Demonstrating the Use of Optical Fibres in Biomedical Sensing: A Collaborative Approach for Engagement and Education
Abstract
1. Introduction
2. Review: Optical Fibres and Lung Disease Diagnostics
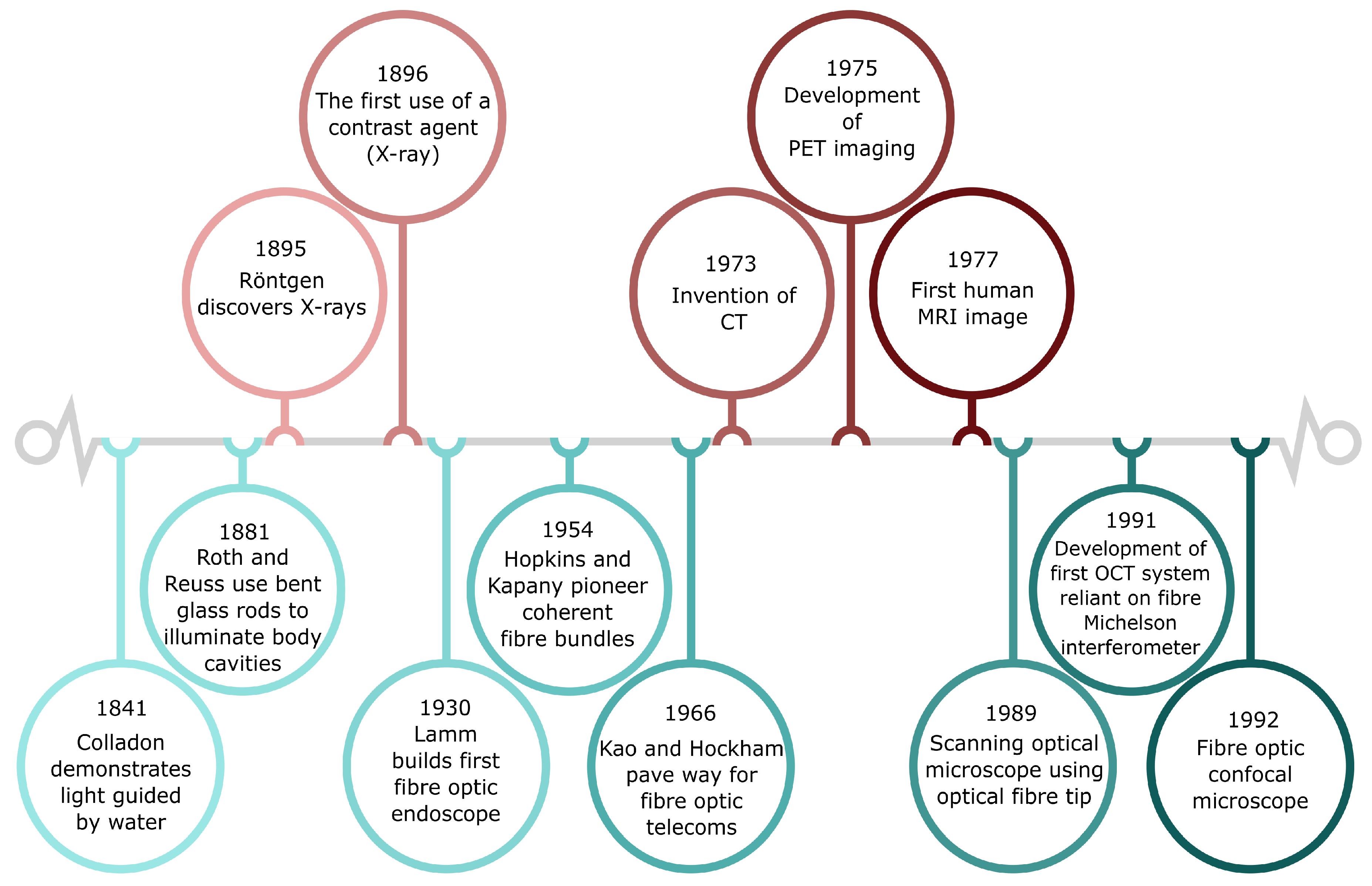
2.1. Optical Fibres: From Telecommunications to Biomedical Imaging and Sensing
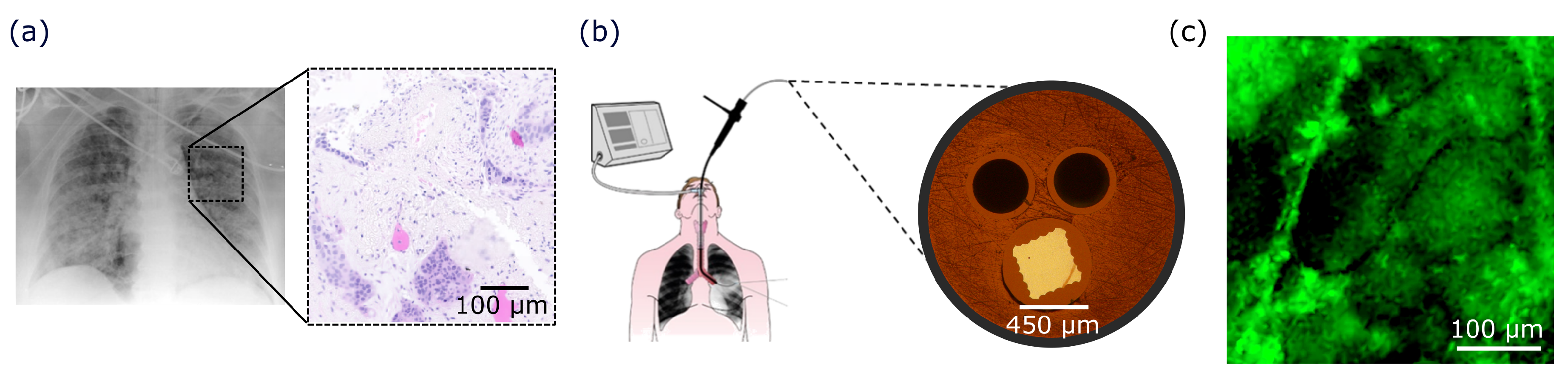
2.2. Diagnostic Challenges of Pulmonary Diseases
3. Translation from Biomedical Research to Public Engagement
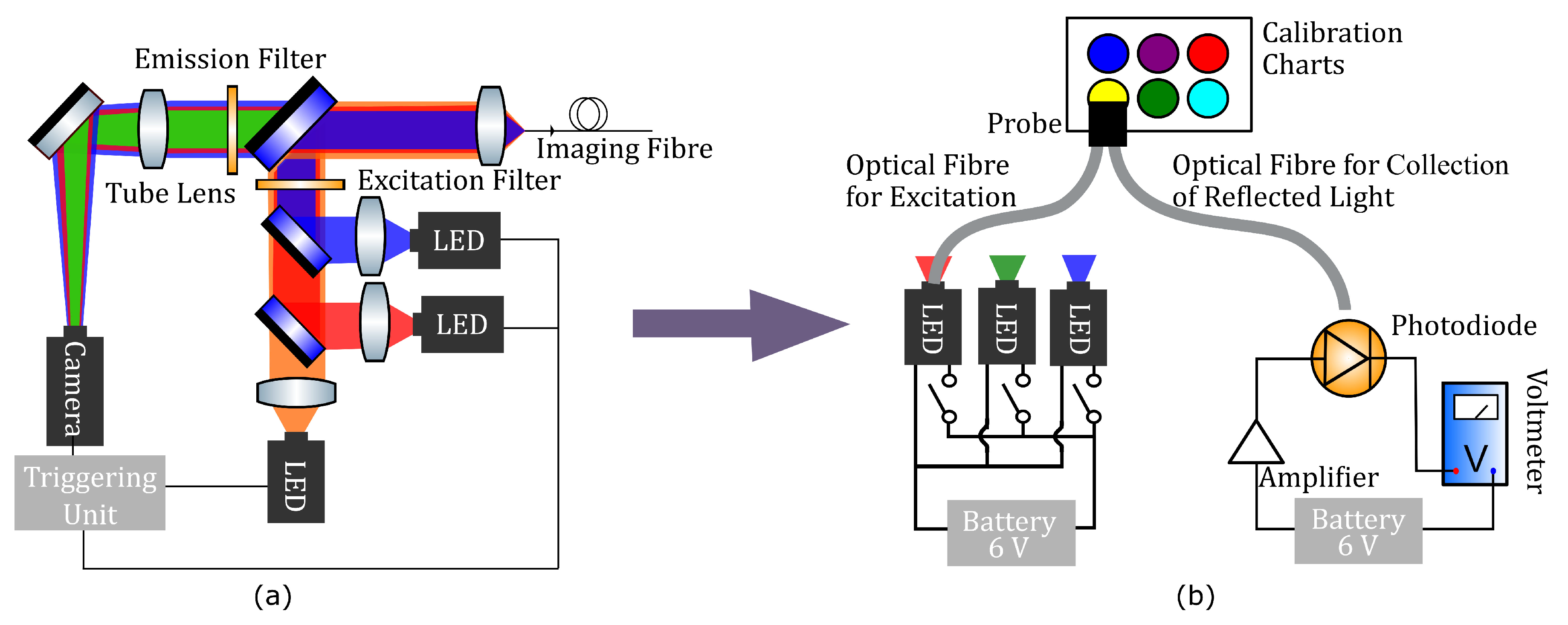
4. Materials and Methods
5. Demonstrating the Use of the OEM Educational Tool
5.1. Using the Education Tool in a Classroom Setting
5.2. Using the Educational Tool in Shorter Engagement Activities
