Cost-Effective Electrochemical Activation of Graphitic Carbon Nitride on the Glassy Carbon Electrode Surface for Selective Determination of Serotonin
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Instruments
2.2. Sensor Preparation
3. Results and Discussion
3.1. Physical Characterization of g-C3N4 Nanosheets
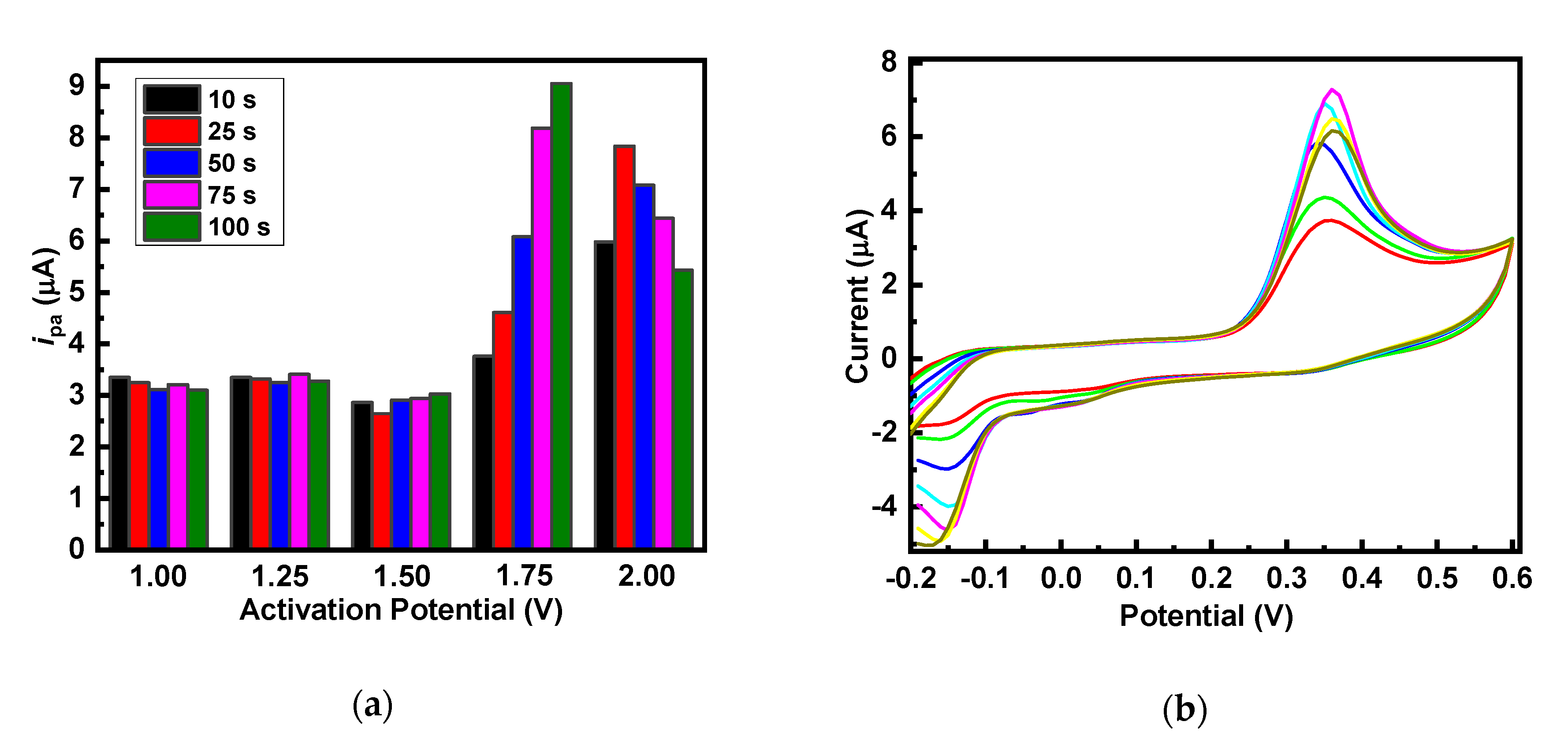
3.2. Optimization of g-C3N4 Nanosheets Synthesis
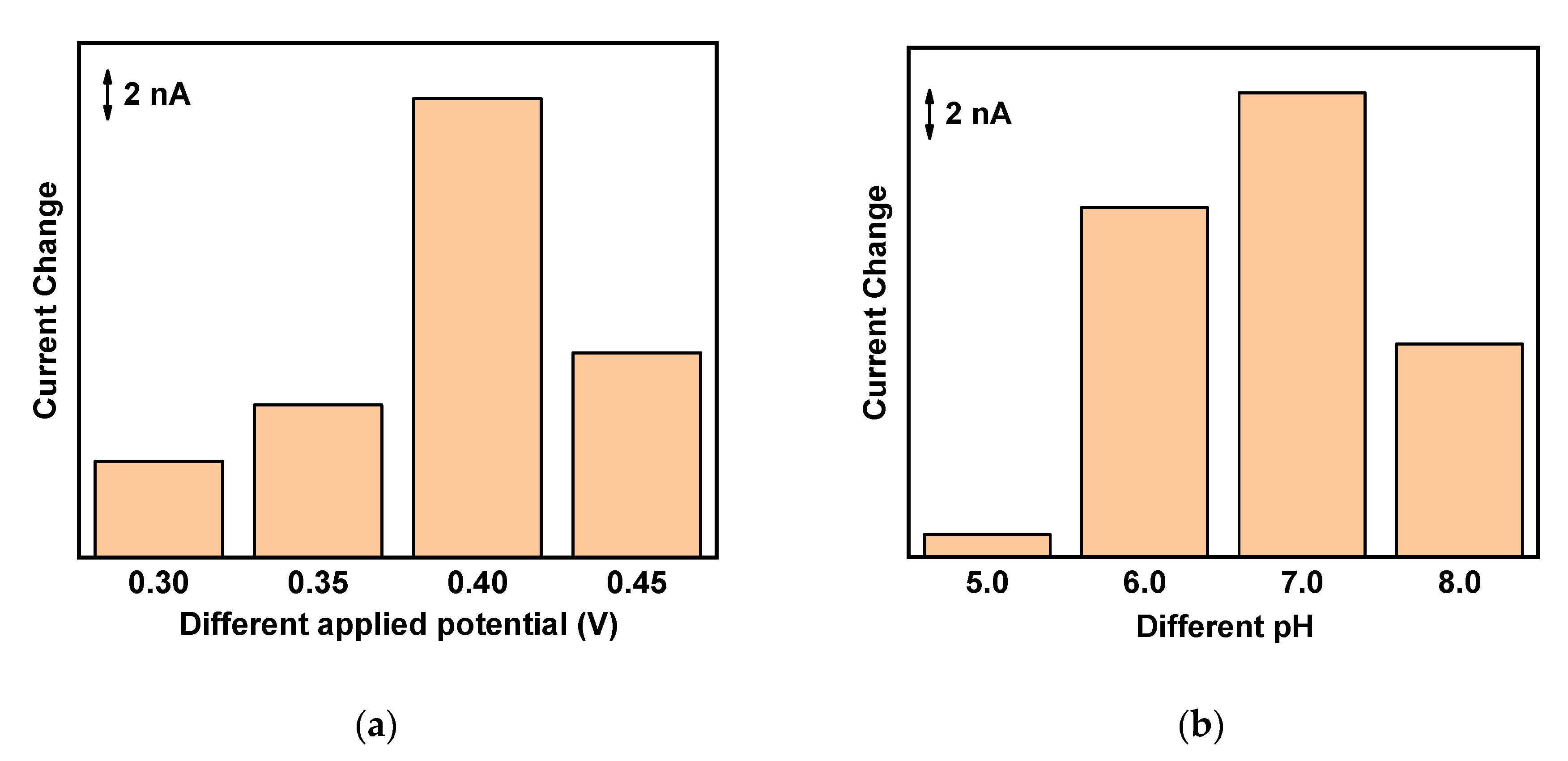
3.3. Optimization of 5-HT Sensors
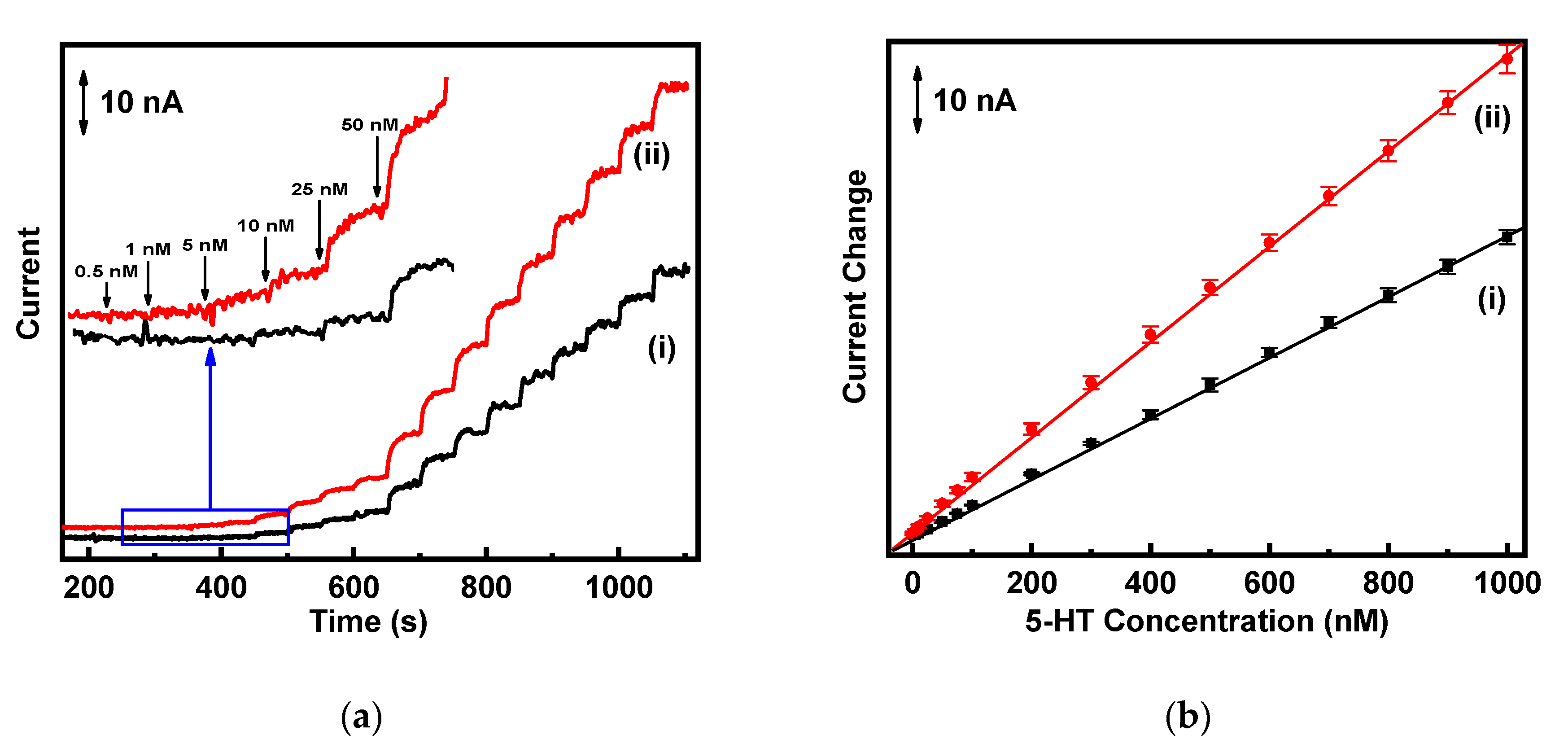
3.4. Calibration Plots
3.5. Selectivity Study
3.6. Reproducibility and Stability
4. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Thanh, T.D.; Balamurugan, J.; Hien, H.V.; Kim, N.H.; Lee, J.H. A novel sensitive sensor for serotonin based on high-quality of AuAg nanoalloy encapsulated graphene electrocatalyst. Biosens. Bioelectron. 2017, 96, 186–193. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, S.; Tao, L.; Min, Q.; Xiang, J.; Wang, Q.; Xie, J.; Yue, Y.; Wu, S.; Li, X.; et al. A disposable electrochemical sensor for simultaneous determination of norepinephrine and serotonin in rat cerebrospinal fluid based on MWNTs-ZnO/chitosan composites modified screen-printed electrode. Biosens. Bioelectron. 2015, 65, 31–38. [Google Scholar] [CrossRef] [PubMed]
- Artigas, F.; Sarrias, M.J.; Martinez, E.; Gelpi, E. Serotonin in body fluids: Characterization of human plasmatic and cerebrospinal fluid pools by means of a new HPLC method. Life Sci. 1985, 37, 441–447. [Google Scholar] [CrossRef]
- Gupta, R.; Gamare, J.S.; Pandey, A.K.; Tyagi, D.; Kamat, J.V. Highly Sensitive Detection of Arsenite Based on Its Affinity toward Ruthenium Nanoparticles Decorated on Glassy Carbon Electrode. Anal. Chem. 2016, 88, 2459–2465. [Google Scholar] [CrossRef] [PubMed]
