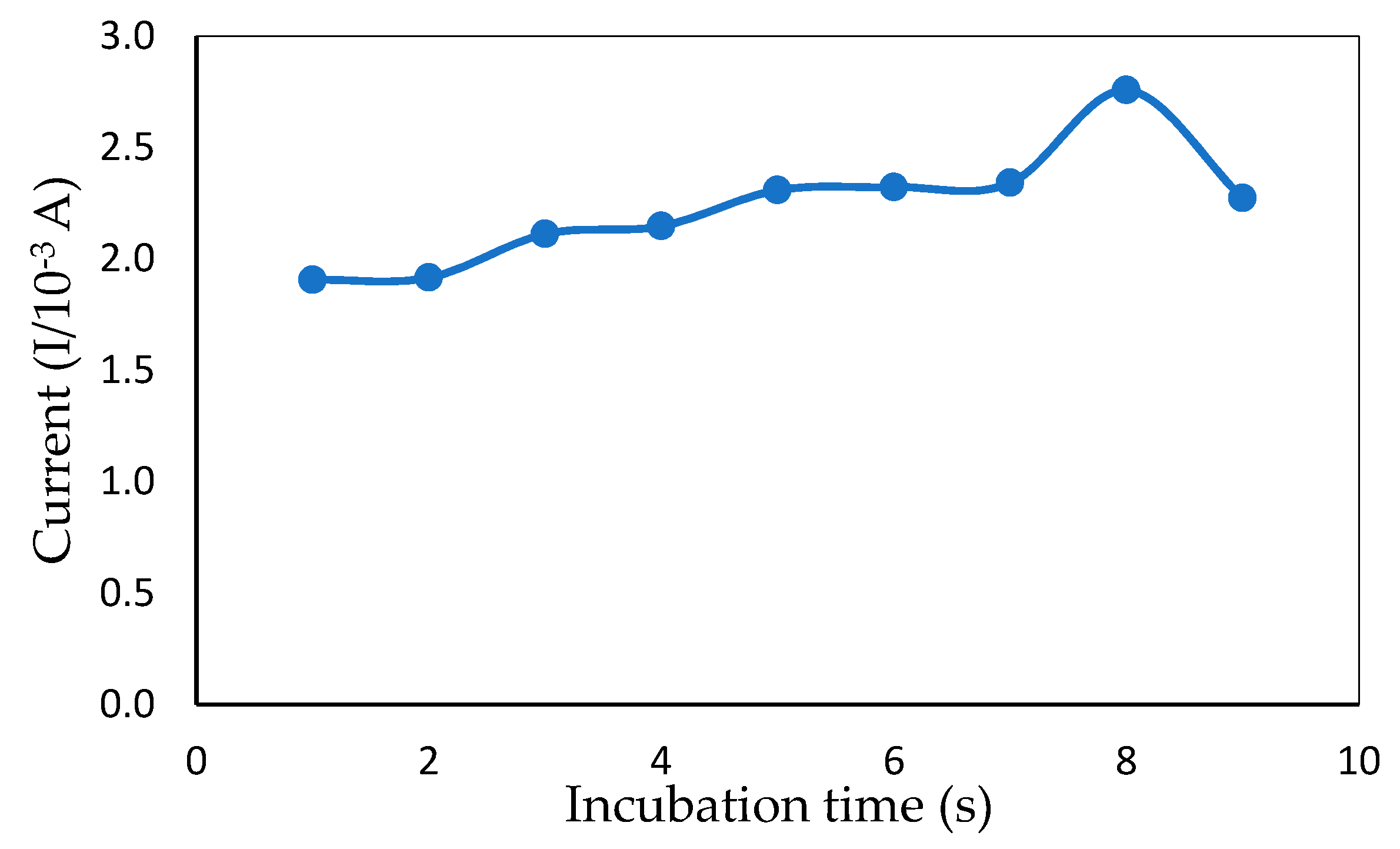
2.5.4. Incubation Times
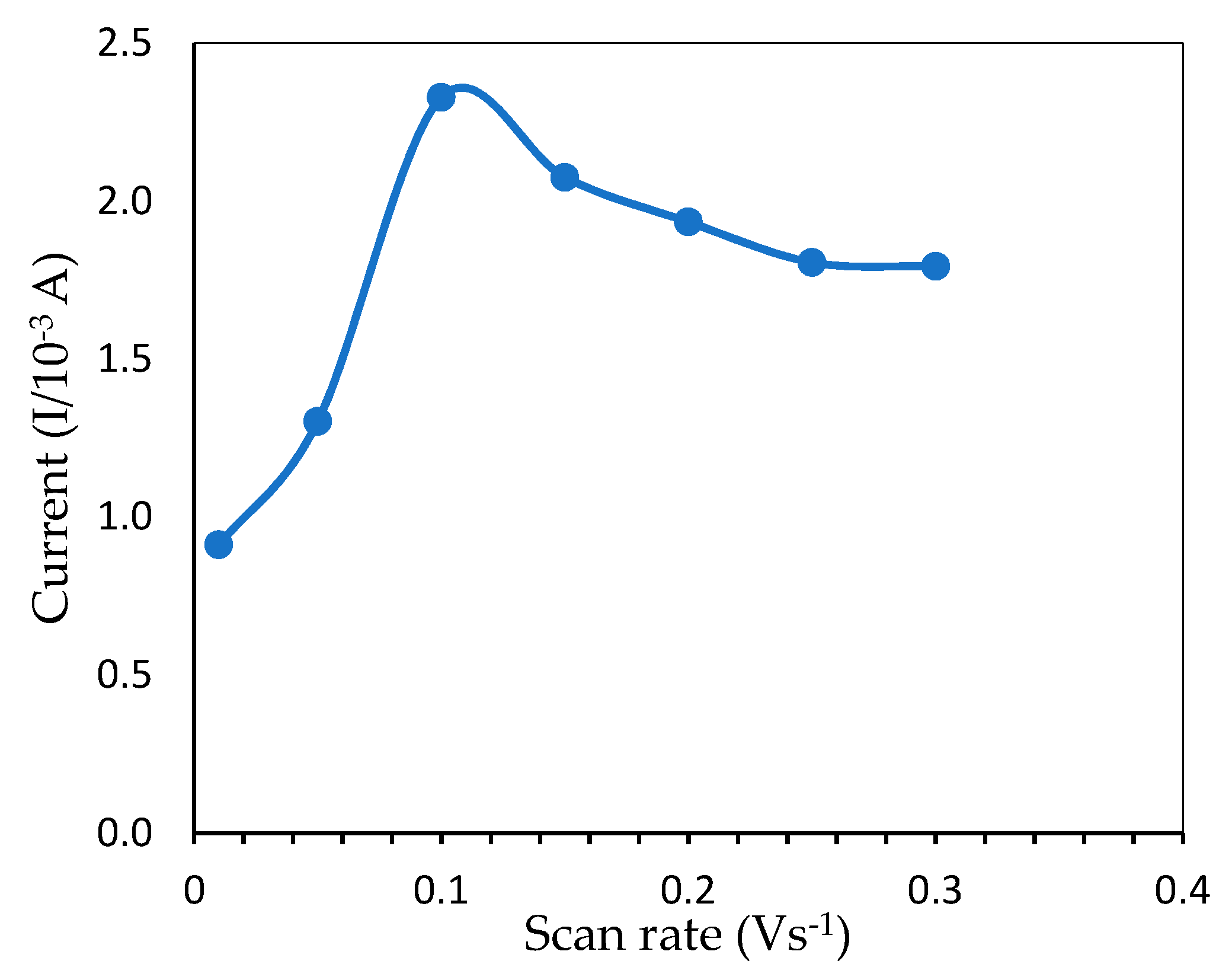
2.5.5. Scan Rates
2.6. Detection of Raw Samples
3. Results and Discussion
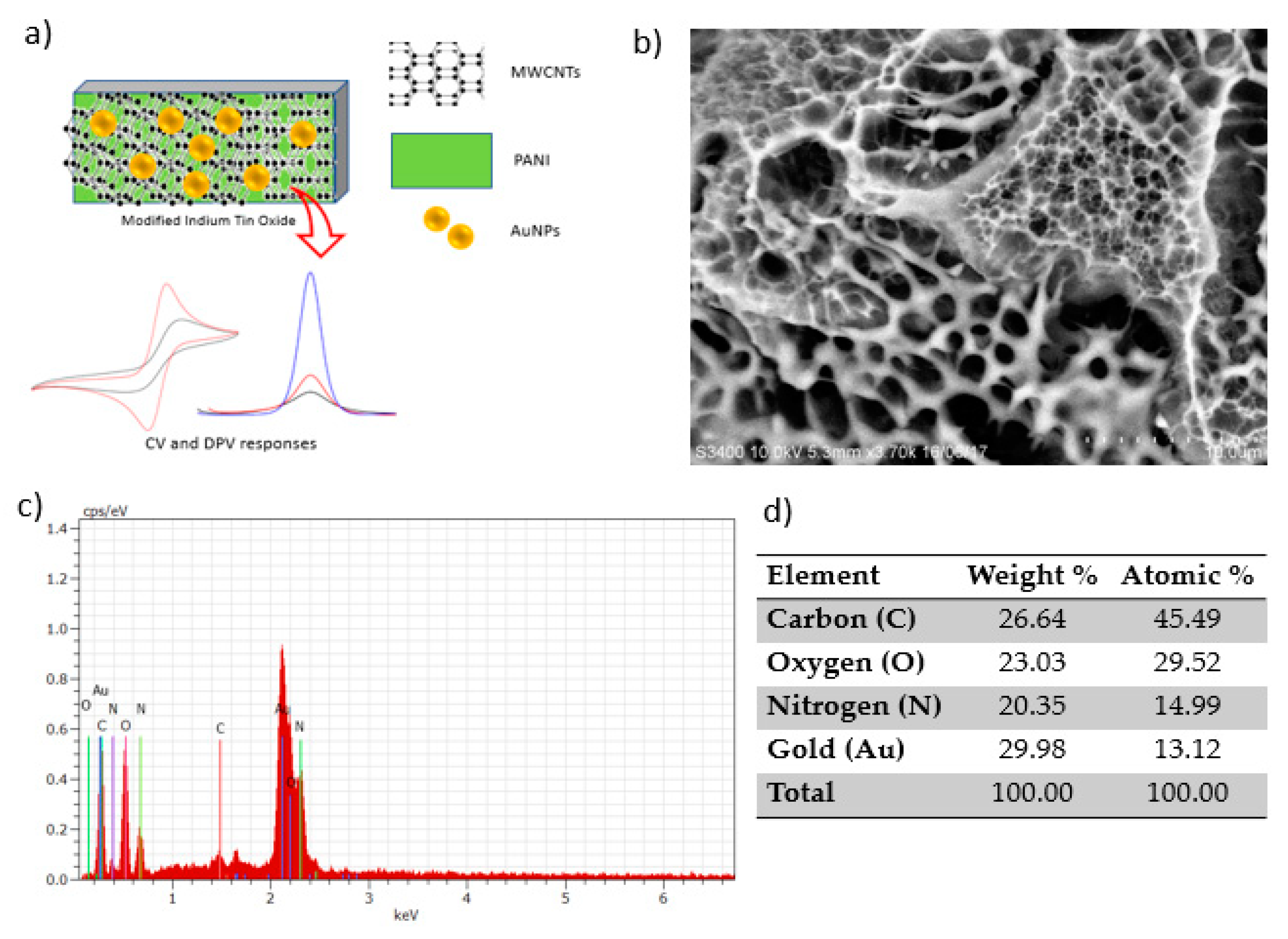
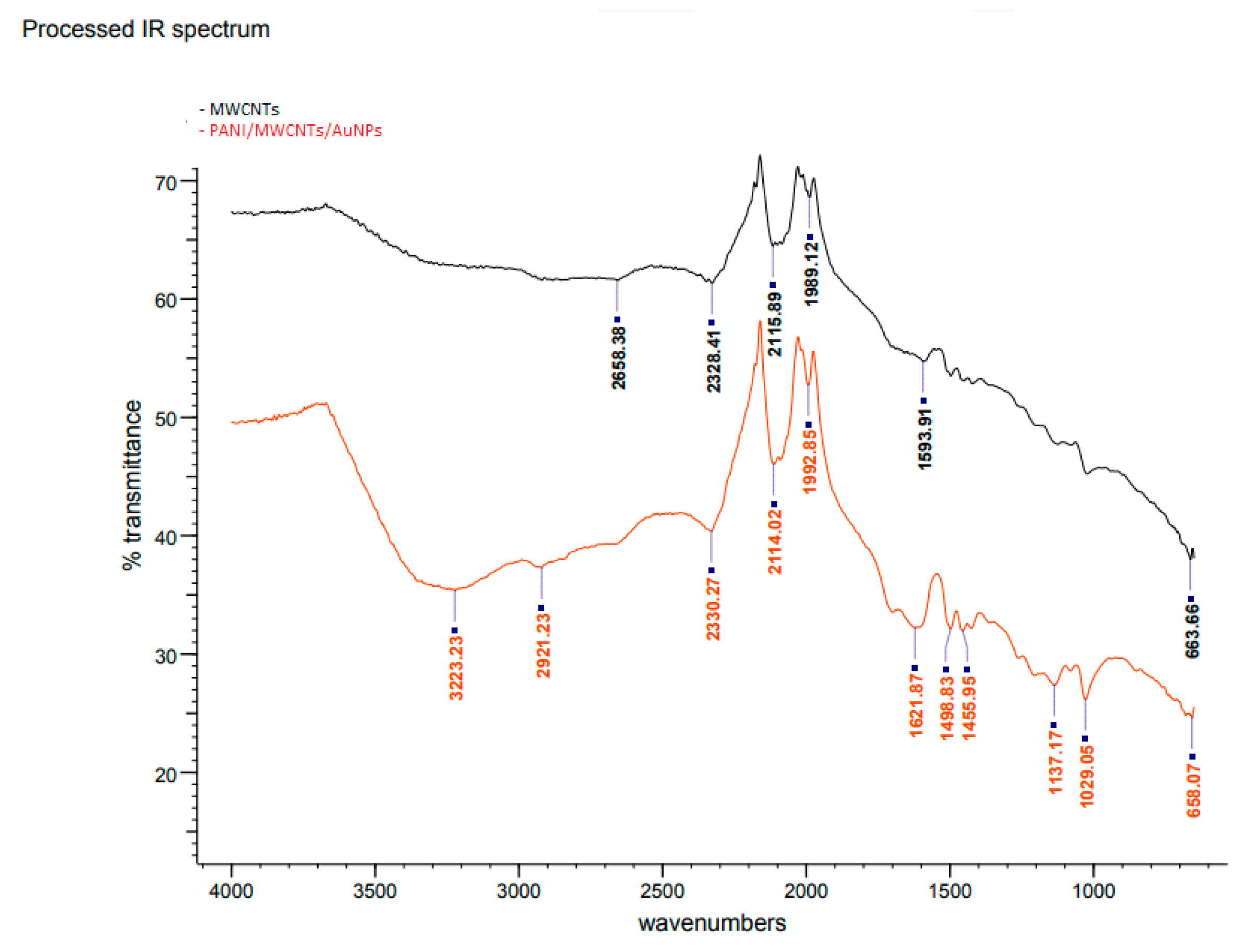
3.1. Characterization of PANI/MWCNTs/AuNPs/ITO
3.2. Optimum Conditions for Mercury Detection
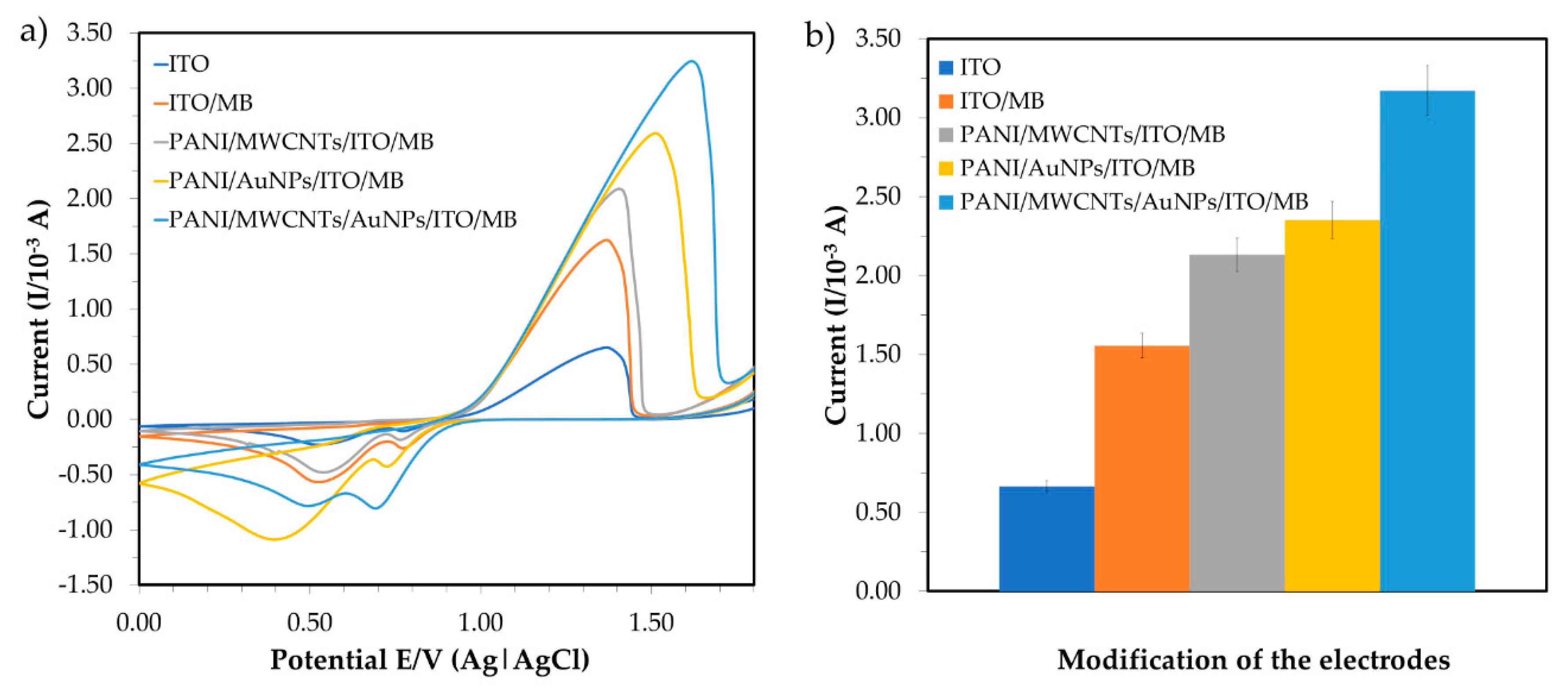