6. Conclusions
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Burney, P.G.; Patel, J.; Newson, R.; Minelli, C.; Naghavi, M. Global and regional trends in COPD mortality, 1990–2010. Eur. Respir. J. 2015, 45, 1239–1247. [Google Scholar] [CrossRef] [PubMed]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Global Tuberculosis Report 2018; WHO: Geneva, Switzerland, 2018. [Google Scholar]
- Owen, R.; Stilgoe, J.; Macnaghten, P.; Gorman, M.; Fisher, E.; Guston, D. A Framework for Responsible Innovation. In Responsible Innovation: Managing the Responsible Emergence of Science and Innovation in Society; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2013. [Google Scholar] [CrossRef]
- Grand, A.; Davies, G.; Holliman, R.; Adams, A. Mapping Public Engagement with Research in a UK University. PLOS ONE 2015, 10, 1–19. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Marris, C.; Rose, N. Open Engagement: Exploring Public Participation in the Biosciences. PLOS Biol. 2010, 8, 1–2. [Google Scholar] [CrossRef] [PubMed]
- Mohr, A.; Raman, S. Representing the Public in Public Engagement: The Case of the 2008 UK Stem Cell Dialogue. PLOS Biol. 2012, 10, 1–4. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Tytler, R.; Osborne, J.; Williams, G.; Tytler, K.; Cripps Clark, J. Opening Up Pathways: Engagement in STEM Across the Primary-Secondary School Transition 2008; DEEWR: Canberra, Australia, 2008.
