Multimodal Non-Contact Luminescence Thermometry with Cr-Doped Oxides
Abstract
1. Introduction
2. Background
3. Methods
4. Results and Discussion
4.1. Temperature Dependence of the Intensity Ratio of Cr3+R-lines
4.2. Wavelength Shift of Cr3+ R-Lines with Temperature
4.3. Temperature Dependence of the Luminescence Decay Time
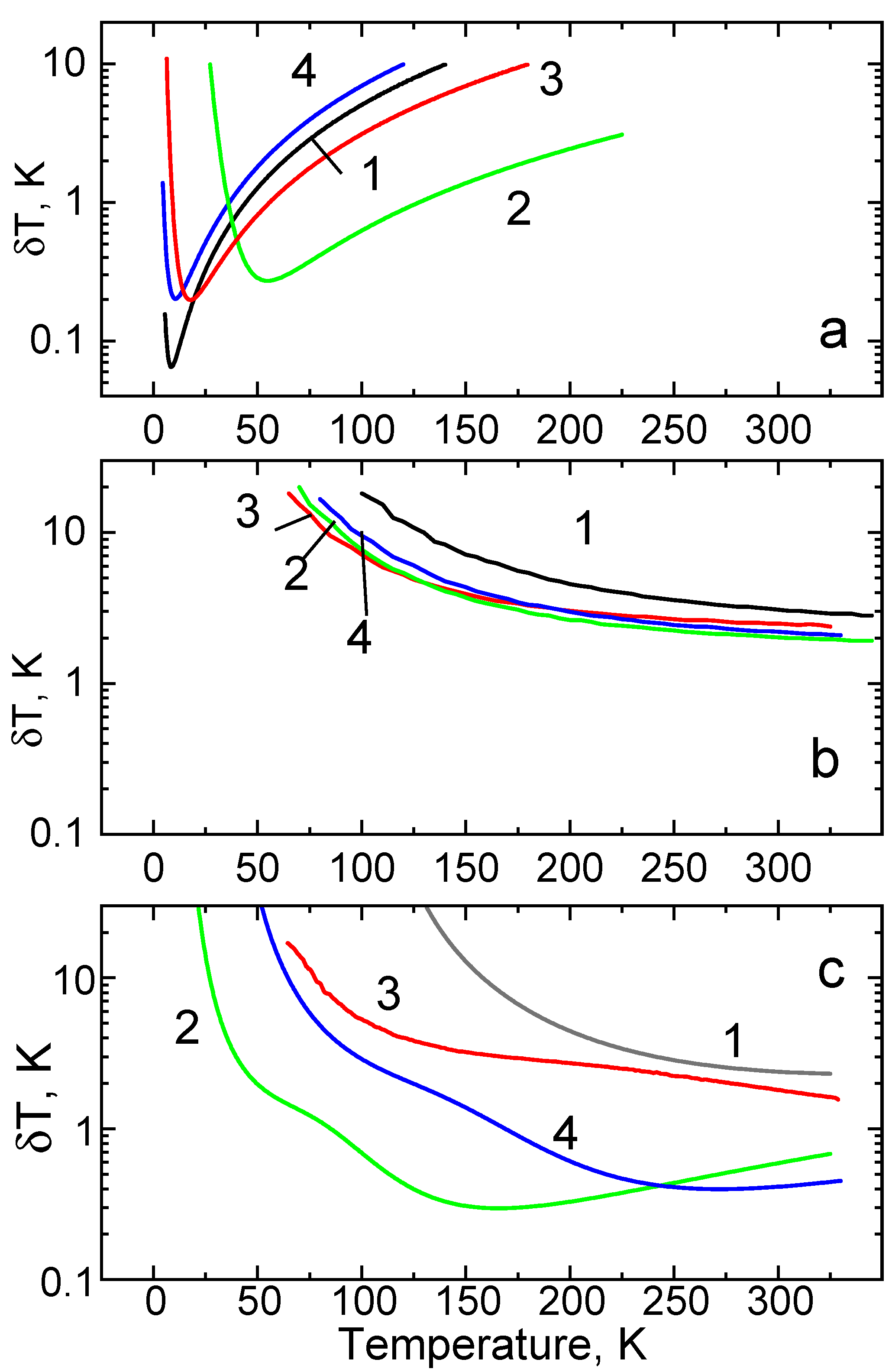
5. Comparison of Temperature Sensing Schemes
6. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Childs, P.R.N.; Greenwood, J.R.; Long, C.A. Review of temperature measurement. Rev. Sci Instrum. 2000, 71, 2959–2978. [Google Scholar] [CrossRef]
- Khalid, A.H.; Kontis, K. Thermographic. Phosphors for high temperature measurements: Principles, current state of the art and recent applications. Sensors 2008, 8, 5673–5744. [Google Scholar] [PubMed]
- Dramićanin, M. Luminescence thermometry. In Methods, Materials, and Applications; Woodhead Publishing: Cambridge, UK, 2018. [Google Scholar]
- Kim, M.M.; Giry, A.; Mastiani, M.; Rodrigues, G.O.; Reis, A.; Mandin, P. Microscale thermometry: A review. Microelectron. Eng. 2015, 148, 129–142. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Lima, P.P.; Silva, N.J.O.; Millan, A.; Amaral, V.S.; Palacio, F.; Carlos, L.D. Thermometry at the nanoscale. Nanoscale 2012, 4, 4799–4829. [Google Scholar] [CrossRef]
- Brubach, J.; Pflitsch, C.; Dreizler, A.; Atakan, B. On surface temperature measurements with thermographic phosphors: A review. Prog. Energ. Combust. 2013, 39, 37–60. [Google Scholar] [CrossRef]
- Wang, X.D.; Wolfbeis, O.S.; Meier, R.J. Luminescent probes and sensors for temperature. Chem. Soc. Rev. 2013, 42, 7834–7869. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Balabhadra, S.; Carlos, L.D. Lanthanide-based thermometers: At the cutting-edge of luminescence thermometry. Adv. Opt. Mater. 2019, 7, 1801239. [Google Scholar] [CrossRef]
- Homeyer, E.; Pailhes, S.; Debord, R.; Jary, V.; Dujardin, C.; Ledoux, G. Diamond contact-less micrometric temperature sensors. Appl. Phys. Lett. 2015, 106, 243502. [Google Scholar] [CrossRef]
- Vlaskin, V.A.; Janssen, N.; van Rijssel, J.; Beaulac, R.; Gamelin, D.R. Tunable dual emission in doped semiconductor nanocrystals. Nano. Lett. 2010, 10, 3670–3674. [Google Scholar] [CrossRef]
- Mahata, M.K.; Koppe, T.; Kumar, K.; Hofsass, H.; Vetter, U. Demonstration of temperature dependent energy migration in dual-mode YVO4: Ho3+/Yb3+ nanocrystals for low temperature thermometry. Sci. Rep. 2016, 6, 36342. [Google Scholar] [CrossRef]
- Wang, J.; Bu, Y.Y.; Wang, X.F.; Seo, H.J. A novel optical thermometry based on the energy transfer from charge transfer band to Eu3+-Dy3+ ions. Sci. Rep. 2017, 7, 6023. [Google Scholar] [CrossRef] [PubMed]
