Microfluidics in Gas Sensing and Artificial Olfaction
Abstract
:1. Introduction
2. Microfluidic Gas Sensing Devices Based on Electrical Transduction
2.1. Coupling of Micro-GC (μGC) and MOS Sensors
2.2. Other Approaches Using MOS Sensors
2.3. Approaches not Using MOS Sensors
3. Microfluidic Gas Sensing Devices Using Optical Transduction
4. Other Approaches
5. Conclusions and Future Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Shahzad, M.; Razzak, S.A.; Hossain, M.M. Catalytic oxidation of volatile organic compounds (VOCs)—A review. Atmos. Environ. 2016, 140, 117–134. [Google Scholar] [CrossRef]
- Zhou, C.; Zhan, Y.; Chen, S.; Xia, M.; Ronda, C.; Sun, M.; Chen, H.; Shen, X. Combined effects of temperature and humidity on indoor VOCs pollution: Intercity comparison. Build. Environ. 2017, 121, 26–34. [Google Scholar] [CrossRef]
- Kampa, M.; Castanas, E. Human health effects of air pollution. Environ. Pollut. 2008, 151, 362–367. [Google Scholar] [CrossRef] [PubMed]
- Palma, S.I.C.J.; Traguedo, A.P.; Porteira, A.R.; Frias, M.J.; Gamboa, H.; Roque, A.C.A. Machine learning for the meta-analyses of microbial pathogens’ volatile signatures. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nakhleh, M.K.; Amal, H.; Jeries, R.; Broza, Y.Y.; Aboud, M.; Gharra, A.; Ivgi, H.; Khatib, S.; Badarneh, S.; Har-Shai, L.; et al. Diagnosis and Classification of 17 Diseases from 1404 Subjects via Pattern Analysis of Exhaled Molecules. ACS Nano 2016, 11, 112–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Das, S.; Pal, M. Review—Non-Invasive Monitoring of Human Health by Exhaled Breath Analysis: A Comprehensive Review. J. Electrochem. Soc. 2020, 167, 037562. [Google Scholar] [CrossRef]
- Semeano, A.T.S.; Maffei, D.F.; Palma, S.; Li, R.W.C.; Franco, B.D.G.M.; Roque, A.C.A.; Gruber, J. Tilapia fish microbial spoilage monitored by a single optical gas sensor. Food Control 2018, 89, 72–76. [Google Scholar] [CrossRef]