- Jimenez, M.; Bridle, H.L. Angry pathogens, how to get rid of them: introducing microfluidics for waterborne pathogen separation to children. Lab Chip 2015, 15, 947–957. [Google Scholar] [CrossRef]
- Wicks, L.C.; Cairns, G.S.; Melnyk, J.; Bryce, S.; Duncan, R.R.; Dalgarno, P.A. EnLightenment: High resolution smartphone microscopy as an educational and public engagement platform. Wellcome Open Res. 2018, 2, 107. [Google Scholar] [CrossRef]
- Esfahani, M.M.N.; Tarn, M.D.; Choudhury, T.A.; Hewitt, L.C.; Mayo, A.J.; Rubin, T.A.; Waller, M.R.; Christensen, M.G.; Dawson, A.; Pamme, N. Lab-on-a-chip workshop activities for secondary school students. Biomicrofluidics 2016, 10, 011301. [Google Scholar] [CrossRef]
- Rackus, D.G.; Riedel-Kruse, I.H.; Pamme, N. “Learning on a chip:” Microfluidics for formal and informal science education. Biomicrofluidics 2019, 13, 041501. [Google Scholar] [CrossRef]
- Kim, H.; Gerber, L.C.; Chiu, D.; Lee, S.A.; Cira, N.J.; Xia, S.Y.; Riedel-Kruse, I.H. LudusScope: Accessible Interactive Smartphone Microscopy for Life-Science Education. PLoS ONE 2016, 11, 1–16. [Google Scholar] [CrossRef]
- Cira, N.J.; Chung, A.M.; Denisin, A.K.; Rensi, S.; Sanchez, G.N.; Quake, S.R.; Riedel-Kruse, I.H. A Biotic Game Design Project for Integrated Life Science and Engineering Education. PLoS Biol. 2015, 13, 1–8. [Google Scholar] [CrossRef] [PubMed]
- Bridle, H.; Morton, J.; Cameron, P.; Desmulliez, M.P.Y.; Kersaudy-Kerhoas, M. Design of problem-based learning activities in the field of microfluidics for 12- to 13-year-old participants—Small Plumbing!: Empowering the next generation of microfluidic engineers. Microfluid. Nanofluid. 2016, 20, 103. [Google Scholar] [CrossRef]
- Hemling, M.; Crooks, J.A.; Oliver, P.M.; Brenner, K.; Gilbertson, J.; Lisensky, G.C.; Weibel, D.B. Microfluidics for High School Chemistry Students. J. Chem. Educ. 2014, 91, 112–115. [Google Scholar] [CrossRef] [PubMed]
- Wong, N.H.L.; Tong, A.S.K.; Posner, M.T. Modular and extensible lesson on optical fibre communication for youths. Phys. Educ. 2019, 54, 055004. [Google Scholar] [CrossRef]
- Colladon, J.D. The Colladon Fountain. Sci. Am. 1884, 51, 359–360. [Google Scholar]
- Kao, K.; Hockham, G. Dielectric-fibre surface waveguides for optical frequencies. Proc. Inst. Electr. Eng. 1966, 113, 1151–1158. [Google Scholar] [CrossRef]
- NobelPrize.org. The Nobel Prize in Physics 2009. Available online: https://www.nobelprize.org/prizes/physics/2009/summary/ (accessed on 6 January 2020).
- Lamm, H. Biegsame optische Geräte. Zeitschrift für Instrumentenkunde 1930, 50, 579–581. [Google Scholar]
- Hopkins, H.H.; Kapany, N.S. A Flexible Fibrescope, using Static Scanning. Nature 1954, 173, 39–41. [Google Scholar] [CrossRef]
- Wallace, M.B.; Fockens, P. Probe-Based Confocal Laser Endomicroscopy. Gastroenterology 2009, 136, 1509–1513. [Google Scholar] [CrossRef]
- Flusberg, B.A.; Cocker, E.D.; Piyawattanametha, W.; Jung, J.C.; Cheung, E.L.M.; Schnitzer, M.J. Fiber-optic fluorescence imaging. Nat. Methods 2005, 2, 941–950. [Google Scholar] [CrossRef]
- Pan, Y.; Volkmer, J.P.; Mach, K.E.; Liu, J.J.; Rouse, R.V.; Sahoo, D.; Chang, T.C.; Van De Rijn, M.; Skinner, E.; Gambhir, S.S.; et al. Endoscopic molecular imaging of human bladder cancer using CD47 antibody. Mol. Imaging Biol. 2013, 15, 2–1590. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.; Zlatev, D.V.; Mach, K.E.; Bui, D.; Liu, J.J.; Rouse, R.V.; Harris, T.; Leppert, J.T.; Liao, J.C. Intraoperative Optical Biopsy during Robotic Assisted Radical Prostatectomy Using Confocal Endomicroscopy. J. Urol. 2016, 195, 1110–1117. [Google Scholar] [CrossRef] [PubMed]
