Opto-Microfluidic System for Absorbance Measurements in Lithium Niobate Device Applied to pH Measurements
Abstract
1. Introduction
2. Materials and Methods
2.1. Device Fabrication
2.1.1. Titanium Diffused Waveguides
2.1.2. Microfabrication of the Channel
2.2. Opto-Microfluidic Setup
2.3. pH Solutions
3. Results and Discussions
3.1. Device Design: Flexibility and Characterization
3.1.1. Waveguides Characterization
3.1.2. Microchannel Quality Fabrication
3.2. pH Sensing Based on Bromothymol Blue Absorbance
3.2.1. Bromothymol Blue Spectral Analysis
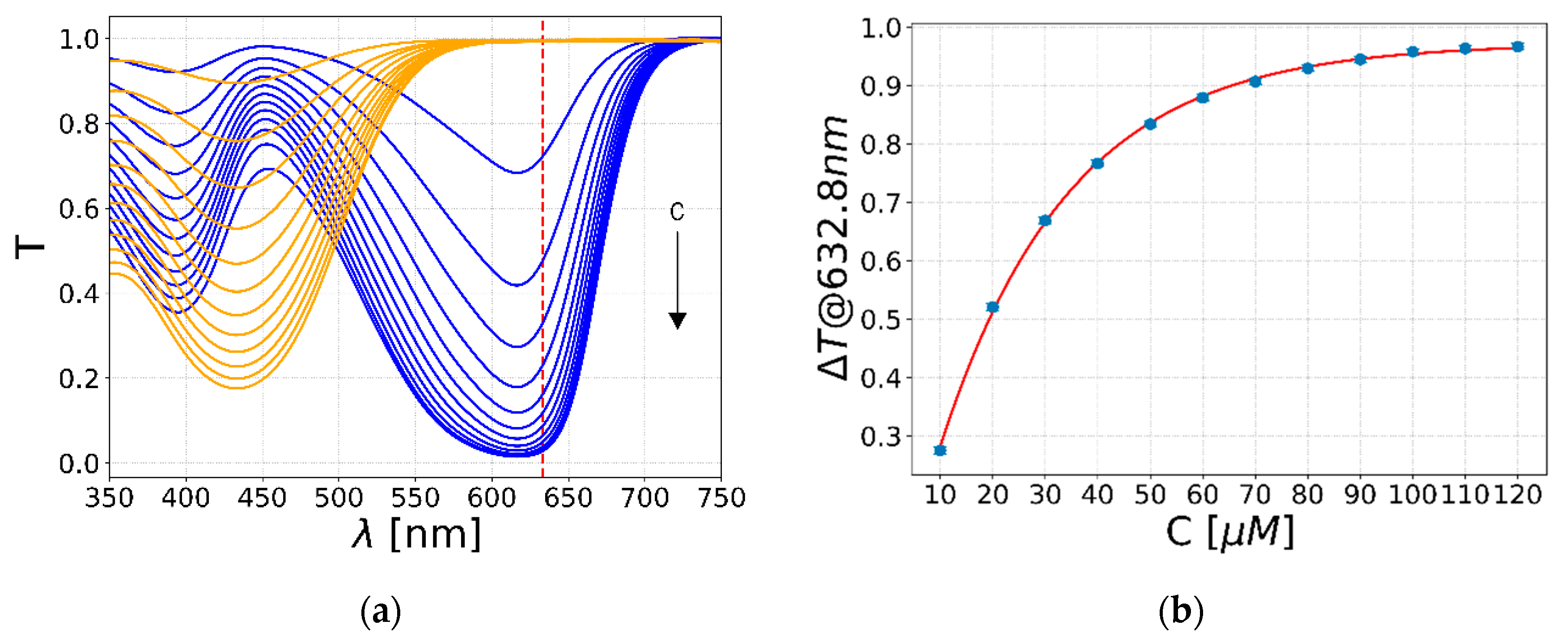
3.2.2. Bromothymol Blue Concentration
3.2.3. Integrated Optical pH Sensing Experiments
4. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Whitesides, G.M. The origins and the future of microfluidics. Nature 2006, 442, 368–373. [Google Scholar] [CrossRef]
- Haeberle, S.; Zengerle, R. Microfluidic platforms for lab-on-a-chip applications. Lab Chip 2007, 7, 1094–1110. [Google Scholar] [CrossRef]
- Mark, D.; Haeberle, S.; Roth, G.; Von Stetten, F.; Zengerle, R. Microfluidic Lab-on-a-Chip Platforms. In Requirements, Characteristics and Applications; Springer: Dordrecht, The Netherlands, 2010; pp. 305–376. [Google Scholar]
- Serra, M.; Ferraro, D.; Pereiro, I.; Viovy, J.L.; Descroix, S. The power of solid supports in multiphase and droplet-based microfluidics: Towards clinical applications. Lab Chip 2017, 17, 3979–3999. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, D.; Lin, Y.; Teste, B.; Talbot, D.; Malaquin, L.; Descroix, S.; Abou-Hassan, A. Continuous chemical operations and modifications on magnetic γ-Fe2O3 nanoparticles confined in nanoliter droplets for the assembly of fluorescent and magnetic SiO2@γ-Fe2O3. Chem. Commun. 2015, 51, 16904–16907. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Serra, M.; Pereiro, I.; Yamada, A.; Viovy, J.-L.; Descroix, S.; Ferraro, D. A simple and low-cost chip bonding solution for high pressure, high temperature and biological applications. Lab Chip 2017, 17, 629–634. [Google Scholar] [CrossRef]
- McDonald, J.C.; Whitesides, G.M. Poly(dimethylsiloxane) as a material for fabricating microfluidic devices. Acc. Chem. Res. 2002, 35, 491–499. [Google Scholar] [CrossRef] [PubMed]
- Tung, Y.C.; Zhang, M.; Lin, C.T.; Kurabayashi, K.; Skerlos, S.J. PDMS-based opto-fluidic micro flow cytometer with two-color, multi-angle fluorescence detection capability using PIN photodiodes. Sens. Actuators B Chem. 2004, 98, 356–367. [Google Scholar] [CrossRef]
- Nguyen, N.T.; Lassemono, S.; Chollet, F.A. Optical detection for droplet size control in microfluidic droplet-based analysis systems. Sens. Actuators B Chem. 2006, 117, 431–436. [Google Scholar] [CrossRef]
- Ferraro, D.; Serra, M.; Filippi, D.; Zago, L.; Guglielmin, E.; Pierno, M.; Descroix, S.; Viovy, J.L.; Mistura, G. Controlling the distance of highly confined droplets in a capillary by interfacial tension for merging on-demand. Lab Chip 2019, 19, 136–146. [Google Scholar] [CrossRef]