- Rusinek, R.; Siger, A.; Gawrysiak-Witulska, M.; Rokosik, E.; Malaga-Toboła, U.; Gancarz, M. Application of an electronic nose for determination of pre-pressing treatment of rapeseed based on the analysis of volatile compounds contained in pressed oil. Int. J. Food Sci. Technol. 2020, 55, 2161–2170. [Google Scholar] [CrossRef]
- Rusinek, R.; Jelen, H.; Malaga-Tobola, U.; Molenda, M.; Gancarz, M. Influence of changes in the level of volatile compounds emitted during rapeseed quality degradation on the reaction of MOS type sensor-array. Sensors 2020, 20, 3135. [Google Scholar] [CrossRef]
- Martínez-García, R.; Moreno, J.; Bellincontro, A.; Centioni, L.; Puig-Pujol, A.; Peinado, R.A.; Mauricio, J.C.; García-Martínez, T. Using an electronic nose and volatilome analysis to differentiate sparkling wines obtained under different conditions of temperature, ageing time and yeast formats. Food Chem. 2021, 334, 127574. [Google Scholar] [CrossRef]
- Qian, K.; Bao, Y.; Zhu, J.; Wang, J.; Wei, Z. Development of a portable electronic nose based on a hybrid filter-wrapper method for identifying the Chinese dry-cured ham of different grades. J. Food Eng. 2021, 290, 110250. [Google Scholar] [CrossRef]
- Rezende, G.C.; Brandner, J.J.; Le Calvé, S.; Brandner, J.J.; Newport, D. Micro Milled Microfluidic Photoionization Detector for Volatile Organic Compounds. Micromachines 2019, 10, 228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbosa, A.J.M.; Oliveira, A.R.; Roque, A.C.A. Protein- and Peptide-Based Biosensors in Artificial Olfaction. Trends Biotechnol. 2018, 36, 1244–1258. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paknahad, M.; Ghafarinia, V.; Hossein-Babaei, F. A microfluidic gas analyzer for selective detection of biomarker gases. In Proceedings of the 2012 IEEE Sensors Applications Symposium, SAS 2012-Proceedings, Brescia, Italy, 7–9 February 2012; pp. 1–5. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Ghafarinia, V. Gas analysis by monitoring molecular diffusion in a microfluidic channel. Anal. Chem. 2010, 82, 8349–8355. [Google Scholar] [CrossRef]
