Recent Advances in Electrochemical Monitoring of Chromium
Abstract
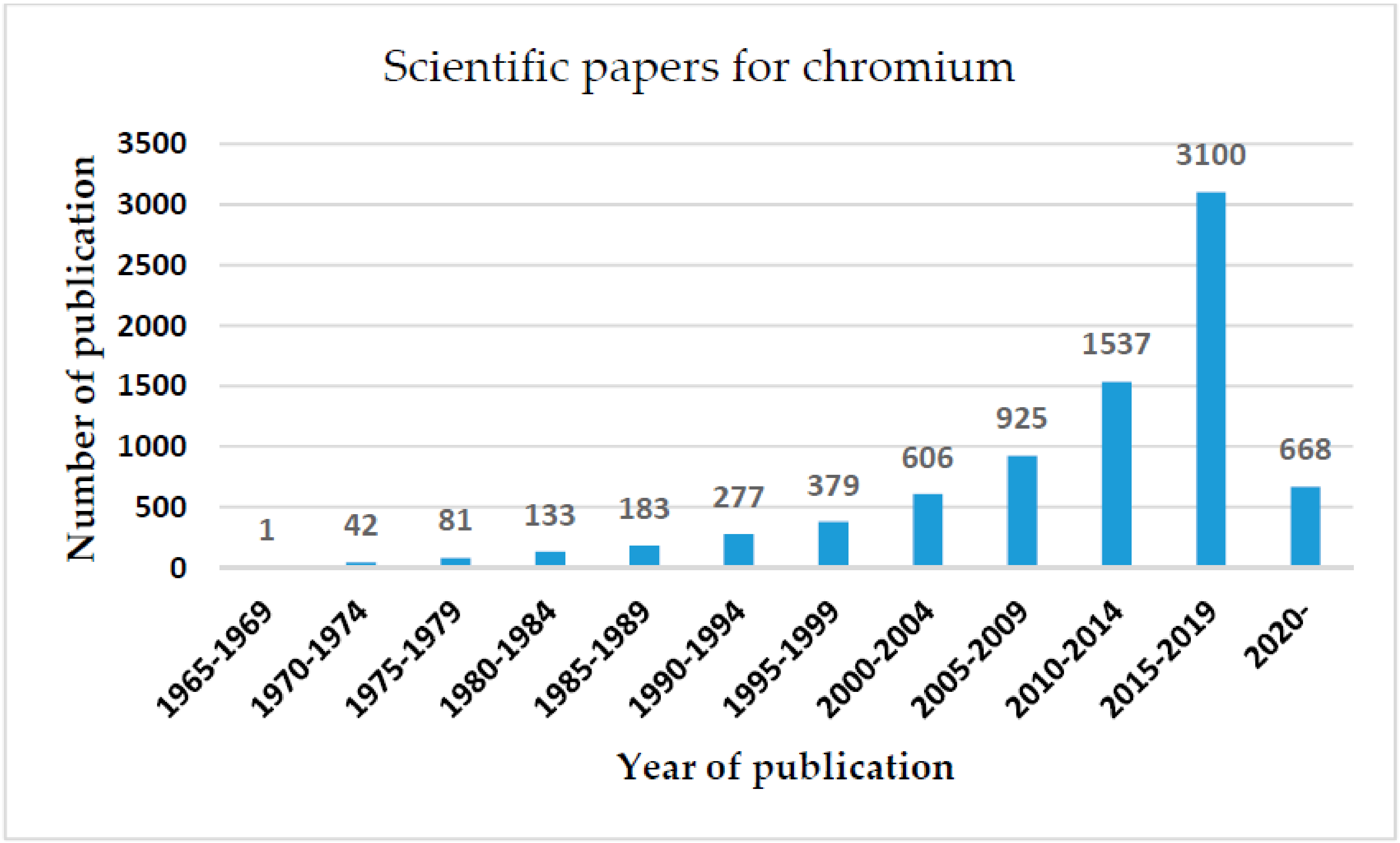
1. Introduction
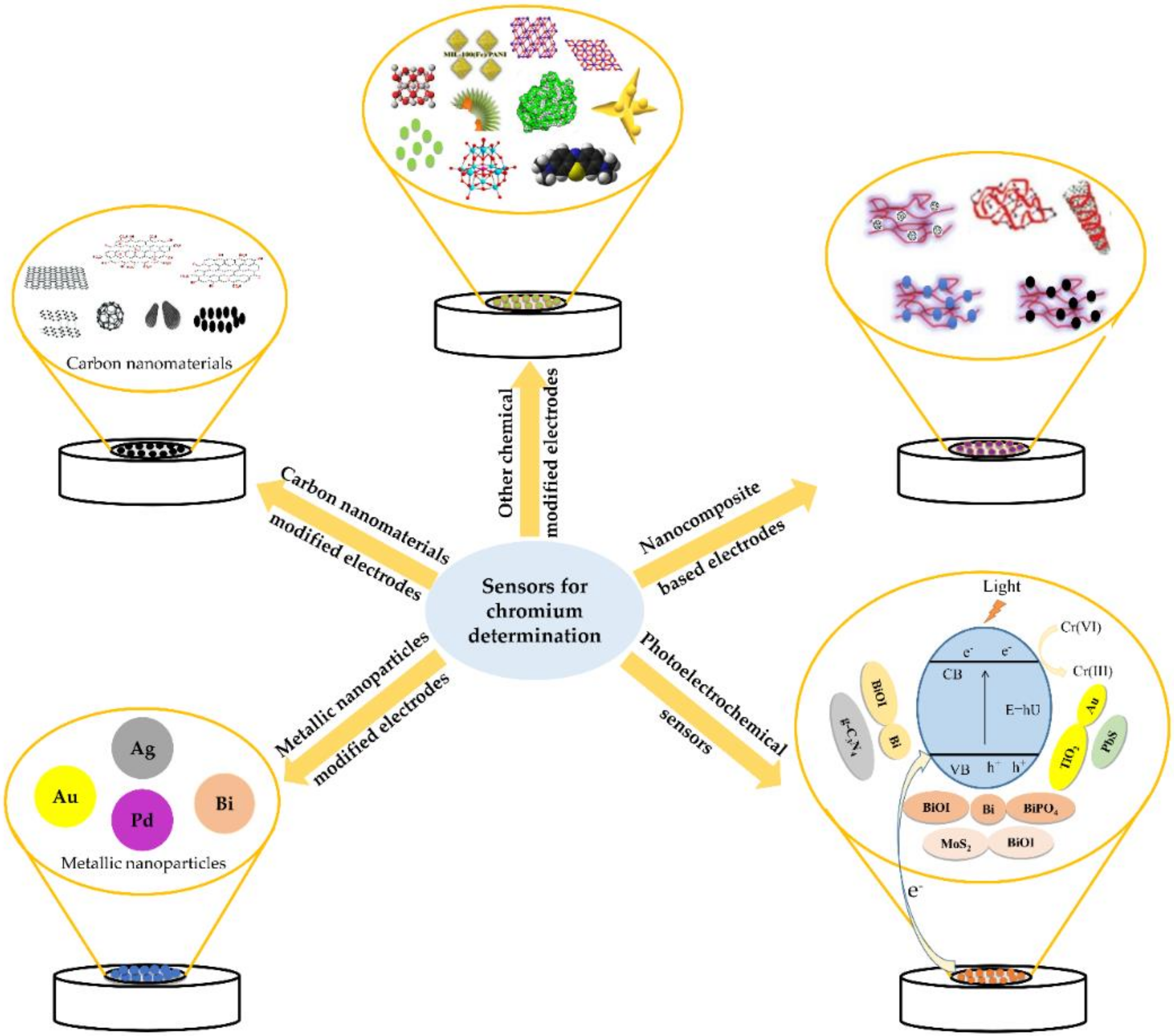
2. Electrochemical Sensors for Hexavalent Chromium Determination
2.1. Carbon Nanomaterials Based-Electrodes
2.2. Metallic Nanoparticles-Based Electrodes
2.3. Nanocomposite Based-Electrodes
2.4. Photoelectrochemical Sensor
2.5. Other Sensors
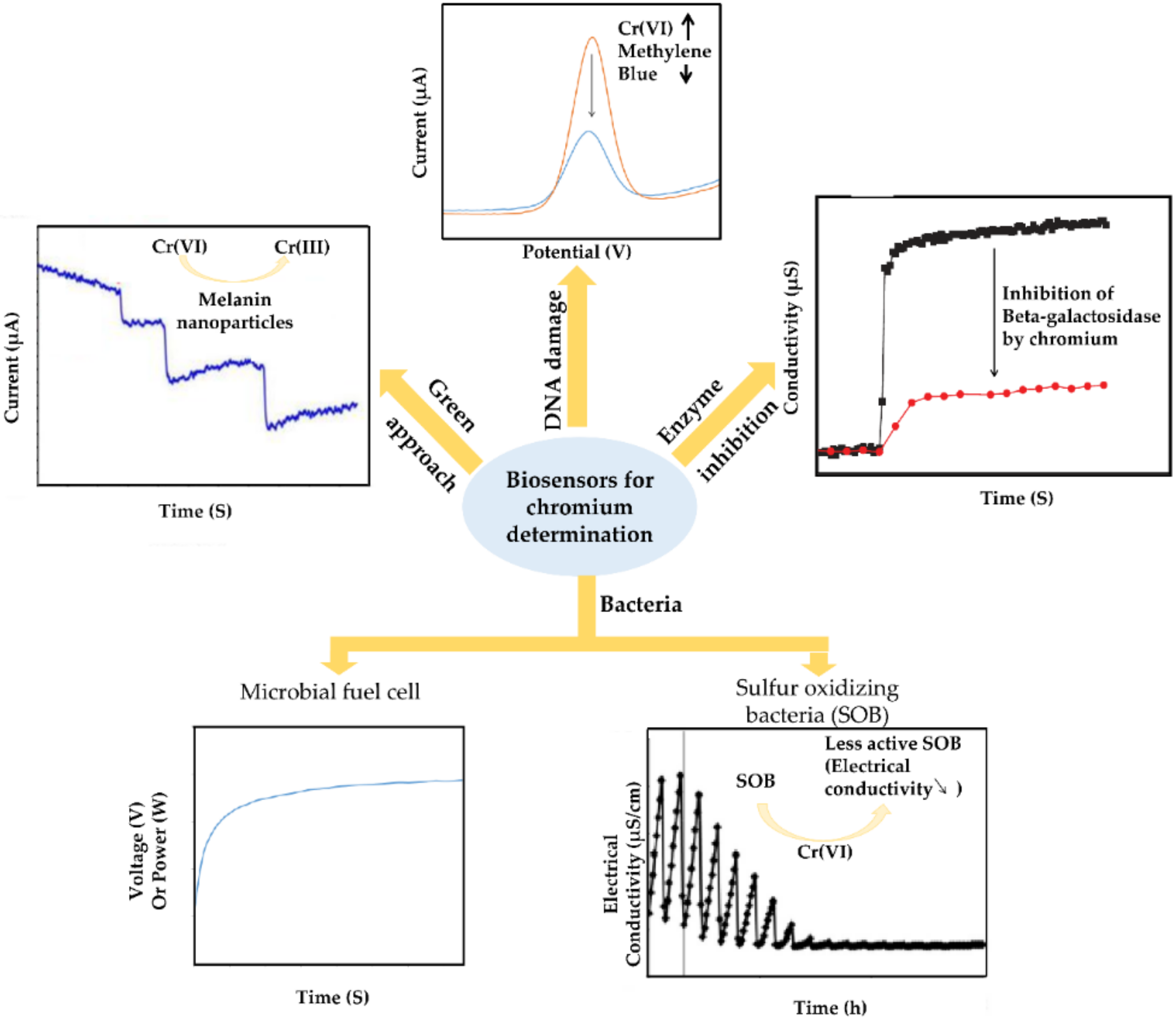
3. Electrochemical Biosensors for Hexavalent Chromium Determination
4. Electrochemical Sensors and Biosensors for Trivalent Chromium Determination
5. Sensors and Biosensors-Based Chromium Speciation
6. Conclusions and Perspectives
Funding
Acknowledgments
Conflicts of Interest
References
- De Oliveira Farias, E.A.; dos Santos, M.C.; de Araujo Dionísio, N.; Quelemes, P.V.; Leite, J.R.D.S.A.; Eaton, P.; Eiras, C. Layer-by-Layer films based on biopolymers extracted from red seaweeds and polyaniline for applications in electrochemical sensors of chromium VI. Mater. Sci. Eng. B 2015, 200, 9–21. [Google Scholar] [CrossRef]
- Arain, M.B.; Ali, I.; Yilmaz, E.; Soylak, M. Nanomaterial’s based chromium speciation in environmental samples: A review. TrAC Trends Anal. Chem. 2018, 103, 44–55. [Google Scholar] [CrossRef]
- Jin, W.; Yan, K. Recent advances in electrochemical detection of toxic Cr(VI). RSC Adv. 2015, 5, 37440–37450. [Google Scholar] [CrossRef]
- Gumpu, M.B.; Sethuraman, S.; Krishnan, U.M.; Rayappan, J.B.B. Areview on detection of heavy metal ions in water-an electrochemical approach. Sens. Actuators B Chem. 2015, 213, 515–533. [Google Scholar] [CrossRef]
- Aarthy, M.; Rajesh, T.; Thirunavoukkarasu, M. Critical review on microbial fuel cells for concomitant reduction of hexavalent chromium and bioelectricity generation. J. Chem. Technol. Biotechnol. 2020, 95, 1298–1307. [Google Scholar] [CrossRef]
- World Health Organization. Guidelines for Drinking-Water Quality Third Edition Incorporating the First and Second Addenda Volume 1 Recommendations, 3rd ed.; World Health Organization: Geneva, Switzerland, 2008; pp. 334–335. [Google Scholar]
- Chromium in Drinking Water. Available online: https://www.epa.gov/sdwa/chromium-drinking-water#:~:text=EPA%20has%20a%20drinking%20water,to%20test%20for%20total%20chromium (accessed on 2 September 2020).
- Arancibia, V.; Nagles, E.; Gomez, M.; Rojas, C. Speciation of Cr(VI) and Cr(III) in water samples by adsorptive stripping voltammetry in the presence of pyrogallol red applying a selective accumulation potential. Int. J. Electrochem. Sci. 2012, 7, 11444–11455. [Google Scholar]
- Grabarczyk, M. Speciation analysis of chromium by adsorptive stripping voltammetry in tap and river water samples. Electroanalysis 2008, 20, 2217–2222. [Google Scholar] [CrossRef]
- Grabarczyk, M.; Kaczmarek, L.; Korolczuk, M. Determination of Cr(VI) in the presence of complexing agents and humic substances by catalytic stripping voltammetry. Electroanalysis 2007, 19, 1183–1188. [Google Scholar] [CrossRef]
- Herrero-Latorre, C.; Barciela-García, J.; García-Martín, S.; Pena-Crecente, R.M. Graphene and carbon nanotubes as solid phase extraction sorbents for the speciation of chromium: A review. Anal. Chim. Acta 2018, 1002, 1–17. [Google Scholar] [CrossRef]
- Milačič, R.; Ščančar, J. Cr speciation in foodstuffs, biological and environmental samples: Methodological approaches and analytical challenges—A critical review. TrAC Trends Anal. Chem. 2020, 115888. [Google Scholar] [CrossRef]
- Özyol, E.; Saçmacı, Ş.; Saçmacı, M.; Ülgen, A. A new turn-on fluorimetric method for the rapid speciation of Cr(III)/Cr(VI) species in tea samples with rhodamine-based fluorescent reagent. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 191, 62–68. [Google Scholar] [CrossRef] [PubMed]
- López-García, I.; Marín-Hernández, J.J.; Hernández-Córdoba, M. Speciation of chromium in waters using dispersive micro-solid phase extraction with magnetic ferrite and graphite furnace atomic absorption spectrometry. Sci. Rep. 2020, 10, 1–8. [Google Scholar] [CrossRef]
- Sadeghi, S.; Moghaddam, A.Z. Solid-phase extraction and HPLC-UV detection of Cr(III) and Cr(VI) using ionic liquid-functionalized silica as a hydrophobic sorbent. Anal. Methods 2014, 6, 4867–4877. [Google Scholar] [CrossRef]
- Aggrawal, M.; Rohrer, J. Determination of Hexavalent Chromium Cr(VI) in Drinking Water by Suppressed Conductivity Detection I; Thermo Fisher Scientific: Sunnyvale, CA, USA, 2016. [Google Scholar]
- Destanoğlu, O.; Gümüş Yılmaz, G. Determination of cyanide, thiocyanate, cyanate, hexavalent chromium, and metal cyanide complexes in various mixtures by ion chromatography with conductivity detection. J. Liquid Chromatogr. Relat. Technol. 2016, 39, 465–474. [Google Scholar] [CrossRef]
- Amin, A.S.; Kassem, M.A. Chromium speciation in environmental samples using a solid phase spectrophotometric method. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2012, 96, 541–547. [Google Scholar] [CrossRef]
- Chang, Q.; Song, S.; Wang, Y.; Li, J.; Ma, J. Application of graphene as a sorbent for preconcentration and determination of trace amounts of chromium (III) in water samples by flame atomic absorption spectrometry. Anal. Methods 2012, 4, 1110–1116. [Google Scholar] [CrossRef]
- He, S.; Lin, X.; Liang, H.; Xiao, F.; Li, F.; Liu, C.; Liu, Y. Colorimetric detection of Cr(VI) using silver nanoparticles functionalized with PVP. Anal. Methods 2019, 11, 5819–5825. [Google Scholar] [CrossRef]
- American Public Health Association. American Water Works Association, Water Pollution Control Federation, Water Environment Federation. Standard Methods for the Examination of Water and Wastewater; American Public Health Association: Michigan, MI, USA, 1915; Volume 2, pp. 420–426. [Google Scholar]
