Portable and Affordable Light Source-Based Photoacoustic Tomography
Abstract
1. Introduction
2. Principles of Photoacoustic Imaging
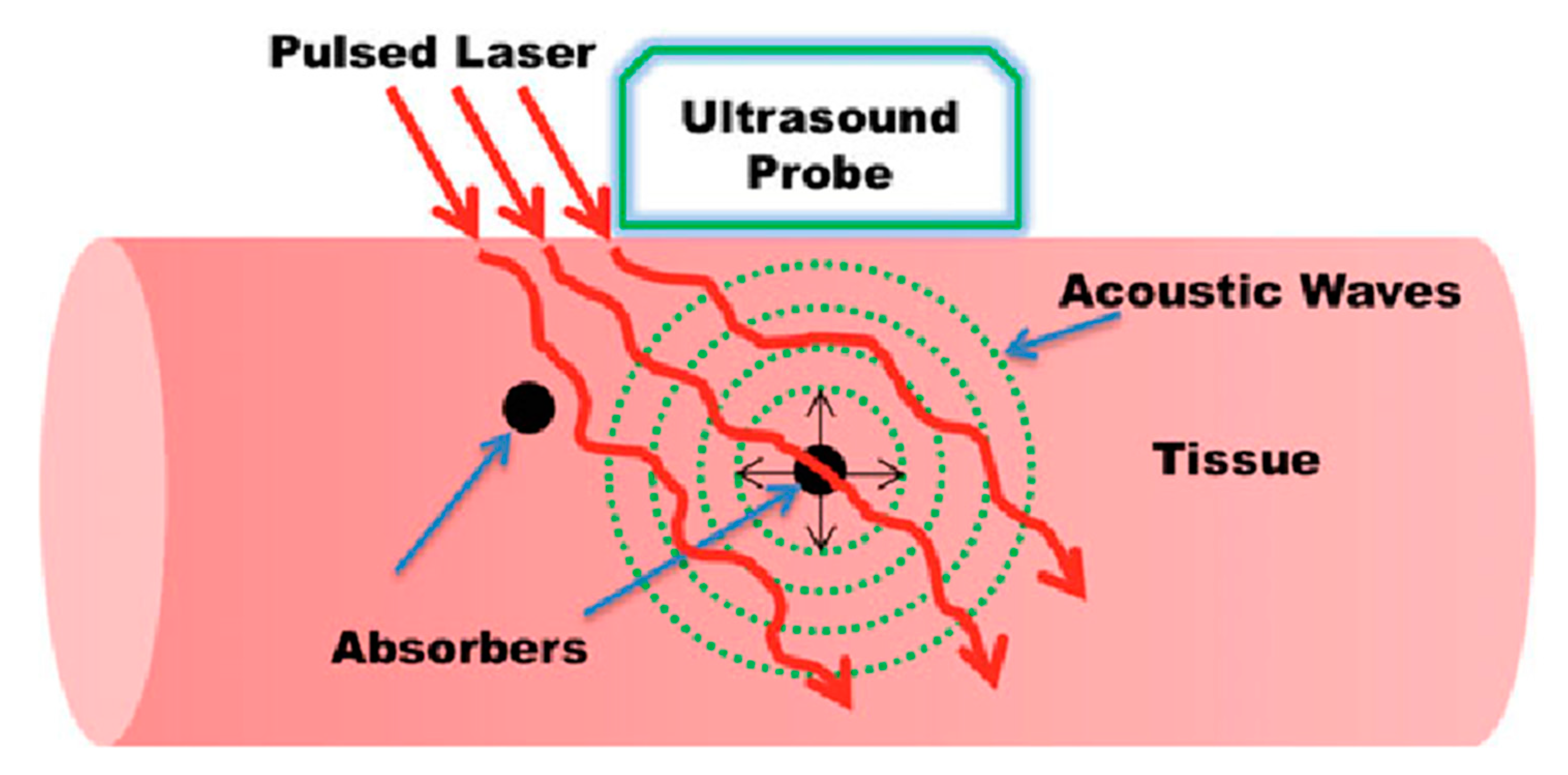
2.1. Generation of Photoacoustic Signals
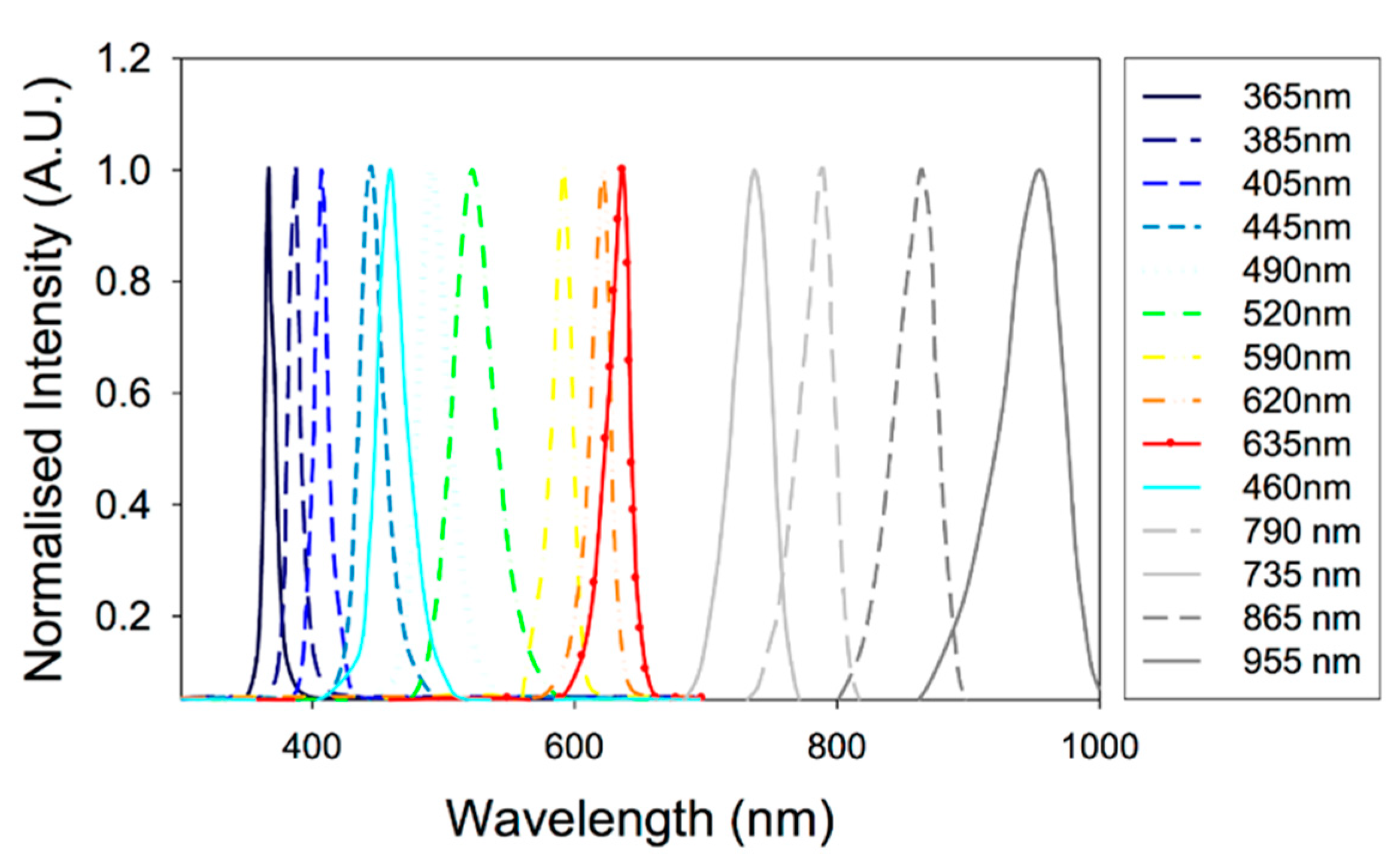
2.2. Illumination Sources
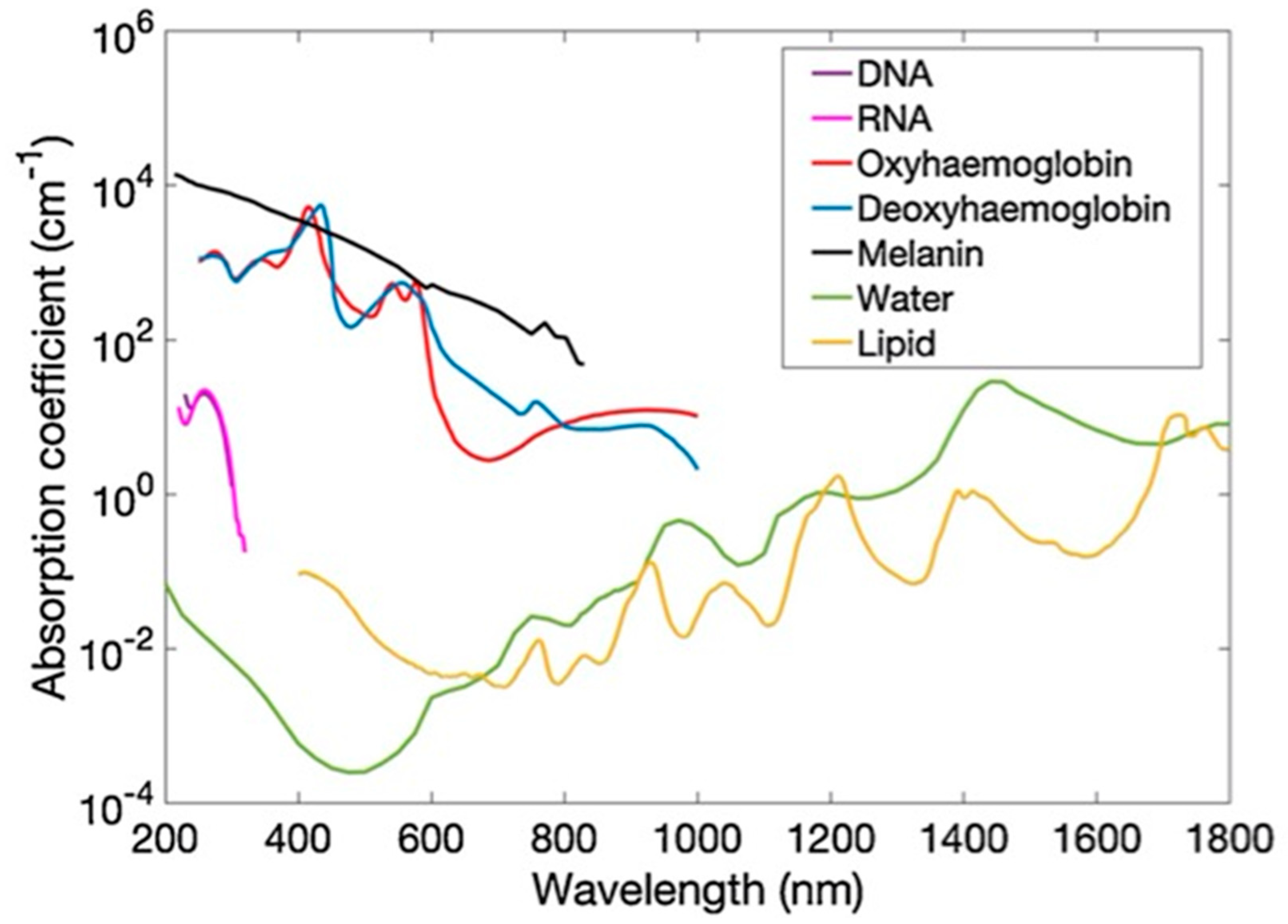
2.3. Optical Absorption
2.4. Ultrasound Propogation and Detection
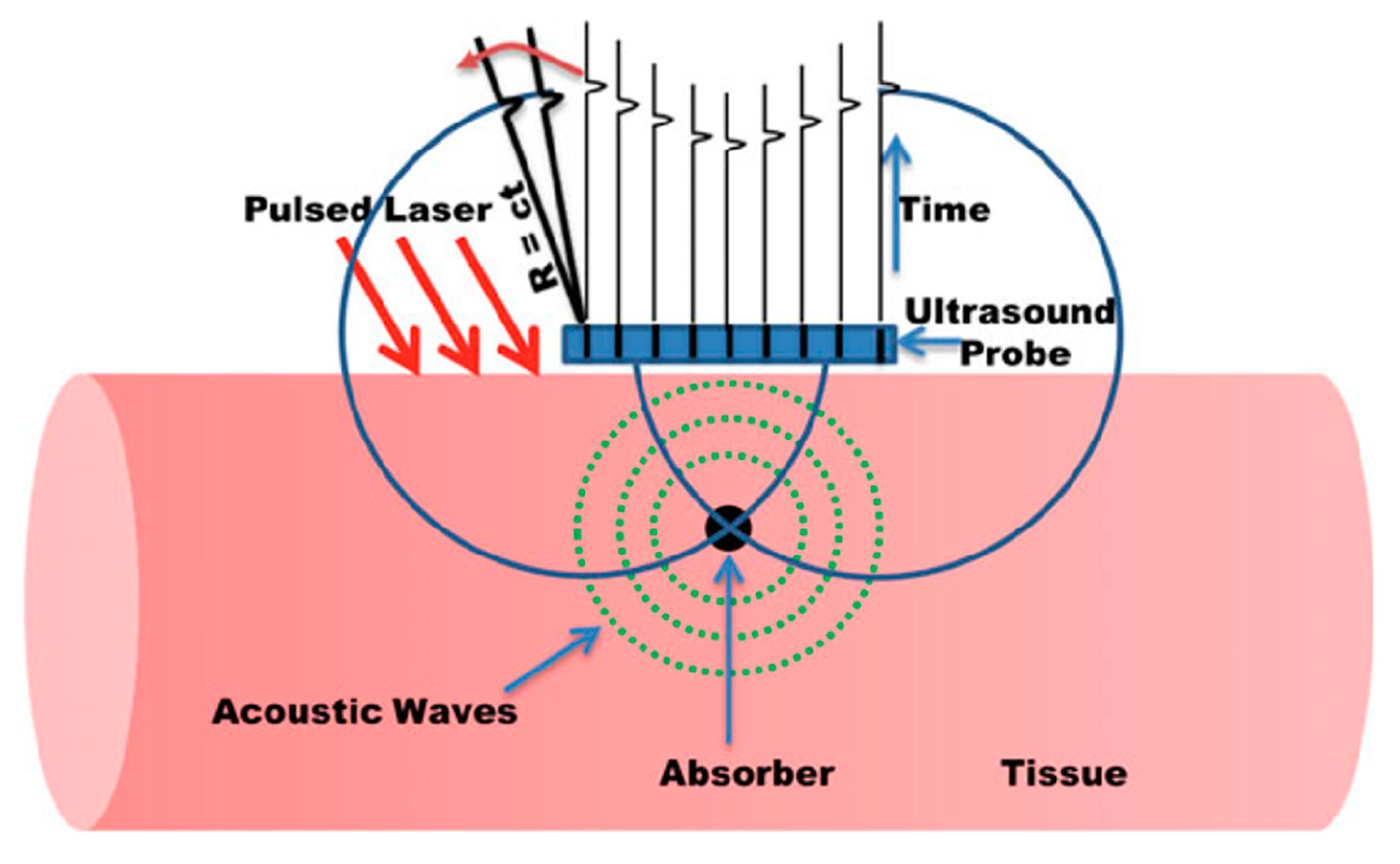
2.5. Image Reconstruction
3. LED-Based Photoacoustic Tomography
3.1. High-Power LEDs Suitable for Photoacoustic Tomography
3.2. High-Power LEDs—Technical Aspects
3.2.1. Emission Wavelength
3.2.2. Overdriving LEDs
3.2.3. Optical Output Power
3.2.4. Pulse Repetition Rate (PRR)
3.2.5. Pulse Width
3.2.6. Spatial Divergence of LEDs
3.3. Technical Developments in LED-Based Photoacoustic Tomography
4. Laser Diode-Based Photoacoustic Tomography
4.1. High-Power Pulsed Laser Diodes
4.2. Pulsed Laser Diodes—Technical Aspects
4.2.1. Emission Wavelengths
4.2.2. Pulsed Laser Diode Drivers
4.2.3. Optical Output Power
4.2.4. Pulse Repetition Rate
4.2.5. Pulse Width
4.2.6. Spatial Divergence of LD
4.3. Technical Developments in LD-Based Photoacoustic Tomography
5. Discussion
6. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef] [PubMed]
- Manohar, S.; Razansky, D. Photoacoustics: A historical review. Adv. Opt. Photonics 2016, 8, 586–617. [Google Scholar] [CrossRef]
- Wang, L.V. Multiscale photoacoustic microscopy and computed tomography. Nat. Photonics 2009, 3, 503–509. [Google Scholar] [CrossRef]
- Omar, M.; Aguirre, J.; Ntziachristos, V. Optoacoustic mesoscopy for biomedicine. Nat. Biomed. Eng. 2019, 3, 354–370. [Google Scholar] [CrossRef]
- Wang, L.V.; Yao, L.V.W.J. A practical guide to photoacoustic tomography in the life sciences. Nat. Methods 2016, 13, 627–638. [Google Scholar] [CrossRef]
- Singh, M.K.A.; Steenbergen, W.; Manohar, S. Handheld Probe-Based Dual Mode Ultrasound/Photoacoustics for Biomedical Imaging. In Frontiers in Biophotonics for Translational Medicine; Progress in Optical Science and Photonics; Springer: Singapore, 2015; Volume 3, pp. 209–247. [Google Scholar] [CrossRef]
- Singh, M.K.A.; Sato, N.; Ichihashi, F.; Sankai, Y. Clinical Translation of Photoacoustic Imaging—Opportunities and Challenges from an Industry Perspective. In LED-Based Photoacoustic Imaging; Progress in Optical Science and Photonics; Springer: Singapore, 2020; Volume 7, pp. 379–393. [Google Scholar] [CrossRef]
- Szabo, T.L. Diagnostic Ultrasound Imaging: Inside Out; Elsevier BV: Amsterdam, The Netherlands, 2014. [Google Scholar]
- Zhao, T.; Desjardins, A.E.; Ourselin, S.; Vercauteren, T.; Xia, W. Minimally invasive photoacoustic imaging: Current status and future perspectives. Photoacoustics 2019, 16, 100146. [Google Scholar] [CrossRef]
- Bell, A.G. On the production and reproduction of sound by light. Am. J. Sci. 1880, 118, 305–324. [Google Scholar] [CrossRef]
- Lutzweiler, C.; Razansky, D. Optoacoustic Imaging and Tomography: Reconstruction Approaches and Outstanding Challenges in Image Performance and Quantification. Sensors 2013, 13, 7345–7384. [Google Scholar] [CrossRef]
- Tian, C.; Xie, Z.; Fabiilli, M.L.; Wang, X. Imaging and sensing based on dual-pulse nonlinear photoacoustic contrast: A preliminary study on fatty liver. Opt. Lett. 2015, 40, 2253–2256. [Google Scholar] [CrossRef]
- Zhu, Y.; Feng, T.; Cheng, Q.; Wang, X.; Du, S.; Sato, N.; Yuan, J.; Singh, M.K.A. Towards Clinical Translation of LED-Based Photoacoustic Imaging: A Review. Sensors 2020, 20, 2484. [Google Scholar] [CrossRef]
- Erfanzadeh, M.; Zhu, Q. Photoacoustic imaging with low-cost sources; A review. Photoacoustics 2019, 14, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Zhong, H.; Duan, T.; Lan, H.; Zhou, M.; Gao, F. Review of Low-Cost Photoacoustic Sensing and Imaging Based on Laser Diode and Light-Emitting Diode. Sensors 2018, 18, 2264. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Tang, Y.; Yao, J. Photoacoustic tomography of blood oxygenation: A mini review. Photoacoustics 2018, 10, 65–73. [Google Scholar] [CrossRef]
- Mallidi, S.; Luke, G.P.; Emelianov, S. Photoacoustic imaging in cancer detection, diagnosis, and treatment guidance. Trends Biotechnol. 2011, 29, 213–221. [Google Scholar] [CrossRef]
- Xia, W.; Singh, M.K.A.; Maneas, E.; Sato, N.; Shigeta, Y.; Agano, T.; Ourselin, S.; West, S.J.; Desjardins, A.E. Handheld Real-Time LED-Based Photoacoustic and Ultrasound Imaging System for Accurate Visualization of Clinical Metal Needles and Superficial Vasculature to Guide Minimally Invasive Procedures. Sensors 2018, 18, 1394. [Google Scholar] [CrossRef]