- Hossein-Babaei, F.; Zare, A.H.; Hooshyar Zare, A. The selective flow of volatile organic compounds in conductive polymer-coated microchannels. Nat. Publ. Gr. 2017, 7, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Paknahad, M.; Bachhal, J.S.; Ahmadi, A.; Hoorfar, M. Characterization of channel coating and dimensions of microfluidic-based gas detectors. Sens. Actuators B Chem. 2017, 241, 55–64. [Google Scholar] [CrossRef]
- Ueno, Y.; Horiuchi, T.; Morimoto, T.; Niwa, O. Microfluidic device for airborne BTEX detection. Anal. Chem. 2001, 73, 4688–4693. [Google Scholar] [CrossRef]
- Zhu, L.; Meier, D.; Boger, Z.; Montgomery, C.; Semancik, S.; DeVoe, D.L. Integrated microfluidic gas sensor for detection of volatile organic compounds in water. Sens. Actuators B Chem. 2007, 121, 679–688. [Google Scholar] [CrossRef]
- Hussain, A.; Semeano, A.T.S.; Palma, S.I.C.J.; Pina, A.S.; Almeida, J.; Medrado, B.F.; Pádua, A.C.C.S.; Carvalho, A.L.; Dionísio, M.; Li, R.W.C.; et al. Tunable Gas Sensing Gels by Cooperative Assembly. Adv. Funct. Mater. 2017, 27, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Potyrailo, R.A.; Bonam, R.K.; Hartley, J.G.; Starkey, T.A.; Vukusic, P.; Vasudev, M.; Bunning, T.; Naik, R.R.; Tang, Z.; Palacios, M.A.; et al. Towards outperforming conventional sensor arrays with fabricated individual photonic vapour sensors inspired by Morpho butterflies. Nat. Commun. 2015, 6, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Persaud, K.C. Towards bionic noses. Sens. Rev. 2017, 37, 165–171. [Google Scholar] [CrossRef]
- Imam, N.; Cleland, T.A. Rapid online learning and robust recall in a neuromorphic olfactory circuit. Nat. Mach. Intell. 2020, 2, 181–191. [Google Scholar] [CrossRef]
- van den Broek, J.; Abegg, S.; Pratsinis, S.E.; Güntner, A.T. Highly selective detection of methanol over ethanol by a handheld gas sensor. Nat. Commun. 2019, 10, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Tiele, A.; Wicaksono, A.; Ayyala, S.K.; Covington, J.A. Development of a compact, iot-enabled electronic nose for breath analysis. Electronics 2020, 9, 84. [Google Scholar] [CrossRef] [Green Version]
