Modeling of the MET Sensitive Element Conversion Factor on the Intercathode Distance
Abstract
:1. Introduction
2. Materials and Methods
3. Results
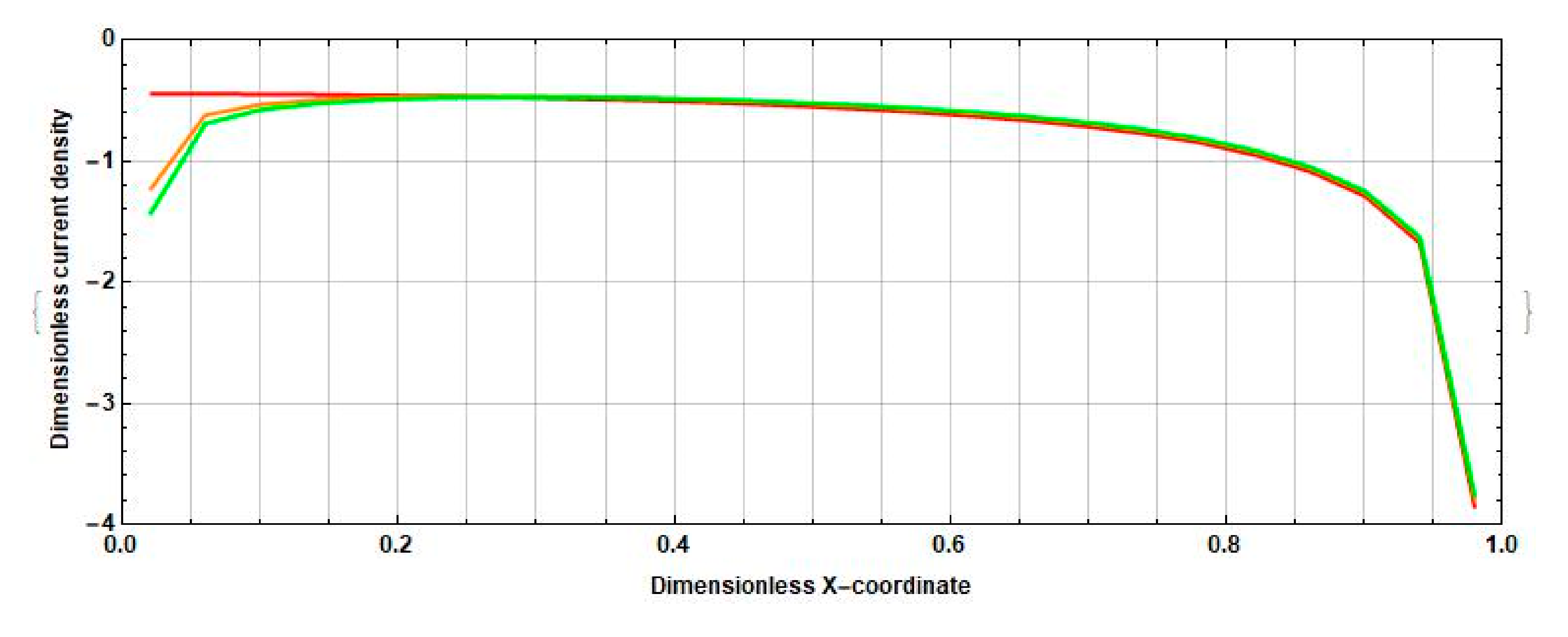
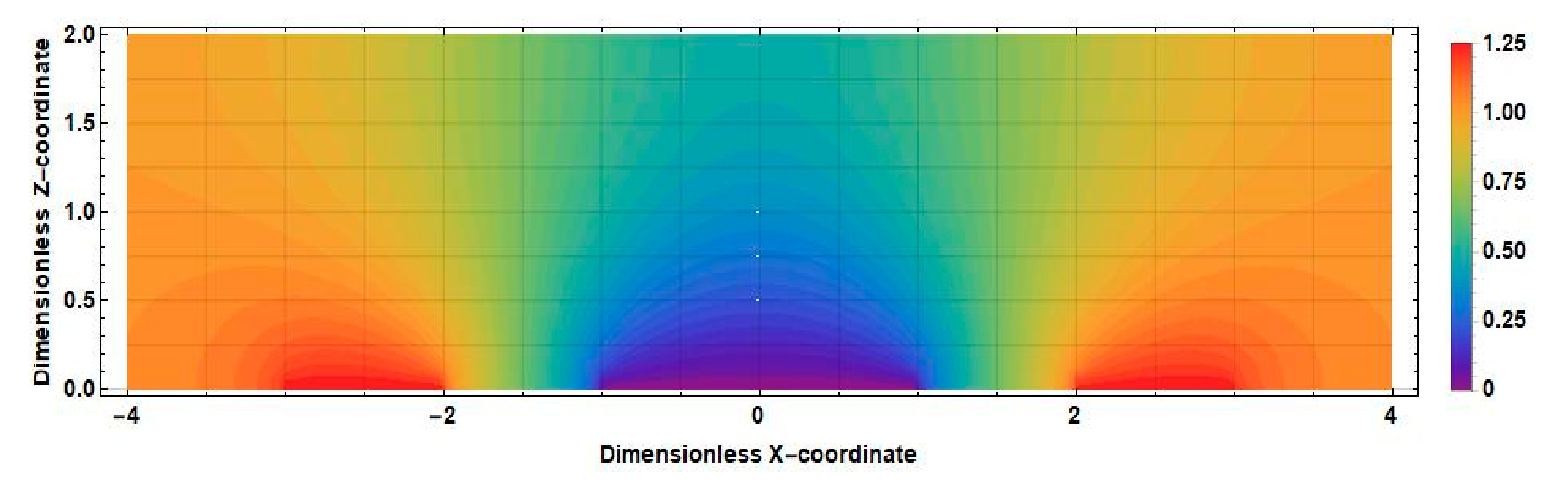
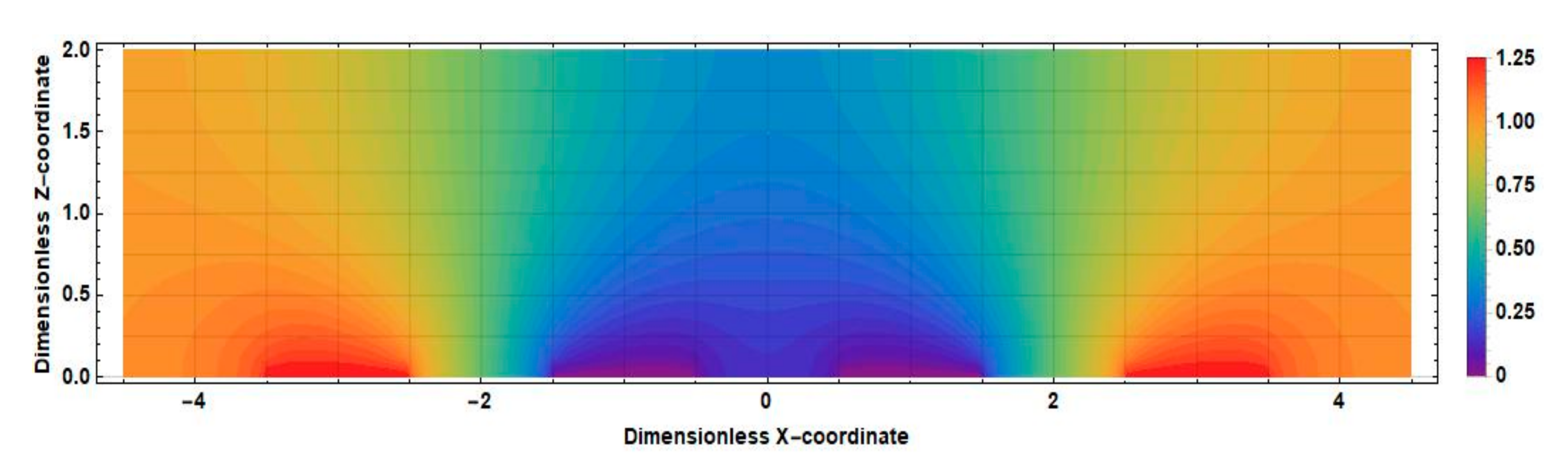
3.1. Stationary Current Density at the Cathodes
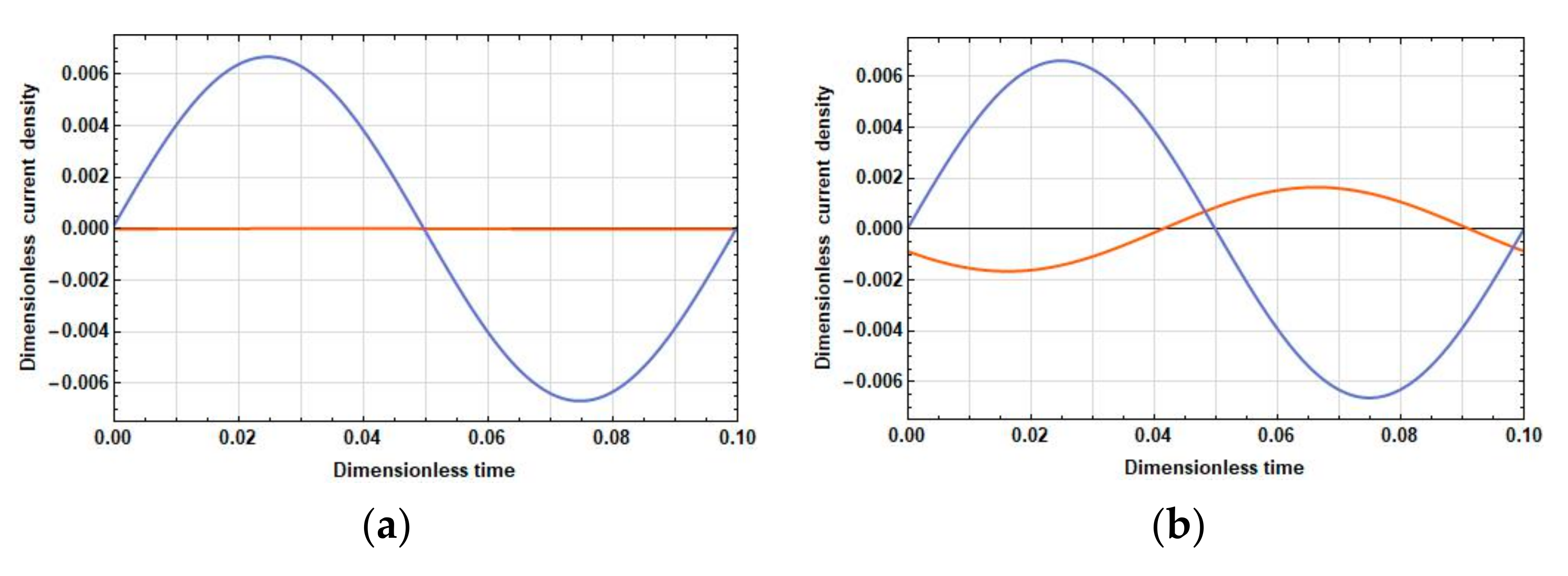
3.2. Density of the Current Linear in Velocity at the Cathodes
- In the field of low frequencies, and are practically in phase.
- In the medium range, the phase difference between and is already quite significant and is about .
- At high frequencies, the oscillations are close to the opposite phase. Thus, from the expression for the response, the following conclusions have been made:
- With an increase in the distance between the cathodes, the difference contribution of the part adjacent to the cathode surface into the total current is positive due to an increase in the amplitude of the current density in this part and the proximity of the phases of the current densities in the parts adjacent to the cathode and the anode.
- At high frequencies, an increase in the amplitude of the current density in the part adjacent to the cathode leads to a decrease in the total current due to the phase difference between the parts adjacent to the anode and the cathode parts, which is close to .
