The Measurement of Nanoparticle Concentrations by the Method of Microcavity Mode Broadening Rate
Abstract
:1. Introduction
2. Materials and Methods
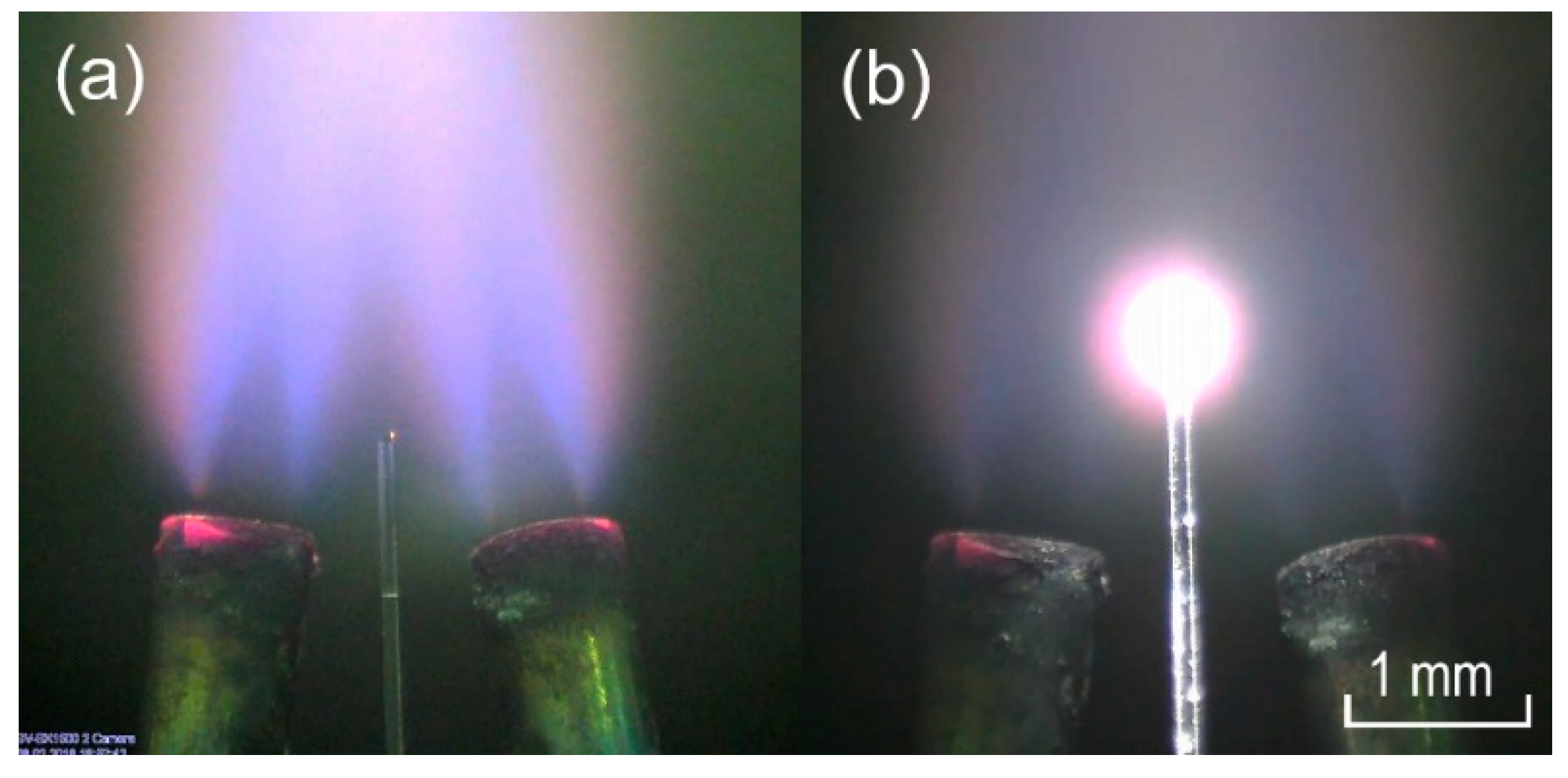
2.1. Manufacturing and Preparation of Microcavities
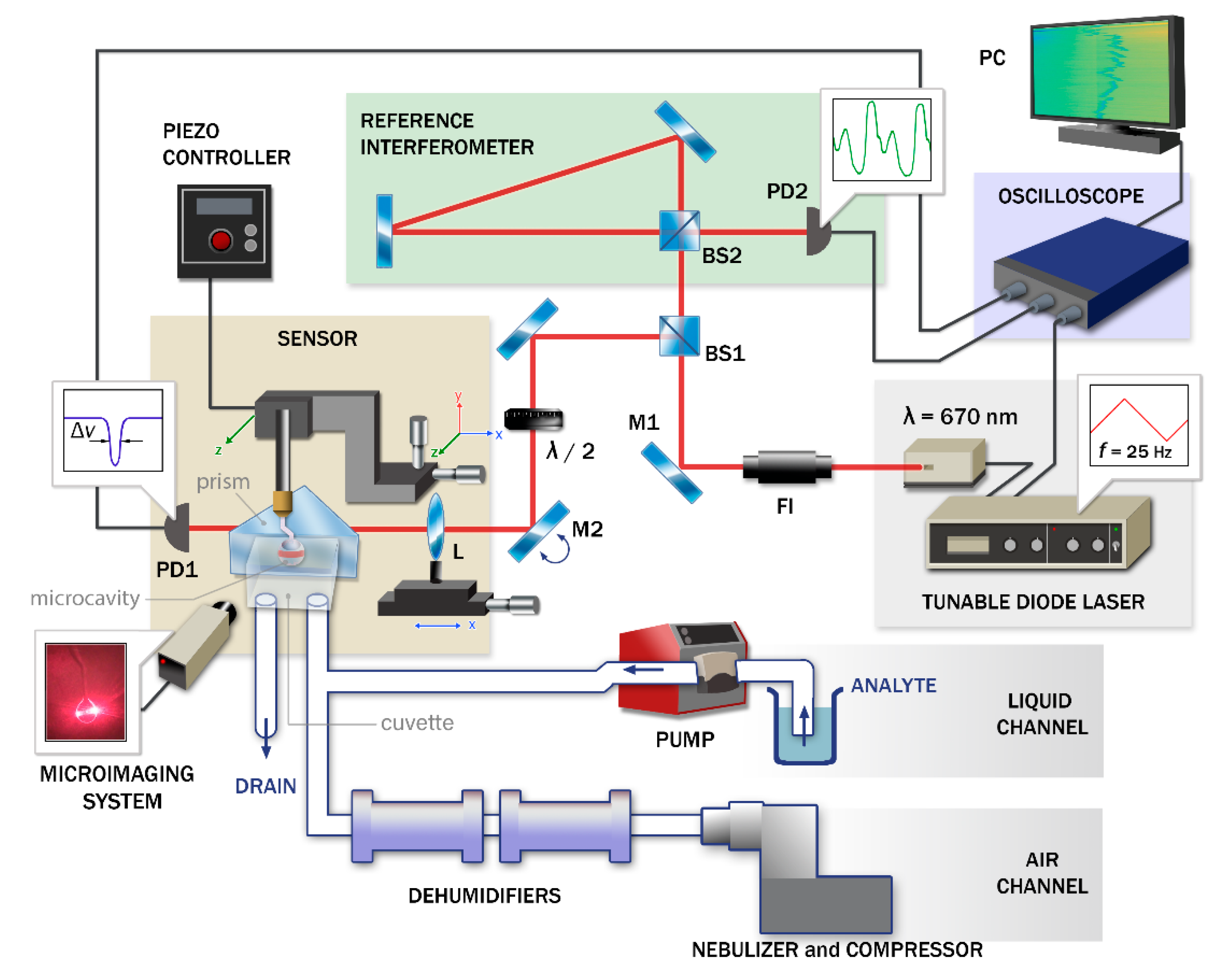
2.2. Measurement System
2.3. Measurement Method and Processing Algorithm
3. Results
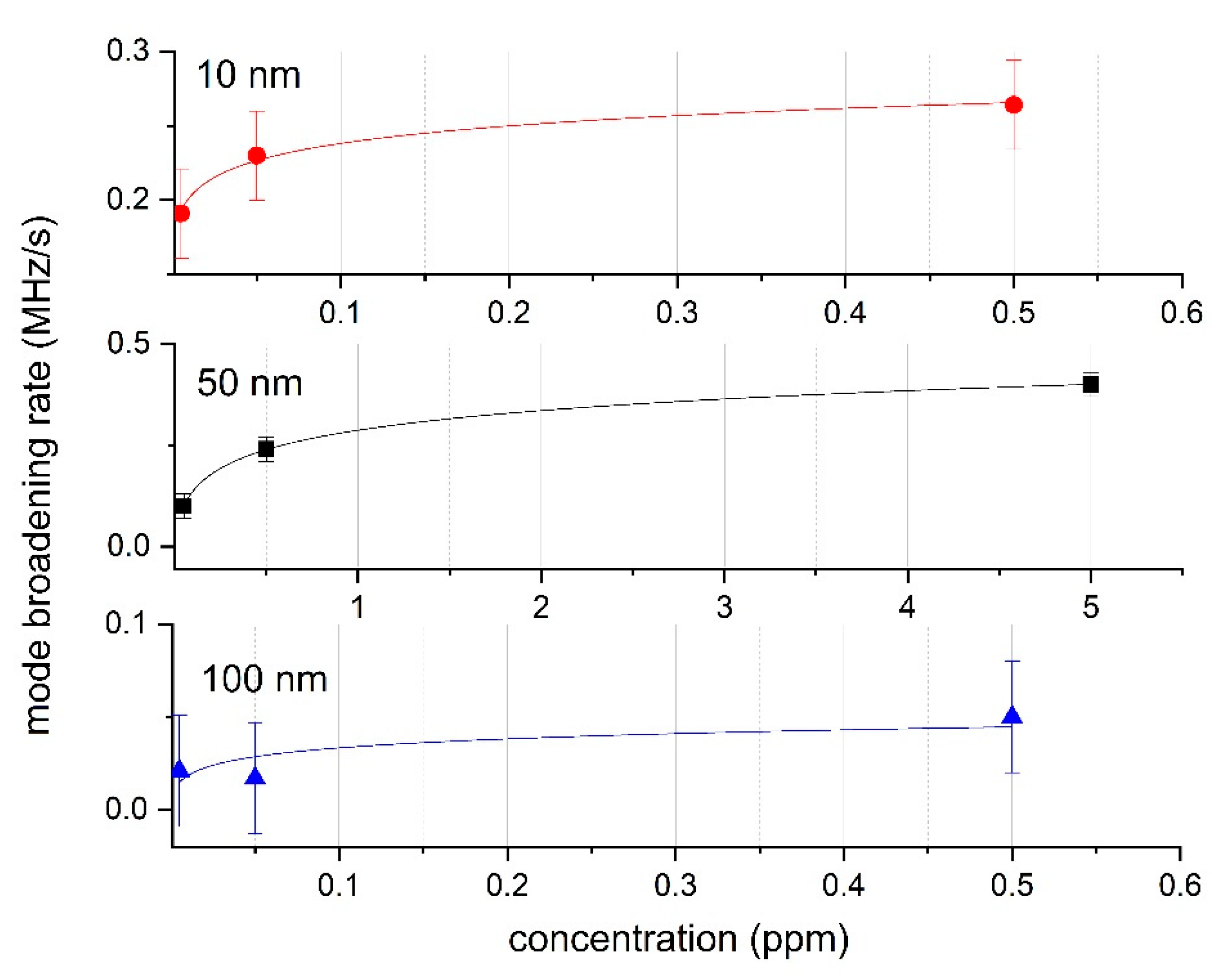
3.1. Graduation and Experiment
3.2. Error Estimation
4. Discussion
- To control the active area of interaction and the diameter of the microcavity. Use microcavities with a small mode index and a WGM belt width of not more than 50 μm (with a microsphere diameter of 300 μm) to measure nanoparticle concentrations below 0.01 ppm;
- To use individual containers for storing microcavities. Protect the surface of the microcavity from accidental contact and pollution. Place microspheres in sealed containers immediately after manufacturing;
- Provide vibration protection. Use rigid clamping of the microcavity and coupling elements relative to each other;
- Use a thermally stabilized measuring cell with temperature control inside the cuvette;
- Use a tunable laser with lower amplitude and frequency noise (less than ±0.45 MHz). Work in the scanning range away from the region of the laser mode hopping where the spectral quality of the laser radiation varies slightly;
- To calibrate the system with particles with a known average size in a medium with a known viscosity coefficient and refractive index.