- Kuchment, P.; Kunyansky, L. Mathematics of thermoacoustic tomography. Eur. J. Appl. Math. 2008, 19, 191–224. [Google Scholar] [CrossRef]
- Jaeger, M.; Schüpbach, S.; Gertsch, A.; Kitz, M.; Frenz, M. Fourier reconstruction in optoacoustic imaging using truncated regularized inverse k -space interpolation. Inverse Probl. 2007, 23, S51–S63. [Google Scholar] [CrossRef]
- Niederhauser, J.J.; Jaeger, M.; Lemor, R.; Weber, P.; Frenz, M. Combined ultrasound and optoacoustic system for real-time high-contrast vascular imaging in vivo. IEEE Trans. Med. Imaging 2005, 24, 436–440. [Google Scholar] [CrossRef]
- Singh, M.K.A. LED-Based Photoacoustic Imaging; Springer Nature Singapore Pte Ltd.: Singapore, 2020. [Google Scholar]
- Allen, T.J. High-Power Light Emitting Diodes; An Alternative Excitation Source for Photoacoustic Tomography. In LED-Based Photoacoustic Imaging; Progress in Optical Science and Photonics; Springer: Singapore, 2020; Volume 7, pp. 23–43. [Google Scholar] [CrossRef]
- Allen, T.J.; Beard, P.C. High power visible light emitting diodes as pulsed excitation sources for biomedical photoacoustics. Biomed. Opt. Express 2016, 7, 1260–1270. [Google Scholar] [CrossRef]
- Singh, M.K.A.; Sato, N.; Sankai, Y.; Ichihashi, F. In vivo demonstration of real-time oxygen saturation imaging using a portable and affordable LED-based multispectral photoacoustic and ultrasound imaging system. Photons Plus Ultrasound: Imaging Sens. 2019 2019, 10878, 108785N. [Google Scholar] [CrossRef]
- Zhu, Y.; Xu, G.; Yuan, J.; Jo, J.; Gandikota, G.; Demirci, H.; Agano, T.; Sato, N.; Shigeta, Y.; Wang, X. Light Emitting Diodes based Photoacoustic Imaging and Potential Clinical Applications. Sci. Rep. 2018, 8, 1–12. [Google Scholar] [CrossRef]
- Singh, M.K.A. Effect of light pulse width on frequency characteristics of photoacoustic signal—An experimental study using a pulse-width tunable LED-based photoacoustic imaging system. Int. J. Eng. Technol. 2018, 7, 4300–4303. [Google Scholar]
- Francis, K.J.; Boink, Y.E.; Dantuma, M.; Singh, M.K.A.; Manohar, S.; Steenbergen, W.; Joseph, F.K. Tomographic imaging with an ultrasound and LED-based photoacoustic system. Biomed. Opt. Express 2020, 11, 2152–2165. [Google Scholar] [CrossRef]
- Francis, K.J.; Boink, Y.E.; Dantuma, M.; Singh, M.K.A.; Manohar, S.; Steenbergen, W. Light Emitting Diodes Based Photoacoustic and Ultrasound Tomography: Imaging Aspects and Applications. In LED-Based Photoacoustic Imaging; Progress in Optical Science and Photonics; Springer: Singapore, 2020; Volume 7, pp. 245–266. [Google Scholar] [CrossRef]
- Xia, W.; Nikitichev, D.I.; Mari, J.M.; West, S.J.; Pratt, R.; David, A.L.; Ourselin, S.; Beard, P.C.; Desjardins, A.E. Performance characteristics of an interventional multispectral photoacoustic imaging system for guiding minimally invasive procedures. J. Biomed. Opt. 2015, 20, 086005. [Google Scholar] [CrossRef]
- Mari, J.M.; Xia, W.; West, S.J.; Desjardins, A.E. Interventional multispectral photoacoustic imaging with a clinical ultrasound probe for discriminating nerves and tendons: An ex vivo pilot study. J. Biomed. Opt. 2015, 20, 110503. [Google Scholar] [CrossRef] [PubMed]
- Singh, M.K.A.; Parameshwarappa, V.; Hendriksen, E.; Steenbergen, W.; Manohar, S. Photoacoustic-guided focused ultrasound for accurate visualization of brachytherapy seeds with the photoacoustic needle. J. Biomed. Opt. 2016, 21, 120501. [Google Scholar] [CrossRef] [PubMed]
- Hansen, R.S. Using high-power light emitting diodes for photoacoustic imaging. SPIE Med. Imaging 2011, 7968, 79680. [Google Scholar] [CrossRef]
- Allen, T.J.; Beard, P.C. Light emitting diodes as an excitation source for biomedical photoacoustics. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2013, San Francisco, CA, USA, 4 March 2013; p. 85811F. [Google Scholar] [CrossRef]
- Agano, T.; Sato, N.; Nakatsuka, H.; Kitagawa, K.; Hanaoka, T.; Morisono, K.; Shigeta, Y. Comparative experiments of photoacoustic system using laser light source and LED array light source. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2015, San Francisco, CA, USA, 11 March 2015; p. 93233X. [Google Scholar] [CrossRef]
- Agano, T.; Sato, N.; Nakatsuka, H.; Kitagawa, K.; Hanaoka, T.; Morisono, K.; Shigeta, Y. Attempts to increase penetration of photoacoustic system using LED array light souce. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2015, San Francisco, CA, USA, 11 March 2015; p. 93233Z. [Google Scholar] [CrossRef]