- Biswas, P.; Karn, A.K.; Balasubramanian, P.; Kale, P.G. Biosensor for detection of dissolved chromium in potable water: A review. Biosens. Bioelectron. 2017, 94, 589–604. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Jin, W. Nanomaterials based electrochemical sensor and biosensor platforms for environmental applications. Trends Environ. Anal. Chem. 2017, 13, 10–23. [Google Scholar] [CrossRef]
- Maduraiveeran, G.; Sasidharan, M.; Ganesan, V. Electrochemical sensor and biosensor platforms based on advanced nanomaterials for biological and biomedical applications. Biosens. Bioelectron. 2018, 103, 113–129. [Google Scholar] [CrossRef]
- Zamora-Galvez, A.; Morales-Narváez, E.; Mayorga-Martinez, C.C.; Merkoçi, A. Nanomaterials connected to antibodies and molecularly imprinted polymers as bio/receptors for bio/sensor applications. Appl. Mater. Today 2017, 9, 387–401. [Google Scholar] [CrossRef]
- Llobet, E. Gas sensors using carbon nanomaterials: A review. Sens. Actuators B Chem. 2013, 179, 32–45. [Google Scholar] [CrossRef]
- El Rhazi, M.; Majid, S.; Elbasri, M.; Salih, F.E.; Oularbi, L.; Lafdi, K. Recent progress in nanocomposites based on conducting polymer: Application as electrochemical sensors. Int. Nano Lett. 2018, 8, 79–99. [Google Scholar] [CrossRef]
- Amine, A.; Mohammadi, H. Amperometry. Ref. Modul. Chem. Mol. Sci. Chem. Eng. 2018. [Google Scholar] [CrossRef]
- Ghanam, A.; Lahcen, A.A.; Amine, A. Electroanalytical determination of Bisphenol A: Investigation of electrode surface fouling using various carbon materials. J. Electroanal. Chem. 2017, 789, 58–66. [Google Scholar] [CrossRef]
- Salimi, A.; Pourbahram, B.; Mansouri-Majd, S.; Hallaj, R. Manganese oxide nanoflakes/multi-walled carbon nanotubes/chitosan nanocomposite modified glassy carbon electrode as a novel electrochemical sensor for chromium (III) detection. Electrochim. Acta 2015, 156, 207–215. [Google Scholar] [CrossRef]
- Wang, C.; Chan, C.K. Carbon nanotube-based electrodes for detection of low ppb-level hexavalent chromium using amperometry. ECS J. Solid State Sci. Technol. 2016, 5, M3026–M3031. [Google Scholar] [CrossRef]
- Rosolina, S.M.; Bragg, S.A.; Ouyang, R.; Chambers, J.Q.; Xue, Z.L. Highly sensitive detection of hexavalent chromium utilizing a sol-gel/carbon nanotube modified electrode. J. Electroanal. Chem. 2016, 781, 120–125. [Google Scholar] [CrossRef]
- Carrington, N.A.; Yong, L.; Xue, Z.L. Electrochemical deposition of sol–gel films for enhanced chromium (VI) determination in aqueous solutions. Anal. Chim. Acta 2006, 572, 17–24. [Google Scholar] [CrossRef]
- Liu, C.; He, C.; Xie, T.; Yang, J. Reduction of graphite oxide using ammonia solution and detection Cr(VI) with graphene-modified electrode. Full-Nanotub. Carbon Nanostruct. 2015, 23, 125–130. [Google Scholar] [CrossRef]
- Chen, Y.; Dong, Y.; Wu, H.; Chen, C.; Chi, Y.; Chen, G. Electrochemiluminescence sensor for hexavalent chromium based on the graphene quantum dots/peroxodisulfate system. Electrochim. Acta 2015, 151, 552–557. [Google Scholar] [CrossRef]
- Cinti, S.; Arduini, F.; Carbone, M.; Sansone, L.; Cacciotti, I.; Moscone, D.; Palleschi, G. Screen-Printed Electrodes Modified with Carbon Nanomaterials: A Comparison among Carbon Black, Carbon Nanotubes and Graphene. Electroanalysis 2015, 27, 2230–2238. [Google Scholar] [CrossRef]
- Arduini, F.; Di Nardo, F.; Amine, A.; Micheli, L.; Palleschi, G.; Moscone, D. Carbon black-modified screen-printed electrodes as electroanalytical tools. Electroanalysis 2012, 24, 743–751. [Google Scholar] [CrossRef]
- Yammouri, G.; Mandli, J.; Mohammadi, H.; Amine, A. Development of an electrochemical label-free biosensor for microRNA-125a detection using pencil graphite electrode modified with different carbon nanomaterials. J. Electroanal. Chem. 2017, 806, 75–81. [Google Scholar] [CrossRef]
- Wang, Y.; Ma, Y.; Zhao, Q.; Hou, L.; Han, Z. Polyoxometalate-based crystalline catalytic materials for efficient electrochemical detection of Cr (VI). Sens. Actuators B Chem. 2020, 305, 127469. [Google Scholar] [CrossRef]
- Liu, D.; Ji, L.; Ding, Y.; Weng, X.; Yang, F.; Zhang, X. Mesoporous carbon black as a metal-free electrocatalyst for highly effective determination of chromium (VI). J. Electroanal. Chem. 2017, 803, 58–64. [Google Scholar] [CrossRef]
- Chen, S.; Yuan, R.; Chai, Y.; Hu, F. Electrochemical sensing of hydrogen peroxide using metal nanoparticles: A review. Microchim. Acta 2013, 180, 15–32. [Google Scholar] [CrossRef]
- Xu, S.; Wang, X.; Zhou, C. A micro electrochemical sensor based on bismuth-modified mesoporous carbon for hexavalent chromium detection. In Proceedings of the 2015 IEEE SENSORS, Busan, Korea, 1–4 November 2015; pp. 1–4. [Google Scholar] [CrossRef]
- Tyszczuk-Rotko, K.; Madejska, K.; Domańska, K. Ultrasensitive hexavalent chromium determination at bismuth film electrode prepared with mediator. Talanta 2018, 182, 62–68. [Google Scholar] [CrossRef]
- Oularbi, L. Etude de Nanocomposites Polypyrrole/Nanoparticule de Carbone par Impédance Électrochimique et Ac-Électrogravimétrie: Application aux Capteurs Électrochimiques. Ph.D. Thesis, Sorbonne and Hassan II Casablanca Universities, Casablanca, Morocco, 29 June 2018. [Google Scholar]
- Stojanović, Z.; Koudelkova, Z.; Sedlackova, E.; Hynek, D.; Richtera, L.; Adam, V. Determination of chromium (VI) by anodic stripping voltammetry using a silver-plated glassy carbon electrode. Anal. Methods 2018, 10, 2917–2923. [Google Scholar] [CrossRef]
- Shahbakhsh, M.; Noroozifar, M. Ultra-trace determination of hexavalent chromium by novel two dimensional biphenol-biphenoquinone nanoribbons/silver nanoparticles. Sens. Actuators B Chem. 2019, 281, 1023–1033. [Google Scholar] [CrossRef]