5. Conclusions
6. Patents
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Riediker, M.; Zink, D.; Kreyling, W.; Oberdörster, G.; Elder, A.; Graham, U.; Lynch, I.; Duschl, A.; Ichihara, G.; Ichihara, S.; et al. Particle toxicology and health—Where are we? Part. Fibre Toxicol. 2019, 16, 19. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sun, T.; Nowack, B. Environmental concentrations of engineered nanomaterials: Review of modeling and analytical studies. Environ. Pollut. 2013, 181, 287–300. [Google Scholar] [CrossRef]
- Gottschalk, F.; Sonderer, T.; Scholz, R.W.; Nowack, B. Modeled Environmental Concentrations of Engineered Nanomaterials (TiO2, ZnO, Ag, CNT, Fullerenes) for Different Regions. Environ. Sci. Technol. 2009, 43, 9216–9222. [Google Scholar] [CrossRef] [PubMed]
- Sondi, I.; Salopek-Sondi, B. Silver nanoparticles as antimicrobial agent: A case study on E. coli as a model for Gram-negative bacteria. J. Colloid Interf. Sci. 2004, 275, 177–182. [Google Scholar]
- Kim, J.S.; Kuk, E.; Yu, K.N.; Kim, J.H.; Park, S.J.; Lee, H.J.; Kim, S.H.; Park, Y.K.; Park, Y.H.; Hwang, C.-Y.; et al. Antimicrobial effects of silver nanoparticles. Nanomedicine 2007, 3, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Rai, M.; Yadav, A.; Gade, A. Silver nanoparticles as a new generation of antimicrobials. Biotechnol. Adv. 2009, 27, 76–83. [Google Scholar] [CrossRef] [PubMed]
- Pal, S.; Tak, Y.K.; Song, J.M. Does the Antibacterial Activity of Silver Nanoparticles Depend on the Shape of the Nanoparticle? A Study of the Gram-Negative Bacterium Escherichia coli. Appl. Environ. Microbiol. 2007, 73, 1712–1720. [Google Scholar] [PubMed] [Green Version]
- Cozzoli, P.D.; Comparelli, R.; Fanizza, E.; Curri, M.L.; Agostiano, A. Photocatalytic activity of organic-capped anatase TiO2 nanocrystals in homogeneous organic solutions. Mater. Sci. Eng. 2003, 23, 707–713. [Google Scholar] [CrossRef]
- Mannes, P.-C.; Smolinski, S.; Blake, D.M.; Hugang, Z.; Wolfrum, E.J.; Jacoby, W. Bactericidal Activity of Photocatalytic TiO2 Reaction: Toward an Understanding of Its Killing Mechanism. Appl. Environ. Microbiol. 1999, 23, 4094–4098. [Google Scholar] [CrossRef] [Green Version]
- Foreman, M.R.; Swaim, J.D.; Vollmer, F. Whispering gallery mode sensors. Adv. Opt. Photonics 2015, 7, 168–240. [Google Scholar] [CrossRef]
- Righini, G.C.; Soria, S. Biosensing by WGM Microspherical Resonators. Sensors 2016, 16, 905. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Qavi, A.J.; Huang, S.H.; Yang, L. Whispering gallery microsensors: A review. arXiv 2018, arXiv:1805.00062. [Google Scholar]
- Vollmer, F. Detecting Single DNA Molecules with Optical Microcavities. SPIE Newsroom 2014, 3. [Google Scholar] [CrossRef]
- Wang, J.; Karnaushenko, D.; Medina-Sanchez, M.; Yin, Y.; Ma, L.; Schmidt, O.G. Three-Dimensional Microtubular Devices for Lab-on-a-Chip Sensing Applications. ACS Sens. 2019, 4, 1476–1496. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.H.; Sheth, S.; Jain, E.; Jiang, X.; Zustiak, S.P.; Yang, L. Whispering gallery mode resonator sensor for in situ measurements of hydrogel gelation. Opt. Express. 2018, 26, 51–62. [Google Scholar] [CrossRef] [PubMed]