- Ananthi, A.; Kumar, S.S.; Phani, K.L. Facile one-step direct electrodeposition of bismuth nanowires on glassy carbon electrode for selective determination of folic acid. Electrochim. Acta 2015, 151, 584–590. [Google Scholar] [CrossRef]
- Yang, C.; Denno, M.E.; Pyakurel, P.; Venton, B.J. Recent trends in carbon nanomaterial-based electrochemical sensors for biomolecules: A review. Anal. Chim. Acta 2015, 887, 17–37. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.L.; Wang, X.F.; Qian, Q.Y.; Wang, F.B.; Xia, X.H. A green approach to the synthesis of graphene nanosheets. ACS Nano 2009, 3, 2653–2659. [Google Scholar] [CrossRef]
- Cao, J.; He, P.; Mohammed, M.A.; Zhao, X.; Young, R.J.; Derby, B.; Kinloch, I.A.; Dryfe, R.A.W. Two-Step Electrochemical Intercalation and Oxidation of Graphite for the Mass Production of Graphene Oxide. J. Am. Chem. Soc. 2017, 139, 17446–17456. [Google Scholar] [CrossRef]
- Zhang, Z.; Yan, J.; Jin, H.; Yin, J. Tuning the reduction extent of electrochemically reduced graphene oxide electrode film to enhance its detection limit for voltammetric analysis. Electrochim. Acta 2014, 139, 232–237. [Google Scholar] [CrossRef]
- Vijayaraj, K.; Dinakaran, T.; Lee, Y.; Kim, S.; Kim, H.S.; Lee, J.; Chang, S.-C. One-step construction of a molybdenum disulfide/multi-walled carbon nanotubes/polypyrrole nanocomposite sensor for the ex-vivo detection of dopamine in mouse brain tissue. Biochem. Biophys. Res. Commun. 2017, 494, 181–187. [Google Scholar] [CrossRef]
- Xiong, M.; Rong, Q.; Meng, H.; Zhang, X. Two-dimensional graphitic carbon nitride nanosheets for biosensing applications. Biosens. Bioelectron. 2017, 89, 212–223. [Google Scholar] [CrossRef] [PubMed]