3.2.1. Effect of the Redox Indicator
3.2.2. Effect of Different Types and pH of Buffer Solution
3.2.3. Effect of Incubation Times
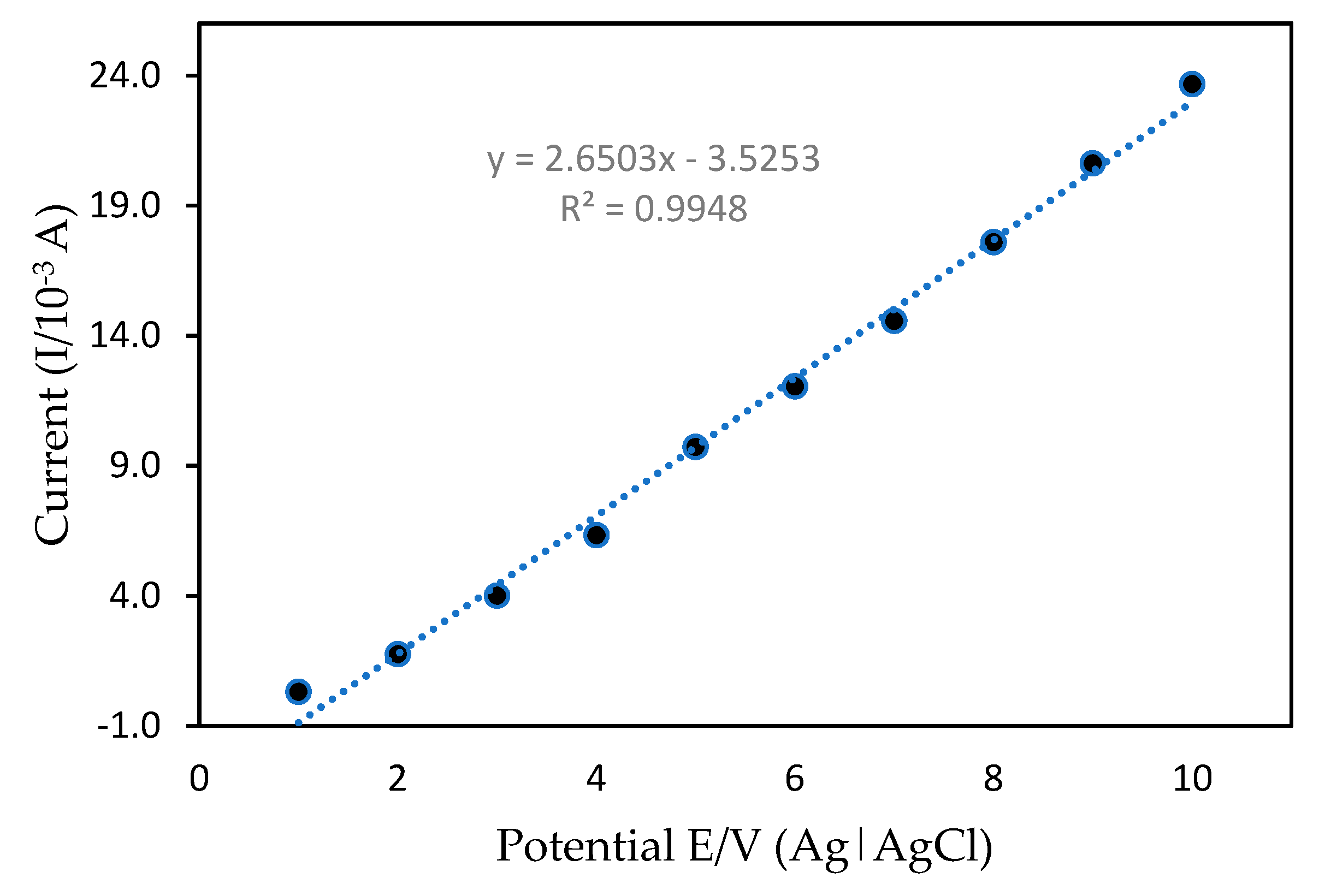
3.2.4. Effects of Scan Rate
3.3. Electrochemical Analysis of Modified ITO
3.3.1. Reproducibility and Repeatability
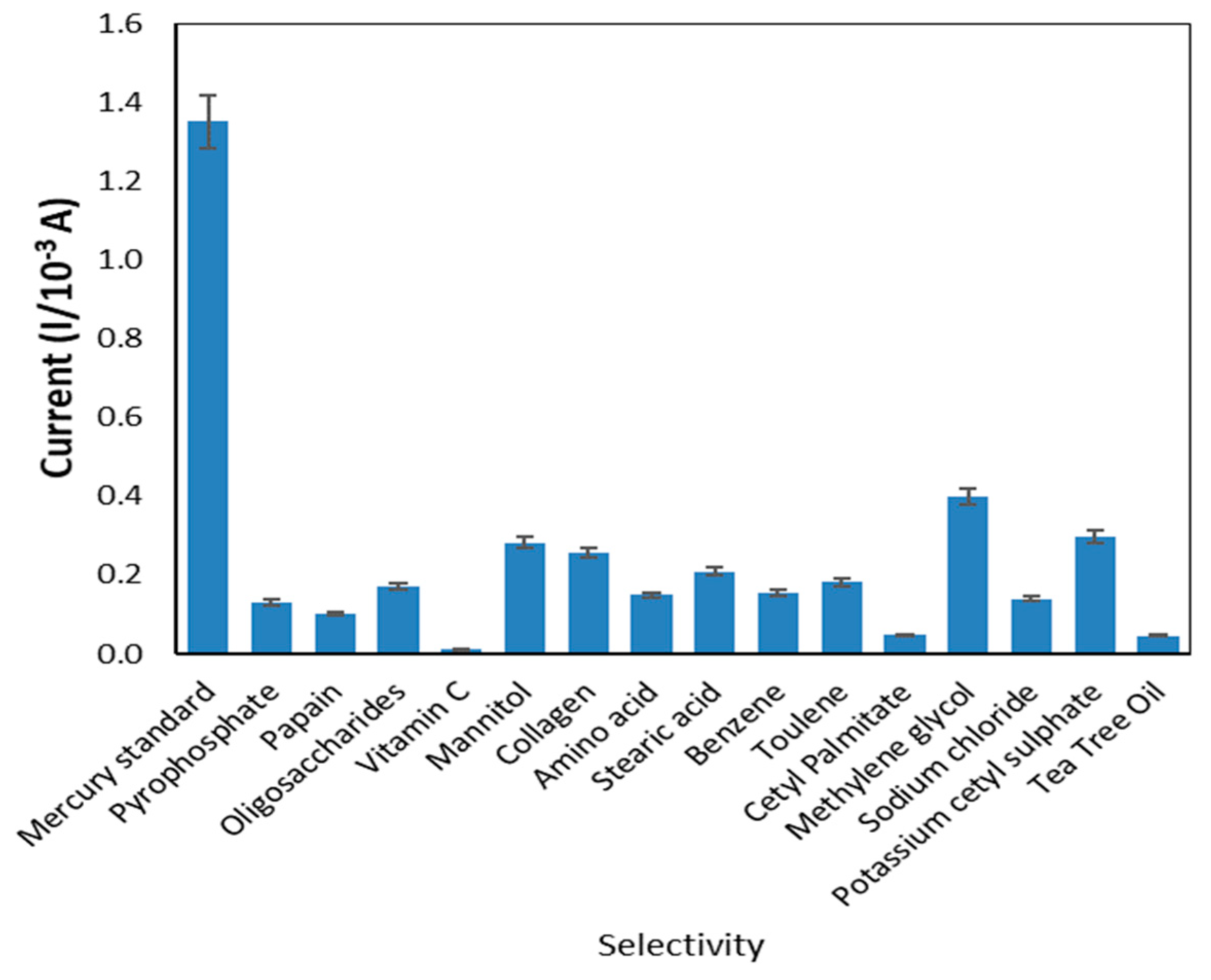
3.3.2. Interference Studies
