Development of a Colorimetric Sensor for Autonomous, Networked, Real-Time Application
Abstract
:1. Introduction
2. Indicators
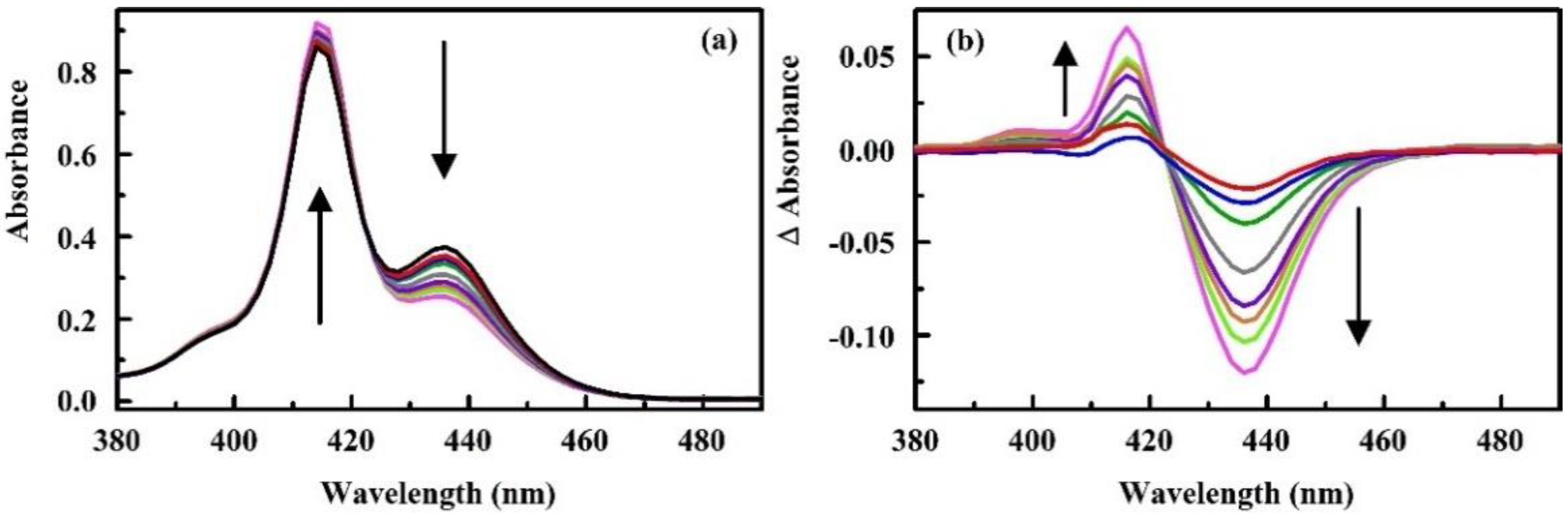
2.1. Porphyrin Based Chemical Detection
2.2. Antimicrobial Peptide Based Biological Detection
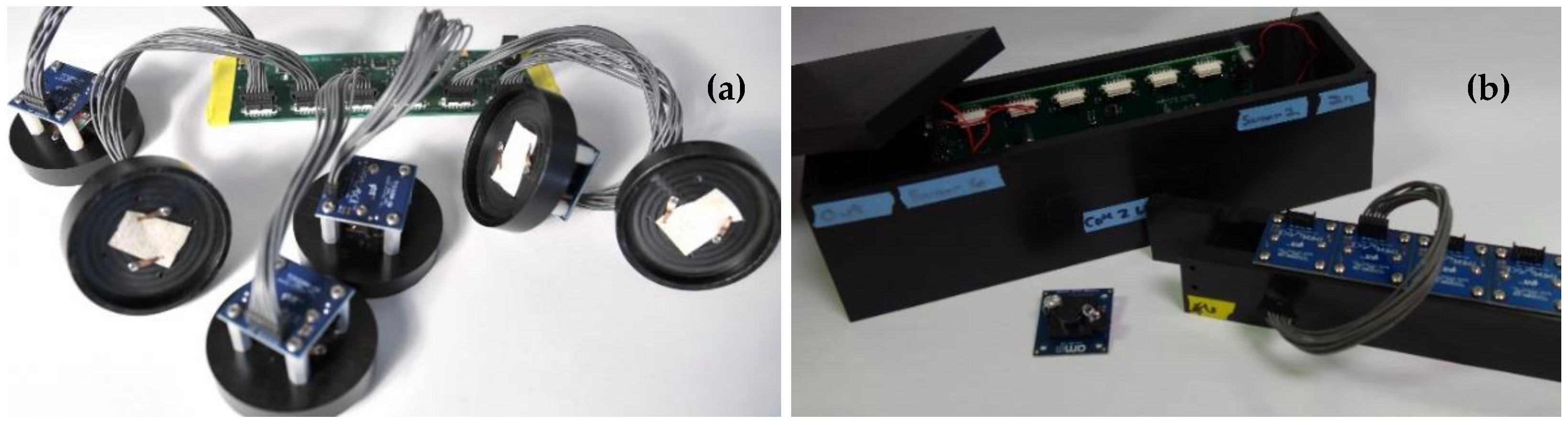
3. Devices
4. Algorithms
4.1. Standard Deviation Algorithm
4.2. Slope Algorithm
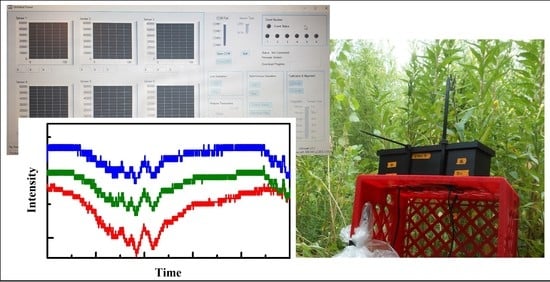
5. Demonstration and Evaluation
6. Ongoing Work
7. Conclusions and Future Outlook
8. Patents
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Choi, S.K.; Jeong, Y.S.; Koh, Y.J.; Lee, J.H.; Nam, H.W.; Lee, J. Analysis of Raman spectral characteristics of chemical warfare agents by using 248-nm UV Raman spectroscopy. Bull. Korean Chem. Soc. 2019, 40, 279–284. [Google Scholar] [CrossRef]
- Corsi, C.; Dundee, A.; Laurenzi, P.; Liberatore, N.; Luciani, D.; Mengali, S.; Mercuri, A.; Pifferi, A.; Simeoni, M.; Tosone, R.; et al. Chemical warfare agents analyzer based on low cost, room temperature, and infrared microbolometer smart sensors. Adv. Opt. Technol. 2012, 2012, 808541. [Google Scholar] [CrossRef] [Green Version]
- Hu, G.X.; Xiong, W.; Luo, H.Y.; Shi, H.L.; Li, Z.W.; Shen, J.; Fang, X.J.; Xu, B.A.; Zhang, J.C. Raman spectroscopic detection for simulants of chemical warfare agents using a spatial heterodyne spectrometer. Appl. Spectrosc. 2018, 72, 151–158. [Google Scholar] [CrossRef]
- Kondo, T.; Hashimoto, R.; Ohrui, Y.; Sekioka, R.; Nogami, T.; Muta, F.; Seto, Y. Analysis of chemical warfare agents by portable Raman spectrometer with both 785 nm and 1064 nm excitation. Forensic Sci. Int. 2018, 291, 23–38. [Google Scholar] [CrossRef]
- Theriault, J.M.; Puckrin, E.; Hancock, J.; Lecavalier, P.; Lepage, C.J.; Jensen, J.O. Passive standoff detection of chemical warfare agents on surfaces. Appl. Opt. 2004, 43, 5870–5885. [Google Scholar] [CrossRef]