- Agano, T.; Sato, N. Photoacoustic Imaging System using LED light source. In Proceedings of the 2016 Conference on Lasers and Electro-Optics (CLEO), San Jose, CA, USA, 5–10 June 2016; The Optical Society: Washington, DC, USA, 2016; p. ATh3N.5. [Google Scholar]
- Hariri, A.; LeMaster, J.; Wang, J.; Jeevarathinam, A.S.; Chao, D.L.; Jokerst, J.V. The characterization of an economic and portable LED-based photoacoustic imaging system to facilitate molecular imaging. Photoacoustics 2018, 9, 10–20. [Google Scholar] [CrossRef]
- Joseph, F.K.; Xavierselvan, M.; Singh, M.K.A.; Mallidi, S.; Van Der Laken, C.; Van De Loo, F.; Steenbergen, W. LED-based photoacoustic imaging for early detection of joint inflammation in rodents: Towards achieving 3Rs in rheumatoid arthritis research. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2020, San Francisco, CA, USA, 17 February 2020; p. 112400M. [Google Scholar] [CrossRef]
- Singh, M.K.A.; Agano, T.; Sato, N.; Shigeta, Y.; Uemura, T. Real-time in vivo imaging of human lymphatic system using an LED-based photoacoustic/ultrasound imaging system. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2018, San Francisco, CA, USA, 19 February 2018; p. 1049404. [Google Scholar] [CrossRef]
- Singh, M.K.A.; Shigeta, Y.; Hanaoka, T.; Agano, T.; Sato, N. High-speed photoacoustic imaging using an LED-based photoacoustic imaging system. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2018, San Francisco, CA, USA, 19 February 2018; p. 104943N. [Google Scholar] [CrossRef]
- Jo, J.; Xu, G.; Zhu, Y.; Burton, M.; Sarazin, J.; Schiopu, E.; Gandikota, G.; Wang, X. Detecting joint inflammation by an LED-based photoacoustic imaging system: A feasibility study. J. Biomed. Opt. 2018, 23, 110501–110504. [Google Scholar] [CrossRef]
- Cheng, Q.; Qian, M.; Wang, X.; Zhang, H.; Wang, P.; Wen, L.; Pan, J.; Gao, Y.; Wu, S.; Zhang, M.; et al. Diagnosis and Treatment Monitoring of Port-Wine Stain Using LED-Based Photoacoustics: Theoretical Aspects and First In-Human Clinical Pilot Study. In LED-Based Photoacoustic Imaging; Progress in Optical Science and Photonics; Springer Nature Singapore Pte Ltd.: Singapore, 2020; pp. 351–377. [Google Scholar]
- Xavierselvan, M.; Mallidi, S. LED-Based Functional Photoacoustics—Portable and Affordable Solution for Preclinical Cancer Imaging. In LED-Based Photoacoustic Imaging; Progress in Optical Science and Photonics; Springer: Singapore, 2020; Volume 7, pp. 303–319. [Google Scholar] [CrossRef]
- Agrawal, S.; Fadden, C.; Dangi, A.; Yang, X.; AlBahrani, H.; Frings, N.; Zadi, S.H.; Kothapalli, S.-R. Light-Emitting-Diode-Based Multispectral Photoacoustic Computed Tomography System. Sensors 2019, 19, 4861. [Google Scholar] [CrossRef]
- Francis, K.J.; Booijink, R.; Bansal, R.; Steenbergen, W. Tomographic Ultrasound and LED-Based Photoacoustic System for Preclinical Imaging. Sensors 2020, 20, 2793. [Google Scholar] [CrossRef]
- Hariri, A.; Chen, F.; Moore, C.; Jokerst, J.V. Noninvasive staging of pressure ulcers using photoacoustic imaging. Wound Repair Regen. 2019, 27, 488–496. [Google Scholar] [CrossRef]
- Maneas, E.; Aughwane, R.; Huynh, N.; Xia, W.; Ansari, R.; Singh, M.K.A.; Hutchinson, J.C.; Sebire, N.J.; Arthurs, O.J.; Deprest, J.; et al. Photoacoustic imaging of the human placental vasculature. J. Biophotonics 2019, 13, e201900167. [Google Scholar] [CrossRef]
- Hariri, A.; Zhao, E.; Jeevarathinam, A.S.; LeMaster, J.; Zhang, J.; Jokerst, J.V. Molecular imaging of oxidative stress using an LED-based photoacoustic imaging system. Sci. Rep. 2019, 9, 11378. [Google Scholar] [CrossRef]
- Xia, W.; Maneas, E.; Huynh, N.T.; Singh, M.K.A.; Brown, N.M.; Ourselin, S.; Gilbert-Kawai, E.; West, S.J.; Desjardins, A.E. Imaging of human peripheral blood vessels during cuff occlusion with a compact LED-based photoacoustic and ultrasound system. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2019, San Francisco, CA, USA, 27 February 2019; p. 1087804. [Google Scholar] [CrossRef]
- Zhu, Y.; Lu, X.; Dong, X.; Yuan, J.; Fabiilli, M.L.; Wang, X. LED-Based Photoacoustic Imaging for Monitoring Angiogenesis in Fibrin Scaffolds. Tissue Eng. Part C Methods 2019, 25, 523–531. [Google Scholar] [CrossRef]
- Kalva, S.K.; Pramanik, M. Photoacoustic Tomography with High-Energy Pulsed Laser Diodes; SPIE Spotlight Series Book; SPIE-Intl Soc Optical Eng: Bellingham, WA, USA, 2020; Volume SL56, ISBN 9781510636620. Available online: https://spie.org/Publications/Book/2566566?SSO=1 (accessed on 28 October 2020).