- Ueno, Y.; Horiuchi, T.; Niwa, O. Air-cooled cold trap channel integrated in a microfluidic device for monitoring airborne BTEX with an improved detection limit. Anal. Chem. 2002, 74, 1712–1717. [Google Scholar] [CrossRef]
- Ueno, Y.; Horiuchi, T.; Tomita, M.; Niwa, O.; Zhou, H.-S.; Yamada, T.; Honma, I. Separate detection of BTX mixture gas by a microfluidic device using a function of nanosized pores of mesoporous silica adsorbent. Anal. Chem. 2002, 74, 5257–5262. [Google Scholar] [CrossRef]
- Ueno, Y.; Horiuchi, T.; Niwa, O.; Zhou, H.-S.; Yamada, T.; Honma, I. Portable automatic BTX measurement system with microfluidic device using mesoporous silicate adsorbent with nano-sized pores. Sens. Actuators B Chem. 2003, 95, 282–286. [Google Scholar] [CrossRef]
- Horiuchi, T.; Ueno, Y.; Camou, S.; Haga, T.; Tate, A. Portable aromatic VOC gas sensor for onsite continuous air monitoring with 10-ppb benzene detection capability. NTT Tech. Rev. 2006, 4, 30–37. [Google Scholar]
- Ueno, Y.; Tate, A.; Niwa, O.; Zhou, H.S.; Yamada, T.; Honma, I. High benzene selectivity of mesoporous silicate for BTX gas sensing microfluidic devices. Anal. Bioanal. Chem. 2005, 382, 804–809. [Google Scholar] [CrossRef]
- Covington, J.A.; Gardner, J.W.; Hamilton, A.; Pearce, T.C.; Tan, S.L. Towards a truly biomimetic olfactory microsystem: An artificial olfactory mucosa. IET Nanobiotechnol. 2007, 1, 15. [Google Scholar] [CrossRef]
- Sánchez-Montañés, M.A.; Gardner, J.W.; Pearce, T.C. Spatio-temporal information in an artificial olfactory mucosa. Proc. R. Soc. A Math. Phys. Eng. Sci. 2008, 464, 1057–1077. [Google Scholar] [CrossRef] [Green Version]
- Dini, F.; Filippini, D.; Paolesse, R.; D’Amico, A.; Lundström, I.; Di Natale, C. Polymers with embedded chemical indicators as an artificial olfactory mucosa. Analyst 2010, 135, 1245–1252. [Google Scholar] [CrossRef] [PubMed]
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Castillo-leo, J. Lab-on-a-Chip Devices and Micro-Total Analysis Systems. Lab-on-a-Chip Devices Micro-Total Anal. Syst. 2014. [Google Scholar] [CrossRef]