- Niernikoski, H.; Soderstrom, M.; Vanninen, P. Detection of chemical warfare agent-related phenylarsenic compounds in marine biota samples by LC-HESI/MS/MS. Anal. Chem. 2017, 89, 11129–11134. [Google Scholar] [CrossRef]
- Subramaniam, R.; Astot, C.; Juhlin, L.; Nilsson, C.; Ostin, A. Direct derivatization and rapid GC-MS screening of nerve agent markers in aqueous samples. Anal. Chem. 2010, 82, 7452–7459. [Google Scholar] [CrossRef]
- Valdez, C.A.; Leif, R.N.; Alcaraz, A. Effective methylation of phosphonic acids related to chemical warfare agents mediated by trimethyloxonium tetrafluoroborate for their qualitative detection and identification by gas chromatography-mass spectrometry. Anal. Chim. Acta 2016, 933, 134–143. [Google Scholar] [CrossRef] [PubMed]
- Young, S.A.; Capacio, B.R. The application of a single-column GC-MS-MS method for the rapid analysis of chemical warfare agents and breakdown products. J. Anal. Toxicol. 2019, 43, 179–187. [Google Scholar] [CrossRef]
- Diauudin, F.N.; Rashid, J.I.A.; Knight, V.F.; Yunus, W.M.Z.W.; Ong, K.K.; Kasin, N.A.M.; Halim, N.; Noor, S.A.M. A review of current advances in the detection of organophosphorus chemical warfare agents based biosensor approaches. Sens. Bio-Sens. Res. 2019, 26, 100305. [Google Scholar] [CrossRef]
- Saito, M.; Uchida, N.; Furutani, S.; Murahashi, M.; Espulgar, W.; Nagatani, N.; Nagai, H.; Inoue, Y.; Ikeuchi, T.; Kondo, S.; et al. Field-deployable rapid multiple biosensing system for detection of chemical and biological warfare agents. Microsyst. Nanoeng. 2018, 4. [Google Scholar] [CrossRef]
- Sferopoulos, R. A Review of Chemical Warfare Agent (CWA) Detector Technologies and Commercial-Off-the-Shelf Items; DSTO-GD-0570; Austrailian Government Defence Science and Technology Organisation, Human Protection and Performance Division, Fishermans Bend: Victoria, Australia, 2009.