- Lin, Z.; Wang, X. Nanostructure engineering and doping of conjugated carbon nitride semiconductors for hydrogen photosynthesis. Angew. Chem. Int. Ed. Engl. 2013, 52, 1735–1738. [Google Scholar] [CrossRef]
- Zhang, Y.; Mori, T.; Ye, J.; Antonietti, M. Phosphorus-doped carbon nitride solid: Enhanced electrical conductivity and photocurrent generation. J. Am. Chem. Soc. 2010, 132, 6294–6295. [Google Scholar] [CrossRef]
- Zhou, D.; Wang, M.; Dong, J.; Ai, S. A Novel Electrochemical Immunosensor Based on Mesoporous Graphitic Carbon Nitride for Detection of Subgroup J of Avian Leukosis Viruses. Electrochim. Acta 2016, 205, 95–101. [Google Scholar] [CrossRef]
- Zhang, J.H.; Wei, M.J.; Wei, Z.W.; Pan, M.; Su, C.-Y. Ultrathin Graphitic Carbon Nitride Nanosheets for Photocatalytic Hydrogen Evolution. ACS Appl. Nano Mater. 2020, 3, 1010–1018. [Google Scholar] [CrossRef]
- Zhang, X.; Xie, X.; Wang, H.; Zhang, J.; Pan, B.; Xie, Y. Enhanced photoresponsive ultrathin graphitic-phase C3N4 nanosheets for bioimaging. J. Am. Chem. Soc. 2013, 135, 18–21. [Google Scholar] [CrossRef]
- Cheng, F.; Wang, H.; Dong, X. The amphoteric properties of g-C3N4 nanosheets and fabrication of their relevant heterostructure photocatalysts by an electrostatic re-assembly route. Chem. Commun. 2015, 51, 7176–7179. [Google Scholar] [CrossRef]
- Lu, Q.; Deng, J.; Hou, Y.; Wang, H.; Li, H.; Zhang, Y. One-step electrochemical synthesis of ultrathin graphitic carbon nitride nanosheets and their application to the detection of uric acid. Chem. Commun. 2015, 51, 12251–12253. [Google Scholar] [CrossRef]
- Jiang, T.; Jiang, G.; Huang, Q.; Zhou, H. High-sensitive detection of dopamine using graphitic carbon nitride by electrochemical method. Mater. Res. Bull. 2016, 74, 271–277. [Google Scholar] [CrossRef]
