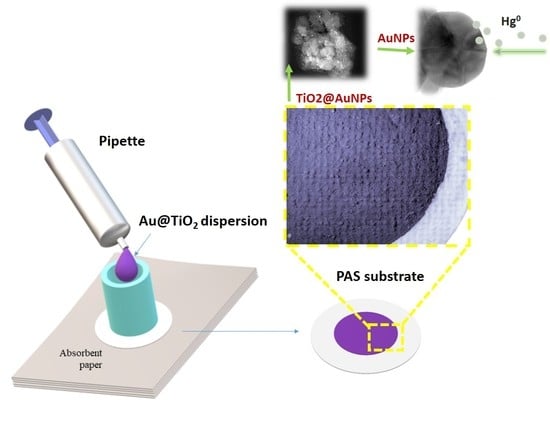
Characteristics and Performances of a Nanostructured Material for Passive Samplers of Gaseous Hg
Abstract
1. Introduction
2. Materials and Method
2.1. Preparation of Au@TiO2 NP Dispersion
2.2. PAS Fabrication
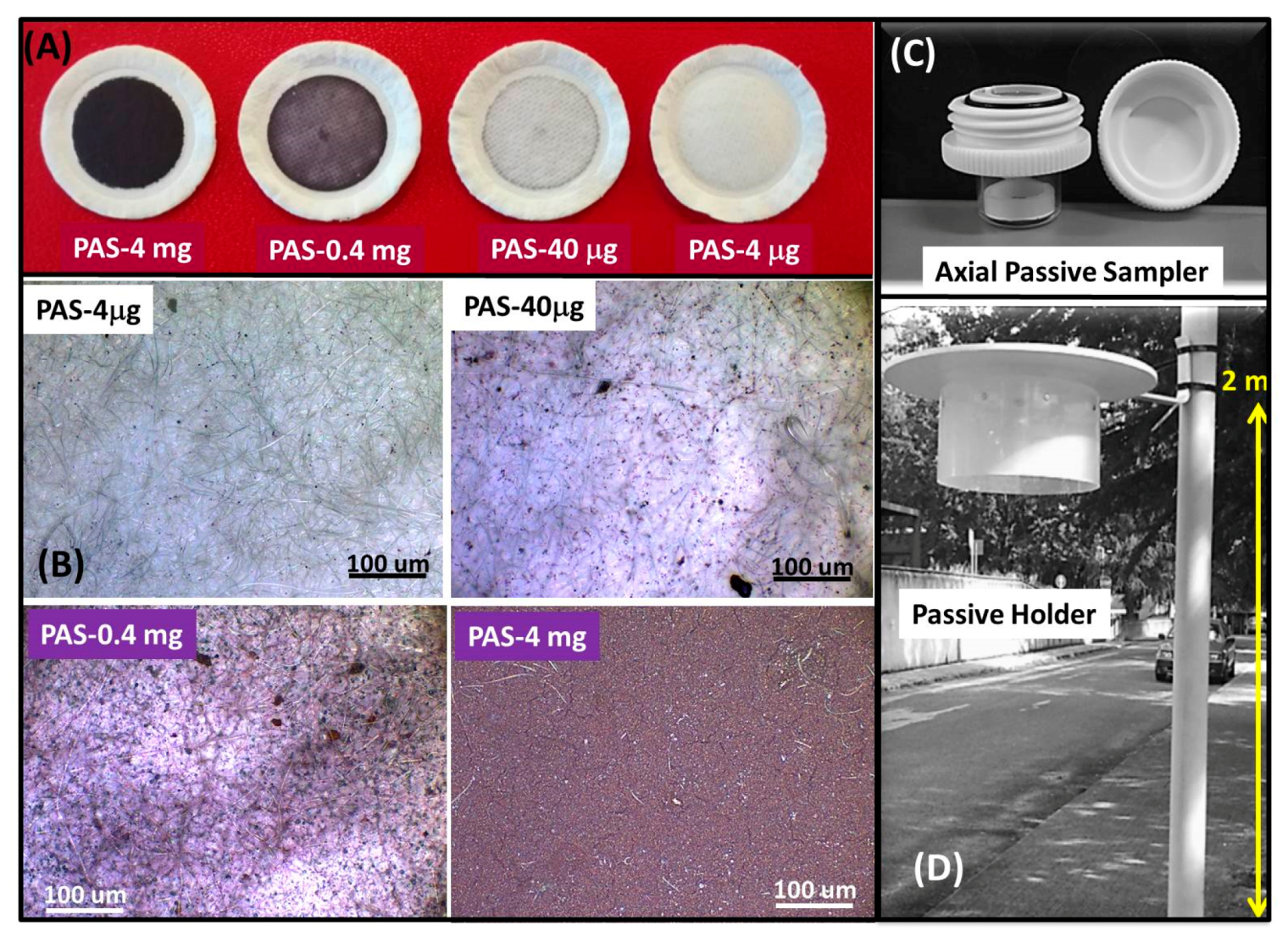
2.2.1. Adsorbing Membrane
2.2.2. PAS Assemblage
2.2.3. Monitoring System Arrangement
2.3. Measurements and Analysis
2.3.1. Measurements
2.3.2. Resampling
2.3.3. Analyses
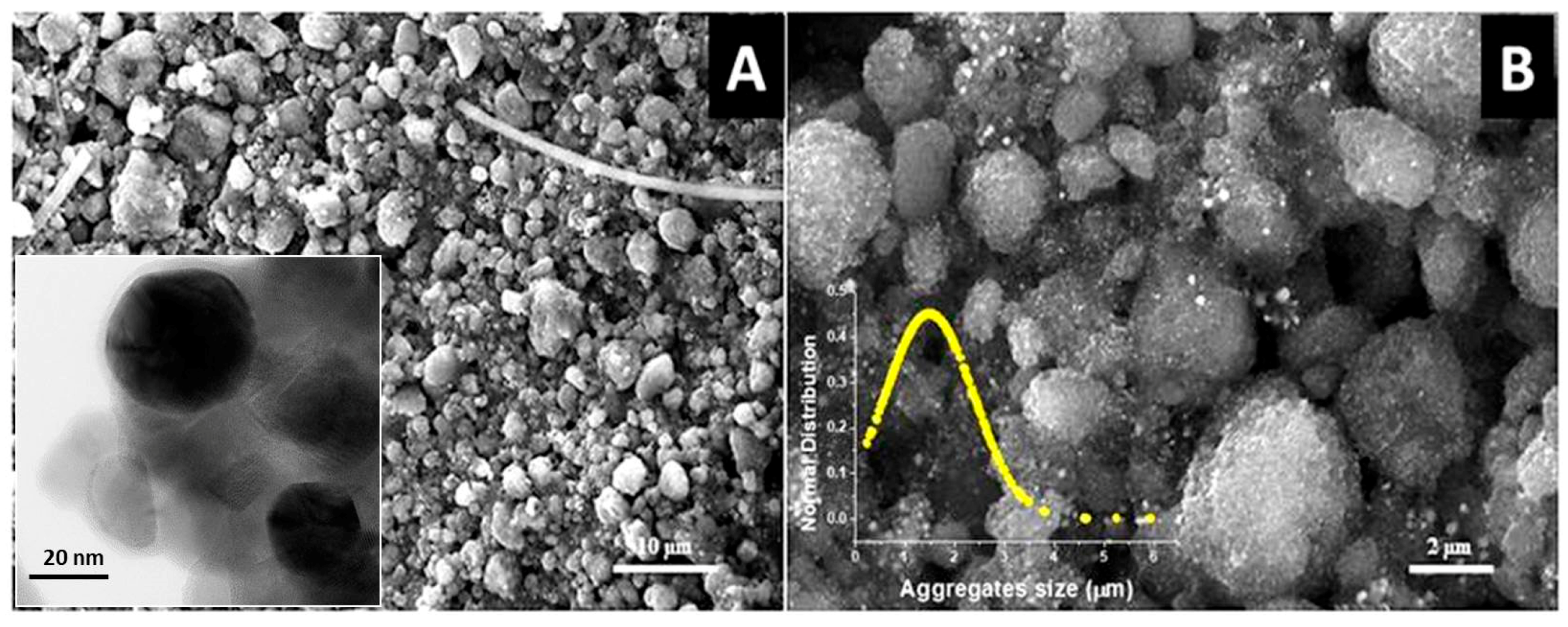
2.4. Material Characterization
3. Results and Discussion
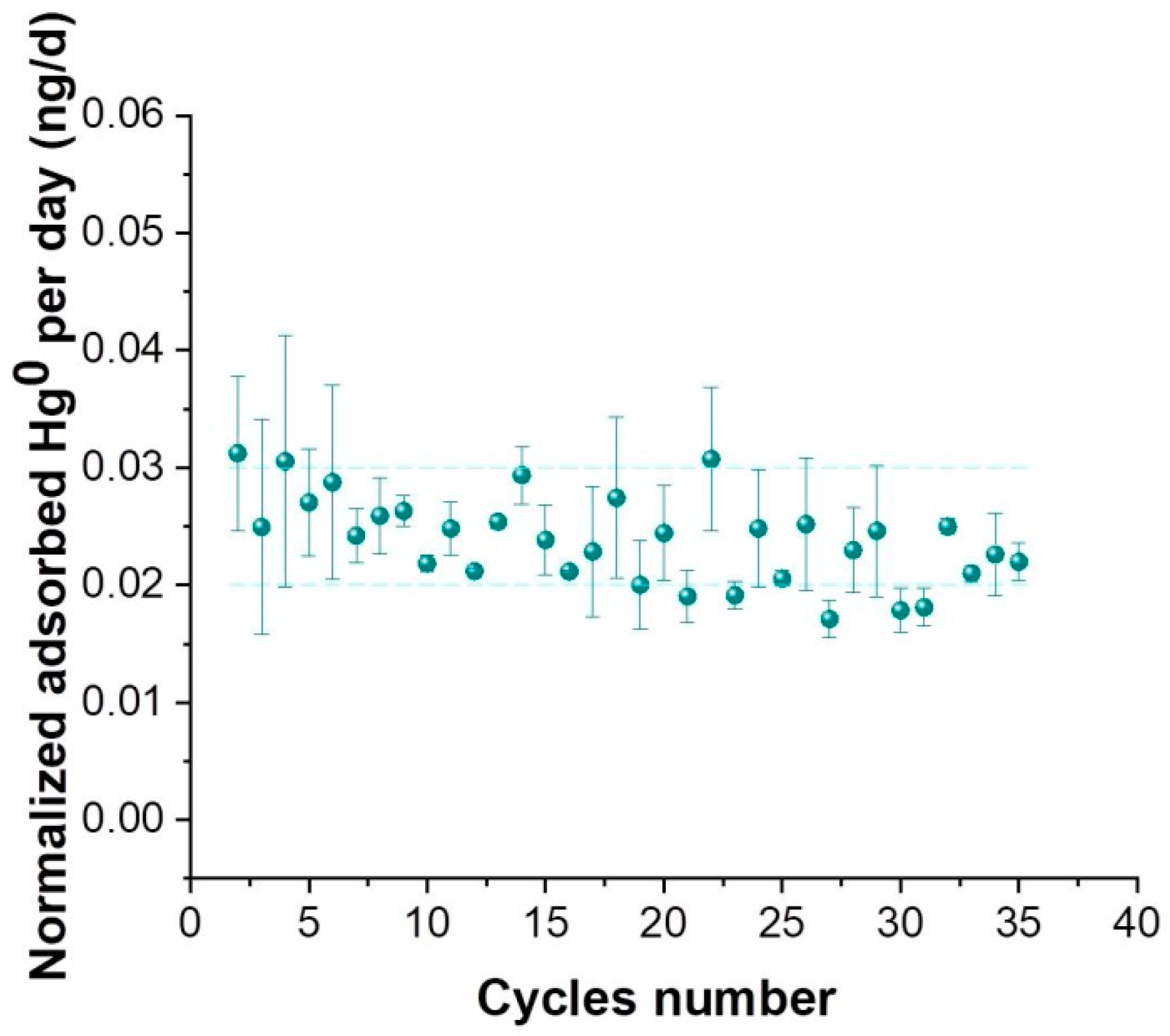
3.1. PAS Characterization
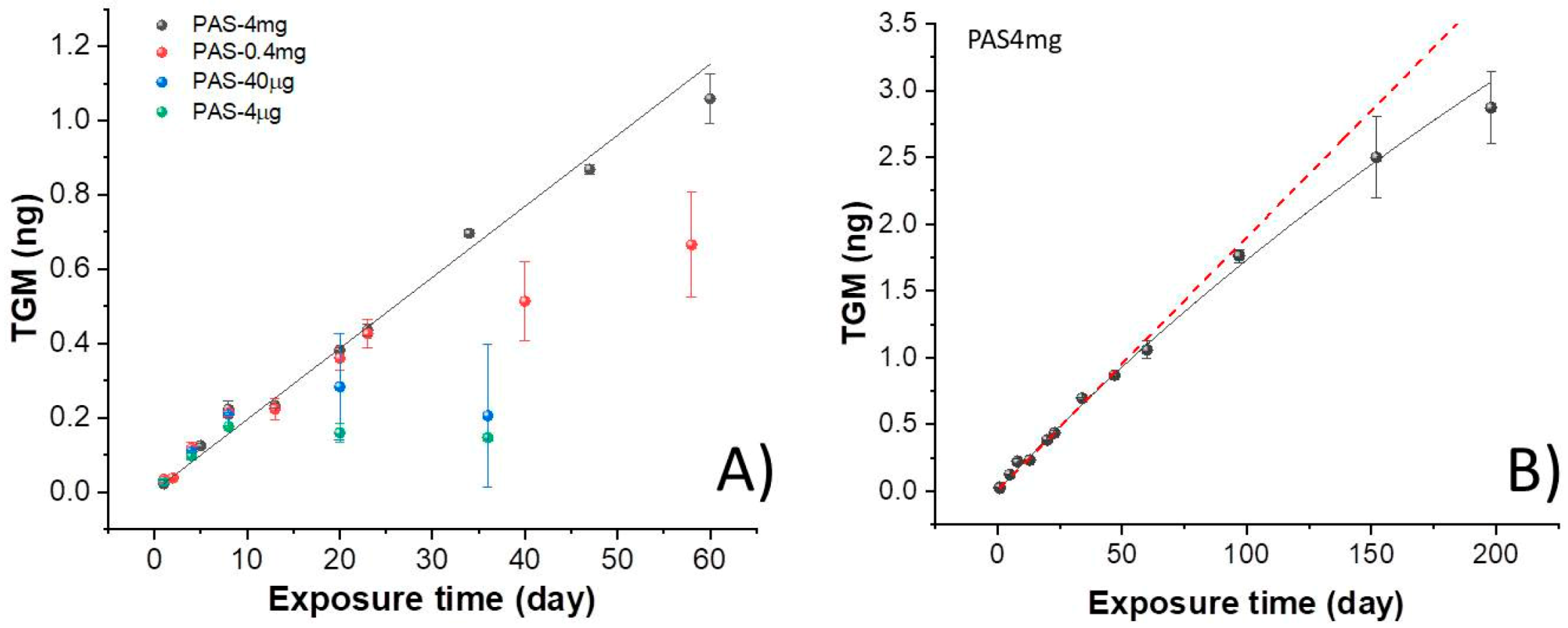
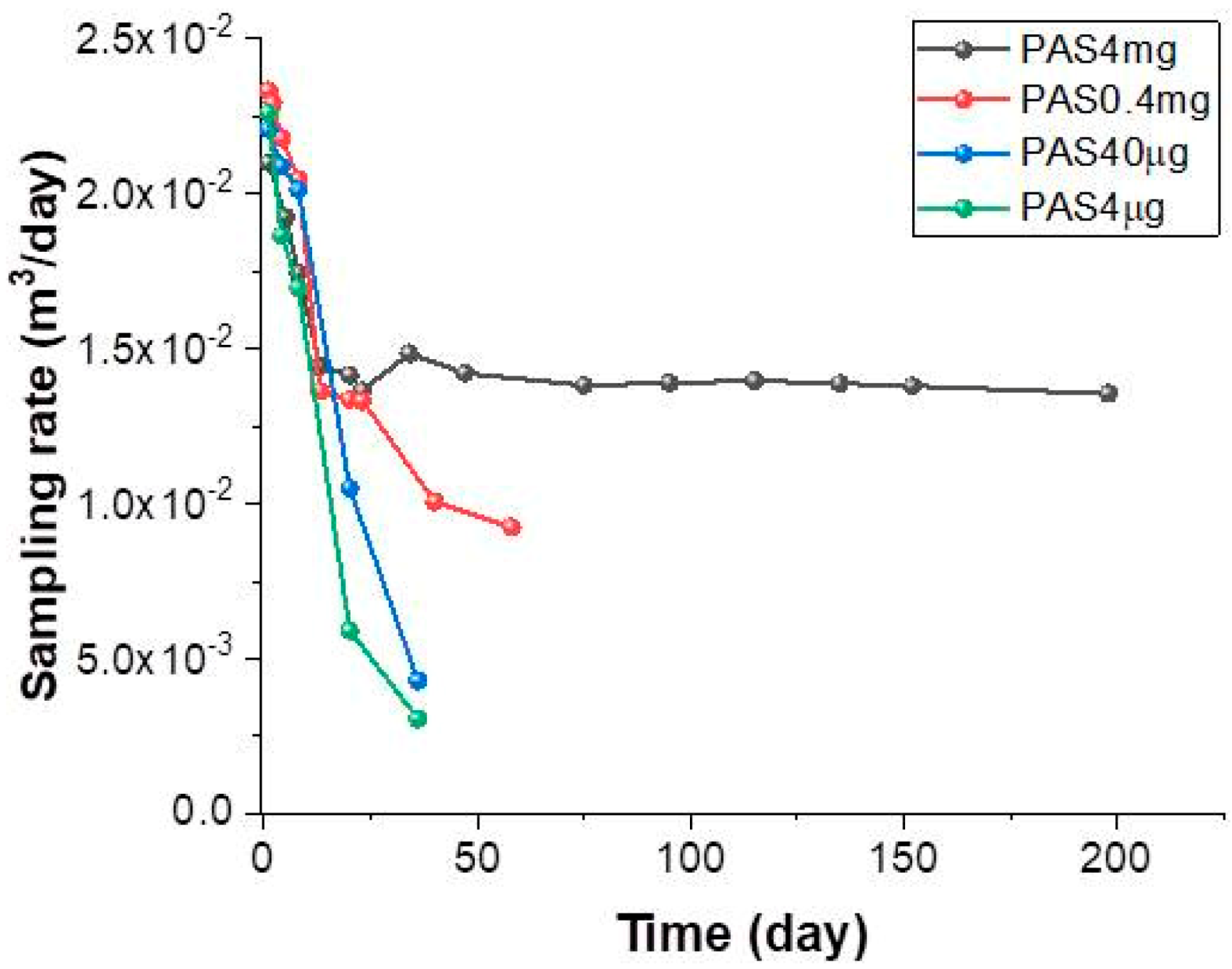
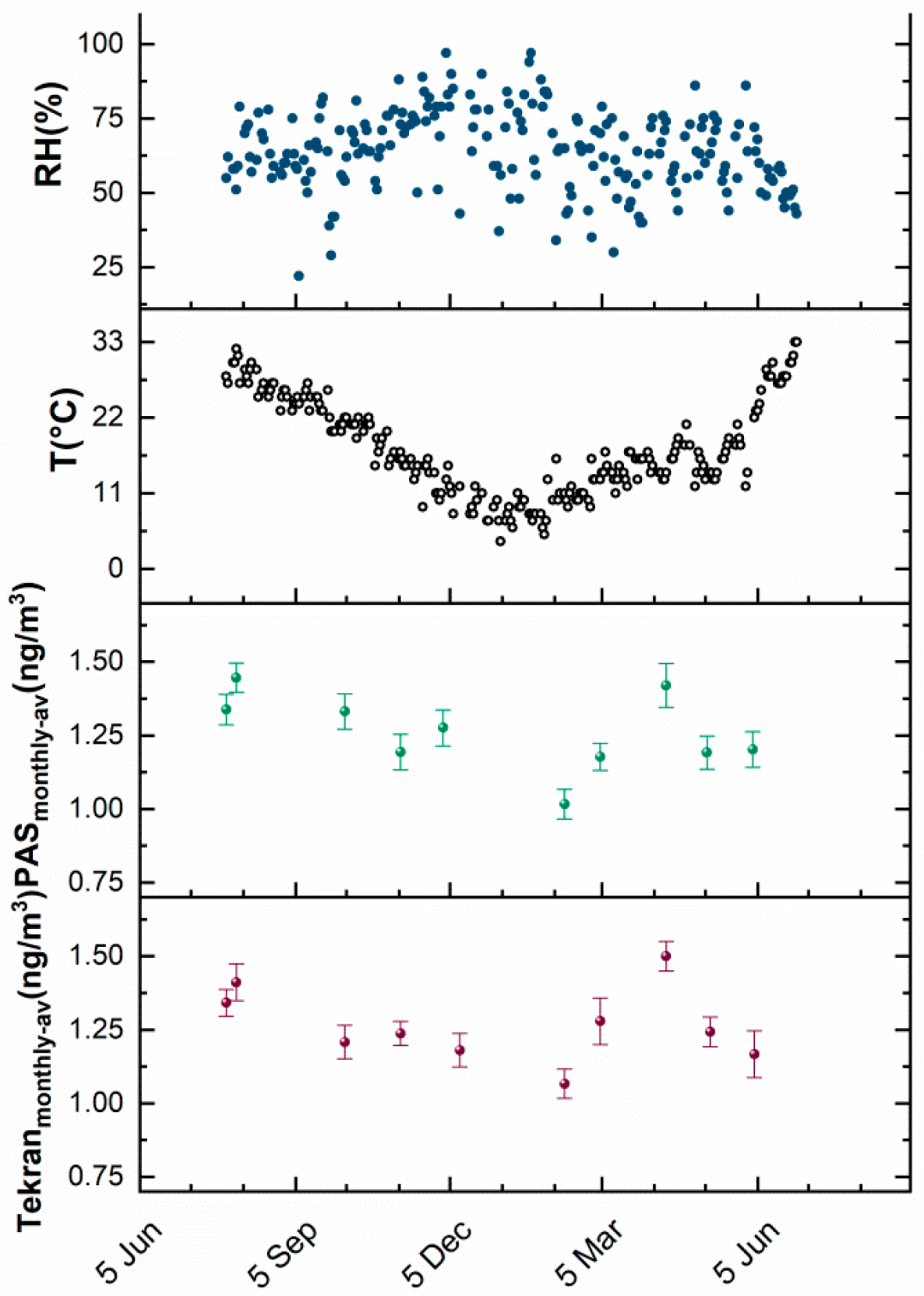
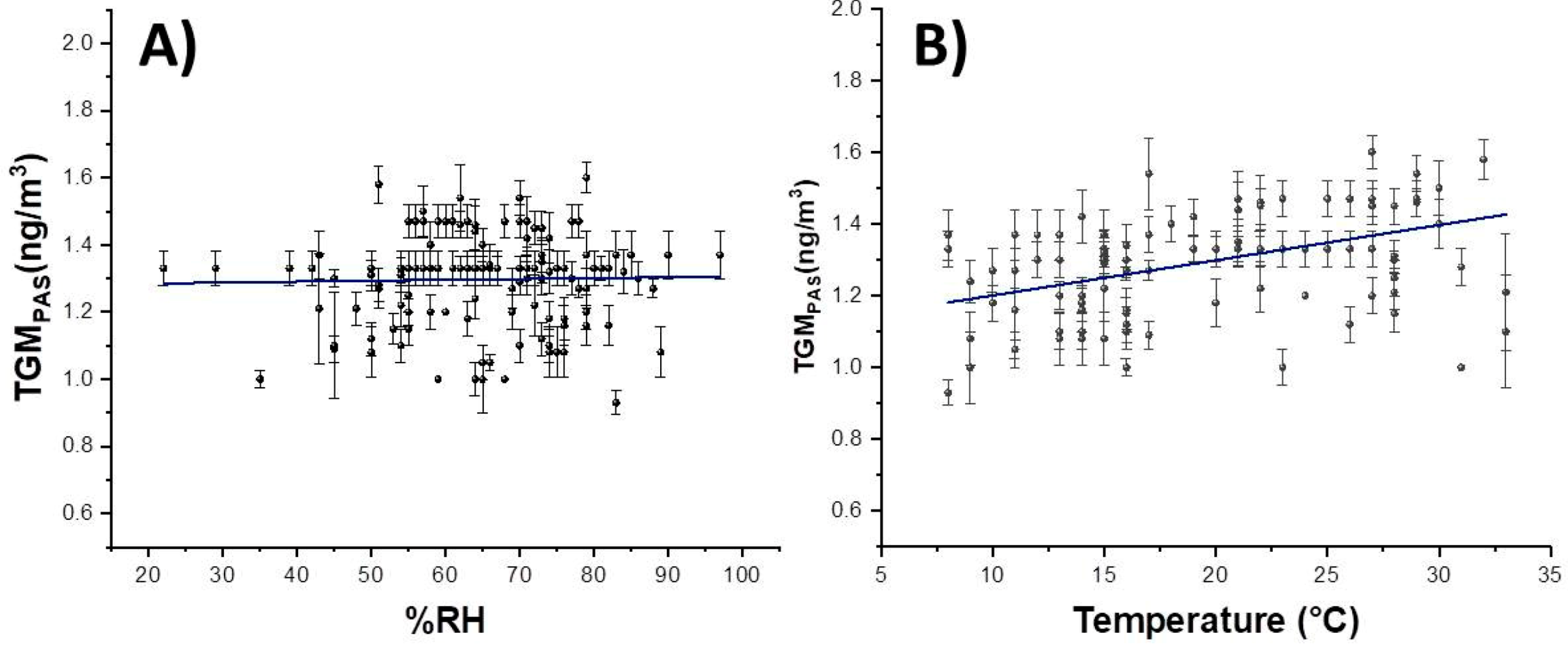
3.2. PAS Exposure
3.3. Resampling