- Perperidis, A.; Parker, H.E.; Karam-Eldaly, A.; Altmann, Y.; Dhaliwal, K.; Thomson, R.R.; Tanner, M.G.; McLaughlin, S. Characterization and modelling of inter-core coupling in coherent fiber bundles. Opt. Express 2017, 25, 11932. [Google Scholar] [CrossRef] [PubMed]
- Eldaly, A.K.; Altmann, Y.; Perperidis, A.; Krstajić, N.; Choudhary, T.R.; Dhaliwal, K.; McLaughlin, S. Deconvolution and Restoration of Optical Endomicroscopy Images. IEEE Trans. Comput. Imaging 2018, 4, 194–205. [Google Scholar] [CrossRef]
- Perperidis, A.; Dhaliwal, K.; McLaughlin, S.; Vercauteren, T. Image computing for fibre-bundle endomicroscopy: A review. arXiv 2018, arXiv:1809.00604. [Google Scholar] [CrossRef]
- Stone, J.M.; Wood, H.A.C.; Harrington, K.; Birks, T.A. Low index contrast imaging fibers. Opt. Lett. 2017, 42, 1484. [Google Scholar] [CrossRef]
- Ukil, A.; Braendle, H.; Krippner, P. Distributed Temperature Sensing: Review of Technology and Applications. IEEE Sens. J. 2012, 12, 885–892. [Google Scholar] [CrossRef]
- Dyer, S.D.; Tanner, M.G.; Baek, B.; Hadfield, R.H.; Nam, S.W. Analysis of a distributed fiber-optic temperature sensor using single-photon detectors. Opt. Express 2012, 20, 3456–3466. [Google Scholar] [CrossRef]
- Correia, R.; James, S.; Lee, S.W.; Morgan, S.P.; Korposh, S. Biomedical application of optical fibre sensors. J. Opt. 2018, 20, 073003. [Google Scholar] [CrossRef]
- Poeggel, S.; Tosi, D.; Duraibabu, D.; Leen, G.; McGrath, D.; Lewis, E. Optical Fibre Pressure Sensors in Medical Applications. Sensors 2015, 15, 17115–17148. [Google Scholar] [CrossRef]
- Coscelli, E.; Sozzi, M.; Poli, F.; Passaro, D.; Cucinotta, A.; Selleri, S.; Corradini, R.; Marchelli, R. Toward A Highly Specific DNA Biosensor: PNA-Modified Suspended-Core Photonic Crystal Fibers. IEEE J. Sel. Top. Quantum Electron. 2010, 16, 967–972. [Google Scholar] [CrossRef]
- Marazuela, M.; Moreno-Bondi, M. Fiber-optic biosensors—An overview. Anal. Bioanal. Chem. 2002, 372, 664–682. [Google Scholar] [CrossRef] [PubMed]
- Queirós, R.; Gouveia, C.; Fernandes, J.; Jorge, P. Evanescent wave DNA-aptamer biosensor based on long period gratings for the specific recognition of E. coli outer membrane proteins. Biosens. Bioelectron. 2014, 62, 227–233. [Google Scholar] [CrossRef] [PubMed]
- Choudhary, T.R.; Tanner, M.G.; Megia-Fernandez, A.; Harrington, K.; Wood, H.A.; Marshall, A.; Zhu, P.; Chankeshwara, S.V.; Choudhury, D.; Monro, G.; et al. High fidelity fibre-based physiological sensing deep in tissue. Sci. Rep. 2019, 9, 7713. [Google Scholar] [CrossRef]
- Ehrlich, K.; Kufcsák, A.; McAughtrie, S.; Fleming, H.; Krstajić, N.; Campbell, C.J.; Henderson, R.K.; Dhaliwal, K.; Thomson, R.R.; Tanner, M.G. pH sensing through a single optical fibre using SERS and CMOS SPAD line arrays. Opt. Express 2017, 25, 30976–30986. [Google Scholar] [CrossRef]
- Tosi, D.; Poeggel, S.; Iordachita, I.; Schena, E. Fiber Optic Sensors for Biomedical Applications. In Opto-Mechanical Fiber Optic Sensors; Elsevier: Amsterdam, The Netherlands, 2018; pp. 301–333. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S. Fiber-Optic Chemical Sensors and Biosensors (2008–2012). Anal. Chem. 2013, 85, 487–508. [Google Scholar] [CrossRef]
