Developing a Silk Fibroin Composite Film to Scavenge and Probe H2O2 Associated with UV-Excitable Blue Fluorescence
Abstract
1. Introduction
2. Materials and Methods
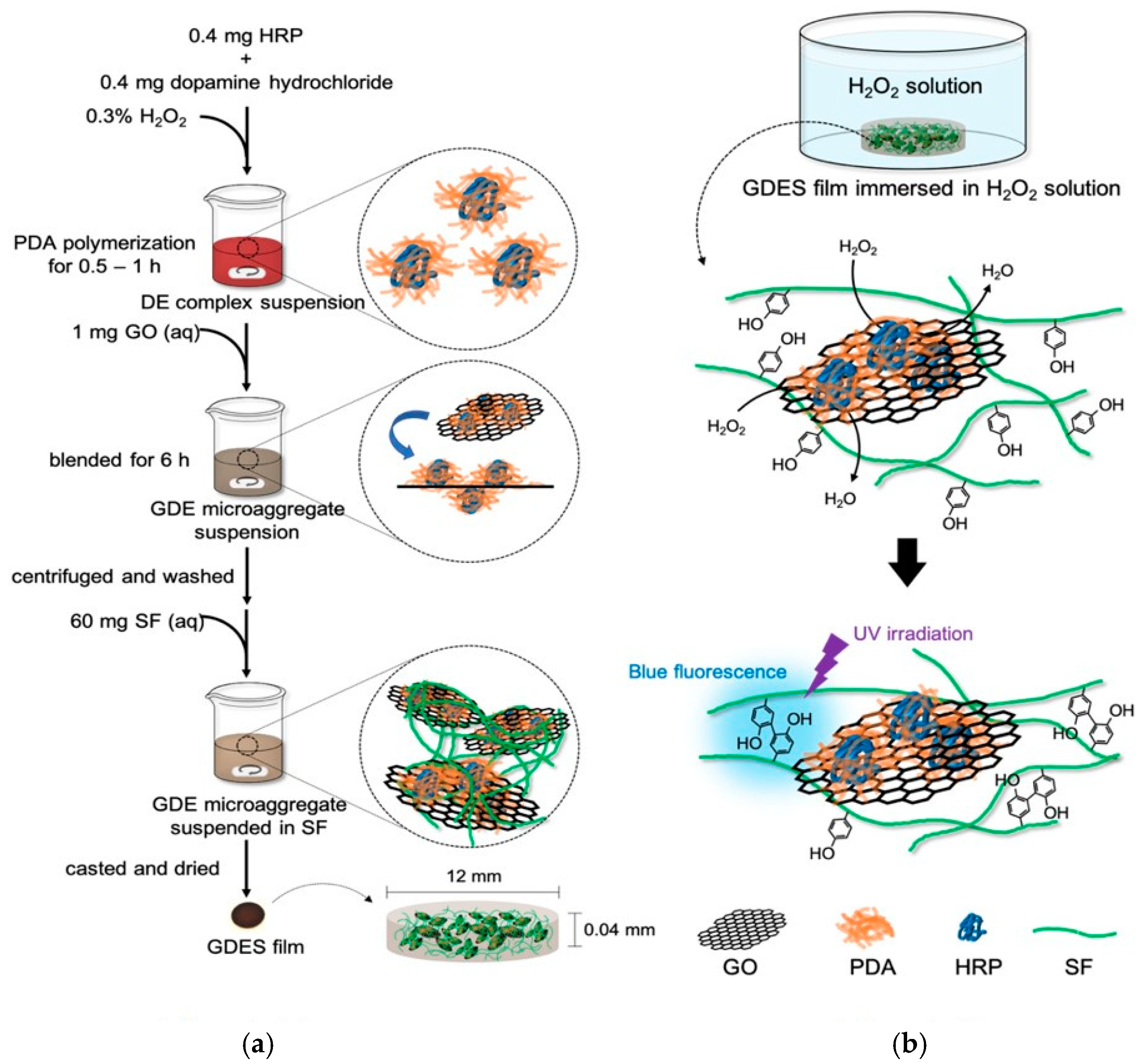
2.1. Fabrication of GDES Films
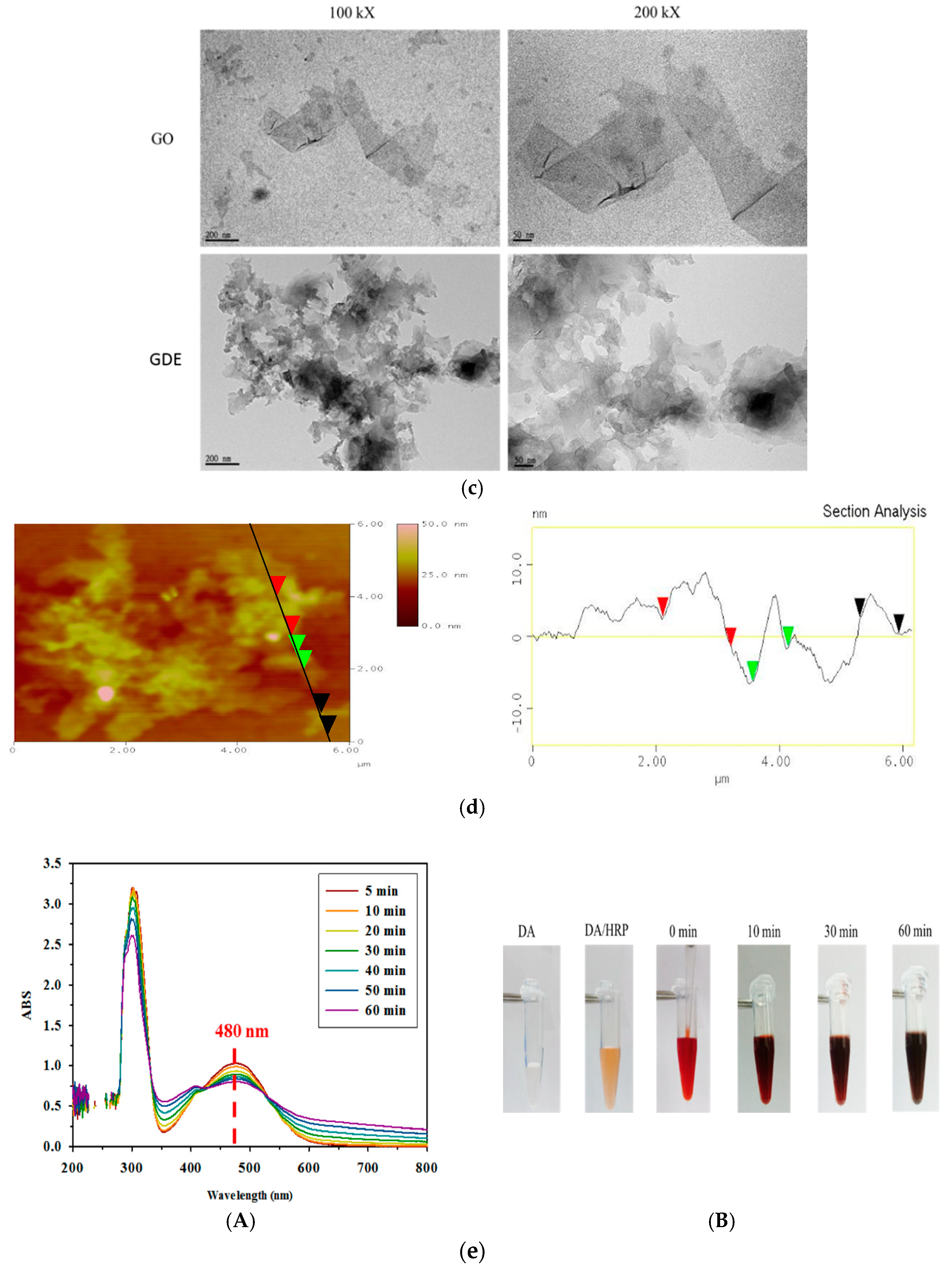
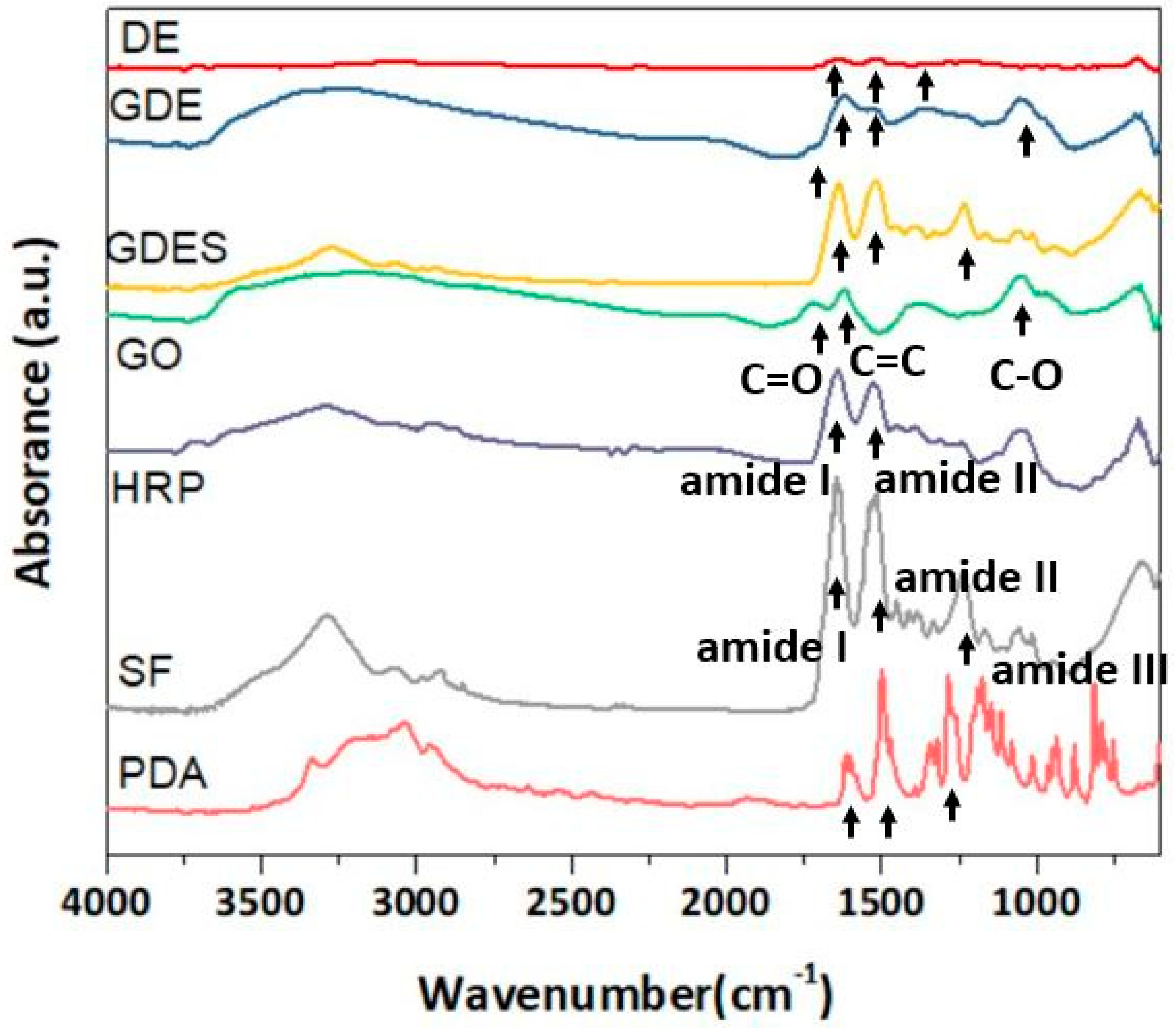
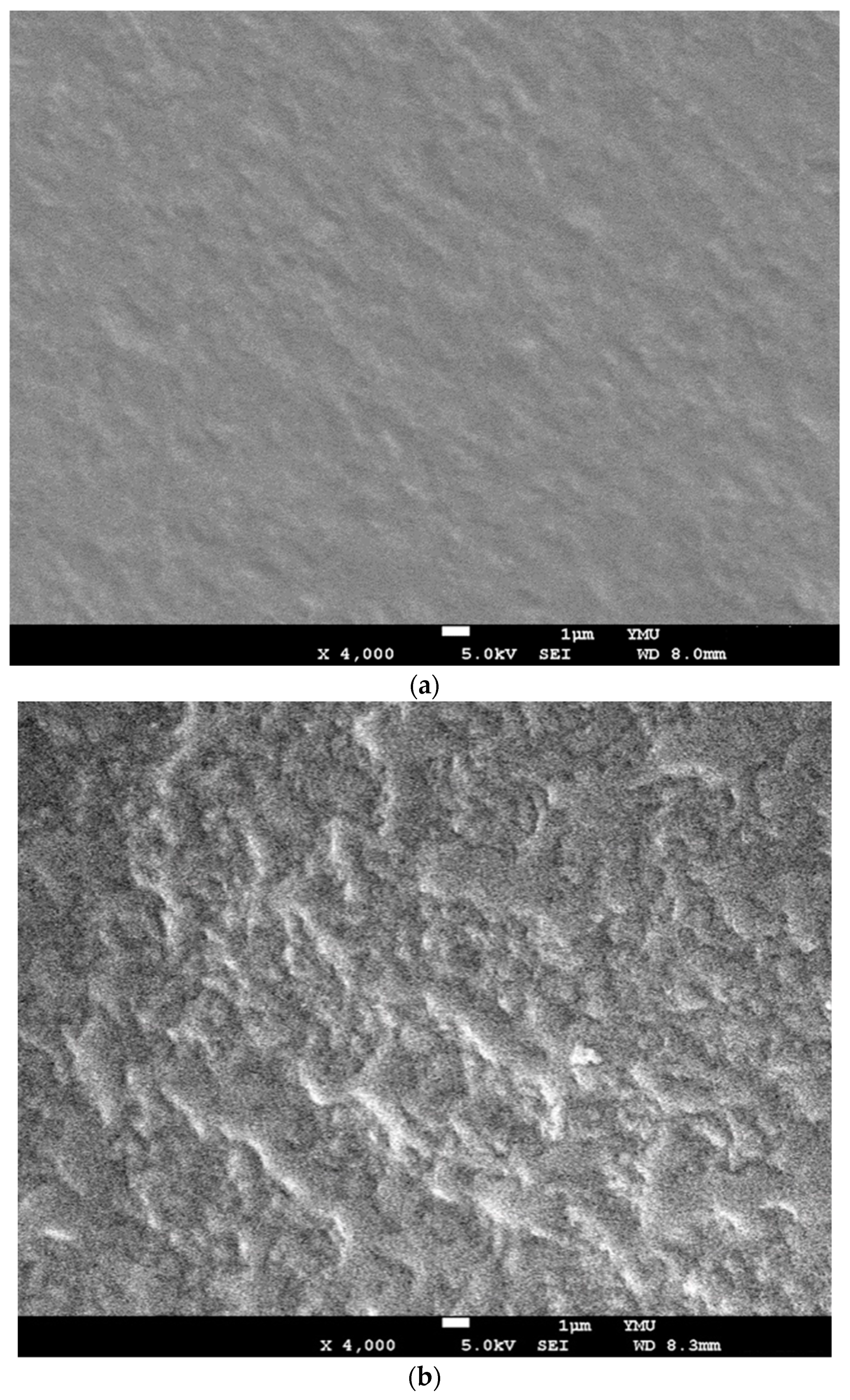
2.2. Characterizations of Components and Films Using ATR-FTIR, SEM and TEM
2.3. H2O2 Scavenging Assay
2.4. Probing H2O2 by Measuring UV-Excitable Blue Fluorescence of GDES Film
2.5. Photothermal Conversion of GDES Films
2.6. Assessment of In Vitro Biocompatibility
2.7. Statistical Analysis
3. Results and Discussion
3.1. Fabricating GDES Films and Analysis of Their Compositions
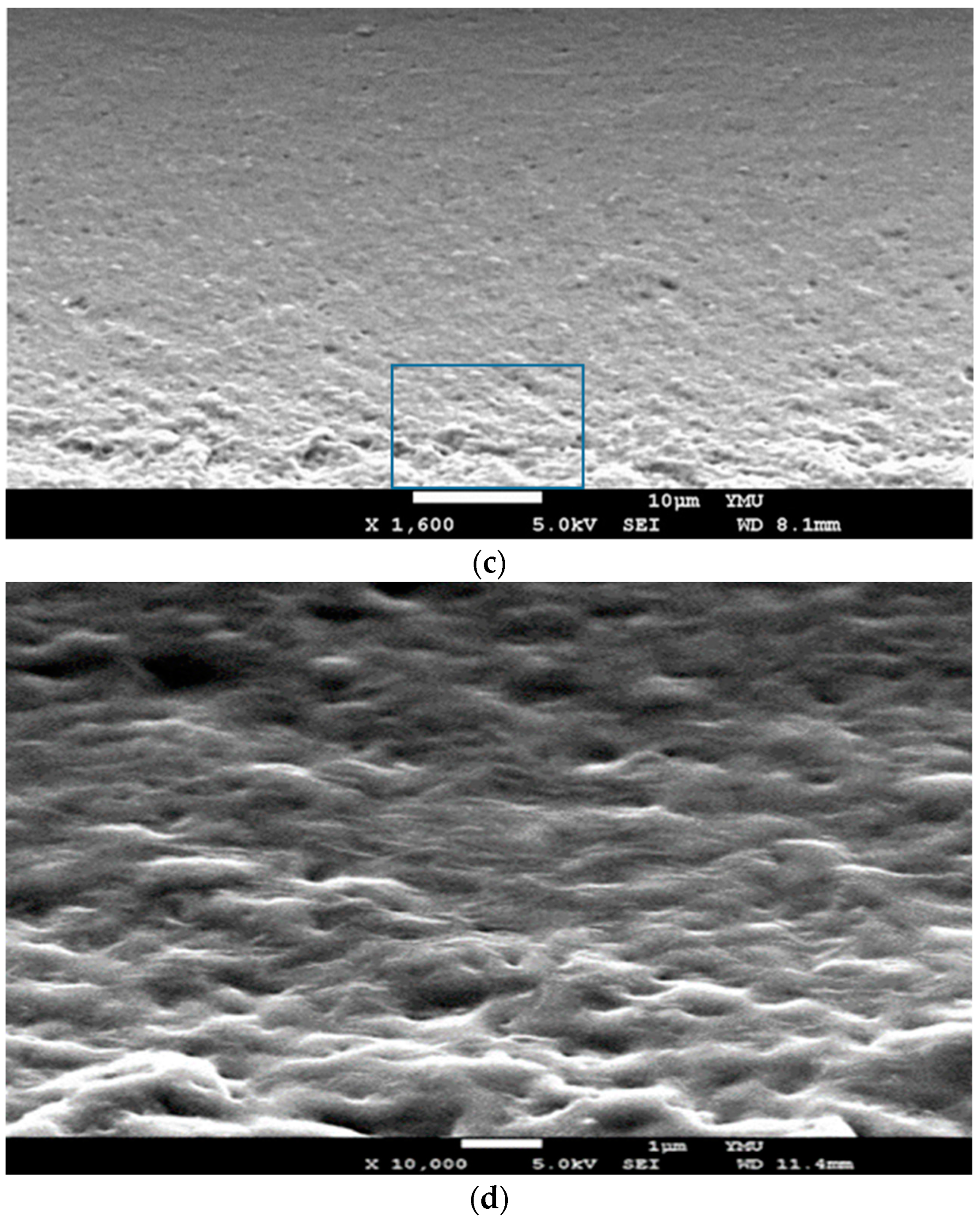
3.2. ATR-FTIR Spectroscopic and SEM Analyses of GDES Films
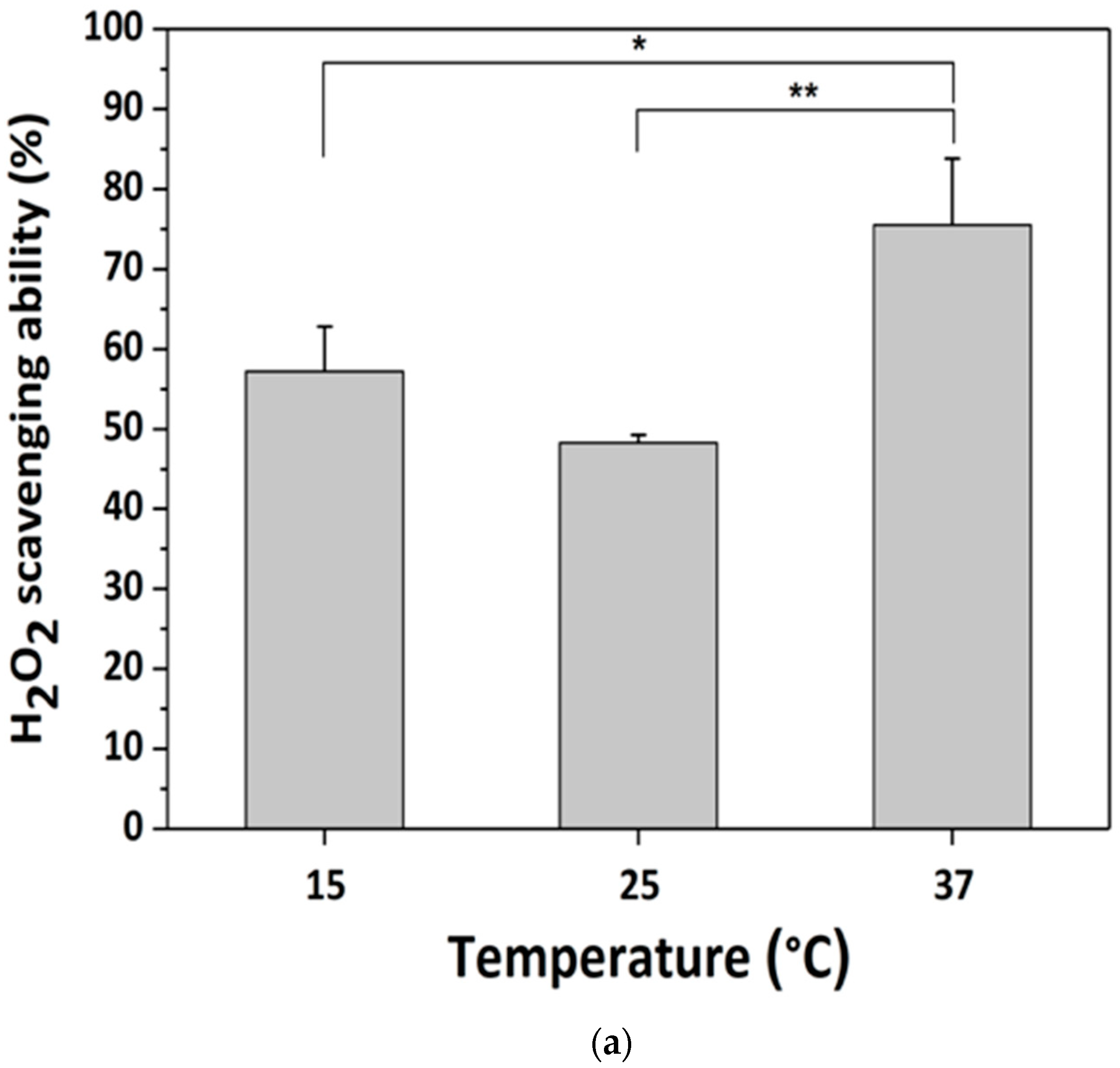
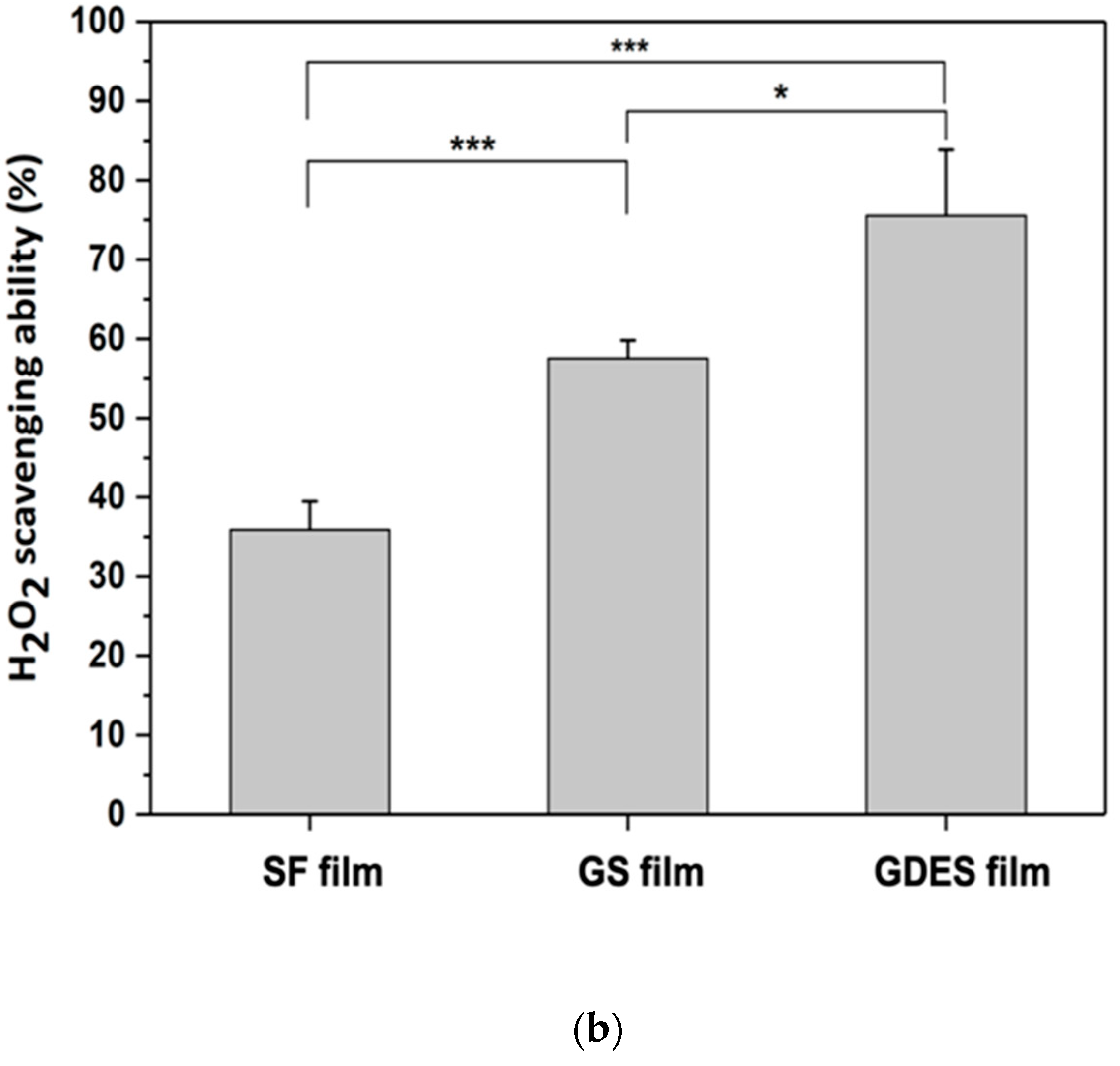
3.3. H2O2-Scavenging by GDES Films
3.4. Use of UV-Excitable Blue Fluorescence of GDES Films to Probe H2O2
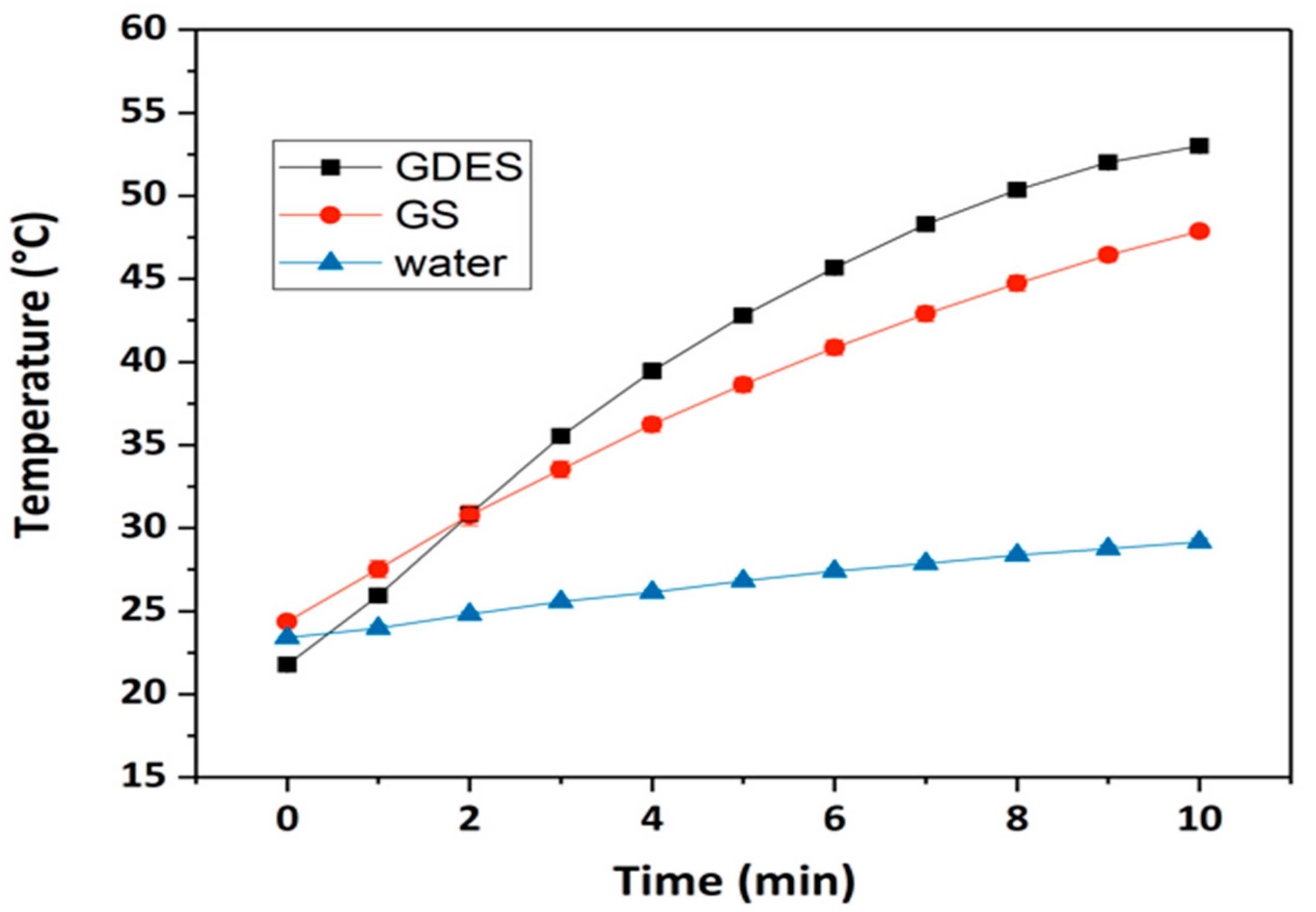
3.5. Photothermal Responses of GDES Films
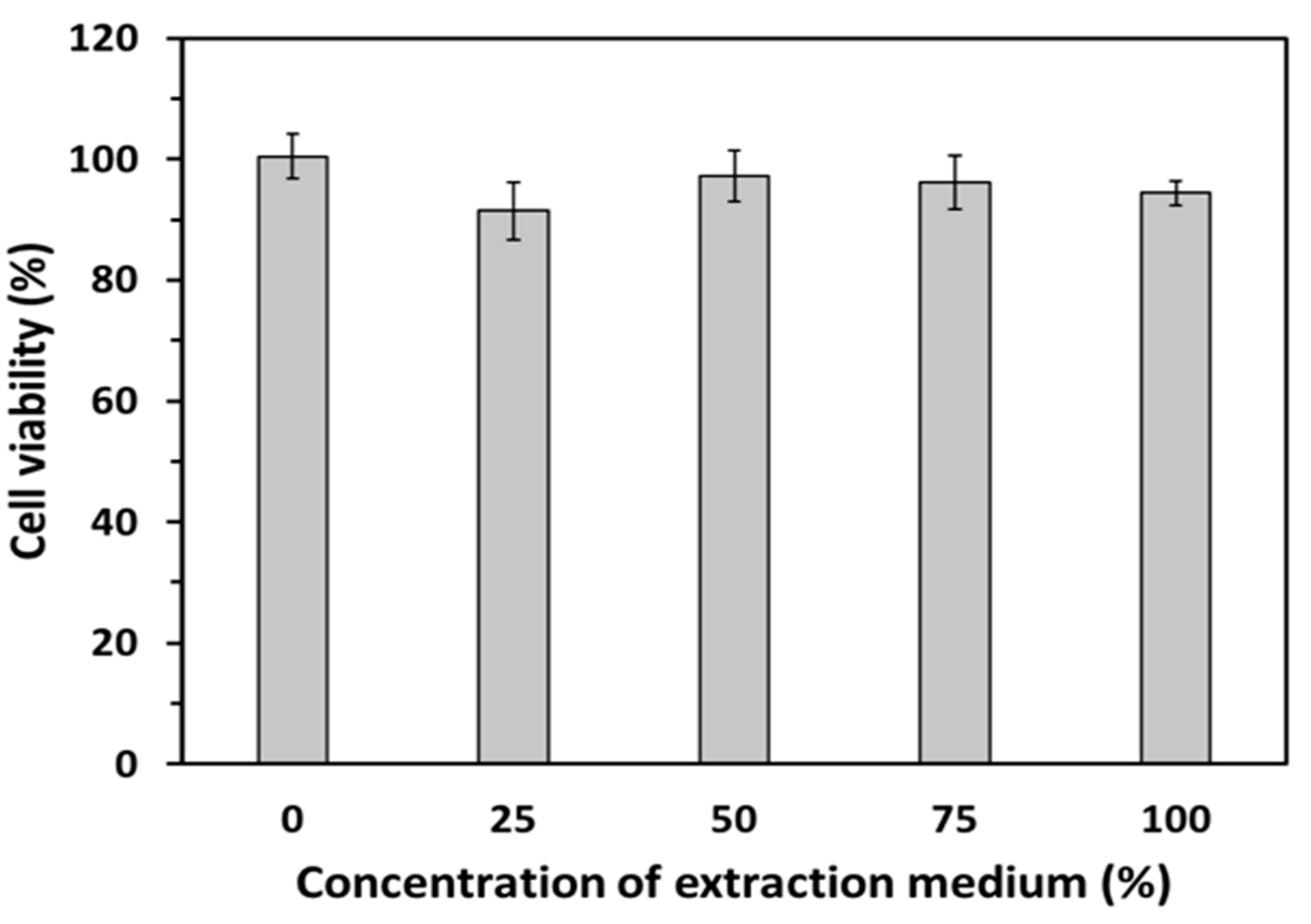
3.6. In Vitro Biocompatibility of GDES Films
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Giorgio, M.; Trinei, M.; Migliaccio, E.; Pelicci, P.G. Hydrogen peroxide: A metabolic by-product or a common mediator of ageing signals? Nat. Rev. Mol. Cell Biol. 2007, 8, 722–728. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Aleyasin, H.; Dickinson, B.C.; Haskew-layton, R.E.; Raton, R.R. Recent advances in hydrogen peroxide imaging for biological applications. Cell Biosci. 2014, 4, 64. [Google Scholar] [CrossRef] [PubMed]