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Committee on the Toxicological Effects of Methylmercury. Toxicological Effects of Methylmercury; National Academies Press: Washington, DC, USA, 2000. [Google Scholar]
- Global Mercury Assessment 2018|UNEP-UN Environment Programme. Available online: https://www.unenvironment.org/resources/publication/global-mercury-assessment-2018 (accessed on 22 July 2020).
- Holmes, C.D.; Jacob, D.J.; Yang, X. Global lifetime of elemental mercury against oxidation by atomic bromine in the free troposphere. Geophys. Res. Lett. 2006, 33. [Google Scholar] [CrossRef]
- Lindberg, S.E.; Stratton, W.J. Atmospheric Mercury Speciation: Concentrations and Behavior of Reactive Gaseous Mercury in Ambient Air. Environ. Sci. Technol. 1998, 32, 49–57. [Google Scholar] [CrossRef]
- Lindberg, S.E.; Lin, C.-J.; Scott, K.J.; Landis, M.S.; Stevens, R.K. Dynamic Oxidation of Gaseous Mercury in the Arctic Troposphere at Polar Sunrise. Environ. Sci. Technol. 2002, 36, 1245–1256. [Google Scholar] [CrossRef]
- Lin, C.-J.; Pehkonen, S.O. The Chemistry of Atmospheric Mercury: A Review. Atmos. Environ. 1999, 33, 2067–2079. [Google Scholar] [CrossRef]
- Duan, L.; Xiu, G.; Feng, L.; Cheng, N.; Wang, C. The mercury species and their association with carbonaceous compositions, bromine and iodine in PM2.5 in Shanghai. Chemosphere 2016. [Google Scholar] [CrossRef]
- Han, Y.J.; Holsen, T.M.; Lai, S.O.; Hopke, P.K.; Yi, S.M.; Liu, W.; Pagano, J.; Falanga, L.; Milligan, M.; Andolina, C. Atmospheric gaseous mercury concentrations in New York State: Relationships with meteorological data and other pollutants. Atmos. Environ. 2004, 38, 6431–6466. [Google Scholar] [CrossRef]