3.3.3. Limit of Detection (LOD)
3.3.4. Analytical Application on Cosmetic Products
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Jelić, D.; Antunovic, V.; Dermanovic, M. Arsenic and mercury content determination in commercial cosmetics products by atomic absorption spectroscopy. Qual. Life 2017, 8, 23–26. [Google Scholar] [CrossRef]
- Saadatzadeh, A.; Afzalan, S.; Zadehdabagh, R.; Tishezan, L.; Najafi, N.; Seyedtabib, M.; Noori, S.M.A. Determination of heavy metals (lead, cadmium, arsenic, and mercury) in authorized and unauthorized cosmetics. Cutan. Ocul. Toxicol. 2019, 38, 207–211. [Google Scholar] [CrossRef]
- Ashrafi, A.M.; Koudelkova, Z.; Sedlackova, E.; Richtera, L.; Adam, V. Review—Electrochemical Sensors and Biosensors for Determination of Mercury Ions. J. Electrochem. Soc. 2018, 165, B824–B834. [Google Scholar] [CrossRef]
- Food and Drug Administration (FDA). Potential Contaminants—FDA’s Testing of Cosmetics for Arsenic, Cadmium, Chromium, Cobalt, Lead, Mercury, and Nickel Content. Available online: https://www.fda.gov/cosmetics/productsingredients/potentialcontaminants/ucm452836.htm (accessed on 17 August 2020).