- Chabinyc, M.L.; Chiu, D.T.; McDonald, J.C.; Stroock, A.D.; Christian, J.F.; Karger, A.M.; Whitesides, G.M. An integrated fluorescence detection system in poly(dimethylsiloxane) for microfluidic applications. Anal. Chem. 2001, 73, 4491–4498. [Google Scholar] [CrossRef]
- Moradi, V.; Akbari, M.; Wild, P. A fluorescence-based pH sensor with microfluidic mixing and fiber optic detection for wide range pH measurements. Sens. Actuators A Phys. 2019, 297, 111507. [Google Scholar] [CrossRef]
- Mogensen, K.B.; El-Ali, J.; Wolff, A.; Kutter, J.P. Integration of polymer waveguides for optical detection in microfabricated chemical analysis systems. Appl. Opt. 2003, 42, 4072–4079. [Google Scholar] [CrossRef] [PubMed]
- Kee, J.S.; Poenar, D.P.; Neuzil, P.; Yobas, L. Monolithic integration of poly(dimethylsiloxane) waveguides and microfluidics for on-chip absorbance measurements. Sens. Actuators B Chem. 2008, 134, 532–538. [Google Scholar] [CrossRef]
- Bilenberg, B.; Nielsen, T.; Clausen, B.; Kristensen, A. PMMA to SU-8 bonding for polymer based lab-on-a-chip systems with integrated optics. J. Micromech. Microeng. 2004, 14, 814. [Google Scholar] [CrossRef]
- Balslev, S.; Bilenberg, B.; Geschke, O.; Jorgensen, A.M.; Kristensen, A.; Kutter, J.P.; Mogensen, K.B.; Snakenborg, D. Fully integrated optical system for lab-on-a-chip applications. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Maastricht, The Netherlands, 25–29 January 2004. [Google Scholar]
- Khoury, M.; Vannahme, C.; Sørensen, K.T.; Kristensen, A.; Berg-Sørensen, K. Monolithic integration of DUV-induced waveguides into plastic microfluidic chip for optical manipulation. Microelectron. Eng. 2014, 121, 5–9. [Google Scholar] [CrossRef]
- Wooten, E.L.; Kissa, K.M.; Yi-Yan, A.; Murphy, E.J.; Lafaw, D.A.; Hallemeier, P.F.; Maack, D.; Attanasio, D.V.; Fritz, D.J.; McBrien, G.J.; et al. Review of lithium niobate modulators for fiber-optic communications systems. IEEE J. Sel. Top. Quantum Electron. 2000, 6, 69–82. [Google Scholar] [CrossRef]
- Bazzan, M.; Sada, C. Optical waveguides in lithium niobate: Recent developments and applications. Appl. Phys. Rev. 2015, 2, 040603. [Google Scholar] [CrossRef]
- Chauvet, M.; Henrot, F.; Bassignot, F.; Devaux, F.; Gauthier-Manuel, L.; Pecheur, V.; Maillotte, H.; Dahmani, B. High efficiency frequency doubling in fully diced LiNbO3 ridge waveguides on silicon. J. Opt. 2016, 18, 085503. [Google Scholar] [CrossRef]
- Vittadello, L.; Zaltron, A.; Argiolas, N.; Bazzan, M.; Rossetto, N.; Signorini, R. Photorefractive direct laser writing. J. Phys. D Appl. Phys. 2016, 49, 125103. [Google Scholar] [CrossRef]
- Chauvet, M.; Al Fares, L.; Guichardaz, B.; Devaux, F.; Ballandras, S. Integrated optofluidic index sensor based on self-trapped beams in LiNbO3. Appl. Phys. Lett. 2012, 101, 181104. [Google Scholar] [CrossRef]
- Zaltron, A.; Bettella, G.; Pozza, G.; Zamboni, R.; Ciampolillo, M.; Argiolas, N.; Sada, C.; Kroesen, S.; Esseling, M.; Denz, C. Integrated optics on Lithium Niobate for sensing applications. In Proceedings of the SPIE—The International Society for Optical Engineering, Prague, Czech Republic, 5 May 2015; Volume 9506. [Google Scholar]