- Tukur, S.A.; Yusof, N.A.; Hajian, R. Linear sweep anodic stripping voltammetry: Determination of Chromium (VI) using synthesized gold nanoparticles modified screen-printed electrode. J. Chem. Sci. 2015, 127, 1075–1081. [Google Scholar] [CrossRef]
- Chirea, M.; Pereira, C.M.; Silva, F. Catalytic effect of gold nanoparticles self-assembled in multilayered polyelectrolyte films. J. Phys. Chem. C 2007, 111, 9255–9266. [Google Scholar] [CrossRef]
- Wyantuti, S.; Ishmayana, S.; Hartati, Y.W. Voltammetric determination of Cr (VI) using gold nanoparticles-modified glassy carbon electrode. Procedia Chem. 2015, 16, 15–23. [Google Scholar] [CrossRef]
- Tu, J.; Gan, Y.; Liang, T.; Wan, H.; Wang, P. A miniaturized electrochemical system for high sensitive determination of chromium (VI) by screen-printed carbon electrode with gold nanoparticles modification. Sens. Actuators B Chem. 2018, 272, 582–588. [Google Scholar] [CrossRef]
- Dutta, S.; Strack, G.; Kurup, P. Gold nanostar-based voltammetric sensor for chromium (VI). Microchim. Acta 2019, 186, 734. [Google Scholar] [CrossRef] [PubMed]
- Du Nguyen, H.; Nguyen, T.T.L.; Nguyen, K.M.; Tran, T.A.T.; Nguyen, A.M.; Nguyen, Q.H. Determination of ppt level chromium (VI) using the gold nano-flakes electrodeposited on platinum rotating disk electrode and modified with 4-thiopyridinium. Am. J. Anal. Chem. 2015, 6, 457. [Google Scholar] [CrossRef][Green Version]
- Mohan, D.; Pittman, C.U., Jr. Activated carbons and low cost adsorbents for remediation of tri-and hexavalent chromium from water. J. Hazard. Mater. 2006, 137, 762–811. [Google Scholar] [CrossRef] [PubMed]
- Sankararamakrishnan, N.; Jaiswal, M.; Verma, N. Composite nanofloral clusters of carbon nanotubes and activated alumina: An efficient sorbent for heavy metal removal. Chem. Eng. J. 2014, 235, 1–9. [Google Scholar] [CrossRef]
- Hilali, N.; Ghanam, A.; Mohammadi, H.; Amine, A.; García-Guzmán, J.J.; Cubillana-Aguilera, L.; Palacios-Santander, J.M. Comparison between modified and unmodified carbon paste electrodes for hexavalent chromium determination. Electroanalysis 2018, 30, 2750–2759. [Google Scholar] [CrossRef]
- Shrivastava, S.; Jadon, N.; Jain, R. Next-generation polymer nanocomposite-based electrochemical sensors and biosensors: A review. TrAC Trends Anal. Chem. 2016, 82, 55–67. [Google Scholar] [CrossRef]
- Shoaie, N.; Daneshpour, M.; Azimzadeh, M.; Mahshid, S.; Khoshfetrat, S.M.; Jahanpeyma, F.; Foruzandeh, M. Electrochemical sensors and biosensors based on the use of polyaniline and its nanocomposites: A review on recent advances. Microchim. Acta 2019, 186, 465. [Google Scholar] [CrossRef] [PubMed]
- Karthika, A.; Nikhil, S.; Suganthi, A.; Rajarajan, M. A facile sonochemical approach based on graphene carbon nitride doped silver molybdate immobilized nafion for selective and sensitive electrochemical detection of chromium (VI) in real sample. Adv. Powder Technol. 2020. [Google Scholar] [CrossRef]
- Liu, J.; Ding, Y.; Ji, L.; Zhang, X.; Yang, F.; Wang, J.; Kang, W. Highly sensitive detection of Cr (VI) in groundwater by bimetallic NiFe nanoparticles. Anal. Methods 2017, 9, 1031–1037. [Google Scholar] [CrossRef]
- Sari, T.K.; Takahashi, F.; Jin, J.; Zein, R.; Munaf, E. Electrochemical determination of chromium (VI) in river water with gold nanoparticles–graphene nanocomposites modified electrodes. Anal. Sci. 2018, 34, 155–160. [Google Scholar] [CrossRef] [PubMed]
- Breslin, C.B.; Branagan, D.; Garry, L.M. Electrochemical detection of Cr (VI) with carbon nanotubes decorated with gold nanoparticles. J. Appl. Electrochem. 2019, 49, 195–205. [Google Scholar] [CrossRef]
- Hussein, M.A.; Ganash, A.A.; Alqarni, S.A. Electrochemical sensor-based gold nanoparticle/poly (aniline-co-o-toluidine)/graphene oxide nanocomposite modified electrode for hexavalent chromium detection: A real test sample. Polym. Plast. Technol. Mater. 2019, 58, 1423–1436. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Wu, Y.; Wang, D.; Peng, Q.; Zhou, G.; Li, Y. Pd-Cu2O and Ag-Cu2O Hybrid Concave Nanomaterials for an Effective Synergistic Catalyst. Angew. Chem. Int. Ed. 2013, 52, 11049–11053. [Google Scholar] [CrossRef]
- Wang, D.; Villa, A.; Porta, F.; Prati, L.; Su, D. Bimetallic gold/palladium catalysts: Correlation between nanostructure and synergistic effects. J. Phys. Chem. C 2008, 112, 8617–8622. [Google Scholar] [CrossRef]
- Prati, L.; Villa, A.; Porta, F.; Wang, D.; Su, D. Single-phase gold/palladium catalyst: The nature of synergistic effect. Catal. Today 2007, 122, 386–390. [Google Scholar] [CrossRef][Green Version]
- Moakhar, R.S.; Hariri, M.B.; Kushwaha, A.; Dolati, A.; Ghorbani, M.; Goh, G.K.L. Au-Pd bimetallic nanoparticle electrodes for direct electroreduction of hexavalent chromium complexes. Aust. J. Chem. 2016, 69, 423–430. [Google Scholar] [CrossRef]
- Sari, T.K.; Jin, J.; Zein, R.; Munaf, E. Anodic Stripping Voltammetry for the Determination of Trace Cr (VI) with Graphite/Styrene-Acrylonitrile Copolymer Composite Electrodes. Anal. Sci. 2017, 33, 801–806. [Google Scholar] [CrossRef] [PubMed]
- Wu, S.; Dai, X.; Cheng, T.; Li, S. Highly sensitive and selective ion-imprinted polymers based on one-step electrodeposition of chitosan-graphene nanocomposites for the determination of Cr (VI). Carbohydr. Polym. 2018, 195, 199–206. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Zhao, M.; Yu, M.; Zhuang, Y.; Cheng, F.; Chen, S. Interfacial potential barrier driven electrochemical detection of Cr6+. Anal. Chim. Acta 2018, 1029, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Nellaiappan, S.; Kumar, A.S. A bipotentiostat based separation-free method for simultaneous flow injection analysis of chromium (III) and (VI) species. Electrochim. Acta. 2018, 273, 248–256. [Google Scholar] [CrossRef]