- Kim, M.K.; Zoh, K.D. Fate and transport of mercury in environmental media and human exposure. J. Prev. Med. Public Health 2012, 45, 335–343. [Google Scholar] [CrossRef] [PubMed]
- Selin, N.E.; Jacob, D.J. Seasonal and spatial patterns of mercury wet deposition in the United States: Constraints on the contribution from North American anthropogenic sources. Atmos. Environ. 2008, 42, 5193–5204. [Google Scholar] [CrossRef]
- Park, J.S.; Oh, S.; Shin, M.Y.; Kim, M.K.; Yi, S.M.; Zoh, K.D. Seasonal variation in dissolved gaseous mercury and total mercury concentrations in Juam Reservoir, Korea. Environ. Pollut. 2008, 154, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Mattoli, V.; Mazzolai, B.; Raffa, V.; Mondini, A.; Dario, P. Design of a new real-time dosimeter to monitor personal exposure to elemental gaseous mercury. Sens. Actuators B Chem. 2007, 123, 158–167. [Google Scholar] [CrossRef]
- Kabir, K.M.M.; Ippolito, S.J.; Kandjani, A.E.; Sabri, Y.M.; Bhargava, S.K. Nano-engineered surfaces for mercury vapor sensing: Current state and future possibilities. TrAC Trends Anal. Chem. 2017, 88, 77–99. [Google Scholar] [CrossRef]
- Salcedo, A.R.M.; Sevilla, F.B. Reversible chemiresistive sensing of ultra-low levels of elemental mercury vapor using thermally reduced graphene oxide. Microchim. Acta 2018, 185. [Google Scholar] [CrossRef] [PubMed]
- Mercury Determination in Natural Gas, Analysis of Mercury in Natural Gas. Available online: https://www.lumexinstruments.com/applications/mercury-determination-in-natural-gas.php (accessed on 22 July 2020).