- Gharouel, S.; Labrador-Paez, L.; Haro-Gonzalez, P.; Horchani-Naifer, K.; Ferid, M. Fluorescence intensity ratio and lifetime thermometry of praseodymium phosphates for temperature sensing. J. Lumin. 2018, 201, 372–383. [Google Scholar] [CrossRef]
- Wang, Y.J.; Tsiumra, V.; Peng, Q.; Liang, H.B.; Zhydachevskyy, Y.; Chaika, M.; Dluzewski, P.; Przybylinska, H.; Suchocki, A. Hole trapping process and highly sensitive ratiometric thermometry over a wide temperature range in Pr3+-Doped Na2La2Ti3O10 layered perovskite microcrystals. J. Phys. Chem. A 2019, 123, 4021–4033. [Google Scholar] [CrossRef] [PubMed]
- Allison, S.W.; Beshears, D.L.; Cates, M.R.; Scudiere, M.B.; Shaw, D.W.; Ellis, A.D. Luminescence of YAG:Dy and YAG:Dy, Er crystals to 1700 °C. Meas. Sci. Technol. 2020, 31, 044001. [Google Scholar] [CrossRef]
- Allison, S.W.; Gillies, G.T. Remote thermometry with thermographic phosphors: Instrumentation and applications. Rev. Sci. Instrum. 1997, 68, 2615–2650. [Google Scholar] [CrossRef]
- Brubach, J.; Kissel, T.; Frotscher, M.; Euler, M.; Albert, B.; Dreizler, A. A survey of phosphors novel for thermography. J. Lumin. 2011, 131, 559–564. [Google Scholar] [CrossRef]
- Chambers, M.D.; Clarke, D.R. Doped oxides for high-temperature luminescence and lifetime thermometry. Annu. Rev. Mater. Res. 2009, 39, 325–359. [Google Scholar] [CrossRef]
- Ahmed, N.; Kraus, H.; Kim, H.J.; Mokina, V.; Tsiumra, V.; Wagner, A.; Zhydachevskyy, Y.; Mykhaylyk, V.B. Characterisation of tungstate and molybdate crystals ABO4 (A = Ca, Sr, Zn, Cd; B = W, Mo) for luminescence lifetime cryothermometry. Materialia 2018, 4, 287–296. [Google Scholar] [CrossRef]
- Pugh-Thomas, D.; Walsh, B.M.; Gupta, M.C. Spectroscopy of BeAl2O4:Cr3+ with application to high-temperature sensing. Appl. Opt. 2010, 49, 2891–2897. [Google Scholar] [CrossRef]
- Wang, X.F.; Liu, Q.; Bu, Y.Y.; Liu, C.S.; Liu, T.; Yan, X.H. Optical temperature sensing of rare-earth ion doped phosphors. RSC Adv. 2015, 5, 86219–86236. [Google Scholar] [CrossRef]
- Fuhrmann, N.; Brubach, J.; Dreizler, A. Phosphor thermometry: A comparison of the luminescence lifetime and the intensity ratio approach. Proc. Combust. Inst. 2013, 34, 3611–3618. [Google Scholar] [CrossRef]
- Yakunin, S.; Benin, B.M.; Shynkarenko, Y.; Nazarenko, O.; Bodnarchuk, M.I.; Dirin, D.N.; Hofer, C.; Cattaneo, S.; Kovalenko, M.V. High-resolution remote thermometry and thermography using luminescent low-dimensional tin-halide perovskites. Nat. Mater. 2019, 18, 846–852. [Google Scholar] [CrossRef]
- Cai, T.; Kim, D.; Kim, M.; Liu, Y.Z.; Kim, K.C. Two-dimensional thermographic phosphor thermometry in a cryogenic environment. Meas. Sci. Technol. 2017, 28, 015201. [Google Scholar] [CrossRef]