- Liu, Y.; Gao, G.; Hu, J.; Zou, X. Electrodeposited AuNPs/rGO nanocomposite as sensor for Cr (VI) determination in water. Int. J. Electrochem. Sci. 2018, 13, 11853–11866. [Google Scholar] [CrossRef]
- Punrat, E.; Maksuk, C.; Chuanuwatanakul, S.; Wonsawat, W.; Chailapakul, O. Polyaniline/graphene quantum dot-modified screen-printed carbon electrode for the rapid determination of Cr (VI) using stopped-flow analysis coupled with voltammetric technique. Talanta 2016, 150, 198–205. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, W.; Huang, X.; Shi, J.; Zou, X.; Li, Z.; Cui, X. Adsorptive stripping voltammetry determination of hexavalent chromium by a pyridine functionalized gold nanoparticles/three-dimensional graphene electrode. Microchem. J. 2019, 149, 104022. [Google Scholar] [CrossRef]
- Han, H.; Pan, D.; Liu, D.; Hu, X.; Lin, M.; Li, F. Cathodic stripping voltammetric determination of chromium in coastal waters on cubic nano-titanium carbide loaded gold nanoparticles modified electrode. Front. Mar. Sci. 2015, 2, 75. [Google Scholar] [CrossRef]
- Ravishankar, T.N.; Muralikrishna, S.; Nagaraju, G.; Ramakrishnappa, T. Electrochemical detection and photochemical detoxification of hexavalent chromium (Cr(vi)) by Ag doped TiO2 nanoparticles. Anal. Methods 2015, 7, 3493–3499. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, P.; Wen, F.; Yuan, B.; Wang, H. Fe3O4 nanospheres on MoS2 nanoflake: Electrocatalysis and detection of Cr (VI) and nitrite. J. Electroanal. Chem. 2016, 761, 14–20. [Google Scholar] [CrossRef]
- Hu, J.; Liu, Y.; Gao, G.; Zou, X. One-step synthesis of AuPdNPs/ERGO nanocomposite for enhanced electrochemical sensing of Cr (VI) in water. J. Electrochem. Soc. 2018, 165, B893. [Google Scholar] [CrossRef]
- Fang, T.; Yang, X.; Zhang, L.; Gong, J. Ultrasensitive photoelectrochemical determination of chromium (VI) in water samples by ion-imprinted/formate anion-incorporated graphitic carbon nitride nanostructured hybrid. J. Hazard. Mater. 2016, 312, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Moakhar, R.S.; Goh, G.K.L.; Dolati, A.; Ghorbani, M. Sunlight-driven photoelectrochemical sensor for direct determination of hexavalent chromium based on Au decorated rutile TiO2 nanorods. Appl. Catal. B Environ. 2017, 201, 411–418. [Google Scholar] [CrossRef]
- Wang, P.; Cao, L.; Wu, Y.; Di, J. A cathodic photoelectrochemical sensor for chromium (VI) based on the use of PbS quantum dot semiconductors on an ITO electrode. Microchim. Acta 2018, 185, 356. [Google Scholar] [CrossRef]
- Li, M.; He, R.; Wang, S.; Feng, C.; Wu, H.; Mei, H. Visible light driven photoelectrochemical sensor for chromium (VI) by using BiOI microspheres decorated with metallic bismuth. Microchim. Acta 2019, 186, 345. [Google Scholar] [CrossRef]
- Li, M.; Zhang, G.; Feng, C.; Wu, H.; Mei, H. Highly sensitive detection of chromium (VI) by photoelectrochemical sensor under visible light based on Bi SPR-promoted BiPO4/BiOI heterojunction. Sens. Actuators B Chem. 2020, 305, 127449. [Google Scholar] [CrossRef]
- Chen, R.; Tang, R.; Chen, C. Photoelectrochemical detection of chromium (VI) using layered MoS 2 modified BiOI. J. Chem. Sci. 2020, 132, 1–9. [Google Scholar] [CrossRef]
- Moakhar, R.S.; Goh, G.K.L.; Dolati, A.; Ghorbani, M. A novel screen-printed TiO2 photoelectrochemical sensor for direct determination and reduction of hexavalent chromium. Electrochem. Commun. 2015, 61, 110–113. [Google Scholar] [CrossRef]
- Ravishankar, T.N.; Vaz, M.D.O.; Ramakrishnappa, T.; Teixeira, S.R.; Dupont, J. Ionic liquid assisted hydrothermal syntheses of Au doped TiO2 NPs for efficient visible-light photocatalytic hydrogen production from water, electrochemical detection and photochemical detoxification of hexavalent chromium (Cr6+). RSC Adv. 2017, 7, 43233–43244. [Google Scholar] [CrossRef]
- Zheng, Q.; Vilà-Nadal, L.; Lang, Z.; Chen, J.J.; Long, D.L.; Mathieson, J.S.; Cronin, L. Self-sorting of heteroanions in the assembly of cross-shaped polyoxometalate clusters. J. Am. Chem. Soc. 2018, 140, 2595–2601. [Google Scholar] [CrossRef]
- Wang, S.S.; Yang, G.Y. Recent advances in polyoxometalate-catalyzed reactions. Chem. Rev. 2015, 115, 4893–4962. [Google Scholar] [CrossRef] [PubMed]
- Blazevic, A.; Rompel, A. The Anderson–Evans polyoxometalate: From inorganic building blocks via hybrid organic–inorganic structures to tomorrows “Bio-POM”. Coord. Chem. Rev. 2016, 307, 42–64. [Google Scholar] [CrossRef]
- Xin, X.; Ma, Y.; Hou, L.; Wang, Y.; Xue, X.; Lin, J.; Han, Z. Krebs-Type {M2 (WO2)2 [B-β-SbW9O33]2}n−(M = SbIII,(WO3)) Tungstoantimonate Possessing Unique Pseudo-Seesaw Sb–O Structure. Inorg. Chem. 2019, 58, 9567–9571. [Google Scholar] [CrossRef]
- Dong, Y.; Dong, Z.; Zhang, Z.; Liu, Y.; Cheng, W.; Miao, H.; Xu, Y. POM constructed from super-sodalite cage with extra-large 24-membered channels: Effective sorbent for uranium adsorption. ACS Appl. Mater. Interfaces 2017, 9, 22088–22092. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Liu, J.; Li, S.L.; Su, Z.M.; Lan, Y.Q. Polyoxometalate-based materials for sustainable and clean energy conversion and storage. EnergyChem 2019, 1, 100021. [Google Scholar] [CrossRef]
- Chen, L.; Chen, W.L.; Wang, X.L.; Li, Y.G.; Su, Z.M.; Wang, E.B. Polyoxometalates in dye-sensitized solar cells. Chem. Soc. Rev. 2019, 48, 260–284. [Google Scholar] [CrossRef] [PubMed]
- Du, N.; Gong, L.; Fan, L.; Yu, K.; Luo, H.; Pang, S.; Zhou, B. Nanocomposites containing keggin anions anchored on pyrazine-based frameworks for use as supercapacitors and photocatalysts. ACS Appl. Nano Mater. 2019, 2, 3039–3049. [Google Scholar] [CrossRef]