- Riley, P.C.; Ince, B.S.; McHugh, V.M.; Hauck, B.C.; Harden, C.S.; Taylor, J.; McIntrye, H.; McSweeney, R.; Long, S. From the warehouse to the field—New applications of existing chemical warfare agent detectors without hardware modification. In Chemical, Biological, Radiological, Nuclear, and Explosives; Guicheteau, J.A., Howle, C.R., Eds.; Spie-Int Soc Optical Engineering: Bellingham, DC, USA, 2019; Volume 11010. [Google Scholar]
- Boris, J. The threat of chemical and biological terrorism: Preparing a response. Comput. Sci. Eng. 2002, 4, 22–32. [Google Scholar] [CrossRef]
- Boris, J.; Patnaik, G.; Obenschain, K.; Moses, A.; Obenschain, M.-Y.; Theodore, Y.; Delaney, J.; Donnelly, J. Fast and accurate prediction of windborne contaminant plumes for civil defense in cities. In Proceedings of the Fifth International Symposium on Computational Wind Engineering (CWE2010), Chapel Hill, CA, USA, 23–27 May 2010. [Google Scholar]
- Britter, R.E.; Hanna, S.R. Flow and dispersion in urban areas. Annu. Rev. Fluid Mech. 2003, 35, 469–496. [Google Scholar] [CrossRef]
- Dekker, A.H.; Skvortsov, A.T. Topological Issues in Sensor Networks. In Proceedings of the 18th World IMACS/MODSIM Congress, Cairns, Australia, 13–17 July 2009; pp. 952–958. [Google Scholar]
- Demetriou, M.A.; Gatsonis, N.A.; IEEE. Scheduling of static sensor networks and management of mobile sensor networks for the detection and containment of moving sources in spatially distributed processes. In Proceedings of the Med 2009: 17th Mediterranean Conference on Control & Automation, Thessaloniki, Greece, 24–26 June 2009; pp. 187–192. [Google Scholar] [CrossRef]
- Jay, P.B. Dust in the wind: Challenges for urban aerodynamics. Proc. SPIE 2007. [Google Scholar] [CrossRef]
- Salim, F.; Moya Castro, R. Parallel Analysis of Urban Aerodynamic Phenomena Using High and Low-Tech Tools. In Proceedings of the 30th International Conference on Education and Research in Computer Aided Architectural Design in Europe, Prague, Czech Republic, 12–14 September 2012; pp. 621–629. [Google Scholar]
- Singh, B.; Pardyjak, E.R.; Norgren, A.; Willemsen, P. Accelerating urban fast response Lagrangian dispersion simulations using inexpensive graphics processor parallelism. Environ. Model. Softw. 2011, 26, 739–750. [Google Scholar] [CrossRef]
- Skvortsov, A.T.; Dawson, P.D.; Roberts, M.D.; Gailis, R.M. Chemical, Biological and Radiological Hazard Assessment: A New Model of a Plume in a Complex Urban Environment. In MODSIM 2007 International Congress on Modelling and Simulation; Modelling and Simulation Society of Australia and New Zealand Inc.: Christchurch, New Zealand, 2007; pp. 645–649. [Google Scholar]
- Truslow, E.; Golowich, S.; Manolakis, D.; Ingle, V.K. Metrics for the comparative evaluation of chemical plume identification algorithms. In Algorithms and Technologies for Multispectral, Hyperspectral, and Ultraspectral Imagery XXI; VelezReyes, M., Kruse, F.A., Eds.; SPIE: Baltimore, MD, USA, 2015; Volume 9472. [Google Scholar]
- De Vito, S. Challenges in Wireless Chemical Sensor Networks; CRC Press: Boca Raton, FL, USA, 2013; pp. 35–55. [Google Scholar]
- Diamond, D.; Coyle, S.; Scarmagnani, S.; Hayes, J. Wireless Sensor networks and chemo-/biosensing. Chem. Rev. 2008, 108, 652–679. [Google Scholar] [CrossRef]
- Do, S.; Lee, M.; Kim, J.S. The effect of a flow field on chemical detection performance of quadrotor drone. Sensors 2020, 20, 3262. [Google Scholar] [CrossRef]
- Fumian, F.; Di Giovanni, D.; Martellucci, L.; Rossi, R.; Gaudio, P. Application of miniaturized sensors to unmanned aerial systems, a new pathway for the survey of polluted areas: Preliminary results. Atmosphere 2020, 11, 471. [Google Scholar] [CrossRef]
- He, X.; Bourne, J.R.; Steiner, J.A.; Mortensen, C.; Hoffman, K.C.; Dudley, C.J.; Rogers, B.; Cropek, D.M.; Leang, K.K. Autonomous chemical-sensing aerial robot for urban/suburban environmental monitoring. IEEE Syst. J. 2019, 13, 3524–3535. [Google Scholar] [CrossRef]