- Vollmer, A.P.; Probstein, R.F.; Gilbert, R.; Thorsen, T. Development of an integrated microfluidic platform for dynamic oxygen sensing and delivery in a flowing medium. Lab Chip 2005, 5, 1059–1066. [Google Scholar] [CrossRef] [Green Version]
- Warden, A.C.; Trowell, S.C.; Gel, M. A Miniature Gas Sampling Interface with Open Microfluidic Channels: Characterization of Gas-to-Liquid Extraction Efficiency of Volatile Organic Compounds. Micromachines 2019, 10, 486. [Google Scholar] [CrossRef] [Green Version]
- Lee, S.H.; Lim, J.H.; Park, J.; Hong, S.; Park, T.H. Bioelectronic nose combined with a microfluidic system for the detection of gaseous trimethylamine. Biosens. Bioelectron. 2015, 71, 179–185. [Google Scholar] [CrossRef]
- Nazemi, H.; Joseph, A.; Park, J.; Emadi, A. Advanced micro-and nano-gas sensor technology: A review. Sensors 2019, 19, 1285. [Google Scholar] [CrossRef] [Green Version]
- Murata, T.; Rahardjo, A.; Fujiyama, Y.; Yamaga, T.; Hanada, M.; Yaegaki, K.; Miyazaki, H. Development of a Compact and Simple Gas Chromatography for Oral Malodor Measurement. J. Periodontol. 2006, 77, 1142–1147. [Google Scholar] [CrossRef]
- Regmi, B.P.; Agah, M. Micro Gas Chromatography: An Overview of Critical Components and Their Integration. Anal. Chem. 2018, 90, 13133–13150. [Google Scholar] [CrossRef] [Green Version]
- Akbar, M.; Restaino, M.; Agah, M. Chip-scale gas chromatography: From injection through detection. Microsyst. Nanoeng. 2015, 1, 1. [Google Scholar] [CrossRef] [Green Version]
- Narayanan, S.; Alfeeli, B.; Agah, M. A micro gas chromatography chip with an embedded non-cascaded thermal conductivity detector. In Proceedings of the Procedia Engineering, Linz, Austria, 5–8 September 2010; Volume 5, pp. 29–32. [Google Scholar] [CrossRef] [Green Version]
- Hossein-Babaei, F.; Hooshyar Zare, A.; Ghafarinia, V.; Erfantalab, S. Identifying volatile organic compounds by determining their diffusion and surface adsorption parameters in microfluidic channels. Sens. Actuators B Chem. 2015, 220, 607–613. [Google Scholar] [CrossRef]