- Zang, D.; Huang, Y.; Li, Q.; Tang, Y.; Wei, Y. Cu dendrites induced by the Anderson-type polyoxometalate NiMo6O24 as a promising electrocatalyst for enhanced hydrogen evolution. Appl. Catal. B Environ. 2019, 249, 163–171. [Google Scholar] [CrossRef]
- Zhu, D.; Bai, Z.; Ma, H.; Tan, L.; Pang, H.; Wang, X. High performance simultaneous detection of β-nicotinamide adenine dinucleotide and l-tryptophan in human serum based on a novel nanocomposite of ferroferric oxide-functionalized polyoxometalates. Sens. Actuators B Chem. 2020, 309, 127787. [Google Scholar] [CrossRef]
- Wang, Q.; Khungwa, J.; Li, L.; Liu, Y.; Wang, X.; Wang, S. Fabrication of polyoxometalate/GO/PDDA hybrid nanocomposite modified electrode and electrocatalysis for nitrite ion, ascorbic acid and dopamine. J. Electroanal. Chem. 2018, 824, 91–98. [Google Scholar] [CrossRef]
- Boussema, F.; Gross, A.J.; Hmida, F.; Ayed, B.; Majdoub, H.; Cosnier, S.; Holzinger, M. Dawson-type polyoxometalate nanoclusters confined in a carbon nanotube matrix as efficient redox mediators for enzymatic glucose biofuel cell anodes and glucose biosensors. Biosens. Bioelectron. 2018, 109, 20–26. [Google Scholar] [CrossRef] [PubMed]
- Xin, X.; Hu, N.; Ma, Y.; Wang, Y.; Hou, L.; Zhang, H.; Han, Z. Polyoxometalate-based crystalline materials as a highly sensitive electrochemical sensor for detecting trace Cr(vi). Dalton Trans. 2020, 49, 4570–4577. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, T.A.; Rodríguez, J.A.; Galán-Vidal, C.A.; Castrillejo, Y.; Barrado, E. Flow based determination of Cr (VI) by adsorptive cathodic stripping voltammetry on an immobilized magnetic poly (ionic liquid) modified electrode. Talanta 2018, 183, 172–176. [Google Scholar] [CrossRef] [PubMed]
- Fu, L.; Liu, Z.; Ge, J.; Guo, M.; Zhang, H.; Chen, F.; Yu, A. (001) plan manipulation of α-Fe2O3 nanostructures for enhanced electrochemical Cr (VI) sensing. J. Electroanal. Chem. 2019, 841, 142–147. [Google Scholar] [CrossRef]
- Wang, W.; Bai, H.; Li, H.; Lv, Q.; Zhang, Q.; Bao, N. Carbon tape coated with gold film as stickers for bulk fabrication of disposable gold electrodes to detect Cr (VI). Sens. Actuators B Chem. 2016, 236, 218–225. [Google Scholar] [CrossRef]
- Tan, F.; Cong, L.; Jiang, X.; Wang, Y.; Quan, X.; Chen, J.; Mulchandani, A. Highly sensitive detection of Cr (VI) by reduced graphene oxide chemiresistor and 1, 4-dithiothreitol functionalized Au nanoparticles. Sens. Actuators B Chem. 2017, 247, 265–272. [Google Scholar] [CrossRef]
- Izadyar, A.; Al-Amoody, F.; Arachchige, D.R. Ion transfer stripping voltammetry to detect nanomolar concentrations of Cr (VI) in drinking water. J. Electroanal. Chem. 2016, 782, 43–49. [Google Scholar] [CrossRef]
- Jaihindh, D.P.; Thirumalraj, B.; Chen, S.M.; Balasubramanian, P.; Fu, Y.P. Facile synthesis of hierarchically nanostructured bismuth vanadate: An efficient photocatalyst for degradation and detection of hexavalent chromium. J. Hazard. Mater. 2019, 367, 647–657. [Google Scholar] [CrossRef]
- Chen, D.D.; Yi, X.H.; Zhao, C.; Fu, H.; Wang, P.; Wang, C.C. Polyaniline modified MIL-100 (Fe) for enhanced photocatalytic Cr (VI) reduction and tetracycline degradation under white light. Chemosphere 2020, 245, 125659. [Google Scholar] [CrossRef]
- Low, J.; Jiang, C.; Cheng, B.; Wageh, S.; Al-Ghamdi, A.A.; Yu, J. A review of direct Z-scheme photocatalysts. Small Methods 2017, 1, 1700080. [Google Scholar] [CrossRef]
- Zeleke, M.A.; Kuo, D.H. Synthesis of oxy-sulfide based nanocomposite catalyst for visible light-driven reduction of Cr (VI). Environ. Res. 2019, 172, 279–288. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, P.R.S.; do Nascimento Marreiro, A.S.; de Oliveira Farias, E.A.; Dionisio, N.A.; Silva Filho, E.C.; Eiras, C. Layer-by-layer hybrid films of phosphate cellulose and electroactive polymer as chromium (VI) sensors. J. Solid State Electrochem. 2015, 19, 2129–2139. [Google Scholar] [CrossRef]
- Ferrigno, R.; Stroock, A.D.; Clark, T.D.; Mayer, M.; Whitesides, G.M. Membraneless vanadium redox fuel cell using laminar flow. J. Am. Chem. Soc. 2002, 124, 12930–12931. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.Y.; Su, J.Y. Membraneless microfluidic microbial fuel cell for rapid detection of electrochemical activity of microorganism. Bioresour. Technol. 2013, 145, 271–274. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Venkataraman, A.; Rosenbaum, M.A.; Angenent, L.T. A Laminar-Flow Microfluidic Device for Quantitative Analysis of Microbial Electrochemical Activity. ChemSusChem 2012, 5, 1119–1123. [Google Scholar] [CrossRef]
- Ye, D.; Yang, Y.; Li, J.; Zhu, X.; Liao, Q.; Zhang, B. A laminar flow microfluidic fuel cell for detection of hexavalent chromium concentration. Biomicrofluidics 2015, 9, 064110. [Google Scholar] [CrossRef]
- Kowsalya, B.; Thampi, V.A.; Sivakumar, V.; Subramanian, B. Electrochemical detection of Chromium (VI) using NiO nanoparticles. J. Mater. Sci. Mater. Electron. 2019, 30, 14755–14761. [Google Scholar] [CrossRef]
- Korshoj, L.E.; Zaitouna, A.J.; Lai, R.Y. Methylene blue-mediated electrocatalytic detection of hexavalent chromium. Anal. Chem. 2015, 87, 2560–2564. [Google Scholar] [CrossRef]
- Wei, J.; Guo, Z.; Chen, X.; Han, D.D.; Wang, X.K.; Huang, X.J. Ultrasensitive and ultraselective impedimetric detection of Cr (VI) using crown ethers as high-affinity targeting receptors. Anal. Chem. 2015, 87, 1991–1998. [Google Scholar] [CrossRef] [PubMed]