- Fatima, A.; Kratkiewicz, K.; Manwar, R.; Zafar, M.; Zhang, R.; Huang, B.; Dadashzadeh, N.; Xia, J.; Avanaki, K. (Mohammad) Review of cost reduction methods in photoacoustic computed tomography. Photoacoustics 2019, 15, 100137. [Google Scholar] [CrossRef]
- Choi, S.S.S.; Mandelis, A. Review of the state of the art in cardiovascular endoscopy imaging of atherosclerosis using photoacoustic techniques with pulsed and continuous-wave optical excitations. J. Biomed. Opt. 2019, 24, 1–15. [Google Scholar] [CrossRef]
- Nissinen, J.; Kostamovaara, J. A 4 a peak current and 2 ns pulse width CMOS laser diode driver for high measurement rate applications. In Proceedings of the 2013 ESSCIRC (ESSCIRC), Bucharest, Romania, 16–20 September 2013; Institute of Electrical and Electronics Engineers (IEEE): Bucharest, Romania, 2013; pp. 355–358. [Google Scholar]
- Canal, C.; Laugustin, A.; Kohl, A.; Rabot, O. Portable multiwavelength laser diode source for handheld photoacoustic devices. Biophotonics: Photonic Solutions for Better Health Care V 2016, 9887, 98872. [Google Scholar] [CrossRef]
- Daoudi, K.; Berg, P.V.D.; Rabot, O.; Kohl, A.; Tisserand, S.; Brands, P.; Steenbergen, W. Handheld probe integrating laser diode and ultrasound transducer array for ultrasound/photoacoustic dual modality imaging. Opt. Express 2014, 22, 26365–26374. [Google Scholar] [CrossRef]
- Allen, T.J.; Cox, B.T.; Beard, P.C. Generating photoacoustic signals using high-peak power pulsed laser diodes. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2005: The Sixth Conference on Biomedical Thermoacoustics, Optoacoustics, and Acousto-optics, San Jose, CA, USA, 25 April 2005; SPIE-Intl Soc Optical Eng: Bellingham, WA, USA, 2005; Volume 5697, pp. 233–243. [Google Scholar]
- Kolkman, R.G.M.; Steenbergen, W.; Van Leeuwen, T.G. In vivo photoacoustic imaging of blood vessels with a pulsed laser diode. Lasers Med Sci. 2006, 21, 134–139. [Google Scholar] [CrossRef] [PubMed]
- Allen, T.J.; Beard, P.C. Pulsed near-infrared laser diode excitation system for biomedical photoacoustic imaging. Opt. Lett. 2006, 31, 3462–3464. [Google Scholar] [CrossRef]
- Berg, P.J.V.D.; Daoudi, K.; Moens, H.J.B.; Steenbergen, W. Feasibility of photoacoustic/ultrasound imaging of synovitis in finger joints using a point-of-care system. Photoacoustics 2017, 8, 8–14. [Google Scholar] [CrossRef]
- Arabul, M.U.; Heres, M.; Rutten, M.C.M.; Van Sambeek, M.R.; Van De Vosse, F.N.; Lopata, R.G.P. Toward the detection of intraplaque hemorrhage in carotid artery lesions using photoacoustic imaging. J. Biomed. Opt. 2016, 22, 41010. [Google Scholar] [CrossRef]
- Heres, H.M.; Arabul, M.U.; Rutten, M.C.M.; Van De Vosse, F.N.; Lopata, R.G.P. Visualization of vasculature using a hand-held photoacoustic probe: Phantom and in vivo validation. J. Biomed. Opt. 2017, 22, 41013. [Google Scholar] [CrossRef]
- Berg, P.J.V.D.; Bansal, R.; Daoudi, K.; Steenbergen, W.; Prakash, J. Preclinical detection of liver fibrosis using dual-modality photoacoustic/ultrasound system. Biomed. Opt. Express 2016, 7, 5081–5091. [Google Scholar] [CrossRef]
- Jaeger, M.; Schwab, H.-M.; Almallouhi, Y.; Canal, C.; Song, M.; Sauget, V.; Sontrop, D.; Mulder, T.; Roumen, P.; Humblet, A.; et al. Deformation-Compensated Averaging for Deep-Tissue LED and Laser Diode-Based Photoacoustic Imaging Integrated with Handheld Echo Ultrasound. In LED-Based Photoacoustic Imaging; Progress in Optical Science and Photonics; Springer: Singapore, 2020; Volume 7, pp. 47–78. [Google Scholar] [CrossRef]