- Gao, W.; Wang, X.; Li, P.; Wu, Q.; Qi, F.; Wu, S.; Yu, Y.; Ding, K. Highly sensitive and selective detection of cadmium with a graphite carbon nitride nanosheets/Nafion electrode. RSC Adv. 2016, 6, 113570–113575. [Google Scholar] [CrossRef]
- Wu, Y.; Yang, Y.; Lei, W.; Li, C.; Hao, Q.; Zhang, C.; Zhang, Y.; Su, J. A Facile Construction of Porous g-C3N4/poly (3,4-ethylenedioxythiophene) Composite Modified Electrode for Ascorbic Acid Determination. J. Electrochem. Soc. 2018, 165, B118–B126. [Google Scholar] [CrossRef]
- Yang, Y.; Hou, H.; Zou, G.; Shi, W.; Shuai, H.; Lib, J.; Ji, X. Electrochemical Exfoliation of Graphene-Like Two-Dimensional Nanomaterials. Nanoscale 2019, 11, 16–33. [Google Scholar] [CrossRef] [PubMed]
- Wang, A.; Wang, C.; Fu, L.; Wong-Ng, W.; Lan, Y. Recent Advances of Graphitic Carbon Nitride-Based Structures and Applications in Catalyst, Sensing, Imaging, and LEDs. Nano-Micro Lett. 2017, 9, 1–21. [Google Scholar] [CrossRef]
- Lin, J.; Pan, Z.; Wang, X. Photochemical Reduction of CO2 by Graphitic Carbon Nitride Polymers. ACS Sustain. Chem. Eng. 2014, 2, 353–358. [Google Scholar] [CrossRef]
- Wang, R.; Li, H.; Zhang, L.; Zeng, Y.-J.; Lv, Z.; Yang, J.-Q.; Mao, J.-Y.; Wang, Z.; Zhou, Y.; Han, S.-T. Graphitic carbon nitride nanosheets for solution processed non-volatile memory devices. J. Mater. Chem. C 2019, 7, 10203–10210. [Google Scholar] [CrossRef]
- Sanchez, S.; Fabregas, E.; Pumera, M. Electrochemical activation of carbon nanotube/polymer composites. Phys. Chem. Chem. Phys. 2009, 11, 182–186. [Google Scholar] [CrossRef]