- Pochwala, S.; Gardecki, A.; Lewandowski, P.; Somogyi, V.; Anweiler, S. Developing of low-cost air pollution sensor-measurements with the unmanned aerial vehicles in Poland. Sensors 2020, 20, 3582. [Google Scholar] [CrossRef]
- Zhou, F.; Pan, S.D.; Chen, W.; Ni, X.P.; An, B.W. Monitoring of compliance with fuel sulfur content regulations through unmanned aerial vehicle (UAV) measurements of ship emissions. Atmos. Meas. Tech. 2019, 12, 6113–6124. [Google Scholar] [CrossRef] [Green Version]
- De Vito, S.; di palma, P.; Ambrosino, C.; Massera, E.; Burrasca, G.; Miglietta, M.L.; Francia, G. Wireless sensor networks for distributed chemical sensing: Addressing power consumption limits with on-board intelligence. Sens. J. IEEE 2011, 11, 947–955. [Google Scholar] [CrossRef] [Green Version]
- Hayes, J.; Beirne, S.; King-Tong, L.; Diamond, D. Evaluation of a low cost wireless chemical sensor network for environmental monitoring. In Proceedings of the SENSORS, 2008 IEEE, Lecce, Italy, 26–29 October 2008; pp. 530–533. [Google Scholar]
- Oikonomou, P.; Botsialas, A.; Olziersky, A.; Hadjigeorgiou, E.P.; Katsikas, S.; Dimas, D.; Sotiropoulos, G.; Raptis, I.; Sanopoulou, M. A self-calibrated wireless sensing system for monitoring the ambient industrial environment. From lab to real-time application. Sens. Actuator B Chem. 2016, 237, 509–520. [Google Scholar] [CrossRef]
- Oikonomou, P.; Botsialas, A.; Olziersky, A.; Kazas, I.; Stratakos, I.; Katsikas, S.; Dimas, D.; Mermikli, K.; Sotiropoulos, G.; Goustouridis, D.; et al. A wireless sensing system for monitoring the workplace environment of an industrial installation. Sens. Actuator B Chem. 2016, 224, 266–274. [Google Scholar] [CrossRef]
- RejinaParvin, J.; Vasanthanayaki, C. Particle swarm optimization-based clustering by preventing residual nodes in wireless sensor networks. IEEE Sens. J. 2015, 15, 4264–4274. [Google Scholar] [CrossRef]
- Iliev, N.; Paprotny, I. Review and comparison of spatial localization methods for low-power wireless sensor networks. IEEE Sens. J. 2015, 15, 5971–5987. [Google Scholar] [CrossRef]
- Kim, B.H.; D’Souza, C.; Voyles, R.M.; Hesch, J.; Roumeliotis, S.I. A reconfigurable computing platform for plume tracking with mobile sensor networks. In Unmanned Systems Technology Viii, Pts 1 and 2; Gerhart, G.R., Shoemaker, C.M., Gage, D.W., Eds.; SPIE: Orlando, FL, USA, 2006; Volume 6230. [Google Scholar]
- Somov, A.; Karpov, E.F.; Karpova, E.; Suchkov, A.; Mironov, S.; Karelin, A.; Baranov, A.; Spirjakin, D. Compact low power wireless gas sensor node with thermo compensation for ubiquitous deployment. IEEE Trans. Ind. Inform. 2015, 11, 1660–1670. [Google Scholar] [CrossRef]
- La, H.M.; Sheng, W.H. Cooperative sensing in mobile sensor networks based on distributed consensus. In Signal and Data Processing of Small Targets 2011; Drummond, O.E., Ed.; SPIE: San Diego, CA, USA, 2011; Volume 8137. [Google Scholar]
- Lepley, J.J.; Lloyd, D.R.; Robins, A.; Rudd, A.; Wilks, A. Dynamic sensor deployment for the monitoring of chemical releases in urban environments (DYCE). In Chemical, Biological, Radiological, Nuclear, and Explosives (CBRNE) Sensing XII; Fountain, A.W., Gardner, P.J., Eds.; SPIE: Orlando, FL, USA, 2011; Volume 8018. [Google Scholar]
- Roth, C.; Knudson, M.; Tumer, K. Agent Fitness Functions for Evolving Coordinated Sensor Networks. In Proceedings of the 13th Annual Conference on Genetic and Evolutionary Computation, Dublin, Ireland 12–16 July 2011; pp. 275–282. [Google Scholar]
- Titouna, C.; Nait-Abdesselam, F.; Khokhar, A.; IEEE. A multivariate outlier detection algorithm for wireless sensor networks. In Proceedings of the ICC 2019 IEEE International Conference on Communications, Shanghai, China, 20–24 May 2019. [Google Scholar]
- Malinski, T. Applications: Past, present, future. In The Porphyrin Handbook, 1st ed.; Kadish, K.M., Smith, K.M., Guilard, R., Eds.; Academic Press: New York, NY, USA, 2000; Volume 6, p. 231. [Google Scholar]