- van der Vliet, A.; Janssen-Heininger, Y.M. Hydrogen peroxide as a damage signal in tissue injury and inflammation: Murderer, mediator, or messenger? J. Cell. Biochem. 2014, 115, 427–435. [Google Scholar] [CrossRef] [PubMed]
- Guo, S.; DiPietro, L.A. Factors affecting wound healing. J. Dent. Res. 2010, 89, 219–229. [Google Scholar] [CrossRef] [PubMed]
- Fitzmaurice, S.D.; Sivamani, R.K.; Isseroff, R.R. Antioxidant therapies for wound healing: A clinical guide to currently commercially available products. Skin Pharmacol. Physiol. 2011, 24, 113–126. [Google Scholar] [CrossRef] [PubMed]
- Zhu, G.; Wang, Q.; Lu, S.; Niu, Y. Hydrogen Peroxide: A potential wound therapeutic target? Med. Princ. Pract. 2017, 26, 301–308. [Google Scholar] [CrossRef]
- Dreyer, D.R.; Park, S.; Bielawski, C.W.; Ruoff, R.S. The chemistry of graphene oxide. Chem. Soc. Rev. 2010, 39, 228–240. [Google Scholar] [CrossRef]
- Chen, Y.W.; Su, Y.L.; Hu, S.H.; Chen, S.Y. Functionalized graphene nanocomposites for enhancing photothermal therapy in tumor treatment. Adv. Drug Deliv. Rev. 2016, 105, 190–204. [Google Scholar] [CrossRef]
- Sun, X.; Liu, Z.; Welsher, K.; Robinson, J.T.; Goodwin, A.; Zaric, S.; Dai, H. Nano-graphene oxide for cellular imaging and drug delivery. Nano Res. 2008, 1, 203–212. [Google Scholar] [CrossRef]
- Morales-Narváez, E.; Merkoçi, A. Graphene oxide as an optical biosensing platform. Adv. Mater. 2012, 24, 3298–3308. [Google Scholar] [CrossRef]
- Zhou, K.; Zhu, Y.; Yang, X.; Luo, J.; Li, C.; Luan, S. A novel hydrogen peroxide biosensor based on Au–graphene–HRP–chitosan biocomposites. Electrochim. Acta 2010, 55, 3055–3060. [Google Scholar] [CrossRef]
- Cao, X.; Li, Y.; Zhang, Z.; Yu, J.; Qian, J.; Liu, S. Catalytic activity and stability of glucose oxidase/horseradish peroxidase co-confined in macroporous silica foam. Analyst 2012, 137, 5785–5791. [Google Scholar] [CrossRef] [PubMed]