- White, I.M.; Fan, X. On the performance quantification of resonant refractive index sensors. Opt. Express. 2008, 16, 1020–1028. [Google Scholar] [CrossRef] [Green Version]
- Corbellini, S.; Ramella, C.; Yu, L.; Pirola, M.; Fernicola, V. Whispering Gallery Mode Thermometry. Sensors 2016, 16, 1814. [Google Scholar] [CrossRef] [Green Version]
- Xu, X.; Jiang, X.; Zhao, G.; Yang, L. Phone-sized whispering-gallery microresonator sensing system. Opt. Express. 2016, 24, 6. [Google Scholar] [CrossRef] [Green Version]
- Jiang, X.; Yang, L. Optothermal dynamics in whispering-gallery microresonators. Light Sci. Appl. 2020, 9, 24. [Google Scholar] [CrossRef] [Green Version]
- Ma, Q.; Huang, L.; Guo, Z.; Rossmann, T. Spectral shift response of optical whispering-gallery modes due to water vapor adsorption and desorption. Meas. Sci. Technol. 2010, 21, 7. [Google Scholar] [CrossRef] [Green Version]
- Singh, P.; Echeveria, L.; Harrison, S.; Reinhardt, C.; Khitrov, V.; Heinz, K.; Chang, A.; Nunzi-Conti, G.; Farnesi, D.; Bond, T. Microresonators for compact optical sensors (μRCOS) for gas detection. In Proceedings of the SPIE, Laser Resonators, Microresonators, and Beam Control XXII, San Francisco, CA, USA, 1–6 February 2020; Volume 11266. [Google Scholar]
- Hu, J.; Liu, S.; Wu, X.; Liu, L.; Xu, L. Orthogonal Demodulation Pound–Drever–Hall Technique for Ultra-Low Detection Limit Pressure Sensing. Sensors 2019, 19, 3223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xia, D.; Huang, L.; Zhao, L. A New Design of an MOEMS Gyroscope Based on a WGM Microdisk Resonator. Sensors 2019, 19, 2798. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kondratiev, N.M.; Lobanov, V.E.; Cherenkov, A.V.; Voloshin, A.S.; Pavlov, N.G.; Koptyaev, S.; Gorodetsky, M.L. Self-injection locking of a laser diode to a high-Q WGM microresonator. Opt. Express 2017, 25, 28167–28178. [Google Scholar] [CrossRef]
- Galiev, R.R.; Pavlov, N.G.; Kondratiev, N.M.; Koptyaev, S.; Lobanov, V.E.; Voloshin, A.S.; Gorodnitskiy, A.S.; Gorodetsky, M.L. Spectrum collapse, narrow linewidth, and Bogatov effect in diode lasers locked to high-Q optical microresonators. Opt. Express. 2018, 26, 30509–30522. [Google Scholar] [CrossRef]
- Del’Haye, P.; Herr, T.; Gavartin, E.; Gorodetsky, M.L.; Holzwarth, R.; Kippenberg, T.J. Octave Spanning Tunable Frequency Comb from a Microresonator. Phys. Rev. Lett. 2011, 107, 063901. [Google Scholar] [CrossRef] [Green Version]
- Kippenberg, T.J.; Gaeta, A.L.; Lipson, M.; Gorodetsky, M.L. Dissipative Kerr solitons in optical microresonators. Science 2018, 361, eaan8083. [Google Scholar] [CrossRef] [Green Version]
- Suh, M.-G.; Yang, Q.-F.; Yang, K.Y.; Yi, X.; Vahala, K.J. Microresonator soliton dual-comb spectroscopy. Science 2016, 354, 600–603. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, N.G.; Lihachev, G.; Koptyaev, S.; Lucas, E.; Karpov, M.; Kondratiev, N.M.; Bilenko, I.A.; Kippenberg, T.J.; Gorodetsky, M.L. Soliton dual frequency combs in crystalline microresonators. Opt. Lett. 2017, 42, 514–517. [Google Scholar] [CrossRef] [Green Version]
- Pavlov, N.G.; Koptyaev, S.; Lihachev, G.V.; Voloshin, A.S.; Gorodnitskiy, A.S.; Ryabko, M.V.; Polonsky, S.V.; Gorodetsky, M.L. Narrow-linewidth lasing and soliton Kerr microcombs with ordinary laser diodes. Nat. Photonics 2018, 12, 694–698. [Google Scholar] [CrossRef] [Green Version]