- Massaroni, C.; Saccomandi, P.; Schena, E. Medical Smart Textiles Based on Fiber Optic Technology: An Overview. J. Funct. Biomater. 2015, 6, 204–221. [Google Scholar] [CrossRef]
- Utzinger, U.; Richards-Kortum, R.R. Fiber optic probes for biomedical optical spectroscopy. J. Biomed. Opt. 2003, 8, 121. [Google Scholar] [CrossRef]
- Santos, I.P.; Barroso, E.M.; Bakker Schut, T.C.; Caspers, P.J.; van Lanschot, C.G.F.; Choi, D.H.; van der Kamp, M.F.; Smits, R.W.H.; van Doorn, R.; Verdijk, R.M.; et al. Raman spectroscopy for cancer detection and cancer surgery guidance: translation to the clinics. Analyst 2017, 142, 3025–3047. [Google Scholar] [CrossRef]
- Matousek, P.; Stone, N. Recent advances in the development of Raman spectroscopy for deep non-invasive medical diagnosis. J. Biophotonics 2013, 6, 7–19. [Google Scholar] [CrossRef]
- Krafft, C.; Dochow, S.; Latka, I.; Dietzek, B.; Popp, J. Diagnosis and screening of cancer tissues by fiber-optic probe Raman spectroscopy. Biomed. Spectrosc. Imaging 2012, 1, 39–55. [Google Scholar] [CrossRef]
- Chin, L.C.; Whelan, W.M.; Vitkin, I.A. Optical Fiber Sensors for Biomedical Applications. In Optical-Thermal Response of Laser-Irradiated Tissue; Springer: Dordrecht, The Netherlands, 2010; pp. 661–712. [Google Scholar] [CrossRef]
- Dochow, S.; Ma, D.; Latka, I.; Bocklitz, T.; Hartl, B.; Bec, J.; Fatakdawala, H.; Marple, E.; Urmey, K.; Wachsmann-Hogiu, S.; et al. Combined fiber probe for fluorescence lifetime and Raman spectroscopy. Anal. Bioanal. Chem. 2015, 407, 8291–8301. [Google Scholar] [CrossRef] [PubMed]
- Sherlock, B.E.; Phipps, J.E.; Bec, J.; Marcu, L. Simultaneous, label-free, multispectral fluorescence lifetime imaging and optical coherence tomography using a double-clad fiber. Opt. Lett. 2017, 42, 3753. [Google Scholar] [CrossRef] [PubMed]
- Fatakdawala, H.; Poti, S.; Zhou, F.; Sun, Y.; Bec, J.; Liu, J.; Yankelevich, D.R.; Tinling, S.P.; Gandour-Edwards, R.F.; Farwell, D.G.; et al. Multimodal in vivo imaging of oral cancer using fluorescence lifetime, photoacoustic and ultrasound techniques. Biomed. Opt. Express 2013, 4, 1724. [Google Scholar] [CrossRef]
- Spiegel, P.K. The first clinical X-ray made in America–100 years. Am. J. Roentgenol. 1995, 164, 241–243. [Google Scholar] [CrossRef]
- Silvestri, G.A.; Gould, M.K.; Margolis, M.L.; Tanoue, L.T.; McCrory, D.; Toloza, E.; Detterbeck, F. Noninvasive staging of non-small cell lung cancer: ACCP evidenced-based clinical practice guidelines. Chest 2007, 132, 178S–201S. [Google Scholar] [CrossRef]
- Daniel, T.M. The history of tuberculosis. Respir. Med. 2006, 100, 1862–1870. [Google Scholar] [CrossRef]
- Haschek, E.; Lindenthal, O. A contribution to the practical use of the photography according to Röntgen. Wiener Klinische Wochenschrift 1896, 9, 63. [Google Scholar]
- Hounsfield, G.N. Computerized transverse axial scanning (tomography): Part 1. Description of system. Br. J. Radiol. 1973, 46, 1016–1022. [Google Scholar] [CrossRef]
- Ter-Pogossian, M.M.; Phelps, M.E.; Hoffman, E.J.; Mullani, N.A. A positron-emission transaxial tomograph for nuclear imaging (PETT). Radiology 1975, 114, 89–98. [Google Scholar] [CrossRef]
- Farwell, M.D.; Pryma, D.A.; Mankoff, D.A. PET/CT imaging in cancer: Current applications and future directions. Cancer 2014, 120, 3433–3445. [Google Scholar] [CrossRef] [PubMed]