- Feng, L.; Musto, C.J.; Kemling, J.W.; Lim, S.H.; Suslick, K.S. A colorimetric sensor array for identification of toxic gases below permissible exposure limits. Chem. Commun. 2010, 46, 2037–2039. [Google Scholar] [CrossRef]
- Johnson, B.J.; Anderson, N.E.; Charles, P.T.; Malanoski, A.P.; Melde, B.J.; Nasir, M.; Deschamps, J.R. Porphyrin-embedded silicate materials for detection of hydrocarbon solvents. Sensors 2011, 11, 886–904. [Google Scholar] [CrossRef] [Green Version]
- Johnson, B.J.; Leska, I.A.; Melde, B.J.; Taft, J.R. Self-reporting materials: Dual use for porphyrin-embedded sorbents. Sens. Actuator B Chem. 2013, 176, 399–404. [Google Scholar] [CrossRef]
- Rakow, N.A.; Suslick, K.S. A colorimetric sensor array for odour visualization. Nature 2000, 406, 710–713. [Google Scholar] [CrossRef] [PubMed]
- Barker, P.S.; Chen, J.R.; Agbor, N.E.; Monkman, A.P.; Mars, P.; Petty, M.C. Vapor recognition using organic films and artificial neural networks. Sensor Actuat B Chem 1994, 17, 143–147. [Google Scholar] [CrossRef]
- Di Natale, C.; Martinelli, E.; Paolesse, R.; D’Amico, A.; Filippini, D.; Lundstrom, I. An artificial olfaction system based on the optical imaging of a large array of chemical reporters. Sensor Actuat B Chem 2009, 142, 412–417. [Google Scholar] [CrossRef]
- Di Natale, C.; Paolesse, R.; Macagnano, A.; Troitsky, V.I.; Berzina, T.S.; D’Amico, A. Pattern recognition approach to the study of the interactions between metalloporphyrin Langmuir-Blodgett films and volatile organic compounds. Anal. Chim. Acta 1999, 384, 249–259. [Google Scholar] [CrossRef]
- Shirsat, M.D.; Sarkar, T.; Kakoullis, J.; Myung, N.V.; Konnanath, B.; Spanias, A.; Mulchandani, A. porphyrin-functionalized single-walled carbon nanotube chemiresistive sensor arrays for VOCs. J. Phys. Chem. C 2012, 116, 3845–3850. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.; Bailey, D.P.; Suslick, K.S. Colorimetric sensor arrays for the analysis of beers: A feasibility study. J. Agric. Food Chem. 2006, 54, 4925–4931. [Google Scholar] [CrossRef]
- Di Natale, C.; Martinelli, E.; Magna, G.; Mandoj, F.; Monti, D.; Nardis, S.; Stefanelli, M.; Paolesse, R. Porphyrins for olfaction mimic: The Rome tor vergata approach. J. Porphyr. Phthalocyanines 2017, 21, 769–781. [Google Scholar] [CrossRef] [Green Version]
- Kladsomboon, S.; Thippakorn, C.; Seesaard, T. Development of organic-inorganic hybrid optical gas sensors for the non-invasive monitoring of pathogenic bacteria. Sensors 2018, 18, 3189. [Google Scholar] [CrossRef] [Green Version]
- Li, L.Q.; Xie, S.M.; Zhu, F.Y.; Ning, J.M.; Chen, Q.S.; Zhang, Z.Z. Colorimetric sensor array-based artificial olfactory system for sensing Chinese green tea’s quality: A method of fabrication. Int. J. Food Prop. 2017, 20, 1762–1773. [Google Scholar] [CrossRef]
- Ngo, H.T.; Minami, K.; Imamura, G.; Shiba, K.; Yoshikawa, G. Effects of center metals in porphines on nanomechanical gas sensing. Sensors 2018, 18, 1640. [Google Scholar] [CrossRef] [Green Version]
- Yan, Q.; Ding, X.Y.; Chen, Z.H.; Xue, S.F.; Han, X.Y.; Lin, Z.Y.; Yang, M.; Shi, G.Y.; Zhang, M. pH-regulated optical performances in organic/inorganic hybrid: A dual-mode sensor array for pattern-recognition-based biosensing. Anal. Chem. 2018, 90, 10536–10542. [Google Scholar] [CrossRef] [PubMed]
- Johnson, B.J.; Taitt, C.R.; Gleaves, A.; North, S.H.; Malanoski, A.P.; Leska, I.A.; Archibong, E.; Monk, S.M. Porphyrin-modified antimicrobial peptide indicators for detection of bacteria. Sens. Bio Sens. Res. 2016, 8, 1–7. [Google Scholar] [CrossRef] [Green Version]
- White, B.J.; Taitt, C.R.; Archibong, E.; Leska, I.A. Colorimetric Biosensor: Crosslinker Variations; NRL/MR/6930-18-9820; US Naval Research Laboratory: Washington, DC, USA, 2018. [Google Scholar]