- Raja, A.S.; Voloshin, A.S.; Guo, H.; Agafonova, S.E.; Liu, J.; Gorodnitskiy, A.S.; Karpov, M.; Pavlov, N.G.; Lucas, E.; Galiev, R.R.; et al. Electrically pumped photonic integrated soliton microcomb. Nat. Commun. 2019, 10, 680. [Google Scholar] [CrossRef] [Green Version]
- Baaske, M.D.; Foreman, M.R.; Vollmer, F. Single-molecule nucleic acid interactions monitored on a label-free microcavity biosensor platform. Nat. Nanotechnol. 2014, 9, 933–939. [Google Scholar] [CrossRef] [PubMed]
- Anderson, M.E.; O’Brien, E.C.; Grayek, E.N.; Hermansen, J.K.; Hunt, H.K. The Detection of Helicobacter hepaticus Using Whispering-Gallery Mode Microcavity Optical Sensors. Biosensors 2015, 5, 562–576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, H.; Dale, P.S.; Calwell, C.W.; Fan, X. Rapid and Label-Free Detection of Breast Cancer Biomarker CA15-3 in Clinical Human Serum Samples with Optofluidic Ring Resonator Sensors. Anal. Chem. 2009, 81, 9858–9865. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Liu, L.; Xu, L. Ultralow sensing limit in optofluidic micro-bottle resonator biosensor by self-referenced differential-mode detection scheme. Appl. Phys. Lett. 2014, 104, 4. [Google Scholar] [CrossRef]
- Arnold, S.; Khoshsima, M.; Teraoka, I. Shift of whispering-gallery modes in microspheres by protein adsorption. Opt. Lett. 2003, 28, 272–274. [Google Scholar] [CrossRef] [Green Version]
- Ozdemir, S.K.; Zhu, J.; He, L.; Yang, L. Estimation of Purcell factor from mode-splitting spectra in an optical microcavity. Phys. Rev. A 2011, 83, 5. [Google Scholar] [CrossRef] [Green Version]
- Hu, Y.; Shao, L.; Arnold, S.; Liu, Y.-C.; Ma, C.-Y.; Xiao, Y.-F. Mode broadening induced by nanoparticles in an optical whispering-gallery microcavity. Phys. Rev. A 2014, 90, 043847. [Google Scholar] [CrossRef] [Green Version]
- Chen, W.; Özdemir, Ş.K.; Zhao, G.; Wiersig, J.; Yang, L. Exceptional points enhance sensing in an optical microcavity. Nature 2017, 548, 192–196. [Google Scholar] [CrossRef]
- Shao, L.; Jiang, X.-F.; Yu, X.-C.; Li, B.-B.; Clements, W.R.; Vollmer, F.; Wang, W.; Xiao, Y.-F.; Gong, Q. Detection of single nanoparticles and lentiviruses using microcavity resonance broadening. Adv. Mater. 2013, 25, 5616–5620. [Google Scholar] [CrossRef]
- Shen, B.-Q.; Yu, X.-C.; Zhi, Y.; Kim, D.; Gong, Q.; Xiao, Y.-F. Detection of single nanoparticles using the dissipative interaction in a high-Q microcavity. Phys. Rev. Appl. 2016, 5, 024011. [Google Scholar] [CrossRef] [Green Version]
- Heylman, K.D.; Knapper, K.A.; Horak, E.H.; Rea, M.T.; Vanga, S.K.; Goldsmith, R.H. Optical Microresonators for Sensing and Transduction: A Materials Perspective. Adv. Mater. 2017, 29, 1700037. [Google Scholar] [CrossRef] [PubMed]
- Cheema, M.I.; Mehrabani, S.; Hayat, A.A.; Peter, Y.-A.; Armani, A.M.; Kirk, A.G. Simultaneous measurement of quality factor and wavelength shift by phase shift microcavity ring down spectroscopy. Opt. Express. 2012, 20, 9090–9098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, A.; Mills, T.; Xu, Y. Nanoscale welding aerosol sensing based on whispering gallery modes in a cylindrical silica resonator. Opt. Express. 2015, 23, 7351–7365. [Google Scholar] [CrossRef] [Green Version]