- Bhanjana, G.; Rana, P.; Chaudhary, G.R.; Dilbaghi, N.; Kim, K.H.; Kumar, S. Manganese oxide nanochips as a novel electrocatalyst for direct redox sensing of hexavalent chromium. Sci. Rep. 2019, 9, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kochana, J.; Starzec, K.; Wieczorek, M.; Knihnicki, P.; Góra, M.; Rokicińska, A.; Kuśtrowski, P. Study on self-assembled monolayer of functionalized thiol on gold electrode forming capacitive sensor for chromium (VI) determination. J. Solid State Electrochem. 2019, 23, 1463–1472. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, B.; Dong, Q.; Lei, Y.; Li, Y.; Ren, J.; Li, B. Flat microliter membrane-based microbial fuel cell as “on-line sticker sensor” for self-supported in situ monitoring of wastewater shocks. Bioresour. Technol. 2015, 197, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Chung, H.; Ju, W.J.; Jho, E.H.; Nam, K. Applicability of a submersible microbial fuel cell for Cr (VI) detection in water. Environ. Monit. Assess. 2016, 188, 613. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.H.; Cheng, C.Y.; Liu, M.H.; Chen, T.Y.; Hsieh, M.C.; Chung, Y.C. Utility of Ochrobactrum anthropi YC152 in a microbial fuel cell as an early warning device for hexavalent chromium determination. Sensors 2016, 16, 1272. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.C.; Tsai, T.H.; Liu, M.H.; Kuo, J.L.; Chang, Y.C.; Chung, Y.C. A green microbial fuel cell-based biosensor for in situ chromium (VI) measurement in electroplating wastewater. Sensors 2017, 17, 2461. [Google Scholar] [CrossRef]
- Xu, Z.; Liu, Y.; Williams, I.; Li, Y.; Qian, F.; Zhang, H.; Li, B. Disposable self-support paper-based multi-anode microbial fuel cell (PMMFC) integrated with power management system (PMS) as the real time “shock” biosensor for wastewater. Biosens. Bioelectron. 2016, 85, 232–239. [Google Scholar] [CrossRef]
- Zhao, S.; Liu, P.; Niu, Y.; Chen, Z.; Khan, A.; Zhang, P.; Li, X. A novel early warning system based on a sediment microbial fuel cell for in situ and real time hexavalent chromium detection in industrial wastewater. Sensors 2018, 18, 642. [Google Scholar] [CrossRef]
- Eom, H.; Hwang, J.H.; Hassan, S.H.; Joo, J.H.; Hur, J.H.; Chon, K.; Oh, S.E. Rapid detection of heavy metal-induced toxicity in water using a fed-batch sulfur-oxidizing bacteria (SOB) bioreactor. J. Microbiol. Methods 2019, 161, 35–42. [Google Scholar] [CrossRef]
- Bachan Upadhyay, L.S.; Verma, N. Enzyme inhibition based biosensors: A review. Anal. Lett. 2013, 46, 225–241. [Google Scholar] [CrossRef]
- Fourou, H.; Zazoua, A.; Braiek, M.; Jaffrezic-Renault, N. An enzyme biosensor based on beta-galactosidase inhibition for electrochemical detection of cadmium (II) and chromium (VI). Int. J. Environ. Anal. Chem. 2016, 96, 872–885. [Google Scholar] [CrossRef]
- Jarczewska, M.; Ziółkowski, R.; Górski, Ł.; Malinowska, E. Electrochemical Detection of Chromium (VI): Induced DNA Damage. J. Electrochem. Soc. 2015, 162, B326–B331. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Amini, M.; Rezaei, B. Detection of DNA damage induced by chromium/glutathione/H2O2 system at MWCNTs–poly (diallyldimethylammonium chloride) modified pencil graphite electrode using methylene blue as an electroactive probe. Sens. Actuators B Chem. 2013, 177, 862–870. [Google Scholar] [CrossRef]
- Dashtian, K.; Ghaedi, M.; Hajati, S. Photo-Sensitive Pb5S2I6 crystal incorporated polydopamine biointerface coated on nanoporous TiO2 as an efficient signal-on photoelectrochemical bioassay for ultrasensitive detection of Cr (VI) ions. Biosens. Bioelectron. 2019, 132, 105–114. [Google Scholar] [CrossRef]
- Liu, Y.; Ma, H.; Zhang, Y.; Pang, X.; Fan, D.; Wu, D.; Wei, Q. Visible light photoelectrochemical aptasensor for adenosine detection based on CdS/PPy/g-C3N4 nanocomposites. Biosens. Bioelectron. 2016, 86, 439–445. [Google Scholar] [CrossRef] [PubMed]
- Wang, G.L.; Shu, J.X.; Dong, Y.M.; Wu, X.M.; Zhao, W.W.; Xu, J.J.; Chen, H.Y. Using G-quadruplex/hemin to “switch-on” the cathodic photocurrent of p-type PbS quantum dots: Toward a versatile platform for photoelectrochemical aptasensing. Anal. Chem. 2015, 87, 2892–2900. [Google Scholar] [CrossRef] [PubMed]
- Piacenti da Silva, M.; Fernandes, J.C.; de Figueiredo, N.B.; Congiu, M.; Mulato, M.; de Oliveira Graeff, C.F. Melanin as an active layer in biosensors. Aip Adv. 2014, 4, 037120. [Google Scholar] [CrossRef]
- Kaleli-Can, G.; Ozlu, B.; Özgüzar, H.F.; Onal-Ulusoy, B.; Kabay, G.; Eom, T.; Mutlu, M. Natural melanin nanoparticle-decorated screen-printed carbon electrode: Performance test for amperometric determination of hexavalent chromium as model trace. Electroanalysis 2020. [Google Scholar] [CrossRef]
- Heidari, Z.; Masrournia, M.; Khoshnood, R.S. Fabrication a composite electrode based on MWCNT/Zeolite for potentiometric determination of Cr3+. Orient. J. Chem. 2016, 32, 627–635. [Google Scholar] [CrossRef]
- Heidari, Z.; Masrournia, M. A novel modified carbon paste electrode for the determination of chromium (III) in water. J. Anal. Chem. 2018, 73, 824–831. [Google Scholar] [CrossRef]
- Wu, S.; Sekar, N.C.; Tan, S.N.; Xie, H.; Ng, S.H. Determination of chromium (III) by differential pulse stripping voltammetry at a chitosan–gold nanocomposite modified screen printed electrode. Anal. Methods 2016, 8, 962–967. [Google Scholar] [CrossRef]
- Wyantuti, S.; Hartati, Y.W.; Firdaus, M.L.; Panatarani, C.; Tjokronegoro, R. Fabrication of gold nanoparticles-modified glassy carbon electrode and its application for voltammetric detection of Cr(III). Int. J. Sci. Technol. Res. 2015, 4, 135–139. [Google Scholar]
- Khan, A.; Khan, A.A.P.; Rahman, M.M.; Asiri, A.M.; Alamry, K.A. Preparation of polyaniline grafted graphene oxide–WO 3 nanocomposite and its application as a chromium (iii) chemi-sensor. RSC Adv. 2015, 5, 105169–105178. [Google Scholar] [CrossRef]
- Hussain, M.M.; Asiri, A.M.; Arshad, M.N.; Rahman, M.M. Synthesis, characterization, and crystal structure of (E)-N′-(4-Bromobenzylidene)-benzenesulfonohydrazide and its application as a sensor of chromium ion detection from environmental samples. J. Mol. Struct. 2020, 1207, 127810. [Google Scholar] [CrossRef]