3.3. Electrochemical Cell Sensitivity
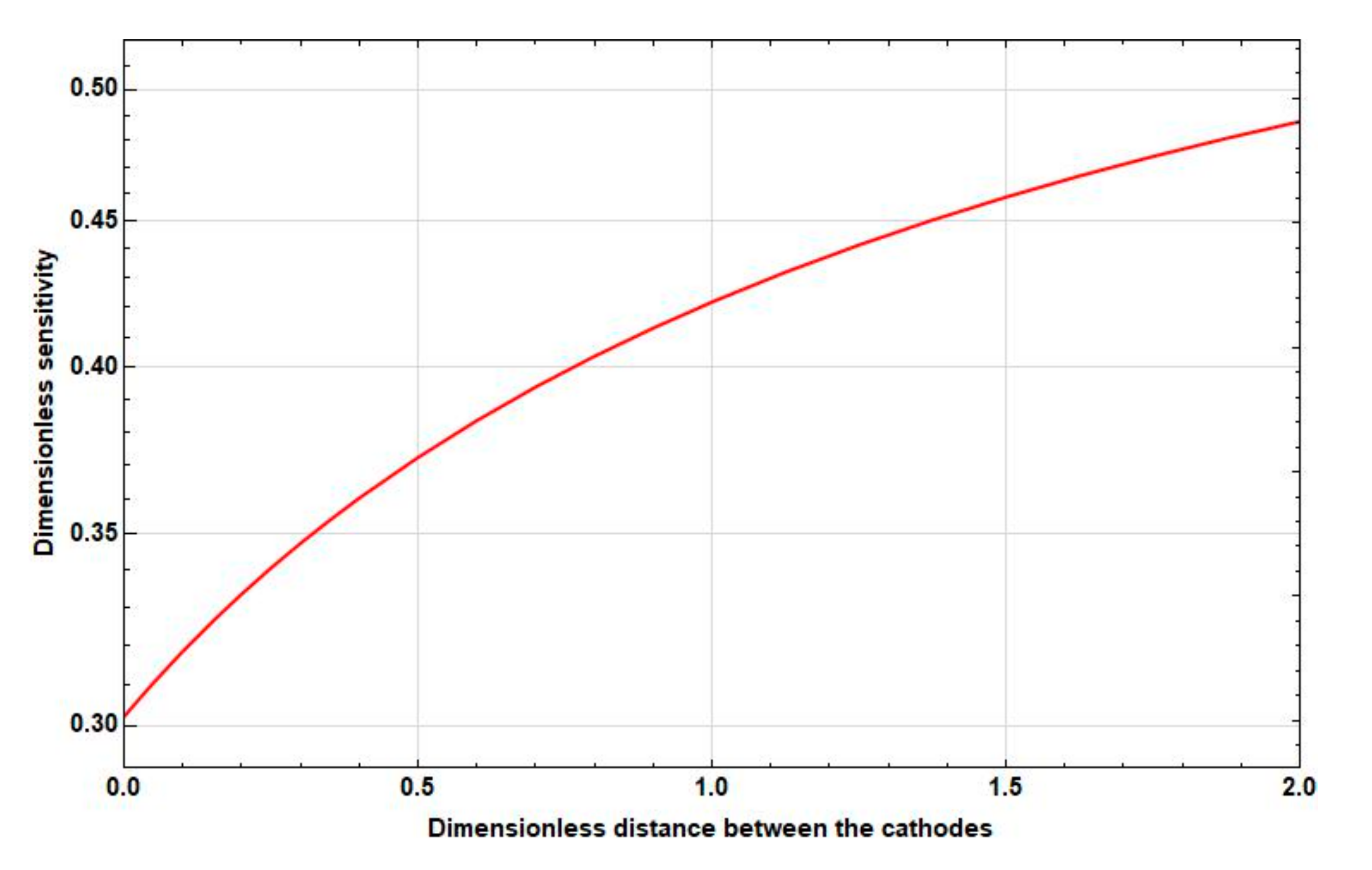
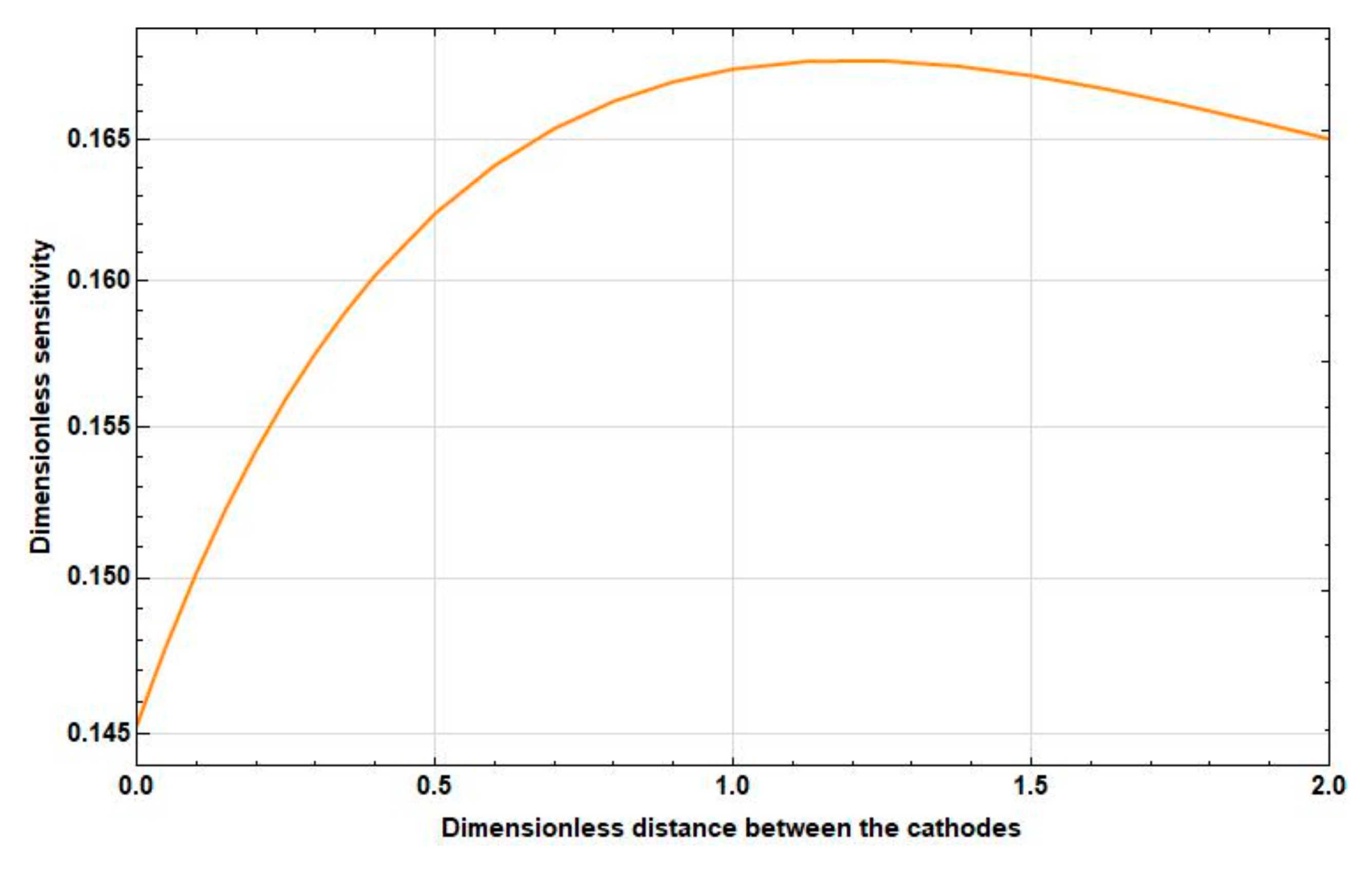
3.3.1. Dependence of the Sensitivity on the Intercathode Distance
- At high frequencies, this increase is negligible and is replaced by a decrease already at small distances between the cathodes ().
- At medium frequencies, the increase goes to the distance between the cathodes . Then, the sensitivity starts to decrease. In this case, the maximum sensitivity is greater than the sensitivity at zero distance between the cathodes at 10–15%.
- At low frequencies, there is a significant, more than , increase in sensitivity in the interval from to , which continues even at .