- Savchenkov, A.A.; Matsko, A.B.; Ilchenko, V.S.; Maleki, L. Optical resonators with ten million finesse. Opt. Express. 2007, 15, 6768–6773. [Google Scholar] [CrossRef] [PubMed]
- Collot, L.; Lefevre-Seguin, V.; Brune, M.; Raimond, J.M.; Haroche, S. Very High-Q Whispering-Gallery Mode Resonances Observed on Fused Silica Microspheres. Europhys. Lett. 1993, 23, 327–334. [Google Scholar] [CrossRef]
- Zhu, H.; Suter, J.D.; White, I.M.; Fan, X. Aptamer Based Microsphere Biosensor for Thrombin Detection. Sensors 2006, 6, 785–795. [Google Scholar] [CrossRef] [Green Version]
- Krioukov, E.; Klunder, D.J.W.; Driessen, A.; Greve, J.; Otto, C. Sensor based on an integrated optical microcavity. Opt. Lett. 2002, 27, 512–514. [Google Scholar] [CrossRef]
- Ilchenko, V.S.; Savchenkov, A.A.; Matsko, A.B.; Maleki, L. Nonlinear Optics and Crystalline Whispering Gallery Mode Cavities. Phys. Rev. Lett. 2004, 92, 043903. [Google Scholar] [CrossRef]
- Yang, G.; White, I.M.; Fan, X. An opto-fluidic ring resonator biosensor for the detection of organophosphorus pesticides. Sens. Actuators B 2008, 133, 105–112. [Google Scholar] [CrossRef]
- Yu, X.-C.; Li, B.-B.; Wang, P.; Tong, L.; Jiang, X.-F.; Li, Y.; Gong, Q.; Xiao, Y.-F. Single nanoparticle detection and sizing using a nanofiber pair in an aqueous environment. Adv. Mater. 2014, 26, 7462–7467. [Google Scholar] [CrossRef]
- Levin, G.G.; Min`kov, K.N.; Ermakov, M.M.; Samoilenko, A.A. Studying the Internal Structure of Microcavities by Means of Optical Tomography. Opt. Spectrosc. 2019, 126, 226–231. [Google Scholar] [CrossRef]
- Barton, J.P. Effects of surface perturbations on the quality and the focused-beam excitation of microsphere resonance. J. Opt. Soc. Am. A 1999, 16, 1974–1980. [Google Scholar] [CrossRef]
- Vernooy, D.W.; Ilchenko, V.S.; Mabuchi, H.E.; Streed, W.; Kimble, H.J. High-Q measurements of fused-silica microspheres in the near infrared. Opt. Lett. 1998, 23, 247–249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grudinin, I.S.; Ilchenko, V.S.; Maleki, L. Ultrahigh optical Q factors of crystalline resonators in the linear regime. Phys. Rev. A 2006, 74, 063806. [Google Scholar] [CrossRef]
- Braginsky, V.B.; Gorodetsky, M.L.; Ilchenko, V.S. Quality-factor and nonlinear properties of optical whispering-gallery modes. Phys. Lett. A 1989, 137, 393–397. [Google Scholar] [CrossRef]
- Lisichkin, G.V.; Kudryavcev, G.V.; Serdan, A.A.; Staroverov, S.M.; Yuffa, A.Y. Modified Silicas in Sorption, Catalysis and Chromatography; Khimiya: Moscow, Russia, 1986; p. 248. [Google Scholar]
- Little, C.J.; Dale, A.D.; Whatley, J.A. Optimization of reaction conditions for the preparation of chemically bonded supports. J. Chrom. 1979, 171, 431–434. [Google Scholar] [CrossRef]
- Min`kov, K.N. Research of Optical Dielectric Microcavities for Detecting Nanoparticles. Ph.D. Thesis, National Research University Higher School of Economics, Moscow, Russia, 2018. [Google Scholar]
- Gorodetsky, M.L.; Ilchenko, V.S. High-Q opti-cal whispering-gallery microresonators: Precession ap-proach for spherical mode analysis and emission pat-terns with prism couplers. Opt. Commun. 1994, 113, 133–143. [Google Scholar] [CrossRef]