- Tang, N.; Zheng, J.; Sheng, Q.; Zhang, H.; Liu, R. A novel H2O2 sensor based on the enzymatically induced deposition of polyaniline at a horseradish peroxide/aligned single-wall carbon nanotubes modified Au electrode. Analyst 2011, 136, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Chakraborty, S.; Hill, A.L.; Shirsekar, G.; Afzal, A.J.; Wang, G.-L.; Mackey, D.; Bonello, P. Quantification of hydrogen peroxide in plant tissues using Amplex Red. Methods 2016, 109, 105–113. [Google Scholar] [CrossRef]
- Xue, J.; Liu, W.; Wu, D.; Liu, W.; Song, H. Enhanced peroxidase-like activity of Mo doped ceria nanoparticles for sensitive colorimetric detection of glucose. Anal. Methods 2018, 10, 76–83. [Google Scholar]
- Dai, M.; Huang, T.; Chao, L.; Xie, Q.; Tan, Y.; Chen, C.; Meng, W. Horseradish peroxidase-catalyzed polymerization of L-DOPA for mono-/bi-enzyme immobilization and amperometric biosensing of H2O2 and uric acid. Talanta 2016, 149, 117–123. [Google Scholar] [CrossRef]
- You, X.; Pak, J.J. Graphene-based field effect transistor enzymatic glucose biosensor using silk protein for enzyme immobilization and device substrate. Sens. Actuator B-Chem. 2014, 202, 1357–1365. [Google Scholar] [CrossRef]
- Lee, H.; Dellatore, S.M.; Miller, W.M.; Messersmith, P.B. Mussel-inspired surface chemistry for multifunctional coatings. Science 2007, 318, 426–430. [Google Scholar] [CrossRef]
- Ju, K.Y.; Lee, Y.; Lee, S.; Park, S.B.; Lee, J.K. Bioinspired polymerization of dopamine to generate melanin-like nanoparticles having an excellent free-radical-scavenging property. Biomacromolecules 2011, 12, 625–632. [Google Scholar] [CrossRef]