- Terse-Thakoor, T.; Komori, K.; Ramnani, P.; Lee, I.; Mulchandani, A. Electrochemically Functionalized Seamless Three-Dimensional Graphene-Carbon Nanotube Hybrid for Direct Electron Transfer of Glucose Oxidase and Bioelectrocatalysis. Langmuir 2015, 31, 13054–13061. [Google Scholar] [CrossRef]
- Hashemi, P.; Dankoski, E.C.; Petrovic, J.; Keithley, R.B.; Wightman, R.M. Voltammetric Detection of 5-Hydroxytryptamine Release in the Rat Brain. Anal. Chem. 2009, 81, 9462–9471. [Google Scholar] [CrossRef]
- Dinesh, B.; Veeramani, V.; Chen, S.-M.; Saraswathi, R. In Situ Electrochemical Synthesis of Reduced Graphene Oxide-Cobalt Oxide Nanocomposite Modified Electrode for Selective Sensing of Depression Biomarker in the Presence of Ascorbic Acid and Dopamine. J. Electroanal. Chem. 2017, 786, 169–176. [Google Scholar] [CrossRef]
- Li, Y.; Ji, Y.; Ren, B.; Jia, L.; Ma, G.; Liu, X. Carboxyl-Functionalized Mesoporous Molecular Sieve/Colloidal Gold Modified Nano-Carbon Ionic Liquid Paste Electrode for Electrochemical Determination of Serotonin. Mater. Res. Bull. 2019, 109, 240–245. [Google Scholar] [CrossRef]
- Ran, G.; Chen, X.; Xia, Y. Electrochemical Detection of Serotonin Based on a Poly(Bromocresol Green) Film and Fe3O4 Nanoparticles in a Chitosan Matrix. RSC Adv. 2017, 7, 1847–1851. [Google Scholar] [CrossRef]
- Zhao, J.; Zhang, W.; Sherrell, P.; Razal, J.M.; Huang, X.-F.; Minett, A.I.; Chen, J. Carbon nanotube nanoweb-bioelectrode for highly selective dopamine sensing. ACS Appl. Mater. Interfaces 2012, 4, 44–48. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, A.C.; Wang, X.; Zhu, Y.; Sombers, L.A. Carbon nanotube yarn electrodes for enhanced detection of neurotransmitter dynamics in live brain tissue. ACS Nano 2013, 7, 7864–7873. [Google Scholar] [CrossRef]




| Modified Electrode | Method | Limit of Detection (nM) | Dynamic Linear Range (nM) | Sensitivity (µA µM−1 cm−2) | Ref. |