- Lu, J.Y.; Schroeder, W.H.; Berg, T.; Munthe, J.; Schneeberger, D.; Schaedlich, F. A Device for Sampling and Determination of Total Particulate Mercury in Ambient Air. Anal. Chem. 1998, 70, 2403–2408. [Google Scholar] [CrossRef] [PubMed]
- Home-Tekran Instruments Corporation Tekran Instruments Corporation. Available online: https://www.tekran.com/ (accessed on 22 July 2020).
- Driscoll, C.T.; Mason, R.P.; Chan, H.M.; Jacob, D.J.; Pirrone, N. Mercury as a global pollutant: Sources, pathways, and effects. Environ. Sci. Technol. 2013, 47, 4967–4983. [Google Scholar] [CrossRef]
- Pandey, S.K.; Kim, K.H.; Brown, R.J.C. Measurement techniques for mercury species in ambient air. TrAC Trends Anal. Chem. 2011, 30, 899–917. [Google Scholar] [CrossRef]
- Renner, R. EPA to strengthen persistent, bioaccumulative, and toxic pollutant controls—Mercury first to be targeted. Environ. Sci. Technol. 1999, 33, 62A. [Google Scholar] [CrossRef]
- Margaret, A. Coulter Minamata Convention on Mercury. Int. Leg. Mater. 2016, 55, 582. [Google Scholar] [CrossRef]
- Zhang, W.; Tong, Y.; Hu, D.; Ou, L.; Wang, X. Characterization of atmospheric mercury concentrations along an urban–rural gradient using a newly developed passive sampler-ScienceDirect. Atmos. Environ. 2012, 47, 26–32. [Google Scholar] [CrossRef]
- McLagan, D.S.; Monaci, F.; Huang, H.; Lei, Y.D.; Mitchell, C.P.J.; Wania, F. Characterization and Quantification of Atmospheric Mercury Sources Using Passive Air Samplers. J. Geophys. Res. Atmos. 2019, 124, 2351–2362. [Google Scholar] [CrossRef]
- Kot-Wasik, A.; Zabiegała, B.; Urbanowicz, M.; Dominiak, E.; Wasik, A.; Namieśnik, J. Advances in passive sampling in environmental studies. Anal. Chim. Acta 2007, 602, 141–163. [Google Scholar] [CrossRef]
- Lai, F.Y.; Rauert, C.; Gobelius, L.; Ahrens, L. A critical review on passive sampling in air and water for per- and polyfluoroalkyl substances (PFASs). TrAC Trends Anal. Chem. 2019, 121, 115311. [Google Scholar] [CrossRef]
- Król, S.; Zabiegała, B.; Namieśnik, J. Monitoring VOCs in atmospheric air II. Sample collection and preparation. TrAC Trends Anal. Chem. 2010, 29, 1101–1112. [Google Scholar] [CrossRef]
- Huang, J.; Lyman, S.N.; Hartman, J.S.; Gustin, M.S. A review of passive sampling systems for ambient air mercury measurements. Environ. Sci. Process. Impacts 2014, 16, 374–392. [Google Scholar] [CrossRef] [PubMed]
- McLagan, D.S.; Mitchell, C.P.J.; Huang, H.; Lei, Y.D.; Cole, A.S.; Steffen, A.; Hung, H.; Wania, F. A High-Precision Passive Air Sampler for Gaseous Mercury. Environ. Sci. Technol. Lett. 2016, 3, 24–29. [Google Scholar] [CrossRef]
- Guo, H.; Lin, H.; Zhang, W.; Deng, C.; Wang, H.; Zhang, Q.; Shen, Y.; Wang, X. Influence of meteorological factors on the atmospheric mercury measurement by a novel passive sampler. Atmos. Environ. 2014, 97, 310–315. [Google Scholar] [CrossRef]
- McLagan, D.; Hussain, B.; Huang, H.; Lei, Y.; Wania, F.; Mitchell, C. Identifying and evaluating urban mercury emission sources through passive sampler-based mapping of atmospheric concentrations-IOPscience. Environ. Res. Lett. 2018, 13, 074008. [Google Scholar] [CrossRef]
- Lin, H.; Zhang, W.; Deng, C.; Tong, Y.; Zhang, Q.; Wang, X. Evaluation of passive sampling of gaseous mercury using different sorbing materials. Environ. Sci. Pollut. Res. 2017, 24, 14190–14197. [Google Scholar] [CrossRef]
- Gustin, M.S.; Lyman, S.N.; Kilner, P.; Prestbo, E. Development of a passive sampler for gaseous mercury. Atmos. Environ. 2011, 45, 5805–5812. [Google Scholar] [CrossRef]
- Santos, E.B.; Ferlin, S.; Fostier, A.H.; Mazali, I.O. Using gold nanoparticles as passive sampler for indoor monitoring of gaseous elemental mercury. J. Braz. Chem. Soc. 2017, 28, 1274–1280. [Google Scholar] [CrossRef]
- De Barros Santos, E.; Moher, P.; Ferlin, S.; Fostier, A.H.; Mazali, I.O.; Telmer, K.; Brolo, A.G. Proof of concept for a passive sampler for monitoring of gaseous elemental mercury in artisanal gold mining. Sci. Rep. 2017, 7, 16513. [Google Scholar] [CrossRef]
- Macagnano, A.; Papa, P.; Avossa, J.; Perri, V.; Marelli, M.; Sprovieri, F.; Zampetti, E.; De Cesare, F.; Bearzotti, A.; Pirrone, N. Passive sampling of gaseous elemental mercury based on a composite tio2np/aunp layer. Nanomaterials 2018, 8, 798. [Google Scholar] [CrossRef] [PubMed]