3.3.2. Dependence of the Sensitivity on the Frequency
3.3.3. The Experimental Validation
4. Discussion
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Larkam, C.W. Theoretical Analysis of the Solion Polarized Cathode Acoustic Linear, Transducer. J. Acoust. Soc. Am. 1965, 37, 664. [Google Scholar]
- Huang, H.; Agafonov, V.; Yu, H. Molecular electric transducers as motion sensors: A review. Sensors 2013, 13, 4581–4597. [Google Scholar]
- Agafonov, V.; Neeshpapa, A.; Shabalina, A. Electrochemical Seismometers of Linear and Angular Motion. In Encyclopedia of Earthquake Engineering SE–403–1; Beer, M., Kougioumtzoglou, I.A., Patelli, E., Au, I.S.-K., Eds.; Springer: Berlin/Heidelberg, Germany, 2015; pp. 944–961. [Google Scholar]
- Koulakov, I.; Jaxybulatov, K.; Shapiro, N.M.; Abkadyrov, I.; Deev, E.; Jakovlev, A.; Kuznetsov, P.; Gordeev, E.; Chebrov, V. Symmetric caldera-related structures in the area of the Avacha group of volcanoes in Kamchatka as revealed by ambient noise tomography and deep seismic sounding. J. Volcanol. Geotherm. Res. 2014, 285, 36–46. [Google Scholar]
- Liu, C.; Hua, Q.; Pei, Y.; Yang, T.; Xia, S.; Xue, M.; Le, B.M.; Huo, D.; Liu, F.; Huang, H. Passive-source ocean bottom seismograph (OBS) array experiment in South China Sea and data quality analyses. Chin. Sci. Bull. 2014, 59, 4524–4535. [Google Scholar]
- Gorbenko, V.I.; Zhostkov, R.A.; Likhodeev, D.V.; Presnov, D.A.; Sobisevich, A.L. Feasibility of using molecular-electronic seismometers in passive seismic prospecting: Deep structure of the Kaluga ring structure from microseismic sounding. Seism. Instrum. 2017, 53, 181–191. [Google Scholar]
- Agafonov, V.M.; Egorov, E.I.; Rice, C.E. Closed-loop frequency MET geophone-operating principles and parameters. In Proceedings of the 72nd European Association of Geoscientists and Engineers Conference and Exhibition 2010: A New Spring for Geoscience. Incorporating SPE EUROPEC 2010, Barcelona, Spain, 14–17 June 2010. [Google Scholar]
- Antonov, A.; Shabalina, A.; Razin, A.; Avdyukhina, S.; Egorov, I.; Agafonov, V. Low-frequency seismic node based on molecular-electronic transfer sensors for marine and transition zone exploration. J. Atmos. Ocean. Technol. 2017, 34, 1743–1748. [Google Scholar]
- Zaitsev, D.; Agafonov, V.; Egorov, E.; Avdyukhina, S. Broadband MET Hydrophone. In Proceedings of the EAGE Conference & Exhibition, Copenhagen, Denmark, 11–14 June 2018. [Google Scholar]
- Egorov, E.V.; Shabalina, A.S.; Zaytsev, D.L.; Velichko, G. Low Frequency Hydrophone for Marine Seismic Exploration Systems. In Proceedings of the 5th International Conference on Sensors Engineering and Electronics Instrumentation Advances (SEIA’2019), Canary Islands, Spain, 25–27 September 2019; pp. 69–70. [Google Scholar]
- Kostylev, D.V.; Bogomolov, L.M.; Boginskaya, N.V. About seismic observations on Sakhalin with the use of molecular- electronic seismic sensors of new type. IOP Conf. Ser. Earth Environ. Sci. 2019, 324, 012009. [Google Scholar]
- Kapustian, N.; Antonovskaya, G.; Agafonov, V.; Neumoin, K.; Safonov, M. Seismic monitoring of linear and rotational oscillations of the multistory buildings in Moscow. 2013, 353–363. [Google Scholar]
- Yudahin, F.G.; Kapustian, N.; Egorov, E.; Klimov, A. An investigation of an external impact conversion into the strained rotation inside ancient boulder structures. In Seismic Behaviour and Design of Irregular and Complex Civil Structures; Springer: Berlin/Heidelberg, Germany, 2012; pp. 3–14. [Google Scholar]
- Zaitsev, D.; Egor, E.; Shabalina, A. High resolution miniature MET sensors for healthcare and sport applications. Proc. Int. Conf. Sens. Technol. ICST 2019, 2018, 287–292. [Google Scholar]
- Liang, M.; Huang, H.; Agafonov, V.; Tang, R.; Han, R.; Yu, H. Molecular Electronic Transducer Based Tilting Sensors. Int. Conf. Micro Electro Mech. Syst. 2020, 2, 765–768. [Google Scholar]