- Janfaza, S.; Kim, E.; O’Brien, A.; Najjaran, H.; Nikkhah, M.; Alizadeh, T.; Hoorfar, M. A Nanostructured Microfluidic Artificial Olfaction for Organic Vapors Recognition. Sci. Rep. 2019, 9, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Martini-Laithier, V.; Graur, I.; Bernardini, S.; Aguir, K.; Perrier, P.; Bendahan, M. Ammonia detection by a novel Pyrex microsystem based on thermal creep phenomenon. Sens. Actuators B Chem. 2014, 192, 714–719. [Google Scholar] [CrossRef] [Green Version]
- Martini, V.; Bernardini, S.; Bendahan, M.; Aguir, K.; Perrier, P.; Graur, I. Microfluidic gas sensor with integrated pumping system. Sens. Actuators B Chem. 2012, 170, 45–50. [Google Scholar] [CrossRef]
- Yang, D.; Kang, K.; Kim, D.; Li, Z.; Park, I. Fabrication of heterogeneous nanomaterial array by programmable heating and chemical supply within microfluidic platform towards multiplexed gas sensing application. Sci. Rep. 2015, 5, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Kim, M.G.; Alrowais, H.; Kim, C.; Yeon, P.; Ghovanloo, M.; Brand, O. All-soft, battery-free, and wireless chemical sensing platform based on liquid metal for liquid- and gas-phase VOC detection. Lab Chip 2017, 17, 2323–2329. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.H.; Oh, E.H.; Park, T.H. Cell-based microfluidic platform for mimicking human olfactory system. Biosens. Bioelectron. 2015, 74, 554–561. [Google Scholar] [CrossRef]
- Esteves, C.; Santos, G.M.C.; Alves, C.; Palma, S.I.C.J.; Porteira, A.R.; Filho, J.; Costa, H.M.A.; Alves, V.D.; Morais Faustino, B.M.; Ferreira, I.; et al. Effect of film thickness in gelatin hybrid gels for artificial olfaction. Mater. Today Bio 2019, 1. [Google Scholar] [CrossRef]
- Esteves, C.; Ramou, E.; Porteira, A.R.P.; Moura Barbosa, A.J.; Roque, A.C.A. Seeing the Unseen: The Role of Liquid Crystals in Gas-Sensing Technologies. Adv. Opt. Mater. 2020, 8. [Google Scholar] [CrossRef]