- Papa, P.; Fratoddi, I.; Venditti, I.; Vichi, F.; Macagnano, A.; Zampetti, E.; Bearzotti, A. Use of gold nanoparticles as substrate for diffusive monitoring of gaseous mercury. Materials 2018, 11, 2119. [Google Scholar] [CrossRef] [PubMed]
- Bearzotti, A.; Papa, P.; Macagnano, A.; Zampetti, E.; Venditti, I.; Fioravanti, R.; Fontana, L.; Matassa, R.; Familiari, G.; Fratoddi, I. Environmental Hg vapours adsorption and detection by using functionalized gold nanoparticles network. J. Environ. Chem. Eng. 2018, 6, 4706–4713. [Google Scholar] [CrossRef]
- Macagnano, A.; Perri, V.; Zampetti, E.; Ferretti, A.M.; Sprovieri, F.; Pirrone, N.; Bearzotti, A.; Esposito, G.; De Cesare, F. Elemental mercury vapor chemoresistors employing TIO2 nanofibers photocatalytically decorated with Au-nanoparticles. Sens. Actuators B Chem. 2017, 247. [Google Scholar] [CrossRef]
- Dumarey, R.; Dams, R.; Hoste, J. Comparison of the Collection and Desorption Efficiency of Activated Charcoal, Silver, and Gold for the Determination of Vapor-Phase Atmospheric Mercury. Anal. Chem. 1985, 57, 2638–2643. [Google Scholar] [CrossRef]
- Lyman, S.N.; Gustin, M.S.; Prestbo, E.M.; Kilner, P.I.; Edgerton, E.; Hartsell, B. Testing and application of surrogate surfaces for understanding potential gaseous oxidized mercury dry deposition. Environ. Sci. Technol. 2009, 43, 6235–6241. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Choi, H.D.; Landis, M.S.; Holsen, T.M. An application of passive samplers to understand atmospheric mercury concentration and dry deposition spatial distributions. J. Environ. Monit. 2012, 14, 2976–2982. [Google Scholar] [CrossRef]
- Wang, C.; Ma, L.; Hossain, M.; Wang, H.; Zou, S.; Hickman, J.J.; Su, M. Direct visualization of molecular scale chemical adsorptions on solids using plasmonic nanoparticle arrays. Sens. Actuators B Chem. 2010, 150, 667–672. [Google Scholar] [CrossRef]
- James, J.Z.; Lucas, D.; Koshland, C.P. Gold nanoparticle films as sensitive and reusable elemental mercury sensors. Environ. Sci. Technol. 2012, 46, 9557–9562. [Google Scholar] [CrossRef]
- McNicholas, T.P.; Zhao, K.; Yang, C.; Hernandez, S.C.; Mulchandani, A.; Myung, N.V.; Deshusses, M.A. Sensitive detection of elemental mercury vapor by gold-nanoparticle- decorated carbon nanotube sensors. J. Phys. Chem. C 2011, 115, 13927–13931. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Macagnano, A.; Perri, V.; Zampetti, E.; Bearzotti, A.; De Cesare, F.; Sprovieri, F.; Pirrone, N. A smart nanofibrous material for adsorbing and detecting elemental mercury in air. Atmos. Chem. Phys 2017, 17, 6883–6893. [Google Scholar] [CrossRef]
- Salcedo, A.R.M.; Sevilla, F.B. Colorimetric determination of mercury vapor using smartphone camera-based imaging. Instrum. Sci. Technol. 2018, 46, 450–462. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. EPA Method 7473 (SW-846): Mercury in Solids and Solutions by Thermal Decompostion, Amalgamation, and Atomic Absorption Spectrophotometry; United States Environmental Protection Agency: Washington, DC, USA, 2019. [Google Scholar]
- McLagan, D.S.; Mitchell, C.P.J.; Steffen, A.; Hung, H.; Shin, C.; Stupple, G.W.; Olson, M.L.; Luke, W.T.; Kelley, P.; Howard, D.; et al. Global evaluation and calibration of a passive air sampler for gaseous mercury. Atmos. Chem. Phys. 2018, 18, 5905–5919. [Google Scholar] [CrossRef]
- McLagan, D.S.; Mazur, M.E.E.; Mitchell, C.P.J.; Wania, F. Passive air sampling of gaseous elemental mercury: A critical review. Atmos. Chem. Phys. 2016, 16, 3061–3076. [Google Scholar] [CrossRef]
- Ghidelli, M.; Mascaretti, L.; Bricchi, B.R.; Brognara, A.; Afifi, T.A.; Russo, V.; Casari, C.S.; Bassi, A.L. Light management in TiO2 thin films integrated with Au plasmonic nanoparticles. Semicond. Sci. Technol. 2020, 35. [Google Scholar] [CrossRef]
- Primo, A.; Corma, A.; García, H. Titania supported gold nanoparticles as photocatalyst. Phys. Chem. Chem. Phys. 2011, 13, 886–910. [Google Scholar] [CrossRef]
- Peeters, H.; Keulemans, M.; Nuyts, G.; Vanmeert, F.; Li, C.; Minjauw, M.; Detavernier, C.; Bals, S.; Lenaerts, S.; Verbruggen, S.W. Plasmonic gold-embedded TiO2 thin films as photocatalytic self-cleaning coatings. Appl. Catal. B Environ. 2020, 267, 118654. [Google Scholar] [CrossRef]