- Cates, M.R.; Beshears, D.L.; Allison, S.W.; Simmons, G.M. Phosphor thermometry at cryogenic temperatures. Rev. Sci. Instrum. 1997, 68, 2412–2417. [Google Scholar] [CrossRef]
- Bresson, F.; Devillers, R. Fluorescence temperature sensing on rotating samples in the cryogenic range. Rev. Sci. Instrum. 1999, 70, 3046–3051. [Google Scholar] [CrossRef]
- Liu, X.; Akerboom, S.; de Jong, M.; Mutikainen, I.; Tanase, S.; Meijerink, A.; Bouwman, E. Mixed-Lanthanoid Metal-O.ganic Framework for Ratiometric Cryogenic Temperature Sensing. Inorg. Chem. 2015, 54, 11323–11329. [Google Scholar] [CrossRef]
- Weik, M.; Colletier, J.P. Temperature-dependent macromolecular X-ray crystallography. Acta. Crystallogr. D 2010, 66, 437–446. [Google Scholar] [CrossRef] [PubMed]
- Ponomarenko, O.; Nikulin, A.Y.; Moser, H.O.; Yang, P.; Sakata, O. Radiation-induced melting in coherent X-ray diffractive imaging at the nanoscale. J. Synchrotron. Radiat. 2011, 18, 580–594. [Google Scholar] [CrossRef] [PubMed]
- Garman, E.F.; Weik, M. X-ray radiation damage to biological macromolecules: Further insights. J. Synchrotron. Radiat. 2017, 24, 1–6. [Google Scholar] [CrossRef]
- Russi, S.; González, A.; Kenner, L.R.; Keedy, D.A.; Fraser, J.S.; Bedem, H.V.D. Conformational variation of proteins at room temperature is not dominated by radiation damage. J. Synchrotron. Radiat. 2017, 24, 73–82. [Google Scholar] [CrossRef]
- Warkentin, M.; Atakişi, H.; Hopkins, J.B.; Walko, D.; Thorne, R.E. Lifetimes and spatio-temporal response of protein crystals in intense X-ray microbeams. IUCrJ 2017, 4, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Mykhaylyk, V.B.; Wagner, A.; Kraus, H. Non-contact luminescence lifetime cryothermometry for macromolecular crystallography. J. Synchrotron. Radiat. 2017, 24, 636–645. [Google Scholar] [CrossRef] [PubMed]
- Mykhaylyk, V.; Kraus, H.; Wägner, A. Non-Contact Luminescence Lifetime Microthermometry using Scintillation Sensors. Acta Phys. Pol. A 2018, 133, 1108–1111. [Google Scholar] [CrossRef]
- Warren, A.J.; Axford, D.; Owen, R.L. Direct measurement of X-ray-induced heating of microcrystals. J. Synchrotron. Radiat. 2019, 26, 991–997. [Google Scholar] [CrossRef]
- Mykhaylyk, V.B.; Kraus, H.; Bobb, L.; Gamernyk, R.; Koronski, K. Megahertz non-contact luminescence decay time cryothermometry by means of ultrafast PbI2 scintillator. Sci. Rep. 2019, 9, 5274. [Google Scholar] [CrossRef]
- Mikhailik, V.B.; Kraus, H. Scintillators for cryogenic applications: State-of-art. J. Phys. Stud. 2010, 14, 4201–4206. [Google Scholar] [CrossRef]