- Rohman, A.; Wijayanti, E. Biochemistry and Bioactive Compounds on Bamboo Shoots as the Main Component in Lumpia Semarang. J. Food Pharm. Sci. 2017, 5, 25–28. [Google Scholar] [CrossRef]
- Prasertboonyai, K.; Liawraungrath, B.; Pojanakaroon, T.; Liawraungrath, S. Mercury(II) determination in commercial cosmetics and local Thai traditional medicines by flow injection spectrophotometry. Int. J. Cosmet. Sci. 2016, 38, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Tan, F.; Cong, L.; Saucedo, N.M.; Gao, J.; Li, X.; Mulchandani, A. An electrochemically reduced graphene oxide chemiresistive sensor for sensitive detection of Hg2+ ion in water samples. J. Hazard. Mater. 2016, 320, 226–233. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.; Chen, B.; He, M.; Huang, T.; Hu, B. Speciation of mercury in water and fish samples by HPLC-ICP-MS after magnetic solid phase extraction. Talanta 2017, 171, 213–219. [Google Scholar] [CrossRef] [PubMed]
- Lo, M.; Seydou, M.; Bensghaïer, A.; Pires, R.; Gningue-Sall, D.; Aaron, J.J.; Mekhalif, Z.; Delhalle, J.; Chehimi, M.M. Polypyrrole-wrapped carbon nanotube composite films coated on diazonium-modified flexible ITO sheets for the electroanalysis of heavy metal ions. Sensors 2020, 20, 580. [Google Scholar] [CrossRef]
- Selvolini, G.; Lazzarini, C.; Marrazza, G. Electrochemical nanocomposite single-use sensor for dopamine detection. Sensors 2019, 19, 3097. [Google Scholar] [CrossRef] [PubMed]
- Maity, D.; Manoharan, M.; Rajendra Kumar, R.T. Development of the PANI/MWCNT Nanocomposite-Based Fluorescent Sensor for Selective Detection of Aqueous Ammonia. ACS Omega 2020, 5, 8414–8422. [Google Scholar] [CrossRef]
- Shaikh, M.O.; Srikanth, B.; Zhu, P.-Y.; Chuang, C.-H. Impedimetric Immunosensor Utilizing Polyaniline/Gold Nanocomposite-Modified Screen-Printed Electrodes for Early Detection of Chronic Kidney Disease. Sensors 2019, 19, 3990. [Google Scholar] [CrossRef] [PubMed]
- Guo, C.; Wang, J.; Chen, X.; Li, Y.; Wu, L.; Zhang, J.; Tao, C.A. Construction of a biosensor based on a combination of cytochrome c, graphene, and gold nanoparticles. Sensors 2019, 19, 40. [Google Scholar] [CrossRef] [PubMed]
- Tavakkoli, N.; Soltani, N.; Tabar, Z.K.; Jalali, M.R. Determination of dopamine using the indium tin oxide electrode modified with direct electrodeposition of gold–platinum nanoparticles. Chem. Pap. 2019, 73, 1377–1388. [Google Scholar] [CrossRef]
- Aydar, S.; Bayraktepe, D.E.; Filik, H.; Yazan, Z. A nano-sepiolite clay electrochemical sensor for the rapid electro–catalytic detection of hydroquinone in cosmetic products. Acta Chim. Slov. 2018, 65, 946–954. [Google Scholar] [CrossRef]
- Md Isa, I.; Idris Saidin, M.; Ahmad, M.; Hashim, N.; Abu Bakar, S.; Mohd Ali, N.; MSi, S. Chloroplatinum(II) complex-modified MWCNTs paste electrode for electrochemical determination of mercury in skin lightening cosmetics. Electrochim. Acta 2017. [Google Scholar] [CrossRef]
- Narang, J.; Chauhan, N.; Pundir, C.S. A non-enzymatic sensor for hydrogen peroxide based on polyaniline, multiwalled carbon nanotubes and gold nanoparticles modified Au electrode. Analyst 2011, 136, 4460–4466. [Google Scholar] [CrossRef]
- Zeng, X.; Zhang, Y.; Du, X.; Li, Y.; Tang, W. A highly sensitive glucose sensor based on a gold nanoparticles/polyaniline/multi-walled carbon nanotubes composite modified glassy carbon electrode. New J. Chem. 2018, 42, 11944–11953. [Google Scholar] [CrossRef]
- Chang, Q.; Zhao, K.; Chen, X.; Li, M.; Liu, J. Preparation of gold/polyaniline/multiwall carbon nanotube nanocomposites and application in ammonia gas detection. J. Mater. Sci. 2008, 43, 5861–5866. [Google Scholar] [CrossRef]