- Liu, Y.; Ai, K.; Liu, J.; Deng, M.; He, Y.; Lu, L. Dopamine-melanin colloidal nanospheres: An efficient near-infrared photothermal therapeutic agent for in vivo cancer therapy. Adv. Mater. 2013, 25, 1353–1359. [Google Scholar] [CrossRef]
- Numata, K.; Yamazaki, S.; Katashima, T.; Chuah, J.A.; Naga, N.; Sakai, T. Silk pectin hydrogel with superior mechanical properties, biodegradability, and biocompatibility. Macromol. Biosci. 2014, 14, 799–806. [Google Scholar] [CrossRef] [PubMed]
- Wenk, E.; Merkle, H.P.; Meinel, L. Silk fibroin as a vehicle for drug delivery applications. J. Control. Release 2011, 150, 128–141. [Google Scholar] [CrossRef] [PubMed]
- Partlow, B.P.; Hanna, C.W.; Rnjak-Kovacina, J.; Moreau, J.E.; Applegate, M.B.; Burke, K.A.; Marelli, B.; Mitropoulos, A.N.; Omenetto, F.G.; Kaplan, D.L. Highly tunable elastomeric silk biomaterials. Adv. Funct. Mater. 2014, 24, 4615–4624. [Google Scholar] [CrossRef] [PubMed]
- Hummers, W.S., Jr.; Offeman, R.E. Preparation of graphitic oxide. J. Am. Chem. Soc. 1958, 80, 1339. [Google Scholar] [CrossRef]
- Chung, T.W.; Lo, H.Y.; Chou, T.H.; Chen, J.H.; Wang, S.S. Promoting cardiomyogenesis of hBMSC with a forming self-assembly hBMSC microtissues/HA-GRGD/SF-PCL cardiac patch is mediated by the synergistic functions of HA-GRGD. Macromol. Biosci. 2017, 17, 160–173. [Google Scholar] [CrossRef]
- Lo, H.Y.; Huang, A.L.; Lee, P.C.; Chung, T.W.; Wang, S.S. Morphological transformation of hBMSC from 2D monolayer to 3D microtissue on low-crystallinity SF-PCL patch with promotion of cardiomyogenesis. J. Tissue Eng. Regen. Med. 2018, 12, e1852–e1864. [Google Scholar] [CrossRef]
- Chung, T.W.; Chou, T.H.; Wu, K.Y. Gelatin/PLGA hydrogel films and their delivery of hydrophobic drugs. J. Taiwan Inst. Chem. Eng. 2016, 60, 8–14. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Arnow, L.E. Colorimetric determination of the components of 3,4-Dihydro-xyphenyl-alanine-Tyrosine mixtures. J. Biol. Chem. 1937, 118, 531–537. [Google Scholar]