- Särner, G.; Richter, M.; Aldén, M. Two-dimensional thermometry using temperature-induced line shifts of ZnO:Zn and ZnO:Ga fluorescence. Opt. Lett. 2008, 33, 1327–1329. [Google Scholar] [CrossRef] [PubMed]
- Powell, R.C. Physics of Solid-State Laser Materials; Springer: Berlin/Heidelberg, Germany, 1998. [Google Scholar]
- Zhang, Z.; Grattan, K.T.V.; Palmer, A.W. Temperature dependences of fluorescence lifetimes in Cr3+-doped insulating crystals. Phys. Rev. B 1993, 48, 7772–7778. [Google Scholar] [CrossRef]
- Grinberg, M. 2E→4A2 fluorescence of Cr3+ in high and intermediate field garnets. J. Lumin. 1993, 54, 369–382. [Google Scholar] [CrossRef]
- McCumber, D.E.; Sturge, M.D. Linewidth and Temperature Shift of the R Lines in Ruby. J. Appl. Phys. 1963, 34, 1682. [Google Scholar] [CrossRef]
- Ragan, D.D.; Gustavsen, R.; Schiferl, D. Calibration of the ruby R1 and R2 fluorescence shifts as a function of temperature from 0 to 600 K. J. Appl. Phys. 1992, 72, 5539–5544. [Google Scholar] [CrossRef]
- Kumari, S.; Khare, A. Optical and structural characterization of pulsed laser deposited ruby thin films for temperature sensing application. Appl. Surf. Sci. 2013, 265, 180–186. [Google Scholar] [CrossRef]
- Weinstein, B.A. Ruby thermometer for cryobaric diamond anvil cell. Rev. Sci. Instrum. 1986, 57, 910–913. [Google Scholar] [CrossRef]
- Grattan, K.T.V.; Selli, R.K.; Palmer, A.W. Ruby fluorescence wavelength division fiber-optic temperature sensor. Rev. Sci. Instrum. 1987, 58, 1231–1234. [Google Scholar] [CrossRef]
- Grattan, K.T.V.; Selli, R.K.; Palmer, A.W. Ruby decay-time fluorescence thermometer in a fiber-optic configuration. Rev. Sci. Instrum. 1988, 59, 1328–1335. [Google Scholar] [CrossRef]
- Anghel, F.; Iliescu, C.; Grattan, K.T.V.; Palmer, A.W.; Zhang, Z.Y. Fluorescent-based lifetime measurement thermometer for use at subroom temperatures (200–300 K). Rev. Sci. Instrum. 1995, 66, 2611–2614. [Google Scholar] [CrossRef]
- Hu, Y.; Zhang, Z.; Grattan, K.; Palmer, A.; Meggitt, B. Ruby-based decay-time thermometry: Effect of probe size on extended measurement range (77–800 K). Sens. Actuators A Phys. 1997, 63, 85–90. [Google Scholar] [CrossRef]
- Seat, H.C.; Sharp, J.H. Dedicated temperature sensing with C-axis oriented single crystal ruby (Cr3+ Al2O3) fibers: Temperature and strain dependences of R-line fluorescence. IEEE Trans. Instrum. Meas. 2004, 53, 140–154. [Google Scholar] [CrossRef]
- Grattan, K.T.V.; Zhang, Z.Y.; Sun, T.; Shen, Y.H.; Tong, L.M.; Ding, Z.C. Sapphire-ruby single-crystal fibre for application in high temperature optical fibre thermometers: Studies at temperatures up to 1500 degrees C. Meas. Sci. Technol. 2001, 12, 981–986. [Google Scholar]