- Brognara, A.; Nasri, I.F.M.A.; Bricchi, B.; Li Bassi, A.; Gauchotte-Lindsay, C.; Ghidelli, M.; Lidgi-Guigui, N. Highly sensitive detection of estradiol by a SERS sensor based on TiO2 covered with gold nanoparticles. Beilstein J. Nanotechnol. 2020, 11, 1026–1035. [Google Scholar] [CrossRef]
- Perera, M.; Wijenayaka, L.A.; Siriwardana, K.; Dahanayake, D.; De Silva, K.M.N. Gold nanoparticle decorated titania for sustainable environmental remediation: Green synthesis, enhanced surface adsorption and synergistic photocatalysis. RSC Adv. 2020, 10, 29594–29602. [Google Scholar] [CrossRef]
- Brosset, C.; Iverfeldt, Å. Interaction of solid gold with mercury in ambient air. Water. Air. Soil Pollut. 1989, 43, 147–168. [Google Scholar] [CrossRef]
- Barghigiani, C.; Ristori, T.; Cortopassi, M. Air mercury measurement and interference of atmospheric contaminants with gold traps. Environ. Technol. (UK) 1991, 12, 935–941. [Google Scholar] [CrossRef]
- Jeon, J.-W.; Han, Y.-J.; Cha, S.-H.; Kim, P.-R.; Kim, Y.-H.; Kim, H.; Seok, G.-S.; Noh, S. Application of the Passive Sampler Developed for Atmospheric Mercury and Its Limitation. Atmosphere 2019, 10, 678. [Google Scholar] [CrossRef]
- Zhang, X.; Wania, F. Modeling the uptake of semivolatile organic compounds by passive air samplers: Importance of mass transfer processes within the porous sampling media. Environ. Sci. Technol. 2012, 46, 9563–9570. [Google Scholar] [CrossRef] [PubMed]
- Brumbaugh, W.G.; Petty, J.D.; May, T.W.; Huckins, J.N. A passive integrative sampler for mercury vapor in air and neutral mercury species in water. Chemosph. Glob. Chang. Sci. 2000, 2, 1–9. [Google Scholar] [CrossRef]
- Brown, R.J.C.; Burdon, M.K.; Brown, A.S.; Kim, K.H. Assessment of pumped mercury vapour adsorption tubes as passive samplers using a micro-exposure chamber. J. Environ. Monit. 2012, 14, 2456–2463. [Google Scholar] [CrossRef]
- McLagan, D.S.; Mitchell, C.P.J.; Huang, H.; Hussain, B.A.; Lei, Y.D.; Wania, F. The effects of meteorological parameters and diffusive barrier reuse on the sampling rate of a passive air sampler for gaseous mercury. Atmos. Meas. Tech. 2017, 10, 3651–3660. [Google Scholar] [CrossRef]
- Hu, Q.H.; Kang, H.; Li, Z.; Wang, Y.S.; Ye, P.P.; Zhang, L.; Yu, J.; Yu, X.W.; Sun, C.; Xie, Z.Q. Characterization of atmospheric mercury at a suburban site of central China from wintertime to springtime. Atmos. Pollut. Res. 2014, 5, 769–778. [Google Scholar] [CrossRef]
- Moore, C.; Carpi, A. Mechanisms of the emission of mercury from soil: Role of UV radiation. J. Geophys. Res. 2005, 110, D24302. [Google Scholar] [CrossRef]
- Schlüter, K. Review: Evaporation of mercury from soils. An integration and synthesis of current knowledge. Environ. Geol. 2000, 39, 249–271. [Google Scholar] [CrossRef]
- Brown, R.J.C.; Kumar, Y.; Brown, A.S.; Kim, K.H. Memory effects on adsorption tubes for mercury vapor measurement in ambient air: Elucidation, quantification, and strategies for mitigation of analytical bias. Environ. Sci. Technol. 2011, 45, 7812–7818. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Avossa, J.; De Cesare, F.; Papa, P.; Zampetti, E.; Bearzotti, A.; Marelli, M.; Pirrone, N.; Macagnano, A. Characteristics and Performances of a Nanostructured Material for Passive Samplers of Gaseous Hg. Sensors 2020, 20, 6021. https://doi.org/10.3390/s20216021
Avossa J, De Cesare F, Papa P, Zampetti E, Bearzotti A, Marelli M, Pirrone N, Macagnano A. Characteristics and Performances of a Nanostructured Material for Passive Samplers of Gaseous Hg. Sensors. 2020; 20(21):6021. https://doi.org/10.3390/s20216021
Chicago/Turabian StyleAvossa, Joshua, Fabrizio De Cesare, Paolo Papa, Emiliano Zampetti, Andrea Bearzotti, Marcello Marelli, Nicola Pirrone, and Antonella Macagnano. 2020. "Characteristics and Performances of a Nanostructured Material for Passive Samplers of Gaseous Hg" Sensors 20, no. 21: 6021. https://doi.org/10.3390/s20216021
APA StyleAvossa, J., De Cesare, F., Papa, P., Zampetti, E., Bearzotti, A., Marelli, M., Pirrone, N., & Macagnano, A. (2020). Characteristics and Performances of a Nanostructured Material for Passive Samplers of Gaseous Hg. Sensors, 20(21), 6021. https://doi.org/10.3390/s20216021