- Agafonov, V.M.; Krishtop, V.G. Diffusion Sensor of Mechanical Signals: Frequency Response at High Frequencies. Russ. J. Electrochem. 2004, 40, 537–541. [Google Scholar]
- Li, G.; Sun, Z.; Wang, J.; Chen, D.; Chen, J.; Chen, L.; Xu, C.; Qi, W.; Zheng, Y. A Flexible Sensing Unit Manufacturing Method of Electrochemical Seismic Sensor. Sensors 2018, 18, 1165. [Google Scholar]
- Deng, T.; Chen, D.; Wang, J.; Chen, J.; He, W. A MEMS Based Electrochemical Vibration Sensor for Seismic Motion Monitoring. Microelectromech. Syst. J. 2014, 23, 92–99. [Google Scholar]
- Yang, D.; Pan, L.; Mu, T.; Zhou, X.; Zheng, F. The fabrication of electrochemical geophone based on FPCB process technology. J. Meas. Eng. 2017, 5, 235–239. [Google Scholar]
- Agafonov, V.; Egorov, E. Influence of the electrical field on the vibrating signal conversion in electrochemical (MET) motion sensor. Int. J. Electrochem. Sci. 2016, 11, 2205–2218. [Google Scholar]
- Agafonov, V.; Egorov, E. Electrochemical accelerometer with DC response, experimental and theoretical study. J. Electroanal. Chem. 2016, 761, 8–13. [Google Scholar]
- Agafonov, A.; Shabalina Ma, D.; Krishtop, V. Modeling and experimental study of convective noise in electrochemical planar sensitive element of MET motion sensor. Sensors Actuators A Phys. 2019, 293, 259–268. [Google Scholar]
- Liang, M.; Huang, H.; Agafonov, V.; Tang, R.; Han, R.; Yu, H. Molecular electronic transducer based planetary seismometer with new fabrication process. In Proceedings of the IEEE International Conference on Micro Electro Mechanical Systems (MEMS), Shanghai, China, 24–28 January 2016; pp. 986–989. [Google Scholar]
- Krishtop, V.G.; Agafonov, V.M.; Bugaev, A.S. Technological principles of motion parameter transducers based on mass and charge transport in electrochemical microsystems. Russ. J. Electrochem. 2012, 48, 746–755. [Google Scholar]
- Krishtop, V.G. Technology and application o f electrochemical motion sensors. Adv. Mater. Proc. 2019, 4, 3–9. [Google Scholar]
- Thomas-Alyea, J.; Newman, K.E. Electrochemical Systems, 3rd ed.; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Xu, Y.; Lin, W.J.; Gliege, M.; Gunckel, R.; Zhao, Z.; Yu, H.; Dai, L.L. A Dual Ionic Liquid-Based Low-Temperature Electrolyte System. J. Phys. Chem. B 2018, 122, 12077–12086. [Google Scholar]
- Agafonov, V. Modeling the Convective Noise in an Electrochemical Motion Transducer. Int. J. Electrochem. Sci. 2018, 13, 11442. [Google Scholar]
- Zhevnenko, D.A.; Vergeles, S.S.; Krishtop, T.V.; Tereshonok, D.V.; Gornev, E.S.; Krishtop, V.G. The simulation model of planar electrochemical transducer. Proceedings of International Conference on Micro- and Nano-Electronics 2016, Zvenigorod, Russia, 3–7 October 2016. [Google Scholar]
- Sun, Z.; Li, G.; Chen, L.; Wang, J.; Chen, D.; Chen, J. High-sensitivity electrochemical seismometers relying on parylene-based microelectrodes. Eng. Mol. Syst. 2017, 1, 714–717. [Google Scholar]









| A, µm | B, µm | C, µm | |
|---|---|---|---|
| Sample 1 | 5 | 20 | 100 |
| Sample 2 | 20 | 20 | 80 |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ryzhkov, M.; Agafonov, V. Modeling of the MET Sensitive Element Conversion Factor on the Intercathode Distance. Sensors 2020, 20, 5146. https://doi.org/10.3390/s20185146
Ryzhkov M, Agafonov V. Modeling of the MET Sensitive Element Conversion Factor on the Intercathode Distance. Sensors. 2020; 20(18):5146. https://doi.org/10.3390/s20185146
Chicago/Turabian StyleRyzhkov, Maksim, and Vadim Agafonov. 2020. "Modeling of the MET Sensitive Element Conversion Factor on the Intercathode Distance" Sensors 20, no. 18: 5146. https://doi.org/10.3390/s20185146
APA StyleRyzhkov, M., & Agafonov, V. (2020). Modeling of the MET Sensitive Element Conversion Factor on the Intercathode Distance. Sensors, 20(18), 5146. https://doi.org/10.3390/s20185146