- Berg, P.J.V.D.; Daoudi, K.; Steenbergen, W. Pulsed photoacoustic flow imaging with a handheld system. J. Biomed. Opt. 2016, 21, 026004. [Google Scholar] [CrossRef]
- Upputuri, P.K.; Pramanik, M. Performance characterization of low-cost, high-speed, portable pulsed laser diode photoacoustic tomography (PLD-PAT) system. Biomed. Opt. Express 2015, 6, 4118–4129. [Google Scholar] [CrossRef]
- Kalva, S.K.; Upputuri, P.K.; Pramanik, M. High-speed, low-cost, pulsed-laser-diode-based second-generation desktop photoacoustic tomography system. Opt. Lett. 2018, 44, 81–84. [Google Scholar] [CrossRef]
- Rajendran, P.; Sahu, S.; Dienzo, R.A.; Pramanik, M. In vivo detection of venous sinus distension due to intracranial hypotension in small animal using pulsed-laser-diode photoacoustic tomography. J. Biophotonics 2020, 13, e201960162. [Google Scholar] [CrossRef]
- Upputuri, P.K.; Pramanik, M. Dynamic in vivo imaging of small animal brain using pulsed laser diode-based photoacoustic tomography system. J. Biomed. Opt. 2017, 22, 1. [Google Scholar] [CrossRef]
- Upputuri, P.K.; Das, D.; Maheshwari, M.; Yaowen, Y.; Pramanik, M. Real-time monitoring of temperature using a pulsed laser-diode-based photoacoustic system. Opt. Lett. 2020, 45, 718–721. [Google Scholar] [CrossRef]
- Upputuri, P.K.; Pramanik, M. Fast photoacoustic imaging systems using pulsed laser diodes: A review. Biomed. Eng. Lett. 2018, 8, 167–181. [Google Scholar] [CrossRef]
- Sivasubramanian, K.; Pramanik, M. High frame rate photoacoustic imaging at 7000 frames per second using clinical ultrasound system. Biomed. Opt. Express 2016, 7, 312–323. [Google Scholar] [CrossRef]
- Hariri, A.; Fatima, A.; Mohammadian, N.; Mahmoodkalayeh, S.; Ansari, M.; Bely, N.; Nasiriavanaki, M. Development of low-cost photoacoustic imaging systems using very low-energy pulsed laser diodes. J. Biomed. Opt. 2017, 22, 75001. [Google Scholar] [CrossRef]
- Zeng, L.; Piao, Z.; Huang, S.; Jia, W.; Chen, Z. Label-free optical-resolution photoacoustic microscopy of superficial microvasculature using a compact visible laser diode excitation. Opt. Express 2015, 23, 31026–31033. [Google Scholar] [CrossRef]
- Wang, T.; Nandy, S.; Salehi, H.S.; Kumavor, P.D.; Zhu, Q. A low-cost photoacoustic microscopy system with a laser diode excitation. Biomed. Opt. Express 2014, 5, 3053–3058. [Google Scholar] [CrossRef]
- Zeng, L.; Liu, G.; Yang, D.; Ji, X. Portable optical-resolution photoacoustic microscopy with a pulsed laser diode excitation. Appl. Phys. Lett. 2013, 102, 053704. [Google Scholar] [CrossRef]
- Erfanzadeh, M.; Kumavor, P.D.; Zhu, Q. Laser scanning laser diode photoacoustic microscopy system. Photoacoustics 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Dai, X.; Yang, H.; Jiang, H. In vivo photoacoustic imaging of vasculature with a low-cost miniature light emitting diode excitation. Opt. Lett. 2017, 42, 1456–1459. [Google Scholar] [CrossRef]
- Steinberg, I.; Huland, D.M.; Vermesh, O.; Frostig, H.E.; Tummers, W.S.; Gambhir, S.S. Photoacoustic clinical imaging. Photoacoustics 2019, 14, 77–98. [Google Scholar] [CrossRef]