- Aravind, A.; Mathew, B. Electrochemical sensor based on nanostructured ion imprinted polymer for the sensing and extraction of Cr (III) ions from industrial wastewater. Polym. Int. 2018, 67, 1595–1604. [Google Scholar] [CrossRef]
- Zhu, L.; Miao, M.; Shao, X.; Du, Z.; Huang, K.; Luo, Y.; Xu, W. A universal electrochemical biosensor using nick-HCR nanostructure as molecular gate of nanochannel for detecting Chromium (III) ions and microRNA. Anal. Chem. 2019, 91, 14992–14999. [Google Scholar] [CrossRef]
- Leimbach, M.; Tschaar, C.; Schmidt, U.; Bund, A. Electrochemical characterization of chromium deposition from trivalent solutions for decorative applications by EQCM and near-surface pH measurements. Electrochim. Acta 2018, 270, 104–109. [Google Scholar] [CrossRef]
- Wyantuti, S.; Hartati, Y.W.; Panatarani, C.; Tjokronegoro, R. Cyclic voltammetric study of Chromium (VI) and Chromium (III) on the gold nanoparticles-modified glassy carbon electrode. Procedia Chem. 2015, 17, 170–176. [Google Scholar] [CrossRef]
- Wang, W.; Bai, H.; Li, H.; Lv, Q.; Wang, Z.; Zhang, Q. Disposable plastic electrode for electrochemical determination of total chromium and hexavalent chromium. J. Electroanal. Chem. 2017, 794, 148–155. [Google Scholar] [CrossRef]
- Ma, H.; Li, X.; Yan, T.; Li, Y.; Liu, H.; Zhang, Y.; Wei, Q. Electrogenerated Chemiluminescence Behavior of Au nanoparticles-hybridized Pb(II) metal-organic framework and its application in selective sensing hexavalent chromium. Sci. Rep. 2016, 6, 22059. [Google Scholar] [CrossRef]
- Prabhakaran, D.C.; Riotte, J.; Sivry, Y.; Subramanian, S. Electroanalytical detection of Cr(VI) and Cr(III) ions using a novel microbial sensor. Electroanalysis 2017, 29, 1222–1231. [Google Scholar] [CrossRef]
- Prabhakaran, D.C.; Ramamurthy, P.C.; Sivry, Y.; Subramanian, S. Electrochemical detection of Cr (VI) and Cr (III) ions present in aqueous solutions using bio-modified carbon paste electrode: A voltammetric study. Int. J. Environ. Anal. Chem. 2020. [Google Scholar] [CrossRef]
- Qambrani, N.A.; Hwang, J.H.; Oh, S.E. Comparison of chromium III and VI toxicities in water using sulfur-oxidizing bacterial bioassays. Chemosphere 2016, 160, 342–348. [Google Scholar] [CrossRef] [PubMed]
- Liu, L.; Chen, C.; Chen, C.; Kang, X.; Zhang, H.; Tao, Y.; Yao, S. Poly (noradrenalin) based bi-enzyme biosensor for ultrasensitive multi-analyte determination. Talanta 2019, 194, 343–349. [Google Scholar] [CrossRef] [PubMed]
| Nano-Sensor | Modification Strategy | Technique | Linear Range (µg·L−1) | LOD (µg·L−1) | Real Sample | Reference |
|---|---|---|---|---|---|---|
| CNT/FTO | Coating by doctor blading | CV, amperometry | 0–100 | 5 | cooling tower blowdown water | [31] |
| CNT/flexible paper electrode | Direct painting on the Whatman cellulose filter paper | |||||
| CNT/SPE | Carbon printed | |||||
| Sol-gel/SWCNTs/GCE | electrodeposition | SWV | 5–300 | 0.8 | ashed swine blood | [32] |
| Graphene/GCE | Drop casting | DPCSV | 10.4–52,000 | 7.8 | Tap water | [34] |
| GQD/peroxo-disulfate system | chemical oxidation method | Electro-chemiluminescence | 2.6–3120 | 1.04 | river water | [35] |
| Phosphomolybdate based crystalline materials-CB/GCE | Drop casting | amperometry | 26–19,656 | 1.35 | Lake water | [39] |
| CB/GCE | Drop casting (15 µL) | amperometry | 1.3–25,127.7 | 0.52 | Not done | [40] |
| Nano-Sensor | Modification Strategy | Technique | Linear Range (µg·L−1) | LOD (µg·L−1) | Real Sample | Reference |
|---|---|---|---|---|---|---|
| Bi/MCE | Spin coating- > Photolithography- > carbonization- > Etching and modification | AdSV | 1–25 | 0.05 | - | [42] |
| BiFE with zinc mediator/GCE | reduction of Bi(III) and Zn (II) to the metallic state at −1.1 V for 60 s Zn: reversible mediator | DPCAdSV | 1.04 × 10−5–6.5 × 10−5 | 3.01 × 10−6 | River Water | [43] |
| Ag plated-GCE | In situ plating | DP-ASV | 18.2–2080 | 5.2 | tap water | [45] |
| Ag NPs-BP-BPQ/GPE | Paste packed into the electrode | DPV | 3 LR 4.16 × 10−3–0.52; 0.52–52; 52–5200 | 1.04 × 10−4 | tap water, river water and electroplating wastewater | [46] |
| AuNPs/SPE | Drop casting 5µL | LSASV | 0.7–35.0 | 1.6 × 10−3 | tap and seawater | [47] |
| AuNPs/GCE | Self-assembly process | DPV | 0.05–0.25 | 2.38 × 10−3 | - | [49] |
| AuNPs/SPCE | Electrodeposition | LSV | 20–200 | 5.4 | River water | [50] |
| AuNS/CPSPE | Drop-casting | LSV | 10–75 | 3.5 | Contaminated groundwater | [51] |
| PET/nano-Au/Pt-RDE | Electrodeposition | DPAdCSV | 2.5 × 10−3–40 × 10−3 | 10−3 | Coastal water | [52] |
| AuNPs/CPE | AuNPs electrochemically deposited | DPV | 40–3000 | 7 | Tap water | [55] |
| AuNPs/CPE | AuNPs drop-casted | 100–3000 | 54 | |||
| CPE | 250–1500 | 26 | ||||
| CPE in presence of DPC | 50–260 | 19 |
| Nanocomposite Sensor | Modification Strategy | Technique | Linear Range (µg·L−1) | LOD (µg·L−1) | Real Sample | Reference |
|---|---|---|---|---|---|---|
| Graphite/styrene-acrylonitrile copolymer composite electrode | Chemical method | SWASV | 0–150 | 4.5 | Tap water, river water, mineral water | [67] |
| IIP-S | One step electrodeposition | DPV | 5.2 × 10−2–5.2 | 3.33 × 10−2 | Tap water, river water | [68] |
| g-C3N4/AgM/Nf/GCE | Drop casting | amperometry | 5.2–36.4 | 8.32 × 10−2 | Tap, drinking, river and Industrial wastewater | [58] |
| NiFe NPs-CB/GCE | Drop casting | amperometry | 1.3–5111.6 | 0.52 | groundwater | [59] |
| 3D NiO/PANI foam | Electrodeposition of PANI | DPV | 0–235.3 | 2.06 × 10−5 | - | [69] |
| AuNPs@CNF-CHIT/GCE | drop coating of 5 µL of CNF-CHIT and Au3+ followed by CV | Flow injection analysis coupled dual electrochemical detector (FIA-DECD) | 100–100,000 | 0.32 | wastewater | [70] |