- Kumar, A.; Krishnamoorthy, E.; Devi, H.M.; Uchoi, D.; Tejpal, C.; Ninan, G.; Zynudheen, A. Influence of sea grapes (Caulerpa racemosa) supplementation on physical, functional, and anti-oxidant properties of semi-sweet biscuits. J. Appl. Phycol. 2018, 30, 1393–1403. [Google Scholar] [CrossRef]
- Mukhopadhyay, D.; Dasgupta, P.; Roy, D.S.; Palchoudhuri, S.; Chatterjee, I.; Ali, S.; Dastidar, S.G. A Sensitive in vitro spectrophotometric hydrogen peroxide scavenging Assay using 1, 10-phenanthroline. Free Radic. Antioxid. 2016, 6, 124–132. [Google Scholar] [CrossRef]
- Zhou, Y.; Liang, K.; Zhang, C.; Li, J.; Yang, H.; Liu, X.; Yin, X.; Chen, D.; Xu, W.; Xiao, P. Photo-crosslinked methacrylated chitosan-based nanofibrous scaffolds as potential skin substitute. Cellulose 2017, 24, 4253–4262. [Google Scholar] [CrossRef]
- Georgakilas, V.; Tiwari, J.N.; Kemp, K.C.; Perman, J.A.; Bourlinos, A.B.; Kim, K.S.; Zboril, R. Noncovalent functionalization of graphene and graphene oxide for energy materials, biosensing, catalytic, and biomedical applications. Chem. Rev. 2016, 116, 5464–5519. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.; Stuart, M.A.C.; Kamperman, M. Jack of all trades: Versatile catechol crosslinking mechanisms. Chem. Soc. Rev. 2014, 43, 8271–8298. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Wang, D.; He, S.; Li, J.; Feng, B.; Ma, P.; Xu, P.; Gao, S.; Zhang, S.; Liu, Q. Graphene-based nanoprobes and a prototype optical biosensing platform. Biosens. Bioelectron. 2013, 50, 251–255. [Google Scholar] [CrossRef] [PubMed]