- White, B.J.; Taitt, C.R.; Archibong, E.; Leska, I.A. Colorimetric Biosensor: Porphyrin Variations; NRL/MR/6930-18-9819; US Naval Research Laboratory: Washington, DC, USA, 2018. [Google Scholar]
- Askim, J.R.; Suslick, K.S. Hand-held reader for colorimetric sensor arrays. Anal. Chem. 2015, 87, 7810–7816. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Q.S.; Liu, A.P.; Zhao, J.W.; Ouyang, Q. Classification of tea category using a portable electronic nose based on an odor imaging sensor array. J. Pharm. Biomed. Anal. 2013, 84, 77–89. [Google Scholar] [CrossRef]
- Huang, X.Y.; Xin, J.W.; Zhao, J.W. A novel technique for rapid evaluation of fish freshness using colorimetric sensor array. J. Food Eng. 2011, 105, 632–637. [Google Scholar] [CrossRef]
- Khulal, U.; Zhao, J.W.; Hu, W.W.; Chen, Q.S. Comparison of different chemometric methods in quantifying total volatile basic-nitrogen (TVB-N) content in chicken meat using a fabricated colorimetric sensor array. RSC Adv. 2016, 6, 4663–4672. [Google Scholar] [CrossRef]
- Lei, J.C.; Hou, C.J.; Huo, D.Q.; Li, Y.J.; Luo, X.G.; Yang, M.; Fa, H.B.; Bao, M.Z.; Li, J.J.; Deng, B. Detection of ammonia based on a novel fluorescent artificial nose and pattern recognition. Atmos. Pollut. Res. 2016, 7, 431–437. [Google Scholar] [CrossRef]
- Roales, J.; Pedrosa, J.M.; Guillen, M.G.; Lopes-Costa, T.; Pinto, S.M.A.; Calvete, M.J.F.; Pereira, M.M. Optical detection of amine vapors using ZnTriad porphyrin thin films. Sens. Actuator B Chem. 2015, 210, 28–35. [Google Scholar] [CrossRef]
- Wu, Y.; Huo, D.Q.; Hou, C.J.; Fa, H.B.; Yang, M.; Luo, X.G. Colorimetric artificial nose for identification of breath volatile organic compounds of patients with lung cancer. Chem. Res. Chin. Univ. 2014, 30, 572–577. [Google Scholar] [CrossRef]
- Maurya, N.; Bhardwaj, S.; Singh, A.K. A modest colorimetric chemosensor for investigation of CN-in semi-aqueous environment with high selectivity and sensitivity. Sens. Actuator B Chem. 2016, 229, 483–491. [Google Scholar] [CrossRef]
- Montes-Robles, R.; Moragues, M.E.; Vivancos, J.L.; Ibanez, J.; Fraile, R.; Martinez-Manez, R.; Garcia-Breijo, E. Colorimetric detection of hazardous gases using a remotely operated capturing and processing system. ISA Trans. 2015, 59, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Park, S.H.; Maruniak, A.; Kim, J.; Yi, G.R.; Lim, S.H. Disposable microfluidic sensor arrays for discrimination of antioxidants. Talanta 2016, 153, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Lopez-Ruiz, N.; Curto, V.F.; Erenas, M.M.; Benito-Lopez, F.; Diamond, D.; Palma, A.J.; Capitan-Vallvey, L.F. Smartphone-based simultaneous pH and nitrite colorimetric determination for paper microfluidic devices. Anal. Chem. 2014, 86, 9554–9562. [Google Scholar] [CrossRef]
- McCracken, K.E.; Yoon, J.Y. Recent approaches for optical smartphone sensing in resource-limited settings: A brief review. Anal. Methods 2016, 8, 6591–6601. [Google Scholar] [CrossRef] [Green Version]
- Su, K.Q.; Zou, Q.C.; Zhou, J.; Zou, L.; Li, H.B.; Wang, T.X.; Hu, N.; Wang, P. High-sensitive and high-efficient biochemical analysis method using a bionic electronic eye in combination with a smartphone-based colorimetric reader system. Sens. Actuator B Chem. 2015, 216, 134–140. [Google Scholar] [CrossRef]
- Johnson, B.J.; Erickson, J.S.; Kim, J.; Malanoski, A.P.; Leska, I.A.; Monk, S.M.; Edwards, D.J.; Young, T.N.; Verbarg, J.; Bovais, C.; et al. Miniaturized reflectance devices for chemical sensing. Meas. Sci. Technol. 2014, 25, 095101. [Google Scholar] [CrossRef]
- Johnson, B.J.; Malanoski, A.P.; Erickson, J.S.; Liu, R.; Remenapp, A.R.; Stenger, D.A.; Moore, M.H. Reflectance-based detection for long term environmental monitoring. Heliyon 2017, 3, e00312. [Google Scholar] [CrossRef] [Green Version]
- Malanoski, A.P.; Johnson, B.J.; Erickson, J.S.; Stenger, D.A. Development of a detection algorithm for use with reflectance-based, real-time chemical sensing. Sensors 2016, 16, 1927. [Google Scholar] [CrossRef] [Green Version]