- Savchenkov, A.A.; Borri, S.; de Cumis, M.S.; Matsko, A.B.; Natale, P.; Maleki, L. Modeling and measuring the quality factor of whispering gallery mode resonators. Appl. Phys. B 2018, 124, 171. [Google Scholar] [CrossRef]
- Min’kov, K.N.; Ivanov, A.D.; Samoilenko, A.A.; Ruzhitskaya, D.D.; Levin, G.G.; Efimov, A.A. Measurement of Low Concentrations of Nanoparticles in Aerosols Using Optical Dielectric Microcavity: The Case of TiO2 Nanoparticles. Nanotechnol. Russ. 2018, 13, 38–44. [Google Scholar] [CrossRef]
- Schaaf, P.; Talbot, J. Surface exclusion effects in adsorption processes. J. Chem. Phys. 1989, 91, 4401–4409. [Google Scholar] [CrossRef]
- Ivanov, A.D.; Min’kov, K.N.; Samoilenko, A.A.; Ruzhitskaya, D.D.; Levin, G.G. Application of Optical Microresonators for Measuring the Concentration of Nanoparticles in Liquids. Meas. Tech. 2018, 61, 566–571. [Google Scholar] [CrossRef]
- Leopold, N.; Lendl, B. A New Method for Fast Preparation of Highly Surface-Enhanced Raman Scattering (SERS) Active Silver Colloids at Room Temperature by Reduction of Silver Nitrate with Hydroxylamine Hydrochloride. J. Phys. Chem. B 2003, 107, 5723–5727. [Google Scholar] [CrossRef]
- Lizunova, A.A.; Kalinina, E.G.; Beketov, I.V.; Ivanov, V.V. Development of Reference Materials for the Diameter of Nanoparticles of Colloidal Solutions of Aluminum Oxide and Titanium Dioxide. Meas. Tech. 2014, 57, 848–854. [Google Scholar] [CrossRef]
- Vollmer, F.; Arnold, S.; Keng, D. Single virus detection from the reactive shift of a whispering-gallery mode. Proc. Natl. Acad. Sci. USA 2008, 105, 20701–20704. [Google Scholar] [CrossRef] [Green Version]
- Vollmer, F.; Yang, L. Label-free detection with high-Q microcavities: A review of biosensing mechanisms for integrated devices. Nanophotonics 2012, 1, 267–291. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, A.D. Measurement System Based on Optical Microresonators for Detecting Small Concentrations of Nanoparticles. Ph.D. Thesis, The All-Russian Research Institute for Optical and Physical Measurements, Moscow, Russia, 2018. [Google Scholar]
- Ruzhitskaya, D.D.; Samoilenko, A.A.; Ivanov, A.D.; Min`kov, K.N. Analysis of the Transmission Spectra of Optical Microcavities Using the Mode Broadening Method. Optoelectron. Instrum. Data Process. 2018, 54, 8. [Google Scholar] [CrossRef]



| Factors Affecting Error | Peak Width Value | Broadening Rate Value |
|---|---|---|
| Reference interferometer | ±0.65 MHz | ±0.03 MHz/s |
| Total instability of tunable laser [70] | ±0.45 MHz | ±0.02 MHz/s |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, A.; Min`kov, K.; Samoilenko, A.; Levin, G. The Measurement of Nanoparticle Concentrations by the Method of Microcavity Mode Broadening Rate. Sensors 2020, 20, 5950. https://doi.org/10.3390/s20205950
Ivanov A, Min`kov K, Samoilenko A, Levin G. The Measurement of Nanoparticle Concentrations by the Method of Microcavity Mode Broadening Rate. Sensors. 2020; 20(20):5950. https://doi.org/10.3390/s20205950
Chicago/Turabian StyleIvanov, Alexey, Kirill Min`kov, Alexey Samoilenko, and Gennady Levin. 2020. "The Measurement of Nanoparticle Concentrations by the Method of Microcavity Mode Broadening Rate" Sensors 20, no. 20: 5950. https://doi.org/10.3390/s20205950