- Lai, G.; Cheng, H.; Xin, D.; Zhang, H.; Yu, A. Amplified inhibition of the electrochemical signal of ferrocene by enzyme-functionalized graphene oxide nanoprobe for ultrasensitive immunoassay. Anal. Chim. Acta 2016, 902, 189–195. [Google Scholar] [CrossRef]
- Hu, H.; Dyke, J.C.; Bowman, B.A.; Ko, C.-C.; You, W. Investigation of dopamine analogues: Synthesis, mechanistic understanding, and structure–property relationship. Langmuir 2016, 32, 9873–9882. [Google Scholar] [CrossRef]
- Marcano, D.C.; Kosynkin, D.V.; Berlin, J.M.; Sinitskii, A.; Sun, Z.; Slesarev, A.; Alemany, L.B.; Lu, W.; Tour, J.M. Improved synthesis of graphene oxide. ACS Nano 2010, 4, 4806–4814. [Google Scholar] [CrossRef]
- Wang, G.; Wang, B.; Park, J.; Yang, J.; Shen, X.; Yao, J. Synthesis of enhanced hydrophilic and hydrophobic graphene oxide nanosheets by a solvent thermal Method. Carbon 2009, 47, 68–72. [Google Scholar] [CrossRef]
- Wang, G.X.; Bao, W.J.; Wang, J.; Lu, Q.Q.; Xia, X.H. Immobilization and catalytic activity of horseradish peroxidase on molybdenum disulfide nanosheets modified electrode. Electrochem. Commun. 2013, 35, 146–148. [Google Scholar] [CrossRef]
- Zangmeister, R.A.; Morris, T.A.; Tarlov, M.J. Characterization of polydopamine thin films deposited at short times by autoxidation of dopamine. Langmuir 2013, 29, 8619–8628. [Google Scholar] [CrossRef] [PubMed]
- Um, I.C. Effect of molecular weight and concentration on crystallinity and post drawing of wet spun silk fibroin fiber. Fiber Polym. 2014, 15, 153–160. [Google Scholar]
- Hwang, S.H.; Kang, D.; Ruoff, R.S.; Shin, H.S.; Park, Y.B. Poly (vinyl alcohol) reinforced and toughened with poly(dopamine)-treated graphene oxide, and its use for humidity sensing. ACS Nano 2014, 8, 6739–6747. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.; Xi, P.; Xie, G.; Shi, Y.; Hou, F.; Huang, L.; Chen, F.; Zeng, Z.; Shao, C.; Wang, J. Simultaneous reduction and surface functionalization of graphene oxide for hydroxyapatite mineralization. J. Phys. Chem. C 2012, 116, 3334–3341. [Google Scholar] [CrossRef]