| Au NPs/GR/GCE | Graphene/AuNPs (sonochemical method) Modification of GCE by drop-casting | amperometry | 0–1040 | 0.52 | River water | [60] |
| AuNPs/rGO/GCE | Electrodeposition by CV | SWV | 5.2–1560 | 2.392 | Lake water, river water | [71] |
| Ox.MWCNT-Aunano/Au | Drop casting 20 µL | amperometry | 41.6–11,960 | 37.44 | water | [61] |
| AuNPs/poly(aniline-co-o toluidine)/graphene oxide/AuE | Incubation of AuE in the copolymer (precipitation of a thin layer) | SWV | 2600–26,000 | 1.118 | Tap water | [62] |
| PANI/GQD-modified SPCE | electro-polymerization | LSV | 100–10,000 | 97 | Mineral water | [72] |
| Pyridine functionalized AuNPs/3D rGO/GCE | -3D RGO (electrochemical Reduction) -Electrodeposition of AuNPs -self-assembly of pyridine | AdSV | 25–300 | 1.16 | Wastewater (electroplate factories) | [73] |
| AuNPs/Nano-TiC/GCE | -Drop casting 10µL of Nano-TiC Electrodeposition of AuNPs | DPV | 5.2–1040 | 2.08 | coastal water | [74] |
| Ag-doped TiO2/ GCE | Drop casting | Amperometry | 5–155 | 0.52 | Tap water; lake water | [75] |
| Fe3O4/MoS2 /GCE | Drop casting | amperometry | 52–136,760 | 26 | [76] | |
| AuPdNPs/ERGO/GCE | Electrodeposition by 2 ways (CV-amperometry) | DPV | 2.6–260 and 260–52,000 | 0.676 | Lake water, river water | [77] |
| Au-Pd nanoparticles/ITO | Electrodeposition by CV | LSV And chronoamperometry | 0.052–5200 | 1.56 × 10−2 | Tap and river | [66] |
| Sensor | Modification Strategy | Technique | Linear Range (µg·L−1) | LOD (µg·L−1) | Real Sample | Reference |
|---|---|---|---|---|---|---|
| FTO (photoactive electrode) | Formate anion incorporated graphitic-carbon nitride (F-g-C3N4/IIP) Drop casting | Photoelectrochemical measurements | 0.01–100.00 | 0.006 | Water | [78] |
| Bi/BiOI-X/ITO (where x can be 1, 2, 3 or 4) | Drop casting (20µL) | photoelectrochemical detection | 52–11,960 | 15.6 | tap water, lake water | [81] |
| (BiPO4/BiOI/ITO) | Drop casting | Bi-SPR (Bismuth-surface plasmon resonance) | 26–9360 | 7.8 | Tap and lake water | [82] |
| MoS2/BiOI/ITO | Drop casting (20µL) | photoelectrochemical detection | 2.6–520 520–8320 | 0.52 | Tap and lake water | [83] |
| PbS QDs/ITO | Incubation Assembling with the linker poly(diallyl dimethyl ammonium chloride) | Photoelectrochemical detection | 1.04 × 10−3–104 | 5.2 × 10−4 | tap water, lake water | [80] |
| Au-TiO2/FTO | TiO2 nanorods: hydrothermal method AuNPs: electrodeposition by CV | chronoamperometry technique under chopped simulated solar light irradiation (100 mW·cm−2, light on/off cycles: 30 s) | 0.52–2600 | 0.312 | tap and river water | [79] |
| Au-TiO2/SPE | Screen printing of the TiO2 in the paste Electrodeposition of AuNPs by CV | Amperometry | 0.52–5200 | 0.208 | tap and river water | [84] |
| Au/TiO2 NPs/GCE | (Au@TiO2 NPs by hydrothermal method) Drop casting | amperometry | 5200–140,400 | 520 | Tap water, Industrial wastewater | [85] |
| Nano-Sensor | Modification Strategy | Technique | Linear Range (µg·L−1) | LOD (µg·L−1) | Real Sample | Reference |
|---|---|---|---|---|---|---|
| MB/Au | Incubation | CV | 26–52 × 104 | 26 | Not done | [114] |
| azacrown monolayer/Au | Self-assembly | EIS | 1−100 | 0.0014 | river water | [115] |
| Mn3O4/Nf/Au | Mn3O4 coating | CV | 50–400 | 9.5 | Canal water and Sewage water | [116] |
| Thiol monolayer/Au | Self-assembled monolayer | Capacitive measurement | 0.052–0.52; 0.52–2.6; 5.2–26 | 1.612 × 10−2 | synthetic solutions | [117] |
| Nano-Sensor | Modification Strategy | Technique | Linear Range(µg·L−1) | LOD (µg·L−1) | Real Sample | Reference |
|---|---|---|---|---|---|---|
| MWCNT/zeolite/CPE | Incorporation in paste | Potentiometry | 5.2–52 × 104 | 5.2 | Wastewater | [134] |
| Sensor 1: 3-Methylpyrazol-5-one/zeolite/CPE Sensor 2: chlorinated MWCNTs/zeolite/CPE | Incorporation in paste | potentiometry | 52–52 × 104 5.2–52 × 104 | 20.82.6 | Drinking water, river water | [135] |
| MnOxNP/MWCNTs/Chit/GCE | Drop casting of MWCNs/ Chit. deposition of MnOxNP by CV | CV and amperometry | 156–10,400 | 15.6 | Drinking water | [30] |
| AuNPs/GCE | Self-assembly process | DPSV | 0.5–75 | 0.01 | wastewater | [137] |
| Chitosan-gold/SPE | electrodeposition | DPSV | 52–5200 | 20.8 | industrial wastewater | [136] |
| PANI-g-rGO@WO3/AgE | conducting binders: butyl carbitol acetate (BCA) and ethyl acetate (EA) | I-V | 5.2 × 10−3–52 × 104 | 1.612 × 10−3 ± 5.2 × 10−4 | - | [138] |
| Nf/BBBSH/GCE | Drop casting | I-V | 5.2 × 10−3–52 × 104 | 4.97 × 10−3 | Coal water, Industrial effluent, Red seawater, Tap water, Well water | [139] |
| MWCNT-IIP/Pt | Fabrication of paste of MWCNT-IIP on Pt electrode | DPV | 1 × 103–5 × 103 | 266.1 | industrial wastewater | [140] |
| Nanochannels with n-HCR | I-V | 1.04 × 10−5–1.04 | 1.04 × 10−5 | - | [141] | |
| Quartz crystal electrode | Electroplating | EQCM | - | - | - | [142] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hilali, N.; Mohammadi, H.; Amine, A.; Zine, N.; Errachid, A. Recent Advances in Electrochemical Monitoring of Chromium. Sensors 2020, 20, 5153. https://doi.org/10.3390/s20185153
Hilali N, Mohammadi H, Amine A, Zine N, Errachid A. Recent Advances in Electrochemical Monitoring of Chromium. Sensors. 2020; 20(18):5153. https://doi.org/10.3390/s20185153
Chicago/Turabian StyleHilali, Nazha, Hasna Mohammadi, Aziz Amine, Nadia Zine, and Abdelhamid Errachid. 2020. "Recent Advances in Electrochemical Monitoring of Chromium" Sensors 20, no. 18: 5153. https://doi.org/10.3390/s20185153
APA StyleHilali, N., Mohammadi, H., Amine, A., Zine, N., & Errachid, A. (2020). Recent Advances in Electrochemical Monitoring of Chromium. Sensors, 20(18), 5153. https://doi.org/10.3390/s20185153