- Sackmann, E.K.; Fulton, A.L.; Beebe, D.J. The present and future role of microfluidics in biomedical research. Nature 2014, 507, 181–189. [Google Scholar] [CrossRef] [PubMed]
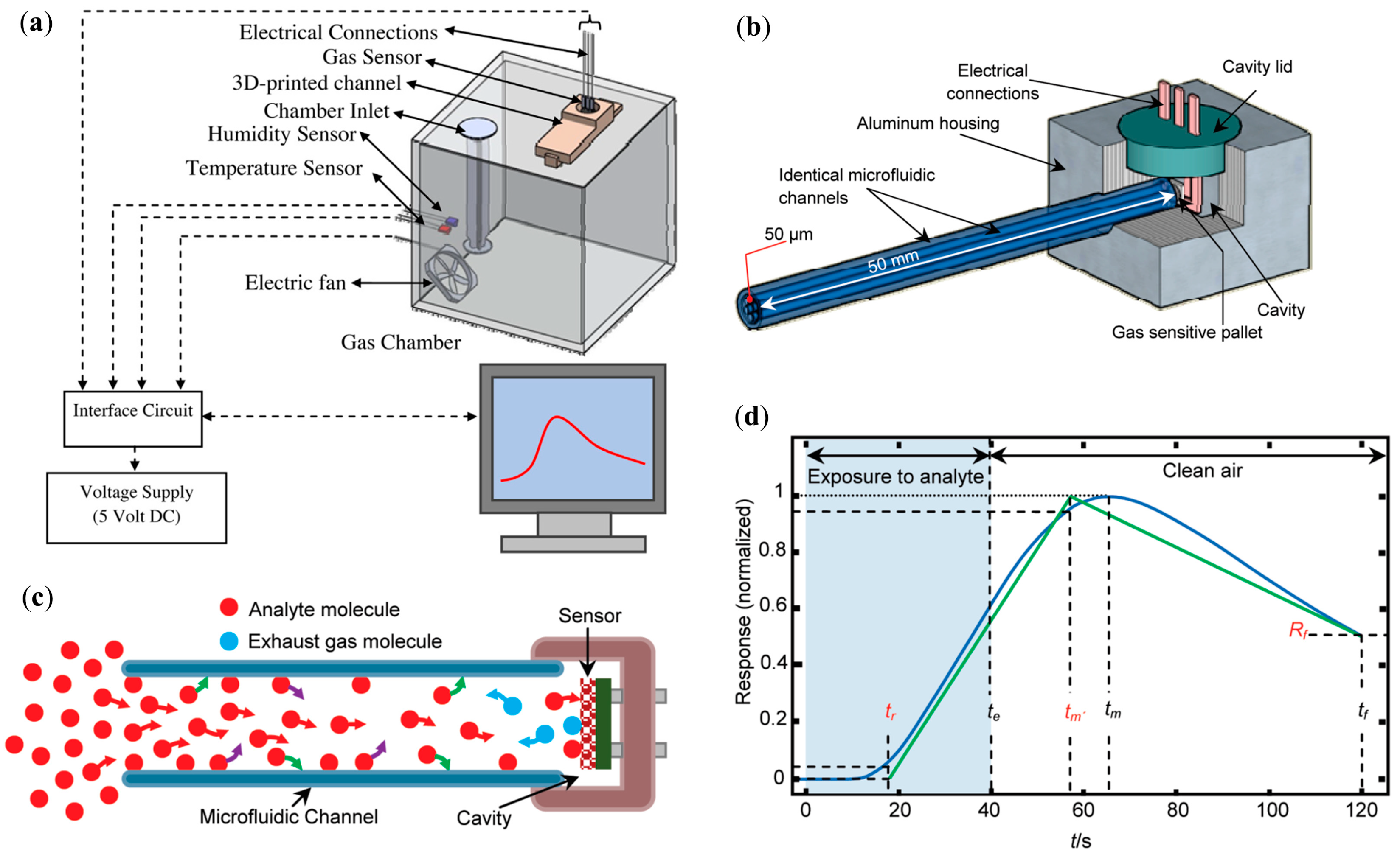
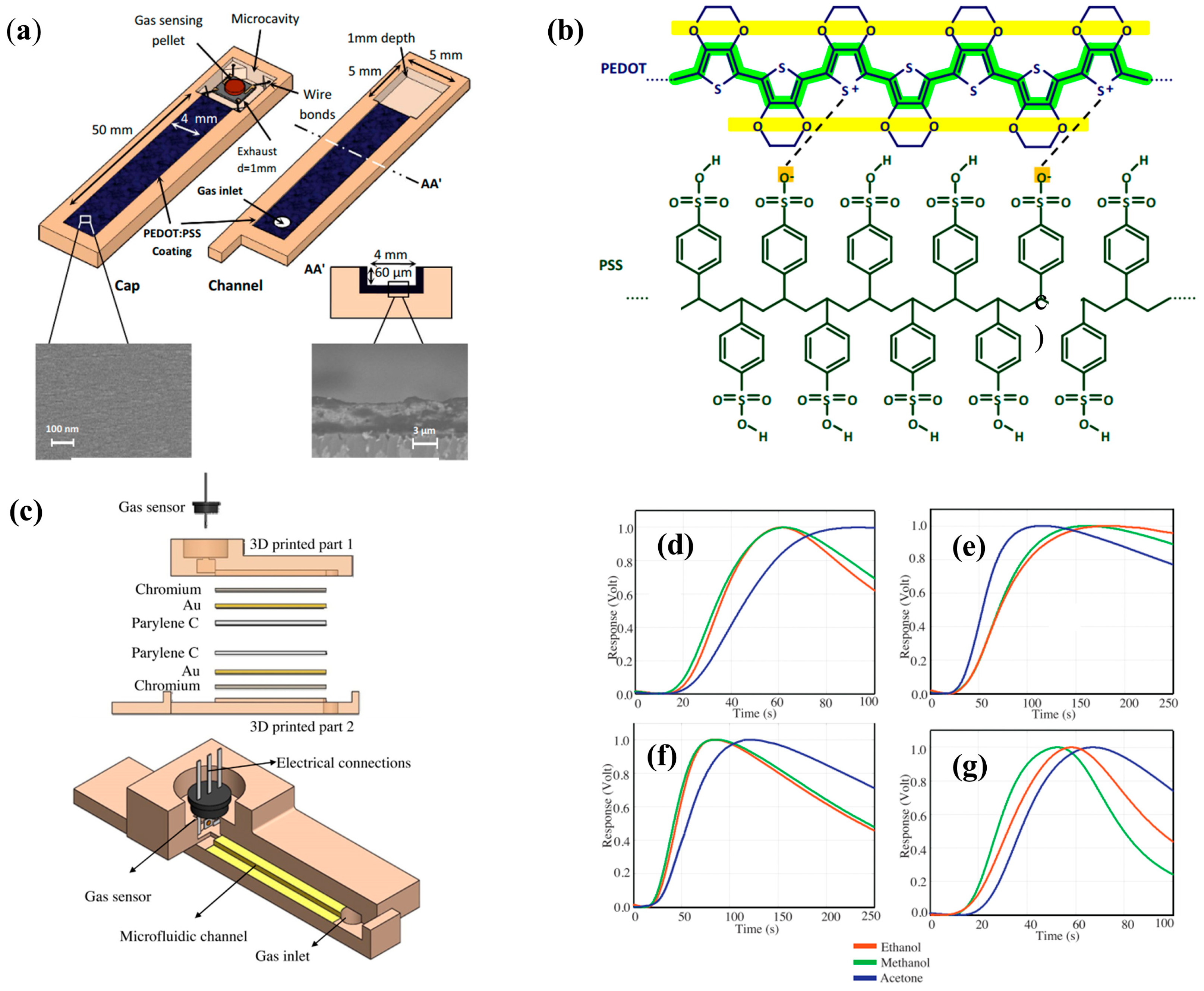
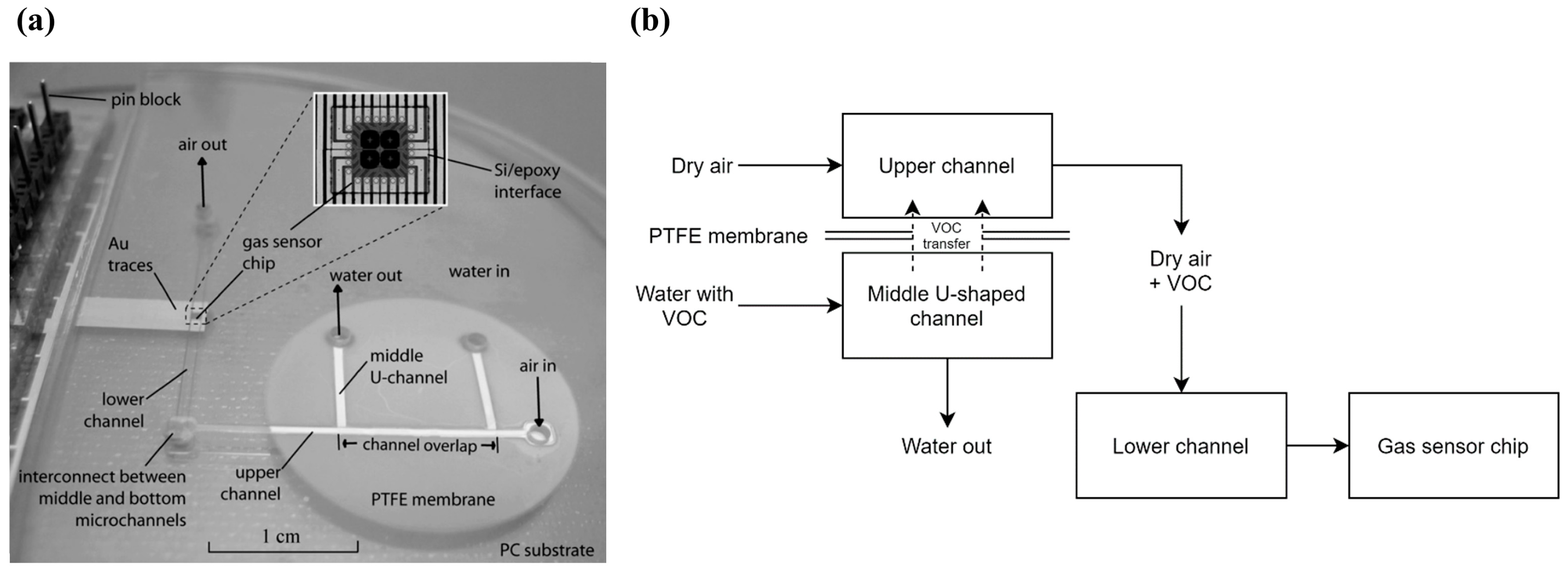

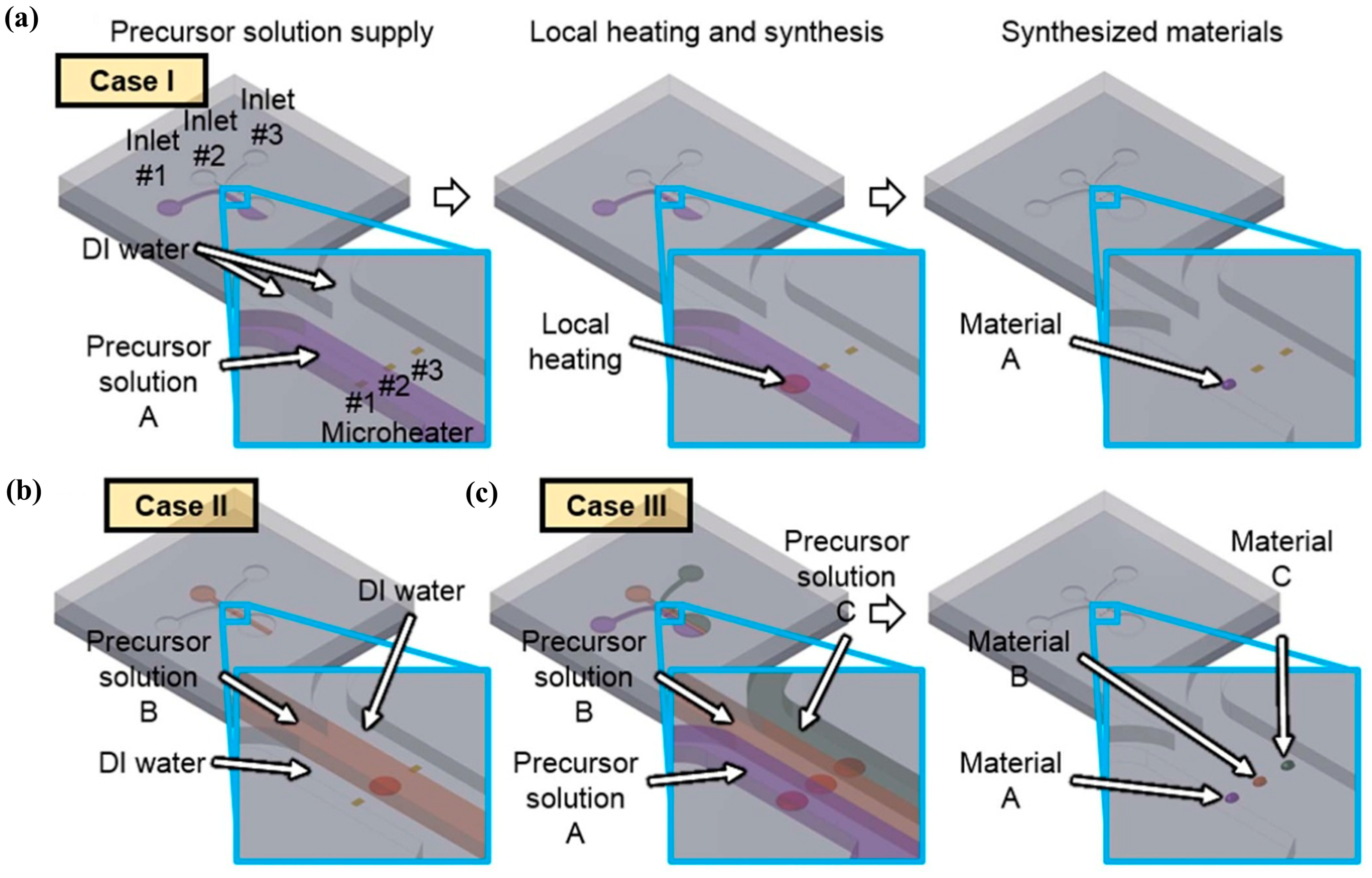



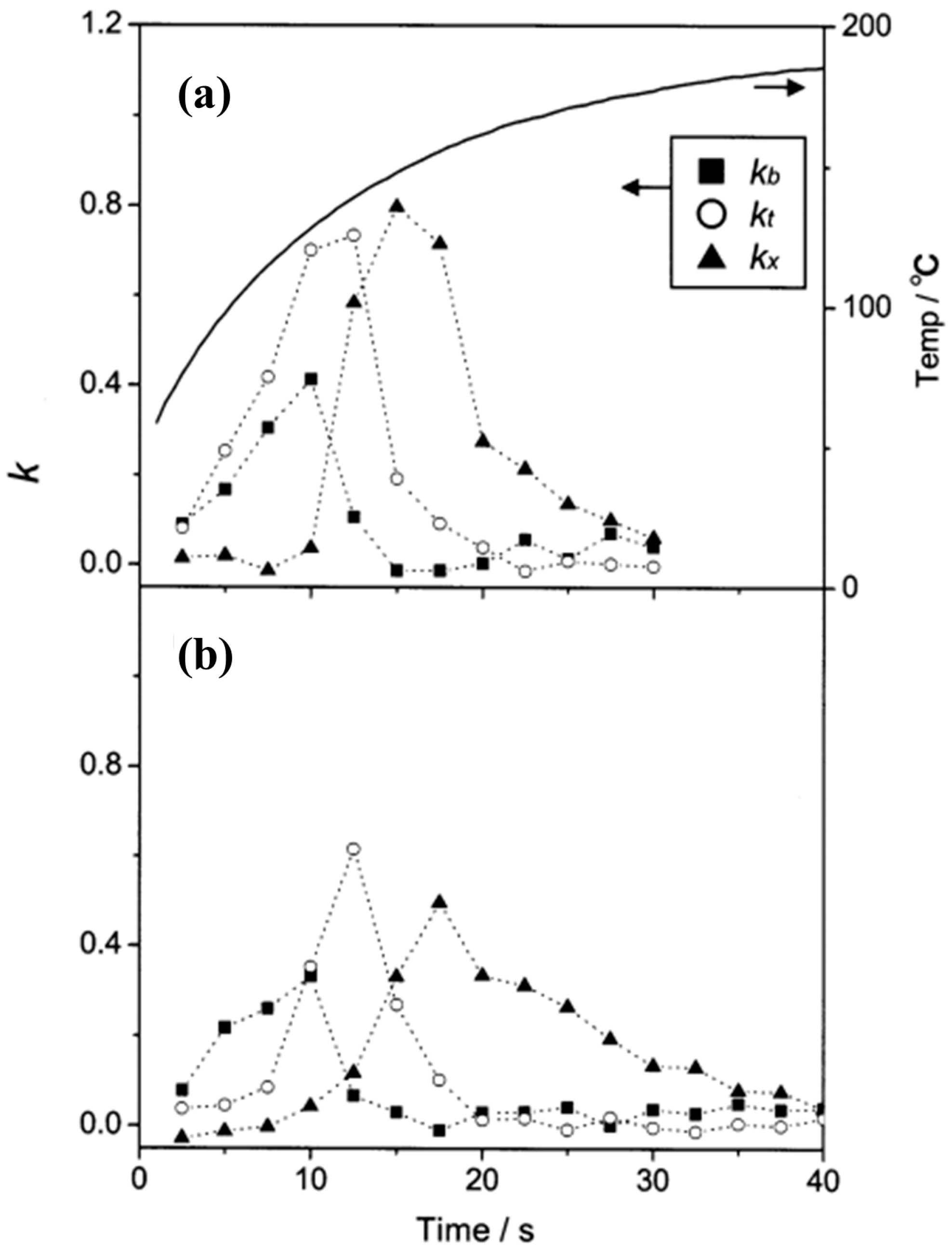
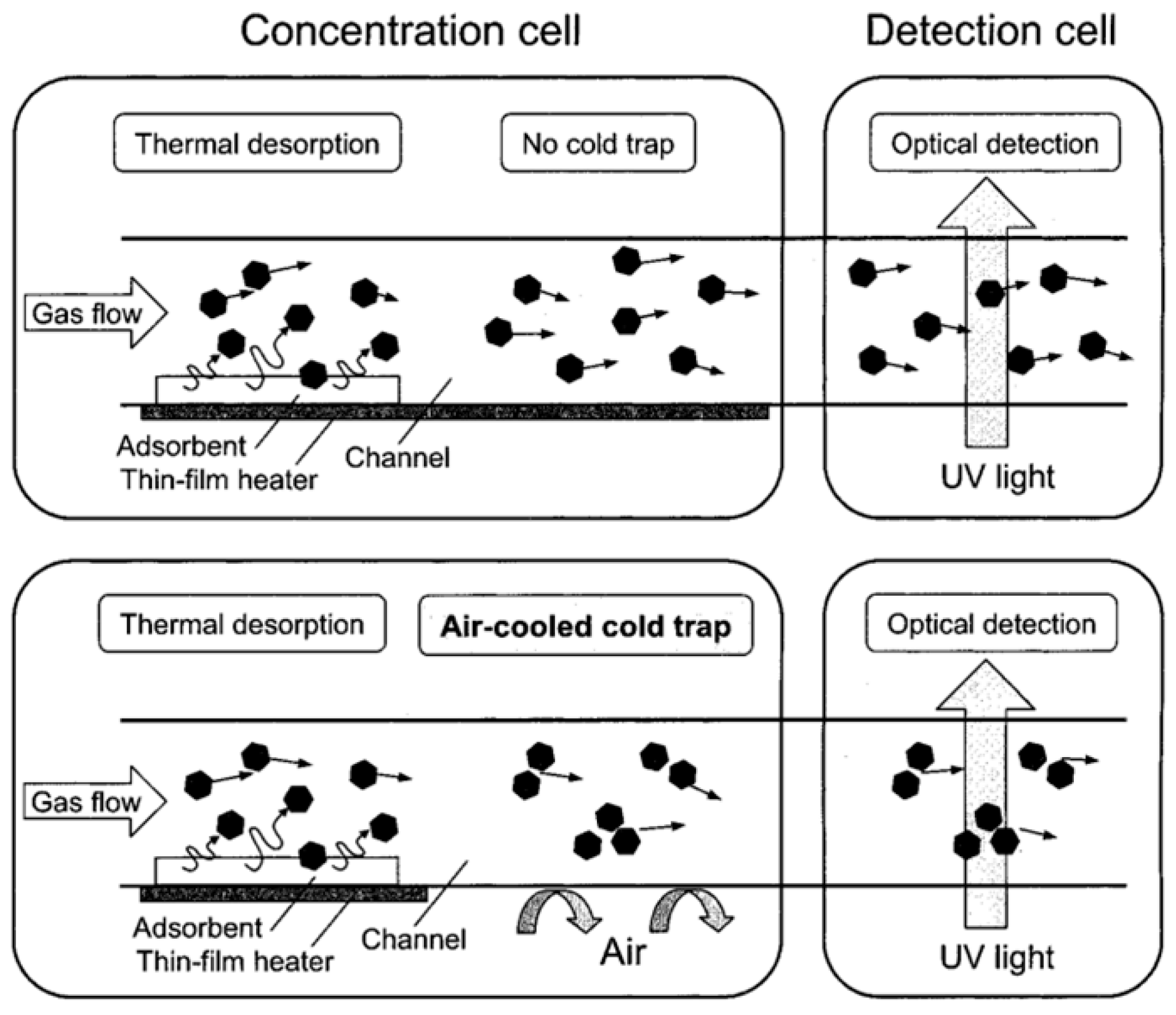

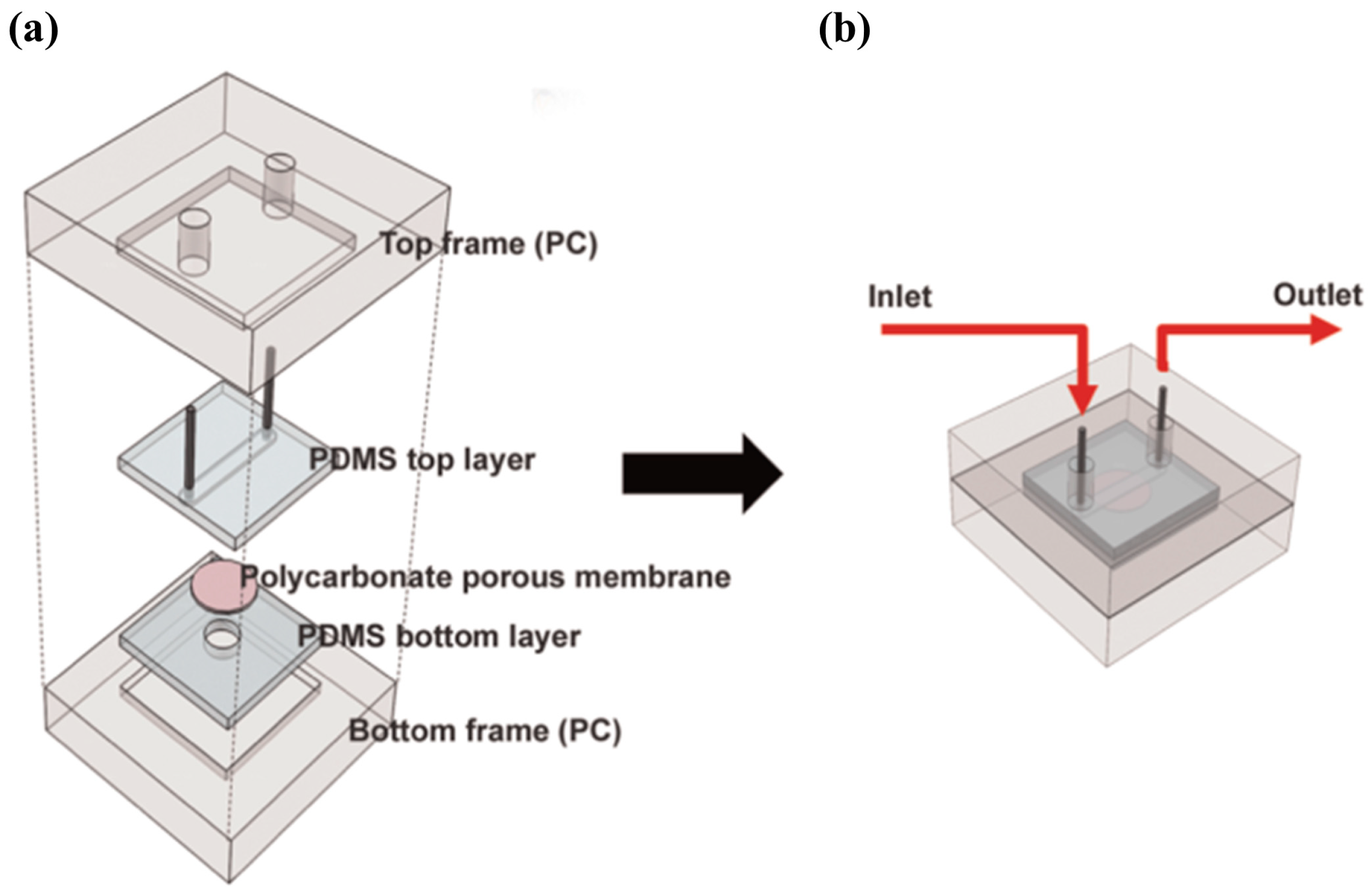


| μGC Column Coating | Sensing Material | Analytes | Range of Detection; LOD | Refs |
|---|---|---|---|---|
| No coating | Tin oxide (SnO2) | Hydrogen, carbon monoxide, argon, oxygen, methanol, ethanol, isopropanol, 1-propanol, tert-butanol, 2-butanol, iso-butanol, 1-butanol, methane, n-butane, n-pentane, acetone, butanone, 2-pentanone, methyl isobutyl ketone, chloroform, toluene, benzene, carbon tetrachloride, and ammonia | 900–1100 ppm; - | [15] |
| No coating | Tin oxide (SnO2) | Ethanol | 500–1000 ppm; 10 ppm | [44] |
| Methanol, iso-propanol, 1-propanol, tert-butanol, iso-butanol, 2-butanol, 1-butanol. | 500–1000 ppm; - | |||
| PEDOT:PSS | Tin oxide (SnO2) | H2, CO, methanol, ethanol, 2-propanol, iso-butanol, acetone, 2-pentanone, hexane, benzene | 1000–10,000 ppm; - | [16] |
| No coating | Tin oxide (SnO2) | Acetone, hydrogen, ethanol, and benzene | 250–3000 ppm; - | [14] |
| Combinations of Cr, Au, Cu, and Parylene C | Tin oxide (SnO2) | Methanol, ethanol, 2-pentanol, acetone, 2-butanone, 2-pentanone. | 250–4000 ppm; - | [17] |
| MIP NPs | Tin oxide (SnO2) | Acetone, ethanol, methanol, butanone, acetonitrile, toluene | 200–4000 ppm; - | [45] |
| System Architecture | Sensing Material | Analytes Tested | Range of Detection; LOD | Measurement | State of Development of the Sensor | Refs |
|---|---|---|---|---|---|---|
| 2-phase microfluidic water monitoring system | SnO2 sensing film | Methanol | 0–100 ppm; 1 ppm | Conductance | Research level | [19] |
| Toluene | 0–100 ppm; 10 ppm | |||||
| 1,2-dichloroethane | 0–1000 ppm; 100 ppm | |||||