- Bettella, G.; Pozza, G.; Kroesen, S.; Zamboni, R.; Baggio, E.; Montevecchi, C.; Zaltron, A.; Gauthier-Manuel, L.; Mistura, G.; Furlan, C.; et al. Lithium niobate micromachining for the fabrication of microfluidic droplet generators. Micromachines 2017, 8, 185. [Google Scholar] [CrossRef]
- Bettella, G.; Zamboni, R.; Pozza, G.; Zaltron, A.; Montevecchi, C.; Pierno, M.; Mistura, G.; Sada, C.; Gauthier-Manuel, L.; Chauvet, M. LiNbO3 integrated system for opto-microfluidic sensing. Sens. Actuators B Chem. 2019, 282, 391–398. [Google Scholar] [CrossRef]
- García-Cabañes, A.; Blázquez-Castro, A.; Arizmendi, L.; Agulló-López, F.; Carrascosa, M. Recent achievements on photovoltaic optoelectronic tweezers based on lithium niobate. Crystals 2018, 8, 65. [Google Scholar] [CrossRef]
- Jubera, M.; García-Cabañes, A.; Olivares, J.; Alcazar, A.; Carrascosa, M. Particle trapping and structuring on the surface of LiNbO3:Fe optical waveguides using photovoltaic fields. Opt. Lett. 2014, 39, 649–652. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Fan, B.; Li, F.; Chen, L.; Shi, L.; Yan, W.; Zhang, Y.; Li, S.; Wang, X.; Wang, X.; Chen, H. Photovoltaic Manipulation of Water Microdroplets on a Hydrophobic LiNbO3 Substrate. Phys. Rev. Appl. 2017, 7, 064010. [Google Scholar] [CrossRef]
- Esseling, M.; Zaltron, A.; Horn, W.; Denz, C. Optofluidic droplet router. Laser Photonics Rev. 2015, 9, 98–104. [Google Scholar] [CrossRef]
- Jubera, M.; Elvira, I.; García-Cabañes, A.; Bella, J.L.; Carrascosa, M. Trapping and patterning of biological objects using photovoltaic tweezers. Appl. Phys. Lett. 2016, 108, 023703. [Google Scholar] [CrossRef]
- Lucchetti, L.; Kushnir, K.; Reshetnyak, V.; Ciciulla, F.; Zaltron, A.; Sada, C.; Simoni, F. Light-induced electric field generated by photovoltaic substrates investigated through liquid crystal reorientation. Opt. Mater. (Amst.) 2017, 73, 64–69. [Google Scholar] [CrossRef]
- Lucchetti, L.; Kushnir, K.; Ciciulla, F.; Zaltron, A.; Bettella, G.; Pozza, G.; Sada, C.; Reshetnyak, V.; Simoni, F. All-optical phase shifter with photovoltaic liquid crystal cell. Proc. SPIE 2016, 9940, 99400G. [Google Scholar]
- Bonfadini, S.; Ciciulla, F.; Criante, L.; Zaltron, A.; Simoni, F.; Reshetnyak, V.; Lucchetti, L. Optofluidic platform using liquid crystals in lithium niobate microchannel. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef]
- Lucchetti, L.; Kushnir, K.; Zaltron, A.; Simoni, F. Light controlled phase shifter for optofluidics. Opt. Lett. 2016, 41, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Grilli, S.; Miccio, L.; Vespini, V.; Finizio, A.; De Nicola, S.; Ferraro, P. Liquid micro-lens array activated by selective electrowetting on lithium niobate substrates. Opt. Express 2008, 16, 8084–8093. [Google Scholar] [CrossRef] [PubMed]
- Ferraro, P.; Coppola, S.; Grilli, S.; Paturzo, M.; Vespini, V. Dispensing nano-pico droplets and liquid patterning by pyroelectrodynamic shooting. Nat. Nanotechnol. 2010, 5, 429–435. [Google Scholar] [CrossRef] [PubMed]