|---|---|---|---|---|---|
| AuAg-GR 1 | Amperometry | 1.6 | 2.7–4820 | 0.766 | [1] |
| RGO-Co3O4 2 | DPV 5 | 48.7 | 100–51,000 | 2.2 | [29] |
| MCM-41-COOH/Au@nano-CILPE 3 | SWV 6 | 100 | 200–20,000 | - | [30] |
| Fe3O4–MWCNT–poly(BCG)/GCE 4 | DPV | 80 | 500–100,000 | - | [31] |
| GCE/g-C3N4 nanosheets | Amperometry | 0.15 | 0.5–1000 | 1.03 | This study |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kathiresan, V.; Rajarathinam, T.; Lee, S.; Kim, S.; Lee, J.; Thirumalai, D.; Chang, S.-C. Cost-Effective Electrochemical Activation of Graphitic Carbon Nitride on the Glassy Carbon Electrode Surface for Selective Determination of Serotonin. Sensors 2020, 20, 6083. https://doi.org/10.3390/s20216083
Kathiresan V, Rajarathinam T, Lee S, Kim S, Lee J, Thirumalai D, Chang S-C. Cost-Effective Electrochemical Activation of Graphitic Carbon Nitride on the Glassy Carbon Electrode Surface for Selective Determination of Serotonin. Sensors. 2020; 20(21):6083. https://doi.org/10.3390/s20216083
Chicago/Turabian StyleKathiresan, Vijayaraj, Thenmozhi Rajarathinam, Seulah Lee, Suhkmann Kim, Jaewon Lee, Dinakaran Thirumalai, and Seung-Cheol Chang. 2020. "Cost-Effective Electrochemical Activation of Graphitic Carbon Nitride on the Glassy Carbon Electrode Surface for Selective Determination of Serotonin" Sensors 20, no. 21: 6083. https://doi.org/10.3390/s20216083
APA StyleKathiresan, V., Rajarathinam, T., Lee, S., Kim, S., Lee, J., Thirumalai, D., & Chang, S.-C. (2020). Cost-Effective Electrochemical Activation of Graphitic Carbon Nitride on the Glassy Carbon Electrode Surface for Selective Determination of Serotonin. Sensors, 20(21), 6083. https://doi.org/10.3390/s20216083