- White, B.J.; Moore, M.H.; Franco, K.; Malanoski, A.P. Colorimetric Environmental Sensor: Aqueous Indicator Screening (Part 1); NRL/MR/6930-18-9806; US Naval Research Laboratory: Washington, DC, USA, 2018. [Google Scholar]
- White, B.J.; Moore, M.H.; Franco, K.; Malanoski, A.P. Colorimetric Environmental Sensor: Aqueous Indicator Screening (Part 2); NRL/MR/6930-18-9808; US Naval Research Laboratory: Washington, DC, USA, 2018. [Google Scholar]
- White, B.J.; Moore, M.H.; Franco, K.; Malanoski, A.P. Colorimetric Environmental Sensor: Aqueous Indicator Screening (Part 3); NRL/MR/6930-18-9810; US Naval Research Laboratory: Washington, DC, USA, 2018. [Google Scholar]
- Kulagina, N.V.; Anderson, G.P.; Ligler, F.S.; Shaffer, K.M.; Taitt, C.R. Antimicrobial peptides: New recognition molecules for detecting botulinum toxins. Sensors 2007, 7, 2808–2824. [Google Scholar] [CrossRef] [Green Version]
- Kulagina, N.V.; Shaffer, K.M.; Anderson, G.P.; Ligler, F.S.; Taitt, C.R. Antimicrobial peptide-based array for Escherichia coli and Salmonella screening. Anal. Chim. Acta 2006, 575, 9–15. [Google Scholar] [CrossRef]
- Ngundi, M.M.; Kulagina, N.V.; Anderson, G.P.; Taitt, C.R. Nonantibody-based recognition: Alternative molecules for detection of pathogens. Expert Rev. Proteom. 2006, 3, 511–524. [Google Scholar] [CrossRef] [PubMed]
- North, S.H.; Wojciechowski, J.; Chu, V.; Taitt, C.R. Surface immobilization chemistry influences peptide-based detection of lipopolysaccharide and lipoteichoic acid. J. Pept. Sci. 2012, 18, 366–372. [Google Scholar] [CrossRef]
- Taitt, C.R.; Shriver-Lake, L.C.; Ngundi, M.M.; Ligler, F.S. Array biosensor for toxin detection: Continued advances. Sensors 2008, 8, 8361–8377. [Google Scholar] [CrossRef] [Green Version]
- Dosselli, R.; Gobbo, M.; Bolognini, E.; Campestrini, S.; Reddi, E. Porphyrin—Apidaecin conjugate as a new broad spectrum antibacterial agent. ACS Med. Chem. Lett. 2010, 1, 35–38. [Google Scholar] [CrossRef] [Green Version]
- Dosselli, R.; Tampieri, C.; Ruiz-Gonzalez, R.; De Munari, S.; Ragas, X.; Sanchez-Garcia, D.; Agut, M.; Nonell, S.; Reddi, E.; Gobbo, M. Synthesis, characterization, and photoinduced antibacterial activity of porphyrin-type photosensitizers conjugated to the antimicrobial peptide apidaecin 1b. J. Med. Chem. 2013, 56, 1052–1063. [Google Scholar] [CrossRef]
- Liu, F.; Ni, A.S.Y.; Lim, Y.; Mohanram, H.; Bhattacharjya, S.; Xing, B.G. Lipopolysaccharide neutralizing peptide-porphyrin conjugates for effective photoinactivation and intracellular imaging of gram-negative bacteria strains. Bioconjug. Chem. 2012, 23, 1639–1647. [Google Scholar] [CrossRef] [PubMed]
- Bourre, L.; Giuntini, F.; Eggleston, I.M.; Mosse, C.A.; MacRobert, A.J.; Wilson, M. Effective photoinactivation of Gram-positive and Gram-negative bacterial strains using an HIV-1 Tat peptide-porphyrin conjugate. Photochem. Photobiol. Sci. 2010, 9, 1613–1620. [Google Scholar] [CrossRef]
- Kuciauskas, D.; Caputo, G.A. Self-assembly of peptide-porphyrin complexes leads to pH-dependent excitonic coupling. J. Phys. Chem. B 2009, 113, 14439–14447. [Google Scholar] [CrossRef] [PubMed]
- Taitt, C.R.; White, B.J. Porphyrin-Peptide Beacons for Microbial Detection. US 9,581,594, 28 February 2017. [Google Scholar]
- Askim, J.R.; Mahmoudi, M.; Suslick, K.S. Optical sensor arrays for chemical sensing: The optoelectronic nose. Chem. Soc. Rev. 2013, 42, 8649–8682. [Google Scholar] [CrossRef] [Green Version]
- Gares, K.L.; Hufziger, K.T.; Bykov, S.V.; Asher, S.A. Review of explosive detection methodologies and the emergence of standoff deep UV resonance Raman. J. Raman Spectrosc. 2016, 47, 124–141. [Google Scholar] [CrossRef]
- Hannon, A.; Lu, Y.J.; Li, J.; Meyyappan, M. A sensor array for the detection and discrimination of methane and other environmental pollutant gases. Sensors 2016, 16, 1163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huerta, R.; Mosqueiro, T.; Fonollosa, J.; Rulkova, N.F.; Rodriguez-Lujan, I. Online decorrelation of humidity and temperature in chemical sensors for continuous monitoring. Chemom. Intell. Lab. Syst. 2016, 157, 169–176. [Google Scholar] [CrossRef] [Green Version]