- Liu, S.; Zhang, X.; Wu, Y.; Tu, Y.; He, L. Prostate-specific antigen detection by using a reusable amperometric immunosensor based on reversible binding and leasing of HRP-anti-PSA from phenylboronic acid modified electrode. Clin. Chim. Acta 2008, 395, 51–56. [Google Scholar] [CrossRef]
- Lin, L.; Song, X.; Chen, Y.; Rong, M.; Zhao, T.; Wang, Y.; Jiang, Y.; Chen, X. Intrinsic peroxidase-like catalytic activity of nitrogen-doped graphene quantum dots and their application in the colorimetric detection of H2O2 and glucose. Anal. Chim. Acta 2015, 869, 89–95. [Google Scholar] [CrossRef]
- Wongkrongsak, S.; Tangthong, T.; Pasanphan, W. Electron beam induced water-soluble silk fibroin nanoparticles as a natural antioxidant and reducing agent fora green synthesis of gold nano-colloid. Radiat. Phys. Chem. 2016, 118, 27–34. [Google Scholar] [CrossRef]
- Lee, P.C.; Zan, B.S.; Chen, L.T.; Chung, T.W. Multifunctional PLGA-based nanoparticles as a controlled release drug delivery system for antioxidant and anticoagulant therapy. Int. J. Nanomed. 2019, 14, 1533–1549. [Google Scholar] [CrossRef]
- Yagi, H.; Tan, J.; Tuan, R.S. Polyphenols suppress hydrogen peroxide-induced oxidative stress in human bone-marrow derived mesenchymal stem cells. J. Cell. Biochem. 2013, 114, 1163–1173. [Google Scholar] [CrossRef]
- Hohnholt, M.C.; Dringen, R. Short time exposure to hydrogen peroxide induces sustained glutathione export from cultured neurons. Free Radic. Biol. Med. 2014, 70, 33–44. [Google Scholar] [CrossRef]