- Miccio, L.; Behal, J.; Mugnano, M.; Memmolo, P.; Mandracchia, B.; Merola, F.; Grilli, S.; Ferraro, P. Biological Lenses as a Photomask for Writing Laser Spots into Ferroelectric Crystals. ACS Appl. Bio Mater. 2019, 2, 4675–4680. [Google Scholar] [CrossRef]
- Langelier, S.M.; Yeo, L.Y.; Friend, J. UV epoxy bonding for enhanced SAW transmission and microscale acoustofluidic integration. Lab Chip 2012, 12, 2970. [Google Scholar] [CrossRef]
- Collins, D.J.; Neild, A.; Ai, Y. Highly focused high-frequency travelling surface acoustic waves (SAW) for rapid single-particle sorting. Lab Chip 2016, 16, 471–479. [Google Scholar] [CrossRef]
- Du, X.Y.; Swanwick, M.E.; Fu, Y.Q.; Luo, J.K.; Flewitt, A.J.; Lee, D.S.; Maeng, S.; Milne, W.I. Surface acoustic wave induced streaming and pumping in 128° Y-cut LiNbO3 for microfluidic applications. J. Micromech. Microeng. 2009, 19, 35016. [Google Scholar] [CrossRef]
- Tseng, W.-K.; Lin, J.-L.; Sung, W.-C.; Chen, S.-H.; Lee, G.-B. Active micro-mixers using surface acoustic waves on Y-cut 128° LiNbO3. J. Micromech. Microeng. 2006, 16, 539. [Google Scholar] [CrossRef]
- Zhang, S.P.; Lata, J.; Chen, C.; Mai, J.; Guo, F.; Tian, Z.; Ren, L.; Mao, Z.; Huang, P.H.; Li, P.; et al. Digital acoustofluidics enables contactless and programmable liquid handling. Nat. Commun. 2018, 9, 1–11. [Google Scholar] [CrossRef]
- Persat, A.; Chambers, R.D.; Santiago, J.G. Basic principles of electrolyte chemistry for microfluidic electrokinetics. Part I: Acid-base equilibria and pH buffers. Lab Chip 2009, 9, 2437–2453. [Google Scholar] [CrossRef]
- Hegab, H.M.; ElMekawy, A.; Stakenborg, T. Review of microfluidic microbioreactor technology for high-throughput submerged microbiological cultivation. Biomicrofluidics 2013, 7, 021502. [Google Scholar] [CrossRef] [PubMed]
- Wencel, D.; Abel, T.; McDonagh, C. Optical chemical pH sensors. Anal. Chem. 2014, 86, 15–29. [Google Scholar] [CrossRef] [PubMed]
- Fouchet, S.; Carenco, A.; Daguet, C.; Guglielmi, R.; Riviere, L. Wavelength Dispersion of Ti Induced Refractive Index Change in LiNbO3 as a Function of Diffusion Parameters. J. Light. Technol. 1987, 5, 700–708. [Google Scholar] [CrossRef]
- Mai, T.D.; Ferraro, D.; Aboud, N.; Renault, R.; Serra, M.; Tran, N.T.; Viovy, J.L.; Smadja, C.; Descroix, S.; Taverna, M. Single-step immunoassays and microfluidic droplet operation: Towards a versatile approach for detection of amyloid-β peptide-based biomarkers of Alzheimer’s disease. Sens. Actuators B Chem. 2018, 255, 2126–2135. [Google Scholar] [CrossRef]
- Khan, W.; Choi, J.H.; Kim, G.M.; Park, S.Y. Microfluidic formation of pH responsive 5CB droplets decorated with PAA-b-LCP. Lab Chip 2011, 11, 3493–3498. [Google Scholar] [CrossRef]
- Keil, R.; Auracher, F. Coupling of single-mode Ti-diffused LiNbO3 waveguides to single-mode fibers. Opt. Commun. 1979, 30, 23–28. [Google Scholar] [CrossRef]
- Irrera, F.; Valli, M. Characterization of planar Ti:LiNbO3 optical waveguides in the visible and near-infrared spectral range. J. Appl. Phys. 1988, 64, 1704–1708. [Google Scholar] [CrossRef]