- Pavey, K.D.; FitzGerald, N.J.; Nielsen, D.J. Making light work: A miniaturised, real-time optical sensor network for the detection of CWA simulant methyl salicylate. Anal. Methods 2012, 4, 2224. [Google Scholar] [CrossRef]
- Seelye, M.; Gupta, G.S.; Bailey, D.; Seelye, J. Low cost colour sensors for monitoring plant growth in a laboratory. In Proceedings of the IEEE Instrumentation and Measurement Technology Conference—I2MTC, Hangzhou, China, 10–12 May 2011; pp. 1–6. [Google Scholar]
- Johnson, B.J.; Liu, R.; Neblett, R.C.; Malanoski, A.P.; Xu, M.; Erickson, J.S.; Zang, L.; Stenger, D.A.; Moore, M.H. Reflectance-based detection of oxidizers in ambient air. Sens. Actuator B Chem. 2016, 227, 399–402. [Google Scholar] [CrossRef] [Green Version]
- Erickson, J.S.; Malanoski, A.P.; White, B.J.; Stenger, D.A.; Tankard, E.R. Practical Implementation of Detection Algorithm for Reflectance-Based, Real-Time Sensing; NRL/MR/6930-18-9812; US Naval Research Laboratory: Washington, DC, USA, 2018. [Google Scholar]
- White, B.J.; Erickson, J.S.; Malanoski, A.P.; Moore, M.H. Reflectance-Based Sensing: Post-Evaluation Analysis of Sensor Responses; NRL/MR/6930-19,9852; US Naval Research Laboratory: Washington, DC, USA, 2019. [Google Scholar]
- White, B.J.; Erickson, J.S.; Malanoski, A.P. Reflectance-Based Sensing: Data and Device Outputs; NRL/MR/6930-20-10,065; US Naval Research Laboratory: Washington, DC, USA, 2020. [Google Scholar]
- Erickson, J.S.; Johnson, B.J.; Malanoski, A.P. Field demonstration of a distributed microsensor network for chemical detection. Sensors 2020, 20, 5424. [Google Scholar] [CrossRef]










| Target | Total Exposures | Initial Algorithm Events | Altered Algorithm Events 1 |
|---|---|---|---|
| Ethylene oxide, 78 ppm | 9 | 1 | 5 |
| Ethylene oxide, 361 ppm | 6 | 0 | 5 |
| Simple Green | 6 | 3 | 0 |
| Sarin, 0.22 mg/m3 (Simple Green) | 7 | 1 | 6 |
| Sulfur Mustard, 1.2 mg/m3 | 6 | 0 | 3 |
| Sulfur Mustard, 2.5 mg/m3 | 7 | 5 | 7 |
| Chlorine gas, 5 ppm | 6 | 6 | 6 |
| Chlorine gas, 100 ppm | 7 | 4 | 4 |
| VX, 0.013 mg/m3 | 6 | 0 | 4 |
| VX, 0.022 mg/m3 | 6 | 0 | 5 |
| Device | Multiplex | ABEAM-6 | ABEAM-15 |
|---|---|---|---|
| Number of Indicators | 6 | 6 | 15 |
| Memory Duration (30 s sampling) | 7.5 days | 60 days | 12 days |
| Size (LxWxH) | Not housed | 27.4 × 7.6 × 7.6 cm | 19.1 × 8.9 × 11.7 cm |
| Weight | 450 g | 1.6 kg | 2.2 kg 1 |
| Software Platform | LabWindows | LabWindows | Java/JavaFX |
| Sensor Hardware | TCS 3200 | TCS 3200, 3400, 34725, and AS 7262 | TCS 34725 |
| 5 s Integration Times | 100 ms | 100–500 ms 2 | 100–600 ms |
| Available Gain | No | Some 3 | Up to 64× |
| Microcontroller | ATMEGA 328-P | XMEGA64-A3U-AU | XMEGA64-A3U-AU |
| Wireless Communications | No | No | Yes |
| USB Communications | Yes | Yes | Yes |
| Power Management | No | No | Yes |
| Fans | No | Yes | No |
| Outdoor Housing | No | Yes | Yes |
| Batteries | No | No | Yes |
| Drip feed, Real-time Detection | No | Yes | Yes |
| Networkable | No | No | Yes |
| Dimmable LEDs | No | No | Yes |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Johnson, B.J.; Malanoski, A.P.; Erickson, J.S. Development of a Colorimetric Sensor for Autonomous, Networked, Real-Time Application. Sensors 2020, 20, 5857. https://doi.org/10.3390/s20205857
Johnson BJ, Malanoski AP, Erickson JS. Development of a Colorimetric Sensor for Autonomous, Networked, Real-Time Application. Sensors. 2020; 20(20):5857. https://doi.org/10.3390/s20205857
Chicago/Turabian StyleJohnson, Brandy J., Anthony P. Malanoski, and Jeffrey S. Erickson. 2020. "Development of a Colorimetric Sensor for Autonomous, Networked, Real-Time Application" Sensors 20, no. 20: 5857. https://doi.org/10.3390/s20205857