- Moloney, F.; Fama, D.; Twomey, M.; O’Leary, R.; Houlihane, C.; Murphy, K.P.; O’Neill, S.B.; O’Connor, O.J.; Breen, D.; Maher, M.M. Cumulative radiation exposure from diagnostic imaging in intensive care unit patients. World J. Radiol. 2016, 8, 419. [Google Scholar] [CrossRef] [PubMed]
- Blandin Knight, S.; Crosbie, P.A.; Balata, H.; Chudziak, J.; Hussell, T.; Dive, C. Progress and prospects of early detection in lung cancer. Open Biol. 2017, 7, 170070. [Google Scholar] [CrossRef] [PubMed]
- Wallmann, J. Antimicrobial resistance: challenges ahead. Vet. Rec. 2014, 175, 323–324. [Google Scholar] [CrossRef]
- Monroe, S.; Polk, R. Antimicrobial use and bacterial resistance. Curr. Opin. Microbiol. 2000, 3, 496–501. [Google Scholar] [CrossRef]
- Yewale, V.N. Antimicrobial resistance—A ticking bomb! Indian Pediatr. 2014, 51, 171–172. [Google Scholar] [CrossRef]
- Parker, H.E.; Stone, J.M.; Marshall, A.D.L.; Choudhary, T.R.; Thomson, R.R.; Dhaliwal, K.; Tanner, M.G. Fibre-based spectral ratio endomicroscopy for contrast enhancement of bacterial imaging and pulmonary autofluorescence. Biomed. Opt. Express 2019, 10, 1856–1869. [Google Scholar] [CrossRef]
- Richards-Kortum, R.; Sevick-Muraca, E. Quantitative optical spectroscopy for tissue diagnoses. Annu. Rev. Phys. Chem. 1996, 47, 555–606. [Google Scholar] [CrossRef]
- Akram, A.R.; Chankeshwara, S.V.; Scholefield, E.; Aslam, T.; McDonald, N.; Megia-Fernandez, A.; Marshall, A.; Mills, B.; Avlonitis, N.; Craven, T.H.; et al. In situ identification of Gram-negative bacteria in human lungs using a topical fluorescent peptide targeting lipid A. Sci. Transl. Med. 2018, 10, eaal0033. [Google Scholar] [CrossRef]
- Zhang, J.; Campbell, R.E.; Ting, A.Y.; Tsien, R.Y. Creating new fluorescent probes for cell biology. Nat. Rev. Mol. Cell Biol. 2002, 3, 906–918. [Google Scholar] [CrossRef]
- Kobayashi, H.; Ogawa, M.; Alford, R.; Choyke, P.L.; Urano, Y. New Strategies for Fluorescent Probe Design in Medical Diagnostic Imaging. Chem. Rev. 2010, 110, 2620–2640. [Google Scholar] [CrossRef]
- Perchant, A.; Le Goualher, G.; Genet, M.; Viellerobe, B.; Berier, F. An integrated fibered confocal microscopy system for in vivo and in situ fluorescence imaging—Applications to endoscopy in small animal imaging. In Proceedings of the 2004 2nd IEEE International Symposium on Biomedical Imaging: Macro to Nano, Arlington, VA, USA, 18 April 2004; Volume 2, pp. 692–695. [Google Scholar] [CrossRef]
- Laemmel, E.; Genet, M.; Le Goualher, G.; Perchant, A.; Le Gargasson, J.F.; Vicaut, E. Fibered Confocal Fluorescence Microscopy (Cell-viZio™) Facilitates Extended Imaging in the Field of Microcirculation. J. Vasc. Res. 2004, 41, 400–411. [Google Scholar] [CrossRef]
- Thiberville, L.; Moreno-Swirc, S.; Vercauteren, T.; Peltier, E.; Cavé, C.; Bourg Heckly, G. In Vivo Imaging of the Bronchial Wall Microstructure Using Fibered Confocal Fluorescence Microscopy. Am. J. Respir. Crit. Care Med. 2007, 175, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Mills, B.; Akram, A.R.; Scholefield, E.; Bradley, M.; Dhaliwal, K. Optical Screening of Novel Bacteria-specific Probes on Ex Vivo Human Lung Tissue by Confocal Laser Endomicroscopy. J. Vis. Exp. 2017, 129, e56284. [Google Scholar] [CrossRef] [PubMed]
- Krstajić, N.; Akram, A.R.; Choudhary, T.R.; McDonald, N.; Tanner, M.G.; Pedretti, E.; Dalgarno, P.A.; Scholefield, E.; Girkin, J.M.; Moore, A.; et al. Two-color widefield fluorescence microendoscopy enables multiplexed molecular imaging in the alveolar space of human lung tissue. J. Biomed. Opt. 2016, 21, 046009. [Google Scholar] [CrossRef] [PubMed]