- Zhou, J.; Lin, S.; Zeng, H.; Liu, J.; Li, B.; Xu, Y.; Zhao, X.; Chen, G. Dynamic intermolecular interactions through hydrogen bonding of water promote heat conduction in hydrogels. Mater. Horiz. 2020. [Google Scholar] [CrossRef]
- Su, Z.; Cheng, Y.; Li, C.; Xiong, Y.; Xiao, L.; Chen, S.; Qin, X. Dispersing gold nanoparticles on thiolated polyaniline-multiwalled carbon nanotubes for development of an indole-3-acetic acid amperometric immunosensor. Nanoscale Adv. 2019, 1, 3607–3613. [Google Scholar] [CrossRef]
- Patel, K.P.; Gayakwad, E.M.; Shankarling, G.S. Graphene Oxide as a Metal-free Carbocatalyst for Direct Amide Synthesis from Carboxylic Acid and Amine Under Solvent-Free Reaction Condition. ChemistrySelect 2020, 5, 8295–8300. [Google Scholar] [CrossRef]
- Gupta, T.K.; Singh, P.; Mathur, R.B.; Dhakate, S.R. Multi-walled carbon nanotube-graphene-polyaniline multiphase nanocomposite with superior electromagnetic shielding effectiveness. Nanoscale 2014. [Google Scholar] [CrossRef]
- Karpuraranjith, M.; Thambidurai, S. Biotemplate-SnO2 particles intercalated PANI matrix: Enhanced photo catalytic activity for degradation of MB and RY-15 dye. Polym. Degrad. Stab. 2016, 133, 108–118. [Google Scholar] [CrossRef]
- David, I.G.; Popa, D.E.; Buleandră, M.; Cheregi, M.C. Electrochemical Methods and (Bio) Sensors for Rosmarinic Acid Investigation. Chemosensors 2020, 8, 74. [Google Scholar] [CrossRef]
- Fu, L.; Xie, K.; Zhang, H.; Zheng, Y.; Su, W.; Liu, Z. Multi-Walled Carbon Nanotube-Assisted Electrodeposition of Silver Dendrite Coating as a Catalytic Film. Coatings 2017, 7, 232. [Google Scholar] [CrossRef]
- Elgrishi, N.; Rountree, K.J.; McCarthy, B.D.; Rountree, E.S.; Eisenhart, T.T.; Dempsey, J.L. A Practical Beginner’s Guide to Cyclic Voltammetry. J. Chem. Educ. 2018, 95, 197–206. [Google Scholar] [CrossRef]
- Jeremiah Aoki, K.; Chen, J. Tips of Voltammetry. Voltammetry 2019. [Google Scholar] [CrossRef]
- Roushani, M.; Saedi, Z.; Hamdi, F.; Dizajdizi, B.Z. Preparation an electrochemical sensor for detection of manganese (II) ions using glassy carbon electrode modified with multi walled carbon nanotube-chitosan-ionic liquid nanocomposite decorated with ion imprinted polymer. J. Electroanal. Chem. 2017, 804, 1–6. [Google Scholar] [CrossRef]
- Bhalla, N.; Jolly, P.; Formisano, N.; Estrela, P. Introduction to biosensors. Essays Biochem. 2016, 60, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Venton, B.J.; Cao, Q. Fundamentals of fast-scan cyclic voltammetry for dopamine detection. Analyst 2020, 145, 1158–1168. [Google Scholar] [CrossRef]
- Brainina, K.Z.; Bukharinova, M.A.; Stozhko, N.Y.; Sokolkov, S.V.; Tarasov, A.V.; Vidrevich, M.B. Electrochemical Sensor Based on a Carbon Veil Modified by Phytosynthesized Gold Nanoparticles for Determination of Ascorbic Acid. Sensors 2020, 20, 1800. [Google Scholar] [CrossRef] [PubMed]
- Online Browsing Platform. ISO 5725-2:2019(en), Accuracy (Trueness and Precision) of Measurement Methods and Results—Part 2: Basic Method for the Determination of Repeatability and Reproducibility of a Standard Measurement Method. Available online: https://www.iso.org/obp/ui/#iso:std:iso:5725:-2:ed-2:v1:en (accessed on 23 October 2020).
- Ahsan, H. The biomolecules of beauty: Biochemical pharmacology and immunotoxicology of cosmeceuticals. J. Immunoass. Immunochem. 2019, 40, 91–108. [Google Scholar] [CrossRef] [PubMed]