- Fares, L.A.; Devaux, F.; Guichardaz, B.; Chauvet, M. Self-trapped beams crossing tilted channels to induce guided polarization separators. Appl. Phys. Lett. 2013, 103, 041111. [Google Scholar] [CrossRef]
- Capel-Cuevas, S.; Cuéllar, M.P.; de Orbe-Payá, I.; Pegalajar, M.C.; Capitán-Vallvey, L.F. Full-range optical pH sensor based on imaging techniques. Anal. Chim. Acta 2010, 681, 71–81. [Google Scholar] [CrossRef]
- Puschett, J.B.; Rao, B.S.; Karandikar, B.M.; Matyjaszewski, K. Indicator characteristics of bromothymol blue derivatives. Talanta 1991, 38, 335–338. [Google Scholar] [CrossRef]
- Benkelfat, B.E.; Ferrière, R.; Wacogne, B.; Mollier, P. Technological implementation of Bragg Grating reflectors in Ti:LiNbO3 waveguides by proton exchange. IEEE Photonics Technol. Lett. 2002, 14, 1430–1432. [Google Scholar] [CrossRef]
- Lincoln, B.; Schinkinger, S.; Travis, K.; Wottawah, F.; Ebert, S.; Sauer, F.; Guck, J. Reconfigurable microfluidic integration of a dual-beam laser trap with biomedical applications. Biomed. Microdevices 2007, 9, 703–710. [Google Scholar] [CrossRef] [PubMed]
- Sridhar, M.; Maurya, D.K.; Friend, J.R.; Yeo, L.Y. Focused ion beam milling of microchannels in lithium niobate. Biomicrofluidics 2012, 6, 012819. [Google Scholar] [CrossRef] [PubMed]
- Welch, D.; Christen, J.B. Real-time feedback control of pH within microfluidics using integrated sensing and actuation. Lab Chip 2014, 14, 1191–1197. [Google Scholar] [CrossRef] [PubMed]








| Blade Thickness | Nominal Width | Resulting Width | Nominal Depth | Resulting Depth |
|---|---|---|---|---|
| 50 µm | 50 µm | 61 ± 1 µm | 100 µm | 98 ± 1 µm |
| 200 µm | 200 µm | 201 ± 1 µm | 100 µm | 99 ± 1 µm |
| Time Interval (min) | Qbasic (µL min−1) | Qacid (µL min−1) |
|---|---|---|
| 0–3 | 100 | 0 |
| 3–6 | 70 | 30 |
| 6–9 | 65 | 35 |
| 9–12 | 60 | 40 |
| 12–15 | 55 | 45 |
| 15–18 | 50 | 50 |
| 18–21 | 45 | 55 |
| 21–24 | 40 | 60 |
| 24–27 | 35 | 65 |
| 27–30 | 30 | 70 |
| 30–33 | 0 | 100 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zamboni, R.; Zaltron, A.; Izzo, E.; Bottaro, G.; Ferraro, D.; Sada, C. Opto-Microfluidic System for Absorbance Measurements in Lithium Niobate Device Applied to pH Measurements. Sensors 2020, 20, 5366. https://doi.org/10.3390/s20185366
Zamboni R, Zaltron A, Izzo E, Bottaro G, Ferraro D, Sada C. Opto-Microfluidic System for Absorbance Measurements in Lithium Niobate Device Applied to pH Measurements. Sensors. 2020; 20(18):5366. https://doi.org/10.3390/s20185366
Chicago/Turabian StyleZamboni, Riccardo, Annamaria Zaltron, Elena Izzo, Gregorio Bottaro, Davide Ferraro, and Cinzia Sada. 2020. "Opto-Microfluidic System for Absorbance Measurements in Lithium Niobate Device Applied to pH Measurements" Sensors 20, no. 18: 5366. https://doi.org/10.3390/s20185366
APA StyleZamboni, R., Zaltron, A., Izzo, E., Bottaro, G., Ferraro, D., & Sada, C. (2020). Opto-Microfluidic System for Absorbance Measurements in Lithium Niobate Device Applied to pH Measurements. Sensors, 20(18), 5366. https://doi.org/10.3390/s20185366