- Atakan, B.; Eckert, C.; Pflitsch, C. Light emitting diode excitation of Cr3+:Al2O3 as thermographic phosphor: Experiments and measurement strategy. Meas. Sci. Technol. 2009, 20, 75304. [Google Scholar] [CrossRef]
- Zhang, Z.Y.; Grattan, K.T.V.; Palmer, A.W.; Fernicola, V.; Crovini, L. Temperature dependence of the YAG:Cr3+ fluorescence lifetime over the range 77 to 900 K. Phys. Rev. B 1995, 51, 2656–2660. [Google Scholar] [CrossRef] [PubMed]
- Fuhrmann, N.; Kissel, T.; Dreizler, A.; Brübach, J. Gd3Ga5O12:Cr—A phosphor for two-dimensional thermometry in internal combustion engines. Meas. Sci. Technol. 2011, 22, 045301. [Google Scholar] [CrossRef]
- Uchiyama, H.; Aizawa, H.; Katsumata, T.; Komuro, S.; Morikawa, T.; Toba, E. Fiber-optic thermometer using Cr-doped YAlO3 sensor head. Rev. Sci. Instrum. 2003, 74, 3883–3885. [Google Scholar] [CrossRef]
- Eldridge, J.I.; Chambers, M.D. Fiber optic thermometer using Cr-doped GdAlO3 broadband emission decay. Meas. Sci. Technol. 2015, 26, 095202. [Google Scholar] [CrossRef]
- Zhiyi, Z.; Grattan, K.T.V.; Palmer, A.W. Fiber-optic high-temperature sensor based on the fluorescence lifetime of alexandrite. J. Appl. Phys. 1992, 63, 3869–3873. [Google Scholar]
- Aizawa, H.; Ohishi, N.; Ogawa, S.; Watanabe, E.; Katsumata, T.; Komuro, S.; Morikawa, T.; Toba, E. Characteristics of chromium doped spinel crystals for a fiber-optic thermometer application. Rev. Sci. Instrum. 2002, 73, 3089–3092. [Google Scholar] [CrossRef]
- Borisov, S.M.; Gatterer, K.; Bitschnau, B.; Klimant, I. Preparation and Characterization of Chromium(III)-Activated Yttrium Aluminum Borate: A New Thermographic Phosphor for Optical Sensing and Imaging at Ambient Temperatures. J. Phys. Chem. C 2010, 114, 9118–9124. [Google Scholar] [CrossRef]
- Back, M.; Trave, E.; Ueda, J.; Tanabe, S. Ratiometric Optical Thermometer Based on Dual Near-Infrared Emission in Cr3+-Doped Bismuth-Based Gallate Host. Chem. Mater. 2016, 28, 8347–8356. [Google Scholar] [CrossRef]
- Li, X.; Jiang, G.; Zhou, S.; Wei, X.; Chen, Y.; Duan, C.-K.; Yin, M. Luminescent properties of chromium(III)-doped lithium aluminate for temperature sensing. Sens. Actuators B Chem. 2014, 202, 1065–1069. [Google Scholar] [CrossRef]
- Chervin, J.C.; Canny, B.; Mancinelli, M. Ruby-spheres as pressure gauge for optically transparent high pressure cells. High Press. Res. 2003, 23, 493. [Google Scholar] [CrossRef]
- Brik, M.G.; Srivastava, A.M. Review—A Review of the Electronic Structure and Optical Properties of Ions with d(3) Electron Configuration (V2+, Cr3+, Mn4+, Fe5+) and Main Related Misconceptions. ECS J. Solid State Sci. Technol. 2018, 7, R3079–R3085. [Google Scholar] [CrossRef]
- Sugano, S. Multiplets of Transition-Metal Ions in Crystals; Academic Press: San Diego, CA, USA, 1970. [Google Scholar]