- Kruger, R.A.; Kuzmiak, C.M.; Lam, R.B.; Reinecke, D.R.; Del Rio, S.P.; Steed, D. Dedicated 3D photoacoustic breast imaging. Med Phys. 2013, 40, 113301. [Google Scholar] [CrossRef]
- Lin, L.; Hu, P.; Shi, J.; Appleton, C.M.; Maslov, K.; Li, L.; Zhang, R.; Wang, L.V. Single-breath-hold photoacoustic computed tomography of the breast. Nat. Commun. 2018, 9, 1–9. [Google Scholar] [CrossRef]
- Abu Anas, E.M.; Zhang, H.K.; Kang, J.; Boctor, E. Enabling fast and high quality LED photoacoustic imaging: A recurrent neural networks based approach. Biomed. Opt. Express 2018, 9, 3852–3866. [Google Scholar] [CrossRef]
- Mozaffarzadeh, M.; Hariri, A.; Moore, C.; Jokerst, J.V. The double-stage delay-multiply-and-sum image reconstruction method improves imaging quality in a LED-based photoacoustic array scanner. Photoacoustics 2018, 12, 22–29. [Google Scholar] [CrossRef]
- Singh, M.K.A.; Sivasubramanian, K.; Sato, N.; Ichihashi, F.; Sankai, Y.; Xing, L. Deep learning-enhanced LED-based photoacoustic imaging. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2020, San Francisco, CA, USA, 17 February 2020; p. 1124038. [Google Scholar] [CrossRef]
- Farnia, P.; Najafzadeh, E.; Hariri, A.; Lavasani, S.N.; Makkiabadi, B.; Ahmadian, A.; Jokerst, J.V. Dictionary learning technique enhances signal in LED-based photoacoustic imaging. Biomed. Opt. Express 2020, 11, 2533–2547. [Google Scholar] [CrossRef]
- Manwar, R.; Hosseinzadeh, M.; Hariri, A.; Kratkiewicz, K.; Noei, S.; Avanaki, K. Photoacoustic Signal Enhancement: Towards Utilization of Low Energy Laser Diodes in Real-Time Photoacoustic Imaging. Sensors 2018, 18, 3498. [Google Scholar] [CrossRef]
- Chandramoorthi, S.; Thittai, A.K. Ultrasound Receive-Side Strategies for Image Quality Enhancement in Low-Energy Illumination Based Photoacoustic Imaging. In LED-Based Photoacoustic Imaging; Progress in Optical Science and Photonics; Springer: Singapore, 2020; Volume 7, pp. 79–112. [Google Scholar] [CrossRef]
- Chandramoorthi, S.; Thittai, A.K. Enhancing Image Quality of Photoacoustic Tomography Using Sub-Pitch Array Translation Approach: Simulation and Experimental Validation. IEEE Trans. Biomed. Eng. 2019, 66, 3543–3552. [Google Scholar] [CrossRef]
- Sowmiya, C.; Thittai, A.K. Noise Reduction in Inherently low-SNR PLD-based PAT images. In Proceedings of the TENCON 2019—2019 IEEE Region 10 Conference (TENCON), Kochi, India, 17–20 October 2019; Institute of Electrical and Electronics Engineers (IEEE): Kochi, India, 2019; pp. 106–108. [Google Scholar]
- Zhang, H.K. Democratizing LED-Based Photoacoustic Imaging with Adaptive Beamforming and Deep Convolutional Neural Network. In LED-Based Photoacoustic Imaging; Progress in Optical Science and Photonics; Springer: Singapore, 2020; Volume 7, pp. 183–202. [Google Scholar] [CrossRef]
- Xia, W.; Ginsberg, Y.; West, S.J.; Nikitichev, D.I.; Ourselin, S.; David, A.L.; Desjardins, A.E. Coded excitation ultrasonic needle tracking: An in vivo study. Med Phys. 2016, 43, 4065. [Google Scholar] [CrossRef]
- Singh, M.K.A.; Jaeger, M.; Frenz, M.; Steenbergen, W. In vivo demonstration of reflection artifact reduction in photoacoustic imaging using synthetic aperture photoacoustic-guided focused ultrasound (PAFUSion). Biomed. Opt. Express 2016, 7, 2955–2972. [Google Scholar] [CrossRef]