- Krstajić, N.; Mills, B.; Murray, I.; Marshall, A. Low-cost high sensitivity pulsed endomicroscopy to visualize tricolor optical signatures. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef] [PubMed]
- Stadler, H.; Duit, R.; Benke, G. Do boys and girls understand physics differently? Phys. Educ. 2000, 35, 417–422. [Google Scholar] [CrossRef]
- Ehrlich, K.; Parker, H.E.; McNicholl, D.K.; Reid, P.; Reynolds, M.; Bussiere, V.; Crawford, G.; Deighan, A.; Garrett, A.; Kufcsák, A.; et al. Edinburgh DataShare, Circuits!—Demonstrating the Use of Optical Fibres in Biomedical Sciences. Available online: https://datashare.is.ed.ac.uk/handle/10283/3374 (accessed on 6 January 2020).

| Learning Intentions | Link from Curriculum to Research | Core Task |
|---|---|---|
| Understanding of organ systems | The impetus for the OEM system modelled is to tackle the lung and its diseases | Engagement with appropriately targeted teaching materials, aimed at student-age readers and providing guidance for teacher-led or peer investigation of these curriculum topics |
| Researching new developments | The OEM system modelled by the educational tool is an ongoing piece of work with regular production of peer-reviewed publications [30,72,73] | |
| Light | The construction and understanding of the educational tool requires knowledge of optical fibres to get light to and from inaccessible spaces, colour theory to interpret what occurs at the distal end, and the use of a number of circuit components to produce a working measurement system | Use of the educational tool itself in classroom environment. The focus on calibration and the reduction of errors facilitates the passive teaching of basic scientific literacy |
| Optical fibres | ||
| Colour mixing | ||
| Building a circuit |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ehrlich, K.; Parker, H.E.; McNicholl, D.K.; Reid, P.; Reynolds, M.; Bussiere, V.; Crawford, G.; Deighan, A.; Garrett, A.; Kufcsák, A.; et al. Demonstrating the Use of Optical Fibres in Biomedical Sensing: A Collaborative Approach for Engagement and Education. Sensors 2020, 20, 402. https://doi.org/10.3390/s20020402
Ehrlich K, Parker HE, McNicholl DK, Reid P, Reynolds M, Bussiere V, Crawford G, Deighan A, Garrett A, Kufcsák A, et al. Demonstrating the Use of Optical Fibres in Biomedical Sensing: A Collaborative Approach for Engagement and Education. Sensors. 2020; 20(2):402. https://doi.org/10.3390/s20020402
Chicago/Turabian StyleEhrlich, Katjana, Helen E. Parker, Duncan K. McNicholl, Peter Reid, Mark Reynolds, Vincent Bussiere, Graham Crawford, Angela Deighan, Alice Garrett, András Kufcsák, and et al. 2020. "Demonstrating the Use of Optical Fibres in Biomedical Sensing: A Collaborative Approach for Engagement and Education" Sensors 20, no. 2: 402. https://doi.org/10.3390/s20020402
APA StyleEhrlich, K., Parker, H. E., McNicholl, D. K., Reid, P., Reynolds, M., Bussiere, V., Crawford, G., Deighan, A., Garrett, A., Kufcsák, A., Norberg, D. R., Spennati, G., Steele, G., Szoor-McElhinney, H., & Jimenez, M. (2020). Demonstrating the Use of Optical Fibres in Biomedical Sensing: A Collaborative Approach for Engagement and Education. Sensors, 20(2), 402. https://doi.org/10.3390/s20020402