- Popa, A.; Toloman, D.; Stan, M.; Silipas, T.D.; Biris, A.R. Optical and electron paramagnetic resonance studies of Cr doped Ga2O3 nanoparticles. In 10th International Conference Processes in Isotopes and Molecules (pim 2015); AIP Publishing: College Park, MD, USA, 2015; p. 060006. [Google Scholar]
- Struve, B.; Huber, G. The effect of the crystal field strength on the optical spectra of Cr3+ in gallium garnet laser crystals. Appl. Phys. A 1985, 36, 195–201. [Google Scholar] [CrossRef]
- Brik, M.; Camardello, S.J.; Srivastava, A.M.; Avram, N.M.; Suchocki, A. Spin-Forbidden Transitions in the Spectra of Transition Metal Ions and Nephelauxetic Effect. ECS J. Solid State Sci. Technol. 2015, 5, R3067–R3077. [Google Scholar] [CrossRef]
- Yamaga, M.; Takeuchi, H.; Han, T.P.J.; Henderson, B. Electron paramagnetic resonance and optical spectra of Cr3+-doped YAlO3. J. Phys. Condens. Matter 1993, 5, 8097–8104. [Google Scholar] [CrossRef]
- Weber, M.J.; Varitimos, T.E. Optical-Spectra and Relaxation of Cr3+ Ions in YAlO3. J. Appl. Phys. 1974, 45, 810–816. [Google Scholar] [CrossRef]
- Walsh, C.G.; Donegan, J.F.; Glynn, T.J.; Morgan, G.P.; Imbusch, G.F.; Remeika, J.P. Luminescence from β -Ga2O3-Cr3+. J. Lumin. 1988, 40–41, 103–104. [Google Scholar] [CrossRef]
- Vivien, D.; Viana, B.; Revcolevschi, J.D.; Barrie, A.; Dunn, B.; Nelson, P.; Stafsudd, O.M. Stafsudd, Optical-Properties of β-Ga2O3-Cr3+ Single-Crystals for Tunable Laser Applications. J. Lumin. 1987, 39, 29–33. [Google Scholar] [CrossRef]
- Tokida, Y.; Adachi, S. Photoluminescence spectroscopy and energy-level analysis of metal-organic-deposited Ga2O3:Cr3+ films. J. Appl. Phys. 2012, 112, 63522. [Google Scholar] [CrossRef]
- Yeom, T.H.; Kim, I.G.; Lee, S.H.; Choh, S.H.; Yu, Y.M. Electron paramagnetic resonance characterization of Cr3+ impurities in a beta-Ga2O3 single crystal. J. Appl. Phys. 2003, 93, 3315–3319. [Google Scholar] [CrossRef]
- Wade, S.; Collins, S.F.; Baxter, G.W. Fluorescence intensity ratio technique for optical fiber point temperature sensing. J. Appl. Phys. 2003, 94, 4743. [Google Scholar] [CrossRef]
- Luchechko, A.; Vasyltsiv, V.; Zhydachevskyy, Y.; Kushlyk, M.; Ubizskii, S.; Suchocki, A.; Zhydachevskii, Y. Luminescence spectroscopy of Cr3+ ions in bulk single crystalline β-Ga2O3. J. Phys. D Appl. Phys. 2020, 53, 354001. [Google Scholar] [CrossRef]
- Dorogokupets, P.I.; Ponomarev, E.M.; Melekhova, E.A. Optimization of experimental data on the heat capacity, volume, and bulk moduli of minerals. Petrology 1999, 7, 574–591. [Google Scholar]
- Senyshyn, A.; Vasylechko, L. Low Temperature Crystal Structure Behaviour of Complex Yttrium Aluminium Oxides YAlO3 and Y3Al5O {12}. Acta Phys. Pol. A 2013, 124, 329–335. [Google Scholar] [CrossRef]