- Wong, T.T.W.; Zhou, Y.; Garcia-Uribe, A.; Li, L.; Maslov, K.; Lin, L.; Wang, L.V. Use of a single xenon flash lamp for photoacoustic computed tomography of multiple-centimeter-thick biological tissue ex vivo and a whole mouse body in vivo. J. Biomed. Opt. 2016, 22, 041003. [Google Scholar] [CrossRef]
- Wang, D.; Wang, Y.; Wang, W.; Luo, D.; Chitgupi, U.; Geng, J.; Zhou, Y.; Wang, L.; Lovell, J.F.; Xia, J. Deep tissue photoacoustic computed tomography with a fast and compact laser system. Biomed. Opt. Express 2016, 8, 112–123. [Google Scholar] [CrossRef] [PubMed]
- Jeng, G.-S.; Li, M.-L.; Kim, M.W.; Yoon, S.J.; Pitre, J.J.; Li, D.S.; Pelivanov, I.; O’Donnell, M. Real-Time Spectroscopic Photoacoustic/Ultrasound (PAUS) Scanning with Simultaneous Fluence Compensation and Motion Correction for Quantitative Molecular Imaging; bioRxiv: New York, NY, USA, 2019. [Google Scholar]
- Allen, T.J.; Alam, S.; Zhang, E.; Laufer, J.; Richardson, D.J.; Beard, P.C. Use of a pulsed fibre laser as an excitation source for photoacoustic tomography. In Proceedings of the Photons Plus Ultrasound: Imaging and Sensing 2011, San Francisco, CA, USA, 28 February 2011; p. 78991V. [Google Scholar] [CrossRef]
- Lashkari, B.; Choi, S.S.S.; Dovlo, E.; Dhody, S.; Mandelis, A. Frequency-Domain Photoacoustic Phase Spectroscopy: A Fluence-Independent Approach for Quantitative Probing of Hemoglobin Oxygen Saturation. IEEE J. Sel. Top. Quantum Electron. 2015, 22, 127–136. [Google Scholar] [CrossRef]
- Lashkari, B.; Mandelis, A. Comparison between pulsed laser and frequency-domain photoacoustic modalities: Signal-to-noise ratio, contrast, resolution, and maximum depth detectivity. Rev. Sci. Instrum. 2011, 82, 094903. [Google Scholar] [CrossRef] [PubMed]
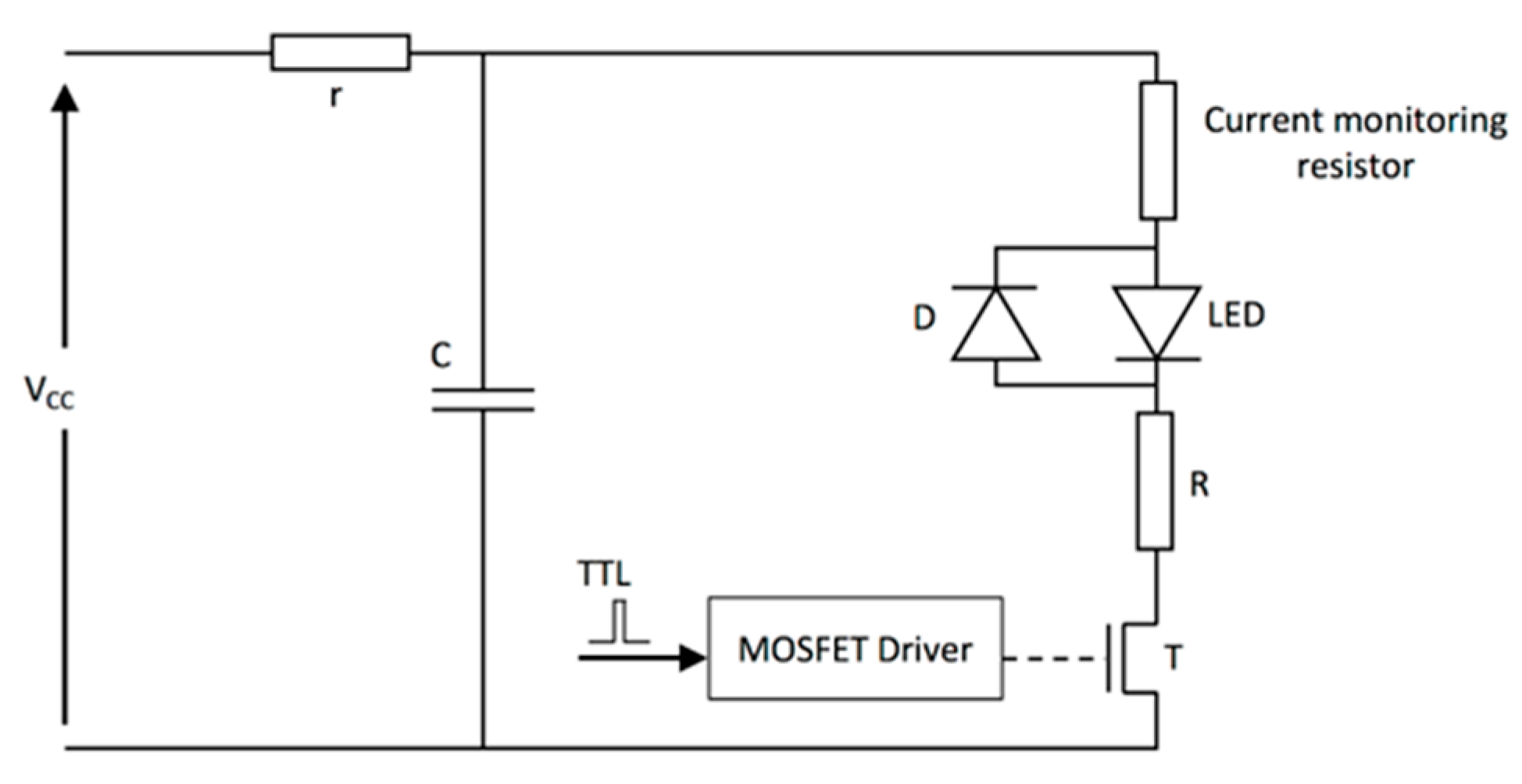
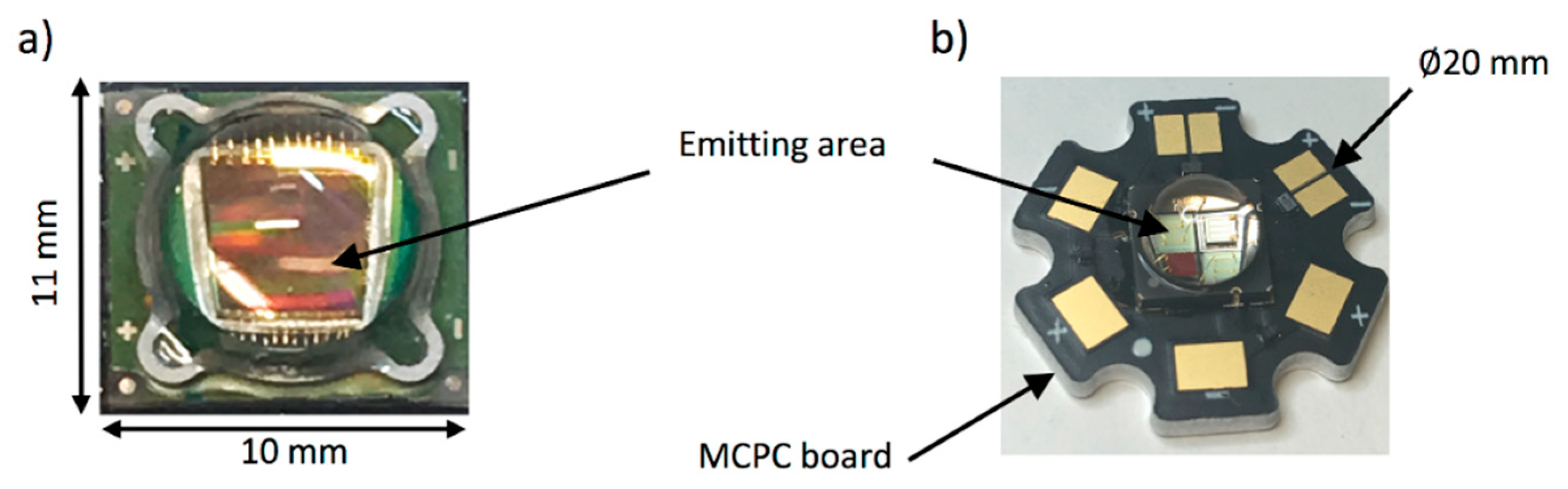

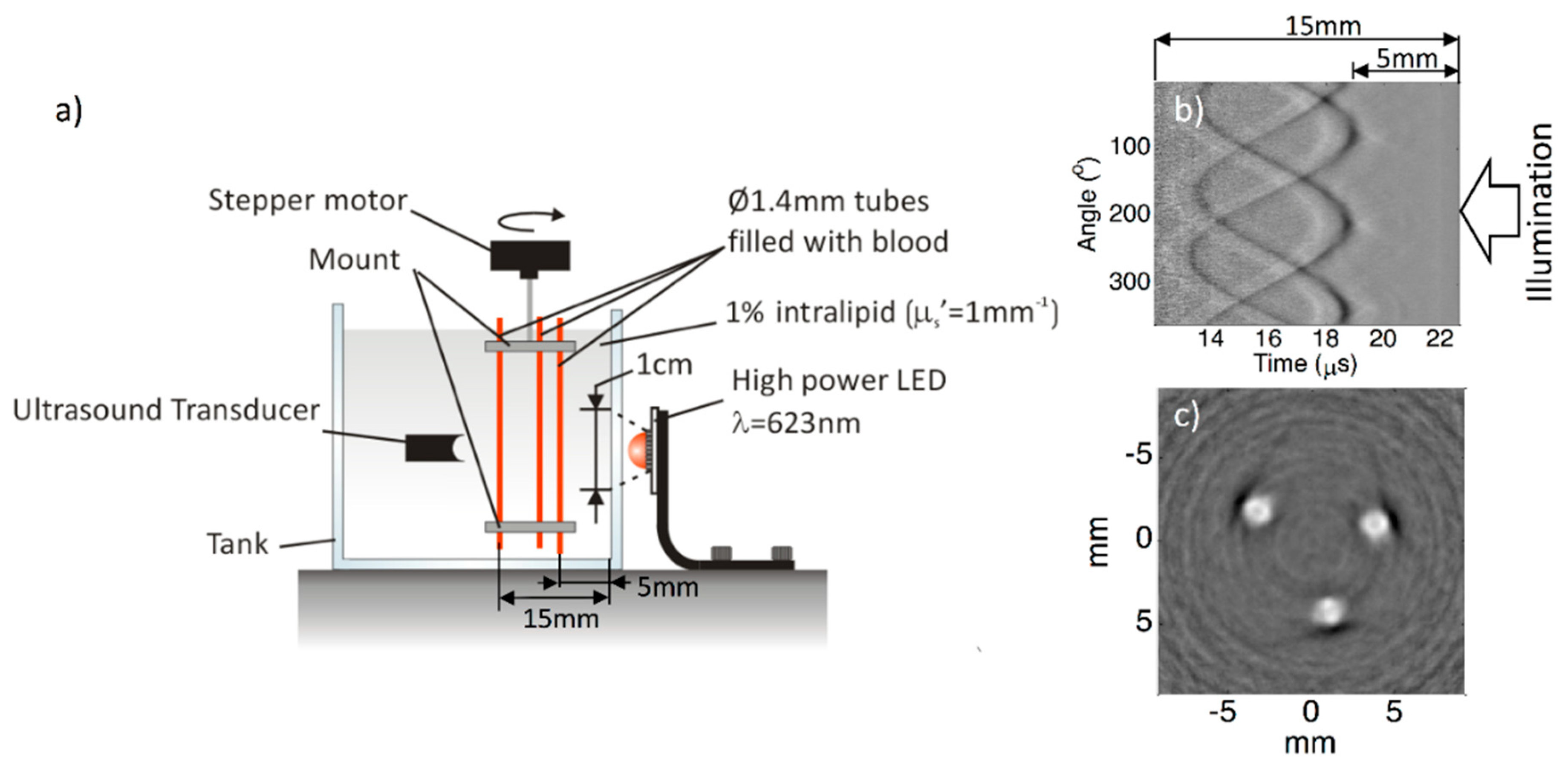
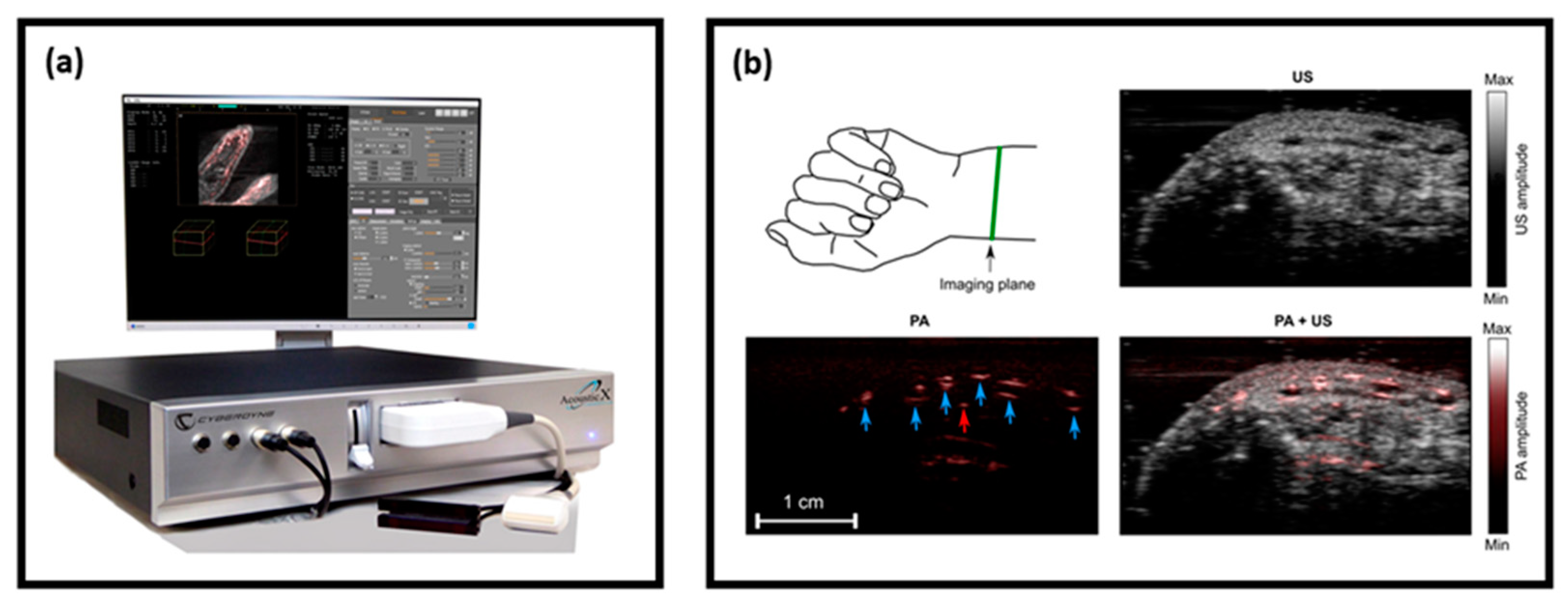
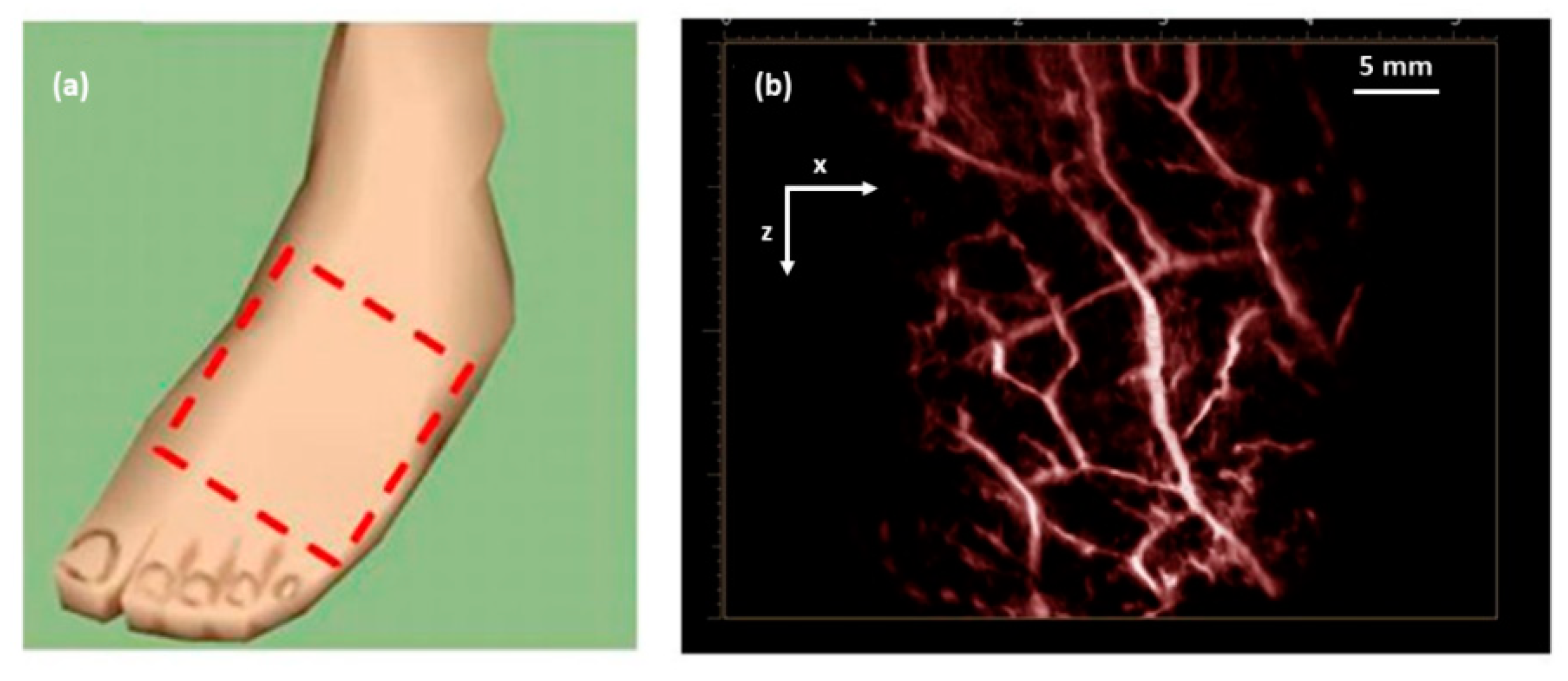

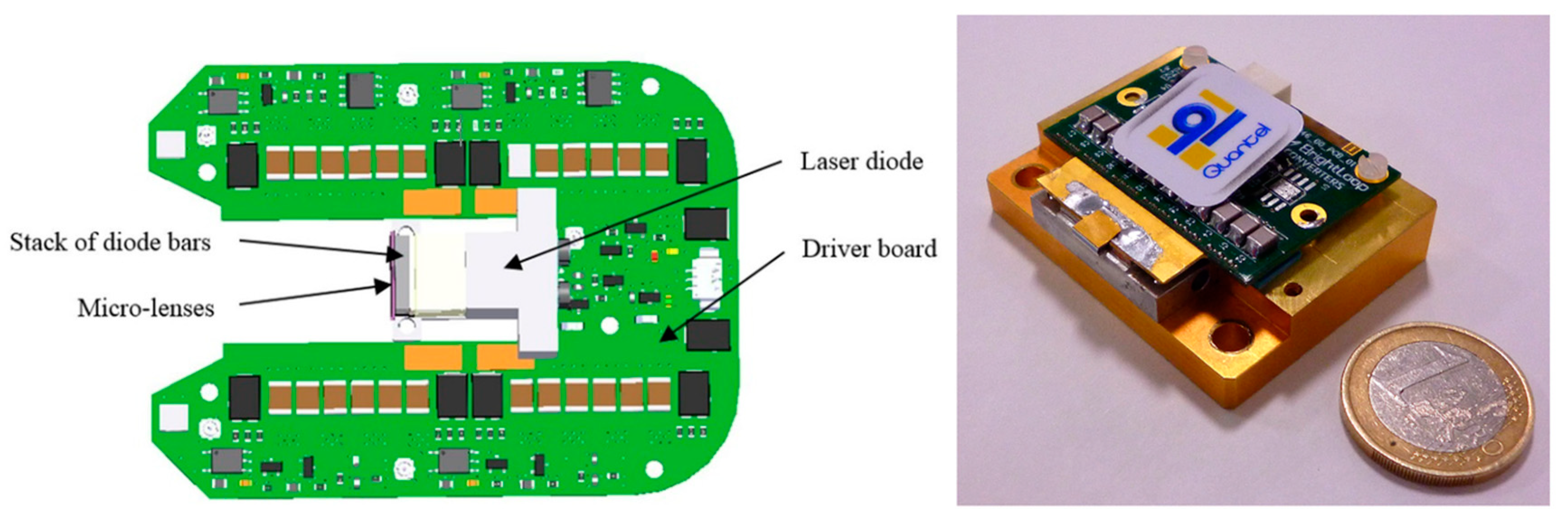
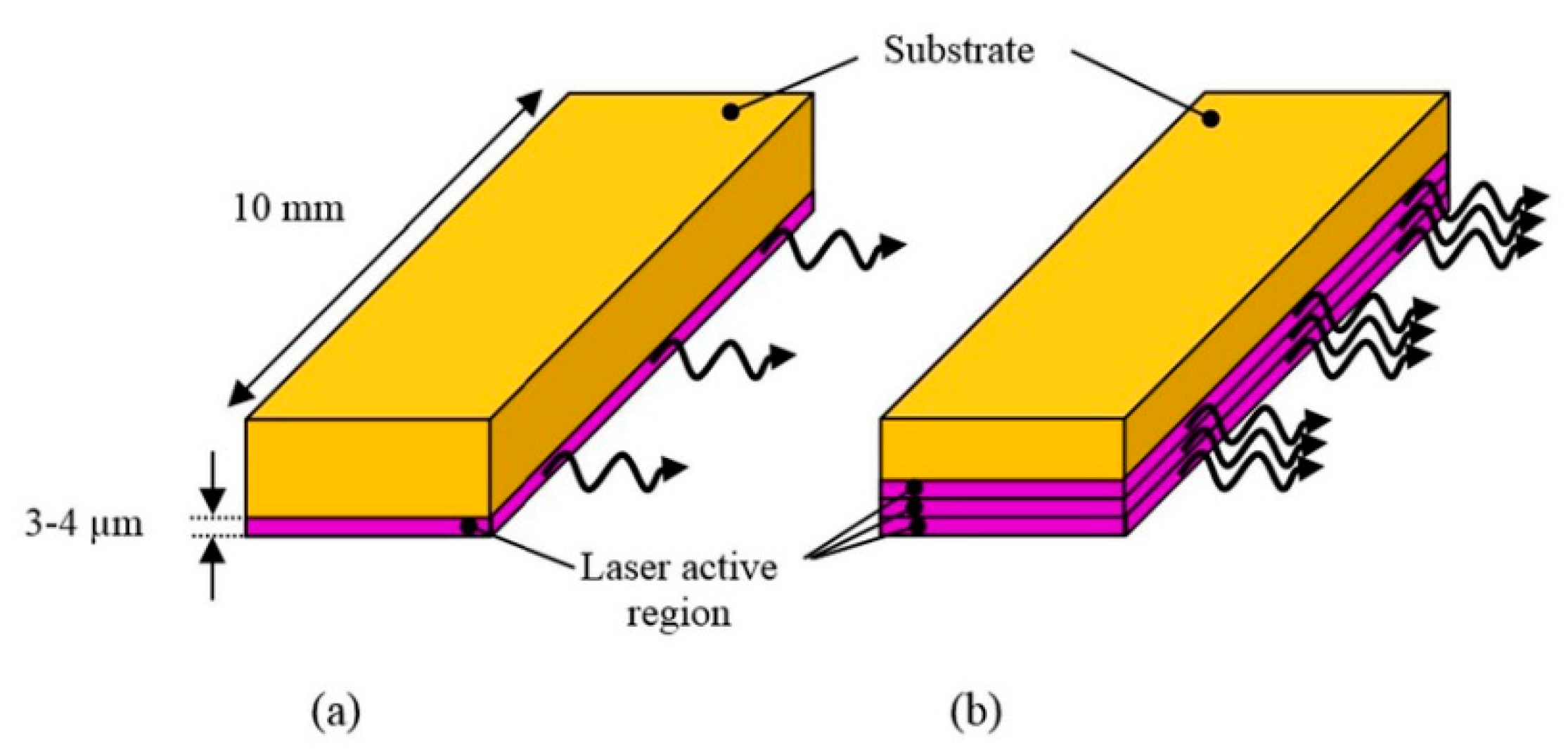

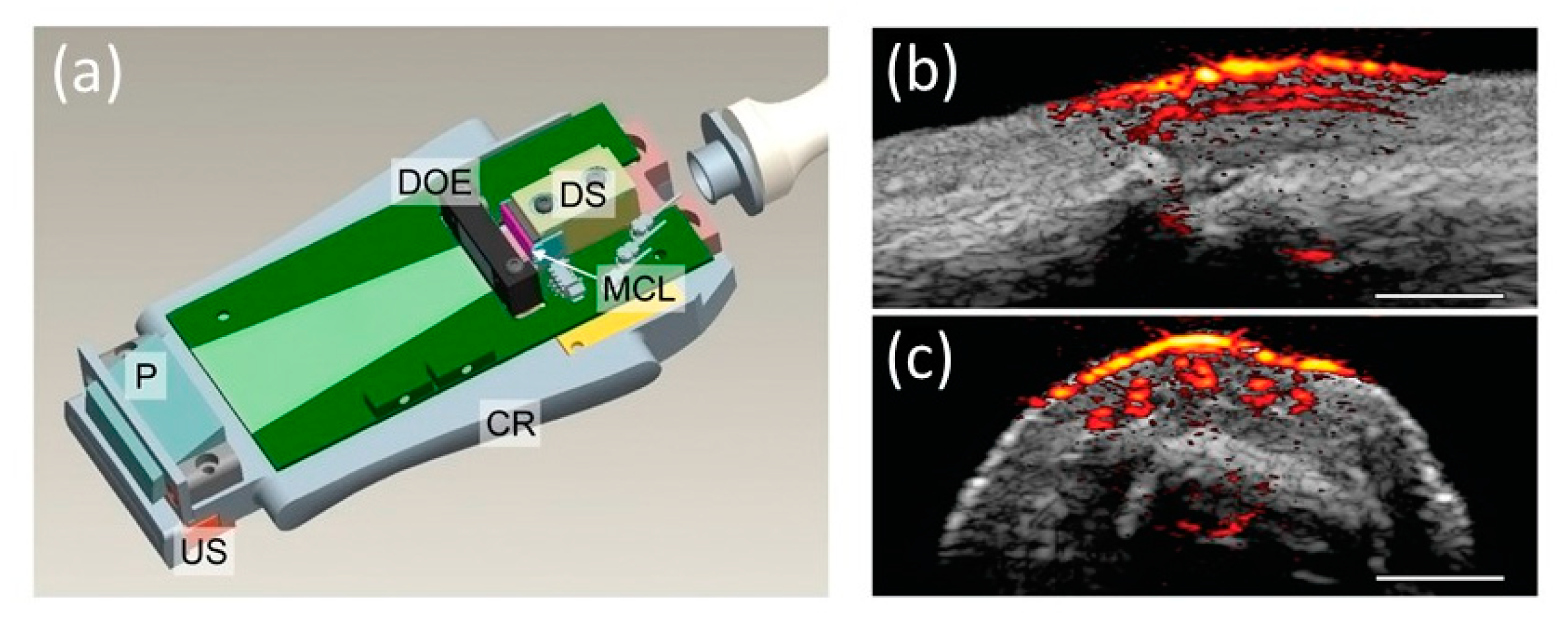
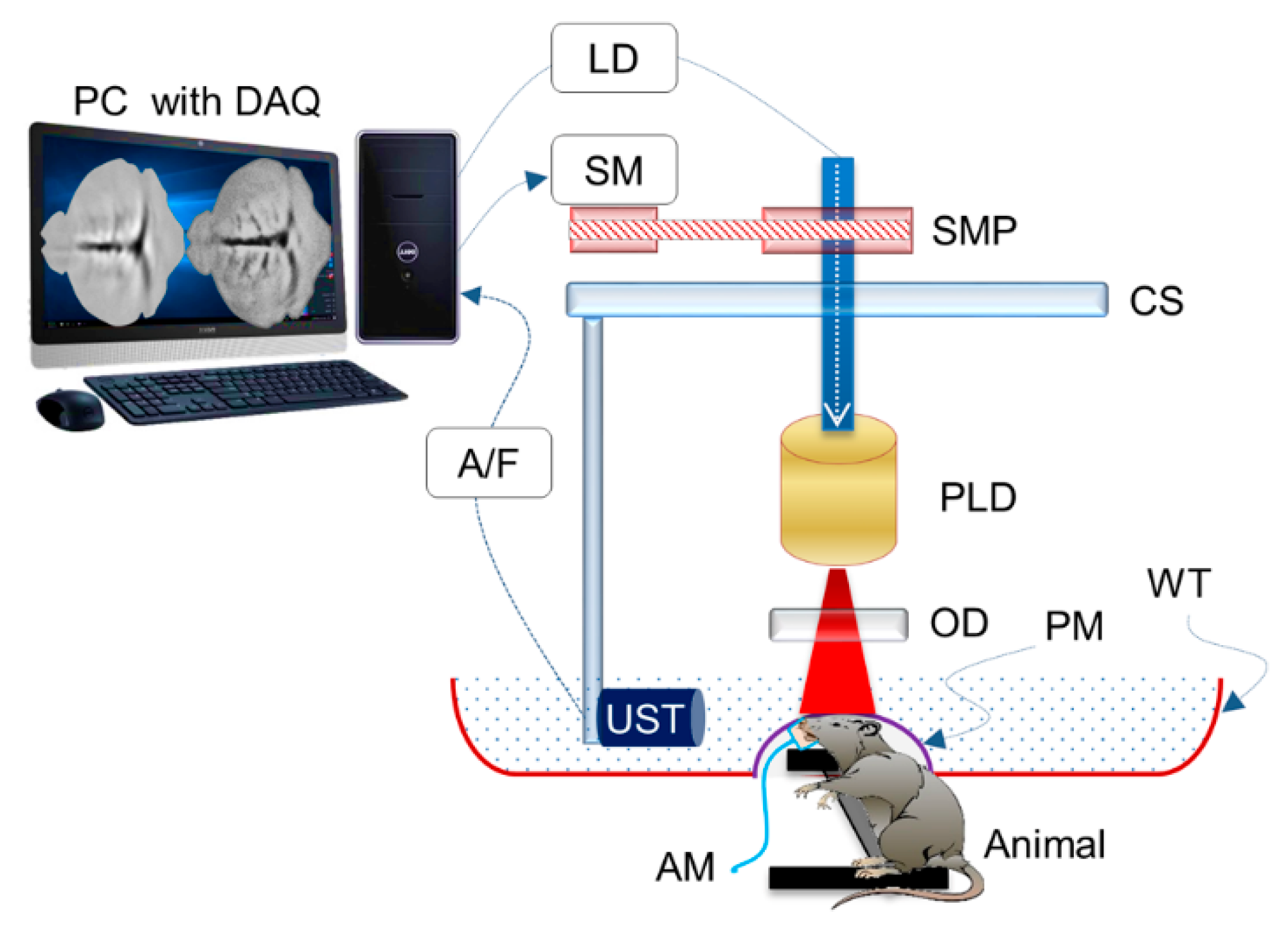
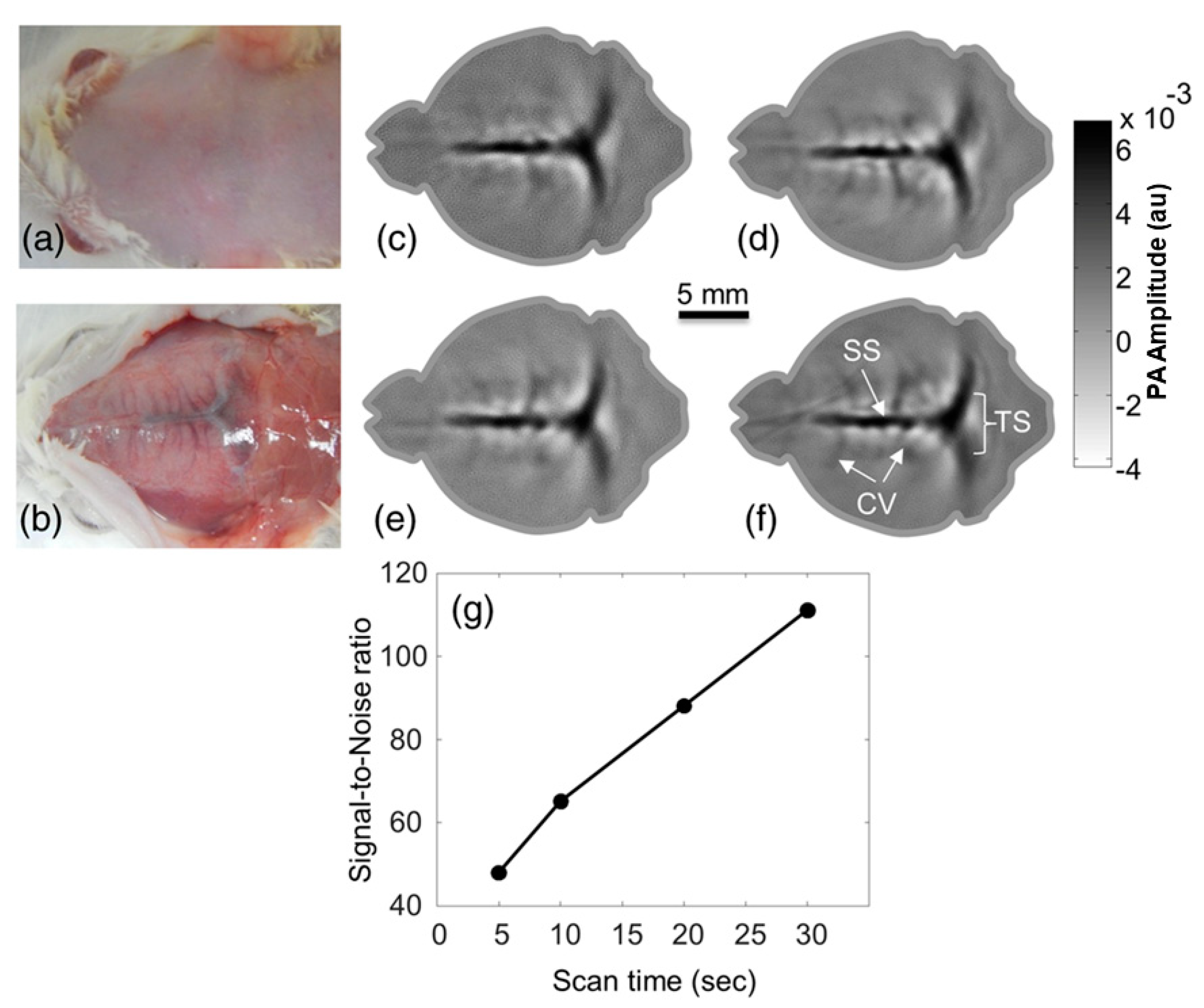
| Energy (mJ) | PRR (Hz) | Pulse Width (ns) | Cost * | Advantages | Disadvantages | |
|---|---|---|---|---|---|---|
| Solid-state lasers | 5–120 | 10–200 | <10 | $70–200 K | Powerful, ~5 cm penetration depth, tunable wavelength | Bulky size, eye protection and laser safe rooms needed |
| LD | 0.5–2.5 | ~1 K–6 K | 30–200 | ~$10–25 K | Integration in a handheld probe feasible, high PRR | Limited penetration depth, eye protection and laser safe rooms needed, wavelength tuning not possible |
| LED | 0.2 | ~200–16 K | 30–100 | $10–15 K | Integration in a handheld probe feasible, high PRR, wide wavelength range, no need of laser-safe rooms and eye-safety goggles | Limited penetration depth, wavelength tuning not possible |
| Q-switched DPSS laser | 1 | 100 K | 2–10 | - | High PRR, low pulse width, Reasonably high optical energy per pulse | Less number of wavelengths (266 nm, 355 nm, 532 nm, 1064 nm) available and spectral tuning may be cumbersome |
| High-energy DPSS laser | 200 | 200 | 10–30 | - | High optical output per pulse, reasonably high PRR | Less number of wavelengths (266 nm, 355 nm, 532 nm, 1064 nm) available and spectral tuning may be cumbersome |
| Wavelength (nm) | 440–550 | 570–650 | 624–920 |
| Material | InGaN | AlGaInP | AlGaAs |