| Pyrex substrate coupled with a silicon cover (etched microchannel) | Sensitive WO3 film | Ammonia | 10–100 ppm; | Resistance | Research level | [46,47] |
| Parallel supply of multiple precursor chemicals within microfluidic channels | ZnO/CuO hybrid nanostructures, CuO nanospikes, and ZnO nanowires | NO2 | 0.1–20 ppm; 0.1 ppm | Resistance | Research level | [48] |
| CO | 20–1000 ppm; 20 ppm |
| System Improvements | Adsorbent Material | Analytes | LOD | LOD Improvement | Refs |
|---|---|---|---|---|---|
| - | Amorphous silicon dioxide powder (SDP) | Toluene | 4 ppm | - | [18] |
| - | Mesoporous silicate powder (SBA-15) | Benzene | 1 ppm | 4-fold | [30] |
| Mesoporous silicate powder (SBA-16) | 100 ppb | 40-fold | |||
| Optimized gas transfer system, increase of signal-to-noise ratio | Mesoporous silicate powder (SBA-16) | Benzene | 10 ppb | 400-fold | [29] |
| Separate detection of the components of BTEX mixture gas (improvement of thermal desorption characteristics) | Mesoporous silicate powder (SBA-15) | Mixture of toluene, benzene, and o-xylene | 1 ppm | 4-fold | [27] |
| Integration of a cold trap (CT) | Amorphous silicon dioxide powder (SDP) | Toluene | 0.05 ppm | 80-fold | [26] |
| Integration of a cold trap (CT), joined the two cells | Mesoporous silicate powder (SBA-15) | Toluene | 10 ppb | 400-fold | [28] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rebordão, G.; Palma, S.I.C.J.; Roque, A.C.A. Microfluidics in Gas Sensing and Artificial Olfaction. Sensors 2020, 20, 5742. https://doi.org/10.3390/s20205742
Rebordão G, Palma SICJ, Roque ACA. Microfluidics in Gas Sensing and Artificial Olfaction. Sensors. 2020; 20(20):5742. https://doi.org/10.3390/s20205742
Chicago/Turabian StyleRebordão, Guilherme, Susana I. C. J. Palma, and Ana C. A. Roque. 2020. "Microfluidics in Gas Sensing and Artificial Olfaction" Sensors 20, no. 20: 5742. https://doi.org/10.3390/s20205742
APA StyleRebordão, G., Palma, S. I. C. J., & Roque, A. C. A. (2020). Microfluidics in Gas Sensing and Artificial Olfaction. Sensors, 20(20), 5742. https://doi.org/10.3390/s20205742