- Aggarwal, R.L.; Ripin, D.J.; Ochoa, J.R.; Fan, T.Y. Measurement of thermo-optic properties of Y3Al5O12, Lu3Al5O12, YAIO(3), LiYF4, LiLuF4, BaY2F8, KGd(WO4)(2), and KY(WO4)(2) laser crystals in the 80–300 K temperature range. J. Appl. Phys. 2005, 98, 103514. [Google Scholar] [CrossRef]
- Guo, Z.; Verma, A.; Wu, X.F.; Sun, F.Y.; Hickman, A.; Masui, T.; Kuramata, A.; Higashiwaki, M.; Jena, D.; Luo, T.F. Anisotropic thermal conductivity in single crystal beta-gallium oxide. Appl. Phys. Lett. 2015, 106, 111909. [Google Scholar] [CrossRef]
- Nelson, D.F.; Sturge, M.D. Relation between Absorption and Emission in the Region of the R Lines of Ruby. Phys. Rev. 1965, 137, A1117–A1130. [Google Scholar] [CrossRef]
- Walling, J.; Peterson, O.; Jenssen, H.; Morris, R.; O’Dell, E. Tunable alexandrite lasers. IEEE J. Quantum. Electron. 1980, 16, 1302–1315. [Google Scholar] [CrossRef]
- Zhang, Z.; Grattan, K. Temperature dependence of YAG: Cr3+ fluorescence lifetime up to 550 K. J. Lumin. 1994, 62, 263–269. [Google Scholar] [CrossRef]
- Shen, Y.R.; Bray, K.L. Effect of pressure and temperature on the lifetime of Cr3+ in yttrium aluminum garnet. Phys. Rev. B 1997, 56, 10882–10891. [Google Scholar] [CrossRef]
- Suchocki, A.; Gilliland, G.D.; Powell, R.C.; Bowen, J.M.; Walling, J.C. Spectroscopic properties of alexandrite crystals II. J. Lumin. 1987, 37, 29–37. [Google Scholar] [CrossRef]
- Zhydachevskii, Y.; Galanciak, D.; Kobyakov, S.; Berkowski, M.; Kaminska, A.; Suchocki, A.; Zakharko, Y.; Durygin, A. Photoluminescence studies of Mn4+ions in YAlO3 crystals at ambient and high pressure. J. Phys. Condens. Matter 2006, 18, 11385–11396. [Google Scholar] [CrossRef]
- Back, M.; Ueda, J.; Xu, J.; Murata, D.; Brik, M.G.; Tanabe, S. Ratiometric Luminescent Thermometers with a Customized Phase-Transition-Driven Fingerprint in Perovskite Oxides. ACS Appl. Mater. Interfaces 2019, 11, 38937–38945. [Google Scholar] [CrossRef] [PubMed]
- Glais, E.; Đorđević, V.; Papan, J.; Viana, B.; Dramićanin, M.D. MgTiO3: Mn4+ a multi-reading temperature nanoprobe. RSC Adv. 2018, 8, 18341–18346. [Google Scholar] [CrossRef]
- Venturini, F.; Bürgi, R.; Borisov, S.M.; Klimant, I. Optical temperature sensing using a new thermographic phosphor. Sens. Actuators A Phys. 2015, 233, 324–329. [Google Scholar] [CrossRef]
- Brites, C.D.S.; Martínez, E.D.; Urbano, R.R.; Rettori, C.; Carlos, L.D. Self-Calibrated Double Luminescent Thermometers Through Upconverting Nanoparticles. Front. Chem. 2019, 7, 267. [Google Scholar] [CrossRef]