- Zou, X.; Zhang, L.; Wang, Z.; Luo, Y. Mechanisms of the antimicrobial activities of graphene materials. J. Am. Chem. Soc. 2016, 138, 2064–2077. [Google Scholar] [CrossRef] [PubMed]
- Liao, K.H.; Lin, Y.S.; Macosko, C.W.; Haynes, C.L. Cytotoxicity of graphene oxide and graphene in human erythrocytes and skin fibroblasts. ACS Appl. Mater. Interfaces 2011, 3, 2607–2615. [Google Scholar] [CrossRef] [PubMed]

| Abbreviation | |
|---|---|
| G | GO, Graphene oxide |
| D | PDA, Polydopamine |
| E | HRP, Horseradish peroxidase |
| S | SF, Silk fibroin |
| DE | PDA-HRP complex |
| GS | GO covered by SF |
| GDE | GO-PDA/HRP microaggregates |
| GDES | GO-PDA/HRP microaggregates covered by SF |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chung, T.-W.; Chang, C.-Y.; Chang, C.-N.; Liao, C.-H.; Jan, Y.-J.; Chen, L.-T.; Chen, W.-P. Developing a Silk Fibroin Composite Film to Scavenge and Probe H2O2 Associated with UV-Excitable Blue Fluorescence. Sensors 2020, 20, 366. https://doi.org/10.3390/s20020366
Chung T-W, Chang C-Y, Chang C-N, Liao C-H, Jan Y-J, Chen L-T, Chen W-P. Developing a Silk Fibroin Composite Film to Scavenge and Probe H2O2 Associated with UV-Excitable Blue Fluorescence. Sensors. 2020; 20(2):366. https://doi.org/10.3390/s20020366
Chicago/Turabian StyleChung, Tze-Wen, Chun-Yi Chang, Chun-Ning Chang, Chiu-Hsun Liao, Yun-Jen Jan, Li-Ting Chen, and Weng-Pin Chen. 2020. "Developing a Silk Fibroin Composite Film to Scavenge and Probe H2O2 Associated with UV-Excitable Blue Fluorescence" Sensors 20, no. 2: 366. https://doi.org/10.3390/s20020366
APA StyleChung, T.-W., Chang, C.-Y., Chang, C.-N., Liao, C.-H., Jan, Y.-J., Chen, L.-T., & Chen, W.-P. (2020). Developing a Silk Fibroin Composite Film to Scavenge and Probe H2O2 Associated with UV-Excitable Blue Fluorescence. Sensors, 20(2), 366. https://doi.org/10.3390/s20020366