- Giménez-Arnau, E. Chemical Compounds Responsible for Skin Allergy to Complex Mixtures: How to Identify Them? Cosmetics 2019, 6, 71. [Google Scholar] [CrossRef]
- Mathias, P.C.; Hayden, J.A.; Laha, T.J.; Hoofnagle, A.N. Evaluation of matrix effects using a spike recovery approach in a dilute-and-inject liquid chromatography-tandem mass spectrometry opioid monitoring assay. Clin. Chim. Acta 2014, 437, 38–42. [Google Scholar] [CrossRef]
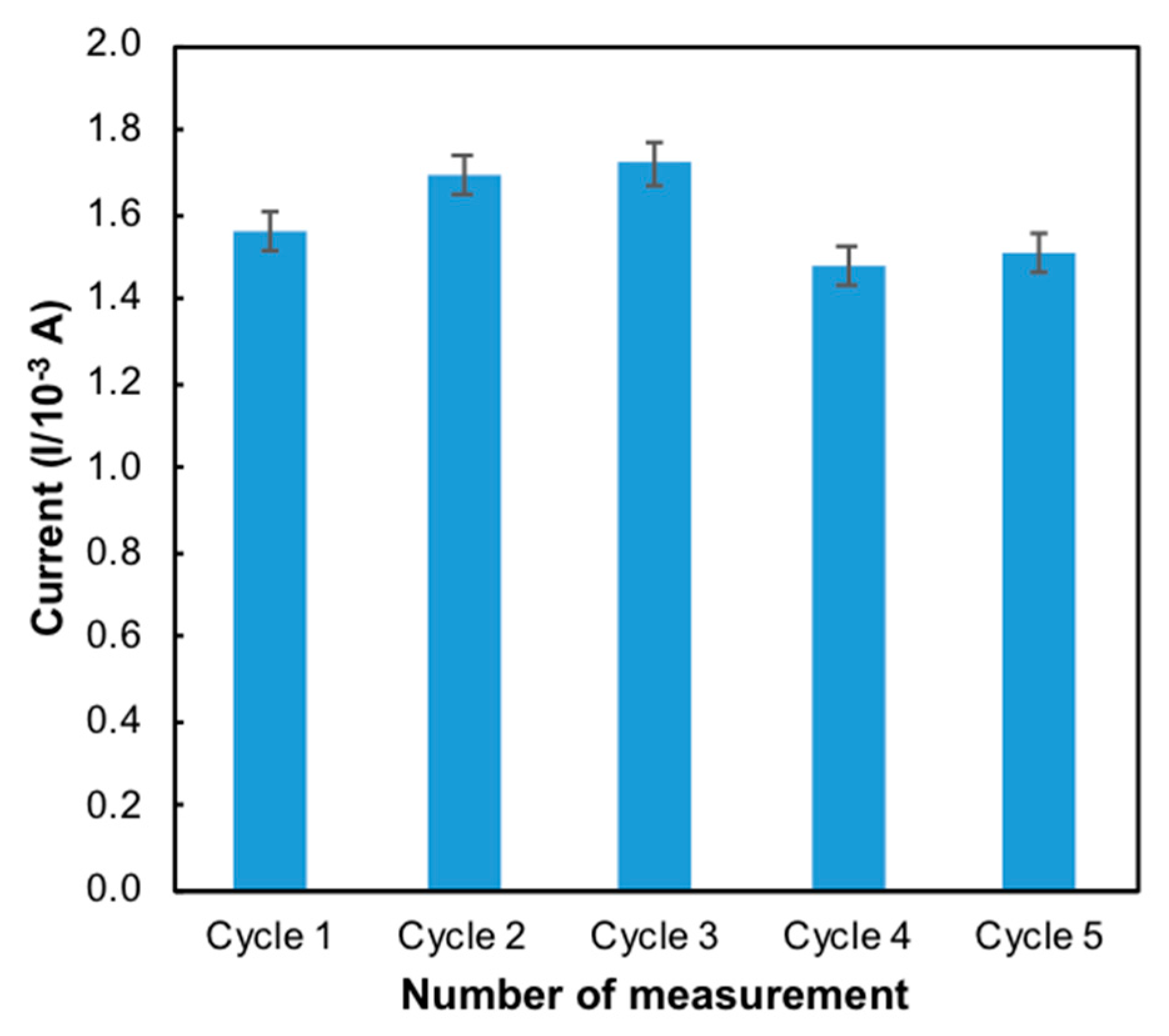
| Characteristic | Mean ± STD | RSD (%) |
|---|---|---|
| Reproducibility | 1.69 × 10−4 ± 2.31 × 10−6 | 2.82 |
| Repeatability | 1.48 × 10−4 ± 3.54 × 10−6 | 1.24 |
| Type of Sensor | Sensing Principle | Detection Limit (mol L−1) | Reference |
|---|---|---|---|
| Electrochemical sensor | Interaction with nanomaterials and nanoparticles | 1.5 × 10−7 mol L−1 (0.03 ppm) | This study |
| Electrochemical sensor | Interaction with nanoparticles and folic acid | 8.43 × 10−6 mol L−1 | [32] |
| Fluorescent optical fiber chemosensor | Photoinduced electron transfer | ~10−6 mol L−1 | [33] |
| Colorimetric sensor | Formation of the colored complex by dithizone ligand | 1.59 × 10−4 mol L−1 | [34] |
| Cosmetic Products | Forms and Properties | Raw Samples | Mean ± STD |
|---|---|---|---|
| Sample 1 Skincare | Lotion | Sample 1 a | <0.01 |
| Serum | Sample 1 b | <0.01 | |
| Moisturizer | Sample 1 c | <0.01 | |
| Sample 2 Suncare | Cream | Sample 2 a | 0.075 ± 0.002 |
| Lotion | Sample 2 b | <0.01 | |
| Gel | Sample 2 c | <0.01 | |
| Sample 3 Haircare | Hair straightener | Sample 3 a | 0.059 ± 0.012 |
| Shampoo | Sample 3 b | <0.01 | |
| Dye | Sample 3 c | 2.129 ± 0.057 | |
| Sample 4 Body care | Soap | Sample 4 a | <0.01 |
| Oil | Sample 4 b | <0.01 | |
| Shower gel | Sample 4 c | <0.01 | |
| Sample 5 Decorative cosmetic | Face powder | Sample 5 a | 0.106 ± 0.028 |
| Foundation | Sample 5 b | 2.135 ± 0.085 | |
| Lipstick | Sample 5 c | 1.060 ± 0.039 | |
| Sample 6 Perfumes | Scented oil | Sample 6 a | <0.01 |
| Deodorant | Sample 6 b | <0.01 | |
| Salve | Sample 6 c | <0.01 |
| Developed Sensor | Added Concentration (ppm) | Found Concentration (ppm) Mean ± STD * | Recovery (%) | RSD (%) |
| 0.03 ppm | 0.03 ± 0.38 | 96.6% | 0.43% | |
| 6 ppm | 5.7 ± 0.45 | 97.5% | 0.52% | |
| 10 ppm | 9.49 ± 0.43 | 97.3% | 0.64% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bohari, N.A.; Siddiquee, S.; Saallah, S.; Misson, M.; Arshad, S.E. Optimization and Analytical Behavior of Electrochemical Sensors Based on the Modification of Indium Tin Oxide (ITO) Using PANI/MWCNTs/AuNPs for Mercury Detection. Sensors 2020, 20, 6502. https://doi.org/10.3390/s20226502
Bohari NA, Siddiquee S, Saallah S, Misson M, Arshad SE. Optimization and Analytical Behavior of Electrochemical Sensors Based on the Modification of Indium Tin Oxide (ITO) Using PANI/MWCNTs/AuNPs for Mercury Detection. Sensors. 2020; 20(22):6502. https://doi.org/10.3390/s20226502
Chicago/Turabian StyleBohari, Noor Aini, Shafiquzzaman Siddiquee, Suryani Saallah, Mailin Misson, and Sazmal Effendi Arshad. 2020. "Optimization and Analytical Behavior of Electrochemical Sensors Based on the Modification of Indium Tin Oxide (ITO) Using PANI/MWCNTs/AuNPs for Mercury Detection" Sensors 20, no. 22: 6502. https://doi.org/10.3390/s20226502
APA StyleBohari, N. A., Siddiquee, S., Saallah, S., Misson, M., & Arshad, S. E. (2020). Optimization and Analytical Behavior of Electrochemical Sensors Based on the Modification of Indium Tin Oxide (ITO) Using PANI/MWCNTs/AuNPs for Mercury Detection. Sensors, 20(22), 6502. https://doi.org/10.3390/s20226502