- Eldridge, J.I. Luminescence decay-based Y2O3:Er phosphor thermometry: Temperature sensitivity governed by multiphonon emission with an effective phonon energy transition. J. Lumin. 2019, 214, 116535. [Google Scholar] [CrossRef]
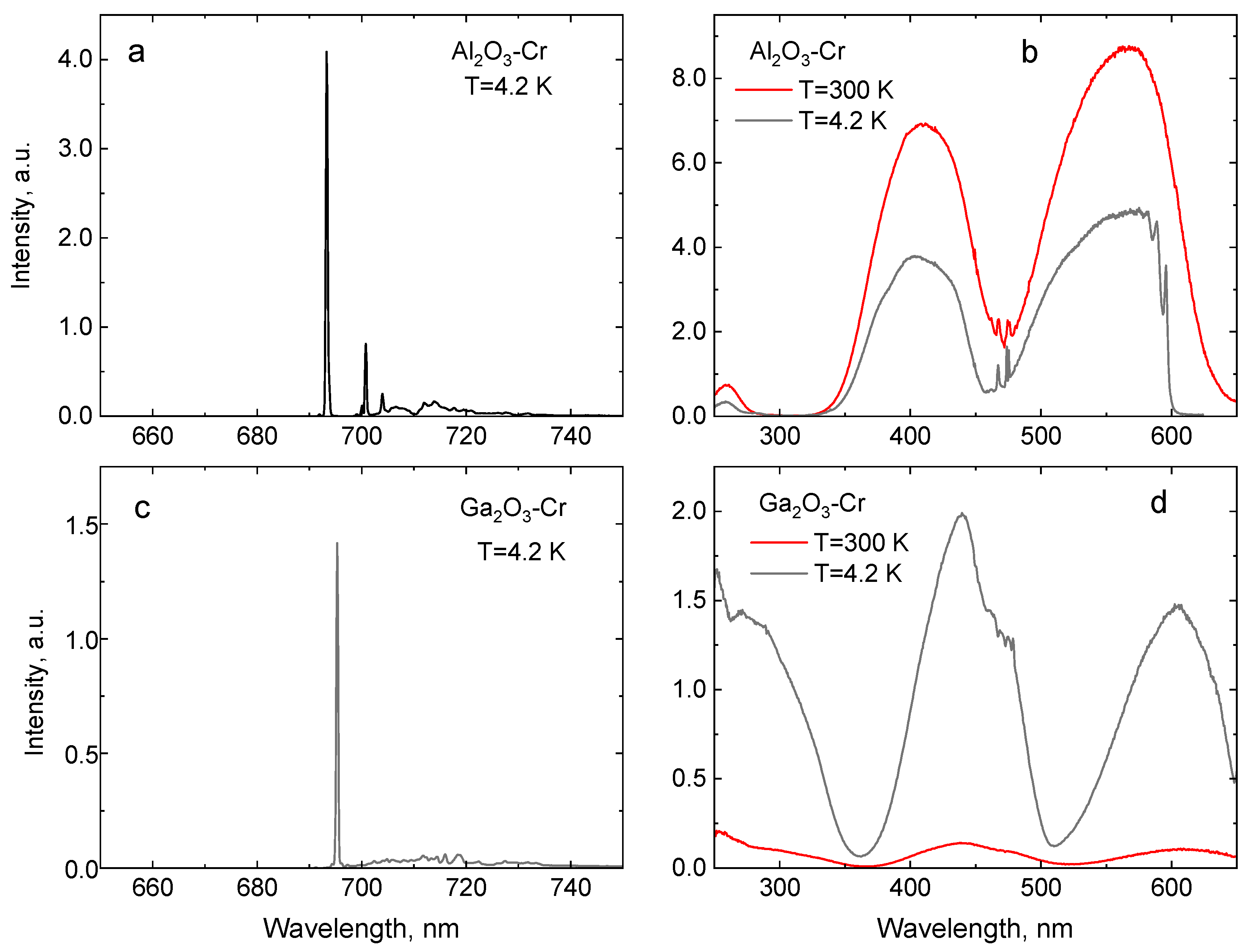
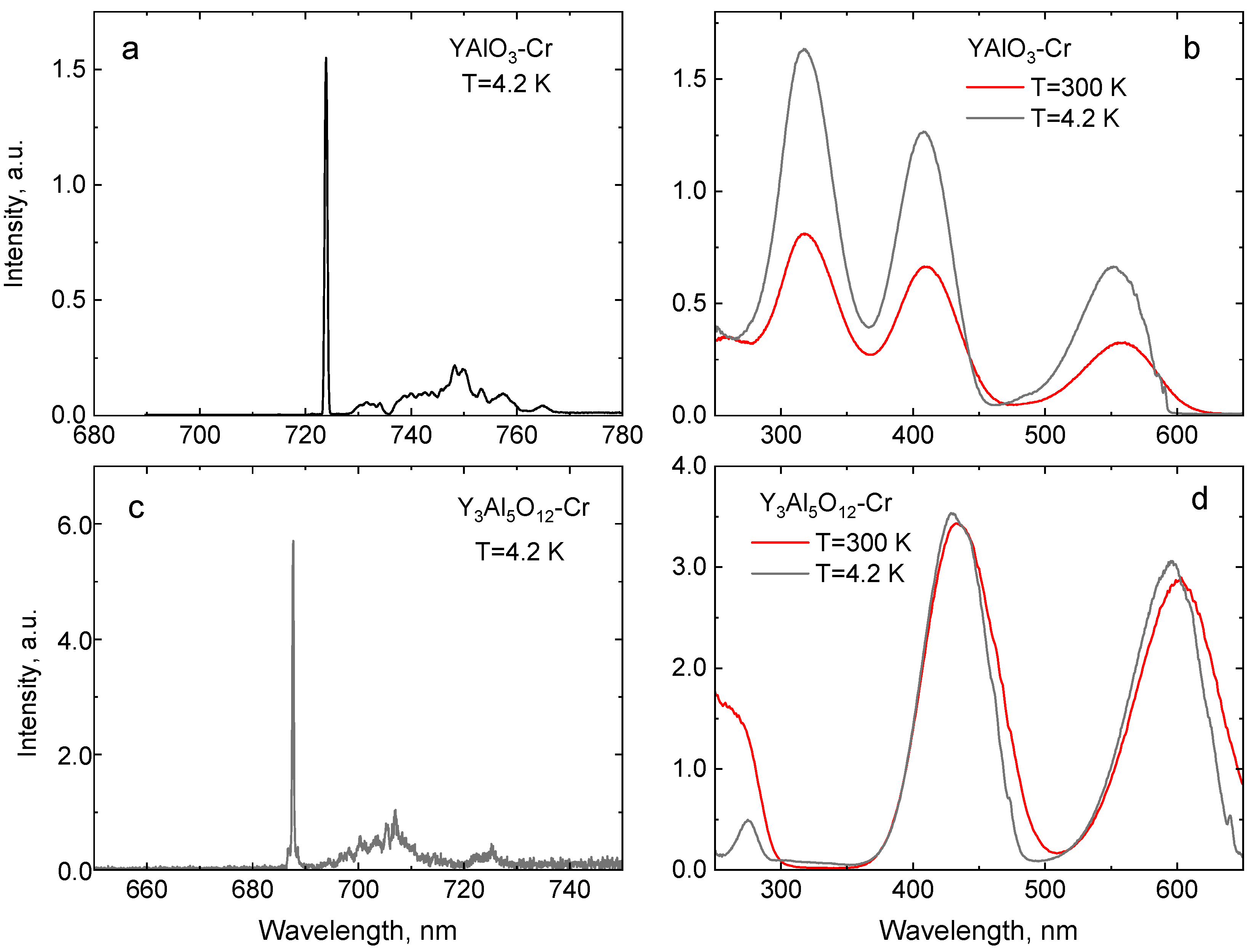
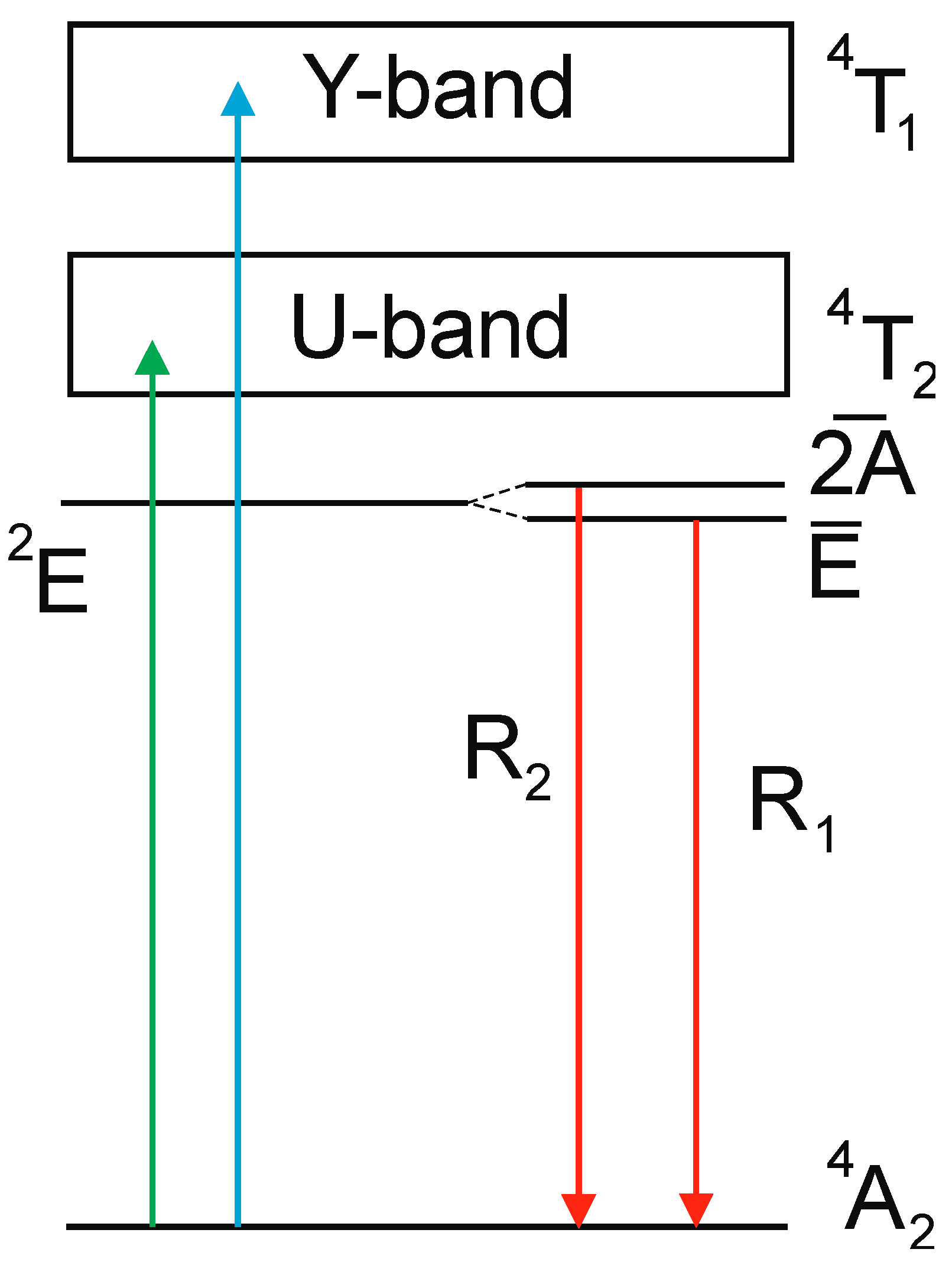
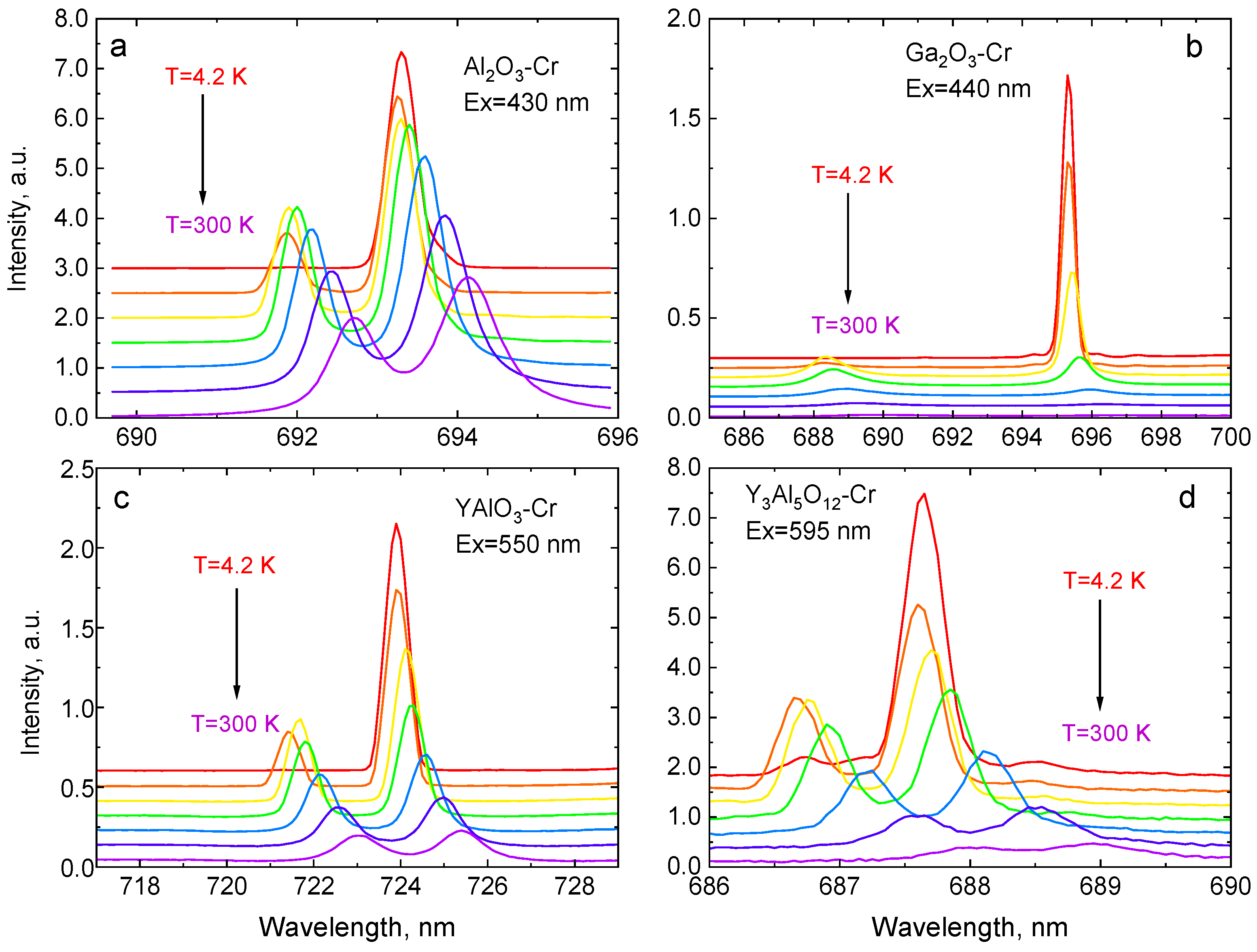
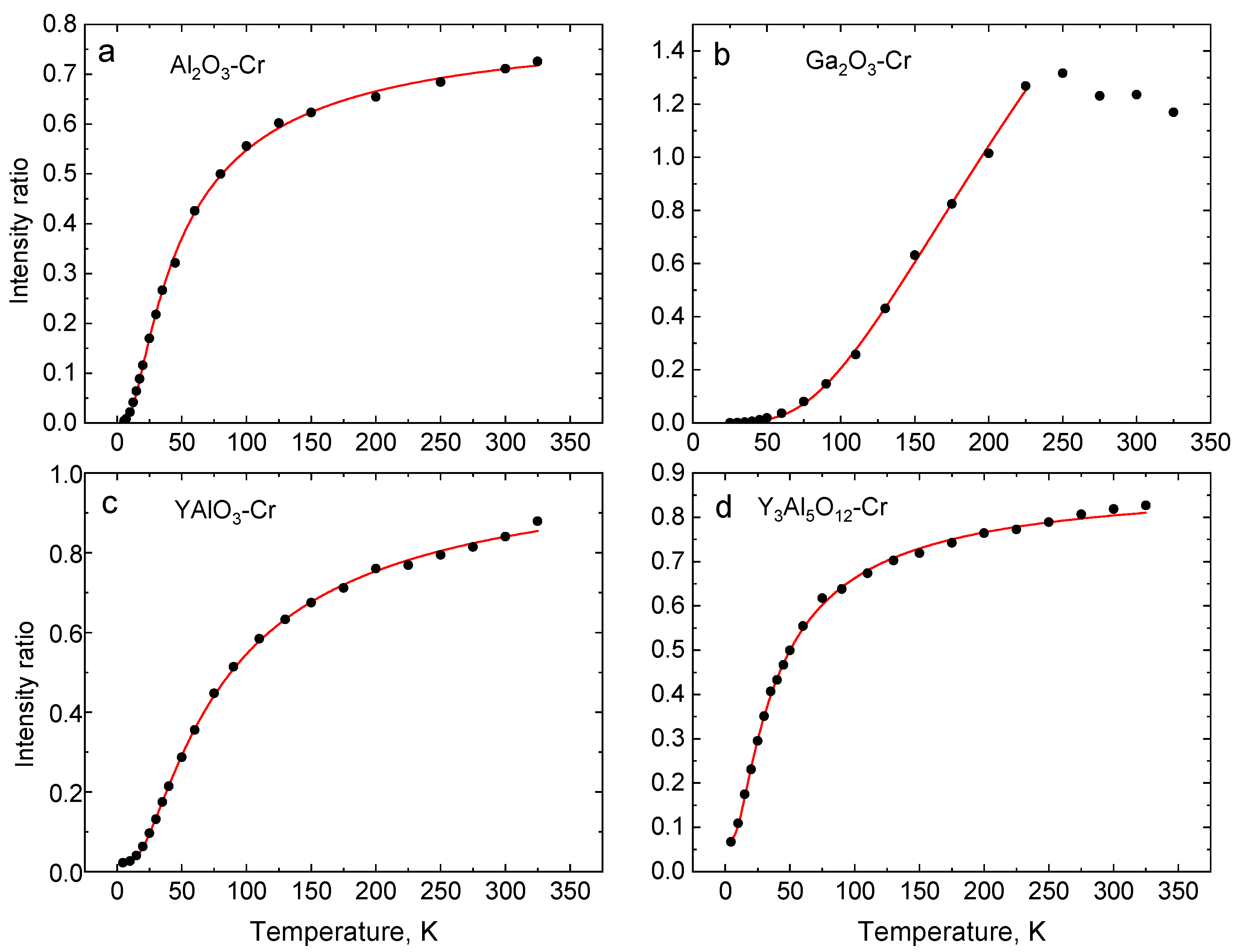
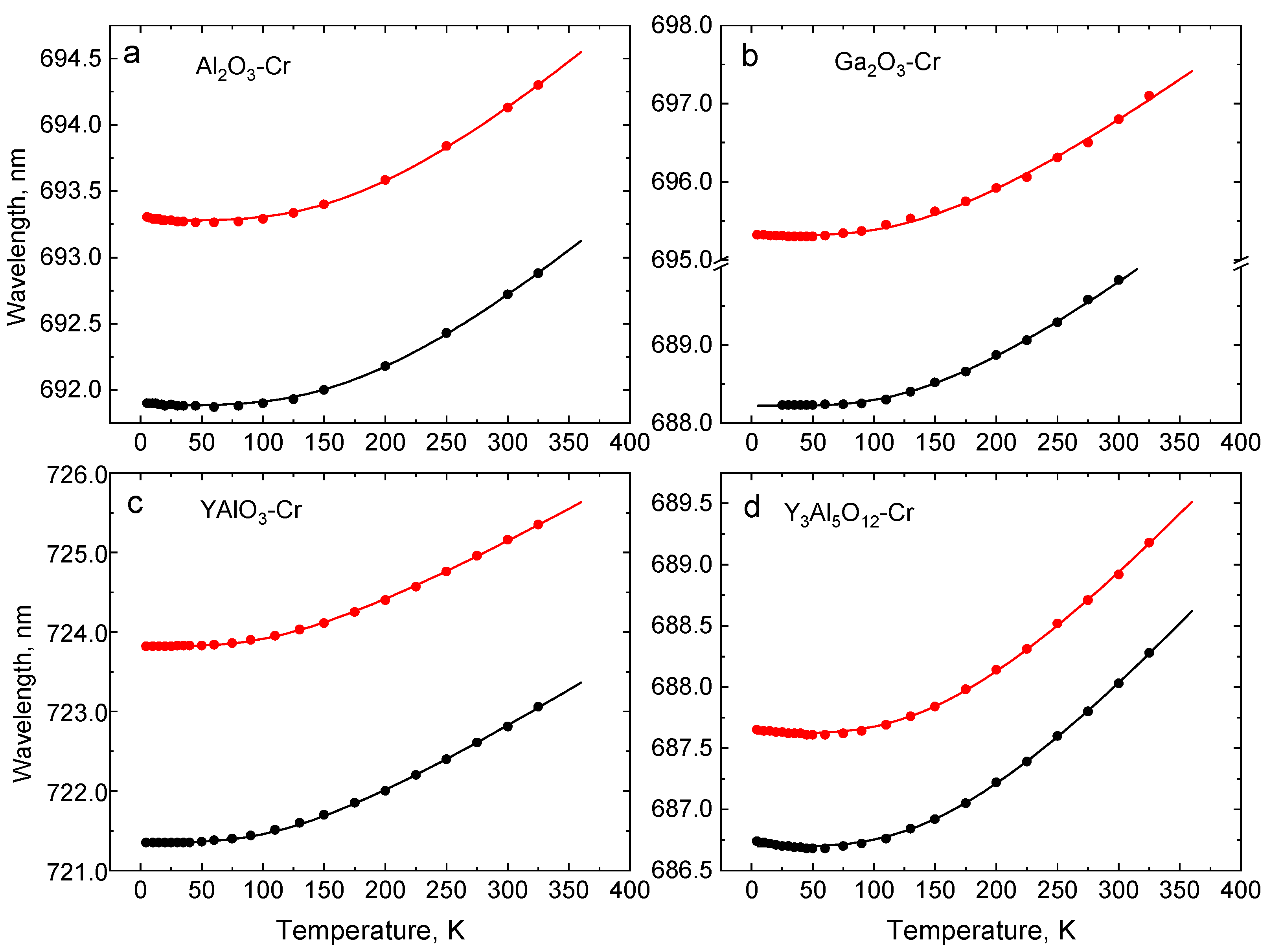

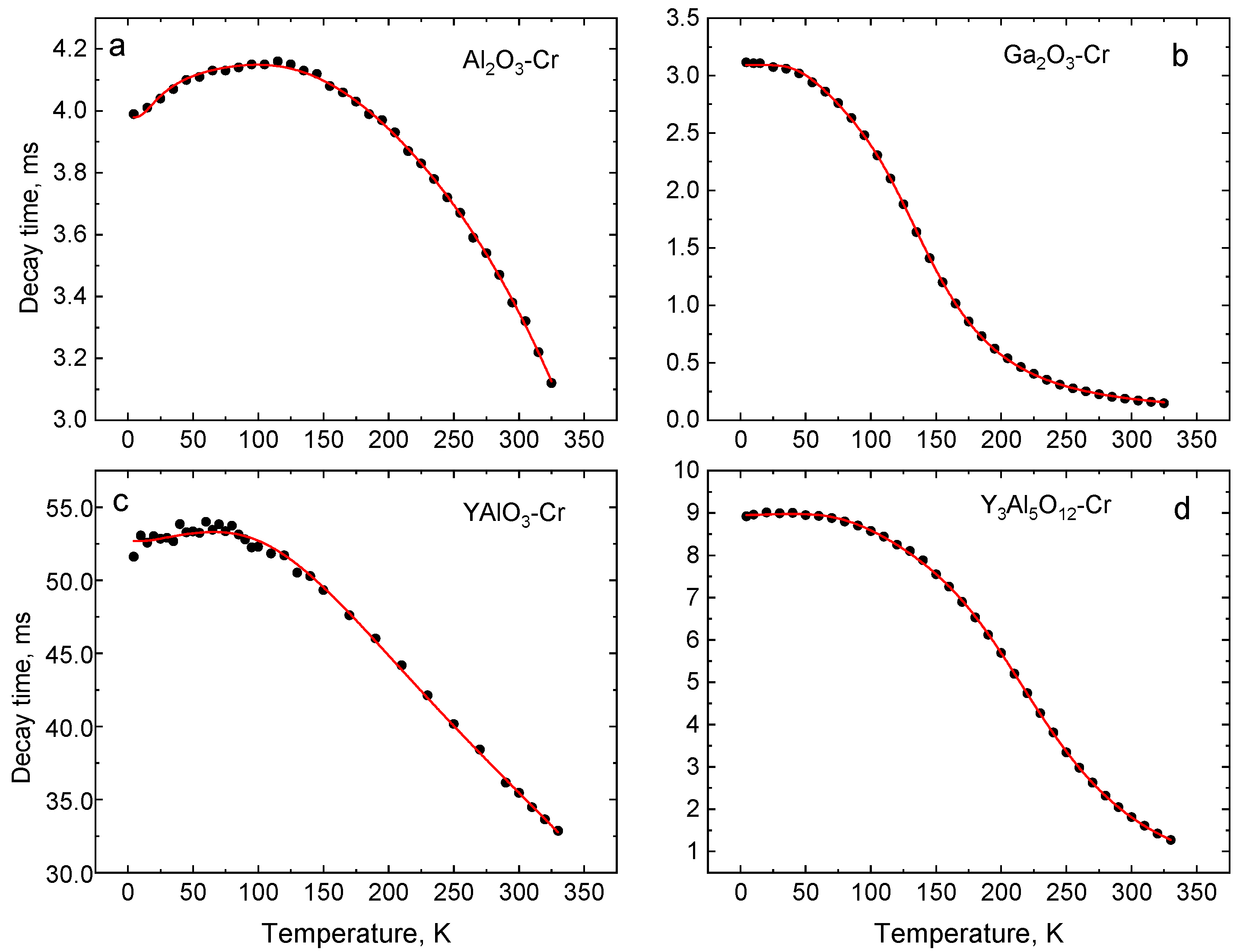
| Crystal | Band Gap, eV | R1-R2 Gap, meV | 4A2→4T1g (Y-BAND), nm | 4A2→4T2g (U-Band), nm | 2E-4T2g Gap, meV | ||
|---|---|---|---|---|---|---|---|
| Al2O3 | 9.4 | 693.3 | 692.7 | 3.6 | 411 | 568 | 290 |
| Ga2O3 | 4.85 | 695.3 | 689.7 | 18.2 | 441 | 608 | 80 |
| YAlO3 | 8.5 | 723.9 | 723.0 | 5.9 | 422 | 564 | 380 |
| Y3Al5O12 | 7.1 | 687.7 | 688.9 | 2.4 | 434 | 601 | 135 |
| Parameter | Al2O3-Cr | Ga2O3-Cr | YAlO3-Cr | Y3Al5O12-Cr |
|---|---|---|---|---|
| A | 0.809 ± 0.004 | 5.39 ± 0.19 | 1.019 ± 0.004 | 0.814 ± 0.008 |
| D, meV | 3.36 ± 0.04 | 28.4 ± 0.6 | 5.8 ± 0.1 | 2.8 ± 0.1 |
| Parameter | Al2O3-Cr | Ga2O3-Cr | YAlO3-Cr | Y3Al5O12-Cr |
|---|---|---|---|---|
| , cm−1 | −518.0 | −534.2 | −284.5 | −570.7 |
| , cm−1 | −507.5 | −582.8 | −317.8 | −581.1 |
| TD, K | 867 | 682 | 541 | 739 |
| Parameter | Al2O3-Cr | Ga2O3-Cr | YAlO3-Cr | Y3Al5O12-Cr |
|---|---|---|---|---|
| 1, ms | 3.98 ± 0.01 | 3.09 ± 0.01 | 52.6 ± 0.2 | 8.94 ± 0.03 |
| 2, ms | 4.46 ± 0.01 | 1.02 ± 0.04 | 55.4 ± 1.0 | 9.04 ± 0.08 |
| Ep, meV | 60.9 ± 0.4 | 35.7 ± 0.3 | 41.4 ± 0.6 | 32.6 ± 0.3 |
| D *, meV | 3.6 | 18.2 | 5.9 | 2.4 |
| 3, μs | 33 ± 25 | 30.1 ± 1.4 | 263 ± 28 | 32.6 ± 0.3 |
| ΔE, meV | 237 ± 23 | 83 ± 1 | 279 ± 36 | 138 ± 0.7 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mykhaylyk, V.; Kraus, H.; Zhydachevskyy, Y.; Tsiumra, V.; Luchechko, A.; Wagner, A.; Suchocki, A. Multimodal Non-Contact Luminescence Thermometry with Cr-Doped Oxides. Sensors 2020, 20, 5259. https://doi.org/10.3390/s20185259
Mykhaylyk V, Kraus H, Zhydachevskyy Y, Tsiumra V, Luchechko A, Wagner A, Suchocki A. Multimodal Non-Contact Luminescence Thermometry with Cr-Doped Oxides. Sensors. 2020; 20(18):5259. https://doi.org/10.3390/s20185259
Chicago/Turabian StyleMykhaylyk, Vitaliy, Hans Kraus, Yaroslav Zhydachevskyy, Volodymyr Tsiumra, Andriy Luchechko, Armin Wagner, and Andrzej Suchocki. 2020. "Multimodal Non-Contact Luminescence Thermometry with Cr-Doped Oxides" Sensors 20, no. 18: 5259. https://doi.org/10.3390/s20185259
APA StyleMykhaylyk, V., Kraus, H., Zhydachevskyy, Y., Tsiumra, V., Luchechko, A., Wagner, A., & Suchocki, A. (2020). Multimodal Non-Contact Luminescence Thermometry with Cr-Doped Oxides. Sensors, 20(18), 5259. https://doi.org/10.3390/s20185259