| Target | Application | Depth (mm) | Contrast Agent | Wavelength (nm) | |
|---|---|---|---|---|---|
| Medical needles, Vasculature | Guidance of minimally invasive procedures with peripheral tissue targets [18] | Phantom and ex vivo studies | 38 | N/A | 850 |
| Vasculature | Imaging of human placental vasculature [48] | 7 | N/A | 850 | |
| Tumor | Imaging of intraocular tumors [26] | 10 | N/A | 850 | |
| Vasculature | Non-invasive monitoring of angiogenesis [51] | Animal in vivo | 10 | N/A | 850 |
| Ulcer | Noninvasive imaging of pressure ulcers [47] | 10 | N/A | 690 | |
| Oxygen saturation | Oxygen saturation imaging in rheumatoid arthritis [39] | 5 | N/A | 750/850 | |
| Molecular | Detection and monitoring of reactive oxygen and nitrogen species [49] | 10 | CyBA | 850 | |
| Tumor/Contrast agents | Imaging of tumor using contrast enhancement [44] | 10 | NC | 850 | |
| Cells/Contrast agents | Imaging of molecular-labelled cells [38] | 10 | DiR | 850 | |
| Vasculature | Imaging of peripheral microvasculature and function [26] | Healthy human | 10 | N/A | 690/850 |
| Vasculature | Simultaneous imaging of veins and lymphatic vessels [40] | 10 | ICG | 940/820 | |
| Finger joints | Full view tomography of finger joints [28] | 5 | N/A | 850 | |
| Finger joints | Imaging of inflammatory arthritis [42] | Patient | 5 | N/A | 850 |
| Skin | Imaging of port wine stain [43] | 10 | N/A | 850 |
| Wavelength (nm) | 630–670 | 720–850 | 900–1100 |
| Material | AlGaInP/GaAs | AlGaAs/GaAs | InGaAs/GaAs |
| Target | Application | Depth (mm) | Contrast Agent | Wavelength (nm) | |
|---|---|---|---|---|---|
| Vasculature | Detection of intraplaque hemorrhage in carotid artery [62] | Phantom and ex vivo studies | 20 | N/A | 808 |
| Vasculature | Dynamic imaging studies (for example in cardiovascular medicine) [73] | 20 | N/A | 803 | |
| Red blood cells | Non-invasive blood flow imaging [66] | 7 | N/A | 805 | |
| Vasculature | Detection of liver fibrosis [64] | Animal in vivo | 5 | N/A | 808 |
| Cortical vasculature | Brain imaging [68] | 5 | ICG | 803 | |
| Cerebro-spinal fluid volume level | Detection of venous sinus distension by measuring intra-cranial hypertension [69] | 5 | N/A | 803 | |
| Vasculature and perfusion | Vascular/dermal pathologies [63] | Healthy human | 5 | N/A | 805 |
| Vasculature | Detection of intraplaque hemorrhage in carotid artery [65] | 15 | N/A | 808 | |
| Finger joints | Imaging of rheumatoid arthritis [57] | 5 | N/A | 808 | |
| Finger joints | Imaging of rheumatoid arthritis [61] | Patient | 5 | N/A | 808 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuniyil Ajith Singh, M.; Xia, W. Portable and Affordable Light Source-Based Photoacoustic Tomography. Sensors 2020, 20, 6173. https://doi.org/10.3390/s20216173
Kuniyil Ajith Singh M, Xia W. Portable and Affordable Light Source-Based Photoacoustic Tomography. Sensors. 2020; 20(21):6173. https://doi.org/10.3390/s20216173
Chicago/Turabian StyleKuniyil Ajith Singh, Mithun, and Wenfeng Xia. 2020. "Portable and Affordable Light Source-Based Photoacoustic Tomography" Sensors 20, no. 21: 6173. https://doi.org/10.3390/s20216173
APA StyleKuniyil Ajith Singh, M., & Xia, W. (2020). Portable and Affordable Light Source-Based Photoacoustic Tomography. Sensors, 20(21), 6173. https://doi.org/10.3390/s20216173






